Chapter 18 Nasal obstruction and sleep- disordered breathing
1 FUNCTIONAL VALVULAR ANATOMY
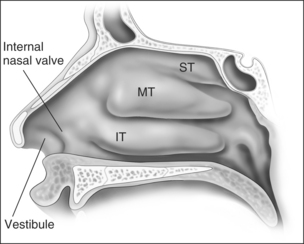
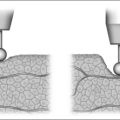
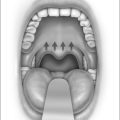
Anatomic models (Figs 18.1 and 18.2) in combination with objective measurements have shown that nasal airflow follows a parabolic curve directed superiorly through the nostril, upwards through the nasal cavity past the turbinates, and posteriorly to the nasopharynx during inspiration.1,2
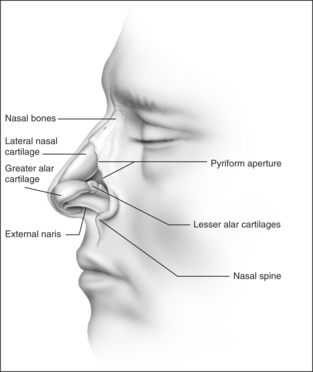
The internal nasal valve area is the narrowest part of the nasal passage and is the major source of nasal resistance in normal patients. Four structures compose the internal nasal valve: the upper lateral cartilage superiorly, nasal septum medially, pyriform aperture inferiorly, and the head of the inferior turbinate posteriorly.3 The narrowest portion of the internal nasal valve area is the region between the septum and the caudal border of the upper lateral cartilage. This structure is approximately 10–15° in Caucasian subjects and can be wider in subjects of African and Asian descent. Patients with internal nasal valve angles of <10° are more prone to internal nasal valve collapse on inspiration.
The external nasal valve is composed of the nares and the nasal vestibule. The nasal vestibule lies just inside the external naris and is located caudal to the internal nasal valve area. The septum is located medially along with the columella, and the alar sidewalls are lateral to the vestibule. Vibrissae are located within the vestibule under the lateral crus and function as a filter for the inspired air. They also serve to direct the air posteriorly into the nasal cavity and to slow the inspired air.4 The nares are composed of the alar margin, the soft tissue triangle, the columella and the nasal sill. The position of the medial crural footplates, the nasal spine and the caudal septum may all change the location and shape of the columella, thereby influencing the nares.
The internal and external nasal valves function together to deliver a smooth air current to the nasal cavities for humidification. The nasal valves can have a greater influence during deep inspiration when the nostrils flare and the diameter of the external nasal valve is increased. The Bernoulli principle is responsible for this effect in that the intraluminal pressure in the internal valve area decreases when the airflow is increased through it. The cartilaginous structure of the nose serves as a counterbalance to this tendency toward internal nasal valve collapse. The investing nasal musculature consists of elevator muscles, including the procerus, levator labii superioris alaeque nasi and anomalous nasi. The depressor muscles include the alar nasalis and the depressor septi nasi while the compressor muscles include the transverse nasalis and the compressor narium minor. There are also minor dilator muscles. The alar muscles contract and dilate the internal nasal valve area in order to keep the lumen open. Throughout normal nasal function, the internal nasal valve area should remain unchanged,5 although collapse demonstrable only during forced inspiration does not require intervention.6
The otolaryngologist should be aware of those characteristics that contribute to nasal obstruction. Those patients who have pre-existing or traumatic septal deviation can suffer nasal obstruction. Insufficient support of the alar rim and alar lobule may lead to external valve collapse on inspiration. Short nasal bones and a long upper cartilaginous vault; narrow, projecting nose; slit-like nostrils; exaggerated supra-alar creases; visible pinching of the lateral wall with inspiration; thin cartilages and skin; and cephalically positioned lateral crura which provide minimal support to the alar margins are all attributes that the surgeon should consider when planning surgical correction.7
2 NASAL OBSTRUCTION AND SDB
