Nasal Airway Considerations in the Evaluation and Treatment of Dentofacial Deformities
• Frequent Causes of Chronic Obstructive Nasal Breathing
• The Effects of Respiratory Pattern and Jaw Posture on Maxillofacial Growth
• Simultaneous Management of Chronic Obstructed Nasal Breathing and the Presenting Dentofacial Deformity
• Approaching the Nasal Airway Through Le Fort I Down-Fracture
• Management of Residual Postoperative Nasal Obstruction
• Effects of Maxillary Arch Expansion and Nasal Floor Recontouring on the Airway
• Comprehensive Approach to Chronic Nasal Airway Obstruction
“Irregularities of the teeth being so frequently associated with some pathologic obstruction of the nasal passages or nasopharynx, this fact should ever be present in the operator’s mind and suitable examination being made; and in case [as is often found] the oral deformity be complicated by the presence of hypertrophied faucal tonsils, adenoid hypertrophies in the vault of the pharynx, or obstruction of the nasal passages, the orthodontist’s work can only be made complete by the assistance of a rhinologist and laryngologist.”2
“The nasal fossae are bounded by the maxillary bones and by bones attached to the maxillae; therefore deformities of maxillary bones are bound to influence the size, shape and patency of the nasal fossae. Nasal obstruction may be due directly to deformity or to lack of development of the maxillae. Any [intranasal] bony [obstructions] are removed [surgically] until ample breathing space is established. If nasal obstruction is due simply to lack of size of the nasal fossae and there are erupted [permanent] molar teeth [on each side], then the treatment consists of placing a jackscrew across the mouth from one upper molar to the other. As the screw is spread, the intermaxillary suture opens, the maxillae separate, and the nasal obstruction is relieved.”10
Frequent Causes of Chronic Obstructive Nasal Breathing
Nasal septoplasty is a generic descriptor of the surgical correction of septal thickenings and deflections frequently carried out to improve nasal airflow and sinus drainage (Figs. 10-1 and 10-2).3,7,20,30,52,53,57,75,80,88,89,91,93,94 Enlarged inferior turbinates also commonly obstruct nasal breathing and interfere with sinus drainage (Fig. 10-3). Hypertrophic inferior turbinates that are only minimally responsive to medical treatment are best managed by partial (surgical) reduction.8,23,77 A tight nasal inlet or constricted pyriform apertures occur in conjunction with a narrow maxillary arch (Fig. 10-4).58,86,90,95 An elevated floor of the nose is a frequent finding in the presence of anterior vertical maxillary excess (i.e., long face growth pattern) (Fig. 10-5).63,81,112 A scarred nasal vestibule seen with a repaired cleft nasal malformation may be another cause of persistent nasal obstruction (Fig. 10-6). Nasal septal deformities, inferior turbinate enlargement, a tight nasal inlet, and an elevated nasal floor all commonly coexist with maxillary deformities (Figs. 10-7 and 10-8).1,12–14 Favorable access to the nasal septum, the inferior turbinates, the pyriform apertures, and the nasal floor to correct these airway obstructions and deformities is possible through the Le Fort I down-fracture osteotomy that is commonly used in orthognathic surgery (Figs. 10-9, 10-10, and 10-11).82–85
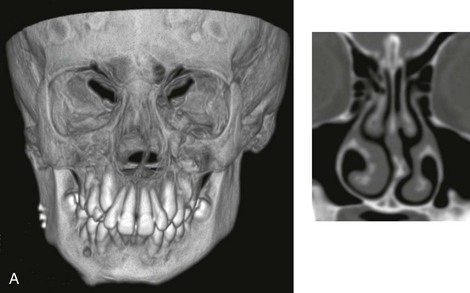
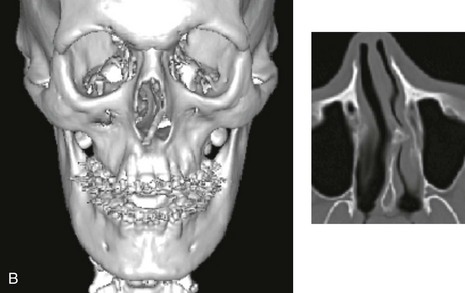
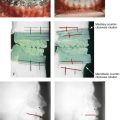
Figure 10-1 A, Three-dimensional maxillofacial and coronal section computed tomography scan views of a child with mandibular deficiency during late mixed dentition. B, Three-dimensional maxillofacial and coronal section computed tomography scan views of a teenager with maxillary deficiency and relative mandibular excess. For both patients, the scans indicate a deviated nasal septum in combination with enlarged inferior turbinates, with an expected increase in nasal airway resistance and diminished breathing space.
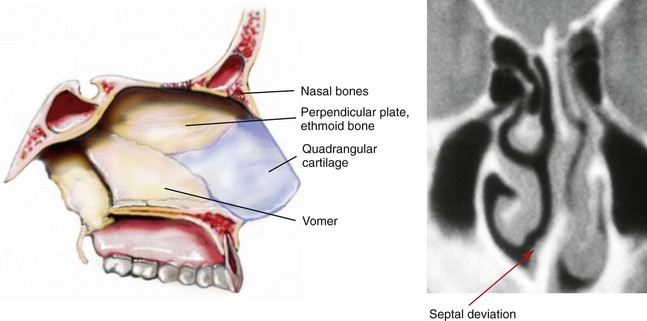
Figure 10-2 Sagittal midfacial illustration and coronal section computed tomography scan view of the skeletal (vomer and perpendicular plate of ethmoid) and cartilage (quadrangular) components of the septum of the nose. The computed tomography scan view indicates deviation and buckling of the septum, which obstructs the left side of the nose. Modified from an original illustration by Bill Winn.