Case reports and case series as early as the 1890s showed an association between the nasal valve and sleep-disordered breathing (SDB). Today, we are all familiar with the fact that nasal obstruction and SDB often co-exist. Among patients referred to sleep clinics, studies have shown that subjective or objective nasal obstruction is a risk factor for SDB.2–4 Approximately 15% of patients with SDB also have nasal obstruction,8 but there is no correlation between the severity of obstructive sleep apnea and an objective measure of nasal resistance – acoustic rhinometry.9 Lofaso analyzed cephalometrics, body mass index and posterior rhinometry and showed that daytime nasal obstruction is an independent risk factor for obstructive sleep apnea.10 Others have explored the relationship between nasal obstruction and SDB showing that there is no association between SDB severity and the severity of the nasal obstruction.11–15
Beyond a simple association, however, nasal obstruction often plays a major role in SDB and its treatment. Nasal obstruction itself may contribute substantially to SDB by being an independent source of airway obstruction. The evidence supporting the role of nasal obstruction in SDB comes from a variety of sources. Normal patients have been shown to experience sleep disturbances – including sleep stage disruption as well as new-onset snoring and even mild obstructive sleep apnea – after acute, complete bilateral nasal obstruction such as abrupt occlusion of their nose.16 One study demonstrated that SDB was related to nasal cross-sectional area objectively assessed using acoustic rhinometry and both titrated nasal continuous positive airway pressure (CPAP) and the Respiratory Disturbance Index (RDI); however, these associations were only present in patients with normal Body Mass Index (BMI).17
Besides the simple effect of nasal obstruction on breathing patterns during sleep, the nose is a major conduit for treatment of obstructive sleep apnea with positive airway pressure therapy. Nasal obstruction can therefore interfere with treatment. Lafond and Series showed that nasal obstruction can increase the required continuous positive airway pressure in obstructive sleep apnea patients.18 They induced an increased nasal resistance with histamine and demonstrated that this increased flow limitation in two commonly used CPAP devices. Zozula and Rosen also showed that nasal obstruction can affect positive airway pressure therapy tolerance and adherence in their study examining and classifying reasons for CPAP non-compliance.19
2.1 INCREASED AIRWAY RESISTANCE
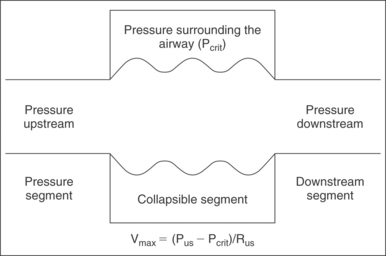
Applying the Starling model to the pharynx (Fig. 18.3), maximal airflow is based on three factors: upstream pressure, resistance in the upstream segment, and the extraluminal pressure. Increased nasal resistance is an increase in resistance of the upstream segment and therefore reduces flow through the collapsible tube, the pharynx. The maximal velocity of air through the nose is proportional to the pressure surrounding the tube (Pcrit) subtracted from the upstream pressure (Pus) and is inversely proportional to the resistance of the upstream segment (Rus).
From a teleological standpoint, nasal resistance functions as an upstream resistor of nasal airflow. In this capacity, nasal resistance matches the impedance of upper and lower airways to prolong inspiration and expiration. On expiration, this prolongation has the beneficial effect of improving pulmonary compliance and increasing gas exchange. On inspiration, however, as suggested by the Starling model, nasal resistance can augment pharyngeal resistance to promote upper airway collapse. The decline in tidal volume and minute ventilation has been shown in several studies.20,21
2.2 UNSTABLE ORAL BREATHING
The second theory describing nasal obstruction and its connection to SDB is based on the instability of oral breathing compared with nasal breathing. With nasal obstruction, patients open their mouth. This reflexive compensation contributes to SDB by narrowing the pharyngeal lumen in two ways. First, the chin and rest of the mandible move posteriorly and inferiorly with mouth opening to displace the tongue in that direction. This directly narrows the pharyngeal airway. Second, this maneuver decreases the length and tension of the muscles surrounding the airway, and the compliance of the pharynx increases.22 It has been shown that opening of the mouth to provide a 1.5 cm separation between incisors moves the angle of the mandible posteriorly 1 cm, a substantial change.23 In fact, two studies have demonstrated that oral breathing is associated with increased airway resistance during sleep compared to nasal breathing.24,25
2.3 IMPAIRED NASAL REFLEXES
The third mechanism focuses on important nasal reflexes. Nasal breathing stimulates ventilation, and the best evidence of this in normal patients comes from a study showing that not only is minute ventilation higher with nasal compared to oral breathing, but also that augmented nasal airflow increases minute ventilation.26 Furthermore, the evidence that this response is due to neural regulation in humans comes from studies which show that application of topical anesthesia in the nose and nasopharynx specifically increases nasal and pharyngeal resistance,27 and that topical nasal anesthesia actually leads to increased SDB – whether this is measured as the number of apneas, apnea duration, or a decrease in genioglossus muscle activity.28
3 EFFECTS OF TREATMENT
In contrast, isolated treatment of nasal obstruction does not successfully treat obstructive sleep apnea for most patients. Verse and Pirsig performed a literature review that showed that medical treatment produced resolution of obstructive sleep apnea in 9% of patients and surgical treatment in 18%.29 Nasal corticosteroids can produce small changes in snoring and the Apnea/Hypopnea Index, but the degree of improvement varies widely.30 A few studies specifically looking at surgical treatment showed that there was minimal to no change in Apnea/Hypopnea Index, but some patients experienced improvements in sleep quality and symptoms of daytime somnolence.31
Because the improvement in SDB associated with isolated treatment of nasal obstruction varies among patients, Series et al. examined 14 patients with nasal obstruction and SDB (Apnea/Hypopnea Index >5) who were treated for nasal obstruction alone. Of the seven patients who had a normal Apnea/Hypopnea Index postoperatively, six out of seven of the patients with good outcomes had mild SDB (Apnea/Hypopnea Index 5–15) at the beginning of the study and had normal cephalometric imaging. This was among the first studies to identify factors that may be associated with better outcomes after nasal surgery designed to treat SDB.32
Finally, there is evidence showing that nasal treatments can facilitate the treatment of SDB by decreasing the magnitude of the positive airway pressure necessary to treat SDB. Friedman showed a reasonable improvement (9.3–6.7 cm water) for septoplasty with or without inferior turbinate reduction,7 and a smaller effect (8.6–8.0 cm water) was seen with an external nasal valve dilator device.33 Two additional studies have shown that nasal surgery can increase the adherence to continuous positive airway pressure devices.34,35
1. Tarabichi M, Fanous N. Finite element analysis of airflow in the nasal valve. Arch Otolaryngol Head Neck Surg. 1993;119(6):638-642.
2. Proctor DF. The upper airways. I. Nasal physiology and defense of the lungs. Am Rev Respir Dis. 1977;115:97-129.
3. Kasperbauer JL, Kern EB. Nasal valve physiology. Implications in nasal surgery. Otolaryngol Clin North Am. 1987;20:699-719.
4. Cottle MH. Structures and function of the nasal vestibule. Arch Otolaryngol Head Neck Surg. 1955;62:173.
5. Cole P. The four components of the nasal valve. Am J Rhinol. 2003;17:107-110.
6. Goode RL. Surgery of the incompetent nasal valve. Laryngoscope. 1985;95:546-555.
7. Constantian MB. Four common anatomic variants that predispose to unfavorable rhinoplasty results: a study based on 150 consecutive secondary rhinoplasties. Plast Reconstr Surg. 2000;105:316-331.
8. Mayer-Brix J, Muller-Marschhausen U, Becker H, Peter JH. How frequent are pathologic ENT findings in patients with obstructive sleep apnea syndrome? HNO. 1989;37(12):511-516.
9. Young T, Finn L, Kim H. Nasal obstruction as a risk factor for sleep disordered breathing. J Allergy Clin Immunol. 1997;99:S757-S762.
10. Lofaso F, Coste A, d’Ortho M, et al. Nasal obstruction as a risk factor for sleep apnea syndrome. Eur Respir J. 2000;16:639-643.
11. Blakley BW, Mahowald M. Nasal resistance and sleep apnea. Laryngoscope. 1987;97:752-754.
12. Miljeteig H, Hoffstein V, Cole P. The effect of unilateral and bilateral nasal obstruction on snoring and sleep apnea. Laryngoscope. 1992;102:1150-1152.
13. Atkins M, Taskar V, Clayton N, Stone P, Woodcock A. Nasal resistance in obstructive sleep apnea. Chest. 1994;105:1133-1135.
14. Riechelmann H, Furst G. Medical ENT diagnosis and therapy of sleep-associated respiratory disorders with obstruction of the upper airways. Pneumologie. 1995;49:523-527.
15. Liistro G, Rombaux P, Belge C, Dury M, Aubert G, Rodenstein DO. High Mallampati score and nasal obstruction are associated risk factors for obstructive sleep apnea. Eur Respir J. 2003;21:248-252.
16. Olsen KD, Kern EB, Westbrook PR. Sleep and breathing disturbance secondary to nasal obstruction. Otolaryngol Head Neck Surg. 1981;89:804-810.
17. Morris LG, Burschtin O, Lebowitz RA, Jacobs JB, Lee KC. Nasal obstruction and sleep-disordered breathing: a study using acoustic rhinometry. Am J Rhinol. 2005;19(1):33-39.
18. Lafond C, Series F. Influence of nasal obstruction on auto-CPAP behaviour during sleep in sleep apnoea/hypopnea syndrome. Thorax. 1998;53:780-783.
19. Zozula R, Rosen R. Compliance with continuous positive airway pressure therapy: assessing and improving treatment outcomes. Curr Opin Pulm Med. 2001;7(6):391-398.
20. Iber C, Berssenbrugge A, Skatrud JB, Dempsey JA. Ventilatory adaptations to resistive loading during wakefulness and non-REM sleep. J Appl Physiol. 1982;52(3):607-614.
21. Henke KG, Sullivan CE. Effects of high-frequency pressure waves applied to upper airway on respiration in central apnea. J Appl Physiol. 1992;73(3):1141-1145.
22. Basner RC, Simon PM, Schwartzstein RM. Breathing route influences upper airway muscle activity in awake normal adults. J Appl Physiol. 1989;66:1766-1771.
23. Kuna ST, Remmers JE. Neural and anatomic factors related to upper airway occlusion during sleep. Med Clin North Am. 1985;69(6):1221-1242.
24. Olsen KD, Suh KW, Staats BA. Sleep and breathing disturbance secondary to nasal obstruction. Otolaryngol Head Neck Surg. 1981;89(5):804-810.
25. Meurice JC, Marc I, Carrier G, Series F. Effects of mouth opening on upper airway collapsibility in normal sleeping subjects. Am J Respir Crit Care Med. 1996;153(1):255-259.
26. McNicholas WT, Coffey M, Boyle T. Effects of nasal airflow on breathing during sleep in normal humans. Am Rev Respir Dis. 1993;147(3):620-623.
27. White D, Cadieux R, Lomard R, et al. The effects of nasal anesthesia on breathing during sleep. Am Rev Respir Dis. 1985;132:972-975.
28. Garpestad E, Basner RC, Ringler J. Phenylephrine-induced hypertension acutely decreases genioglossus EMG activity in awake humans. J Appl Physiol. 1992;72:110-115.
29. Verse T, Pirsig W. Impact of impaired nasal breathing on sleep-disordered breathing. Sleep Breath. 2003;7(2):63-76.
30. Kiely JL, Nolan P, McNicholas WT. Intranasal corticosteroid therapy for obstructive sleep apnoea in patients with co-existing rhinitis. Thorax. 2004;59(1):50-55.
31. Friedman M, Tanyeri H, Lim JW, Landsberg R, Vaidyanathan K, Caldarelli D. Effect of improved nasal breathing on obstructive sleep apnea. Otolaryngol Head Neck Surg. 2000;122(1):71-74.
32. Series F, St Pierre S, Carrier G. Surgical correction of nasal obstruction in the treatment of mild sleep apnoea: importance of cephalometry in predicting outcome. Thorax. 1993;48(4):360-363.
33. Schonhofer B, Kerl J, Suchi S, Kohler D, Franklin KA. Effect of nasal valve dilation on effective CPAP level in obstructive sleep apnea. Respir Med. 2003;97(9):1001-1005.
34. Powell NB, Riley RW, Guilleminault C, Murcia GN. Obstructive sleep apnea, continuous positive airway pressure, and surgery. Otolaryngol Head Neck Surg. 1988;99(4):362-369.
35. Series F, St Pierre S, Carrier G. Effects of surgical correction of nasal obstruction in the treatment of obstructive sleep apnea. Am Rev Respir Dis. 1992;146(5 Pt 1):1261-1265.