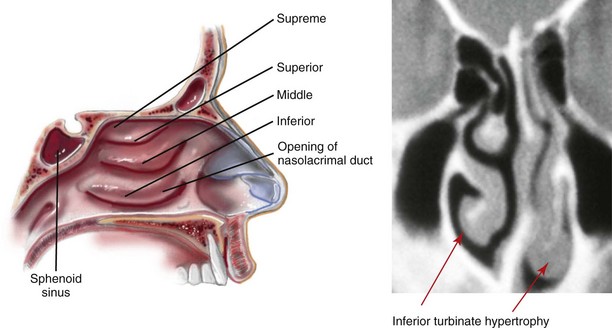
Figure 10-3 Sagittal midfacial illustration and coronal section computed tomography scan showing an axial view of the turbinates. The CT scan view indicates asymmetrical inferior turbinate hypertrophy. Modified from an original illustration by Bill Winn.
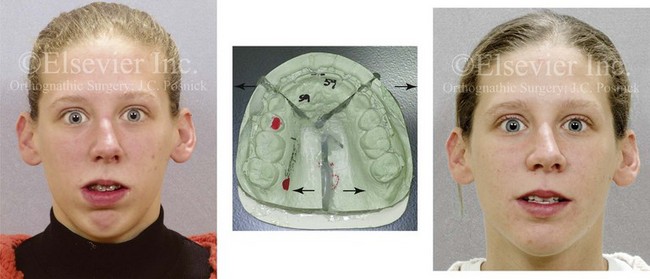
Figure 10-4 A woman in her early 20s shown before and after orthognathic surgery for a long face growth pattern (see Fig. 21-5). Reconstruction also included segmental maxillary osteotomies with arch expansion. This approach also widens the nasal cavity to decrease intranasal airway resistance and improve breathing.
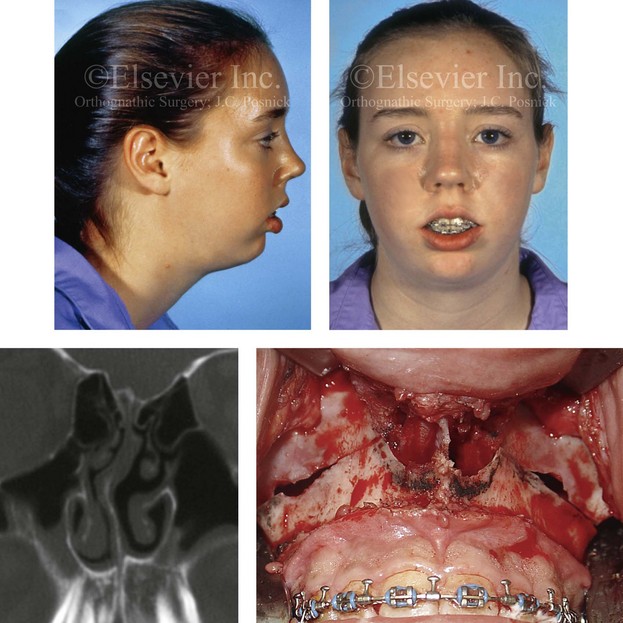
Figure 10-5 A typical teenager with a long face growth pattern demonstrates physical findings that are consistent with a lifelong history of obstructed nasal breathing (see Fig. 7-2). These findings include septal deviations, inferior turbinate hypertrophy, a narrow (tight) nasal aperture, and an elevated nasal floor.
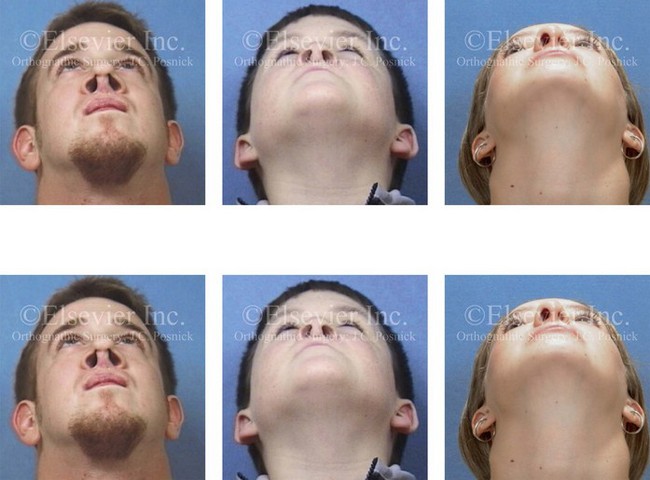
Figure 10-6 Individuals born with cleft lips and palates who have undergone primary repair and secondary reconstruction may present with constrictions of both the internal and external nasal valves. This may be worse as a result of the surgical maneuvers that were previously carried out. Two children with bilateral cleft lip and palate and one with unilateral cleft lip and palate are shown to demonstrate this point. Nasal soft-tissue procedures—including “columella lengthening” in the cases of the bilateral cleft lips and palates and the overzealous resectioning of nasal soft tissues in the case of the unilateral cleft lip and palate—were performed. In all three cases, the nasal valves are constricted (i.e., there is airway obstruction), and the aesthetics are suboptimal.
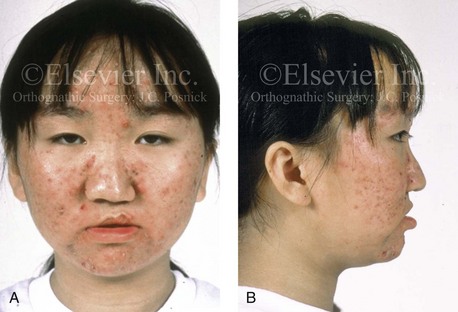
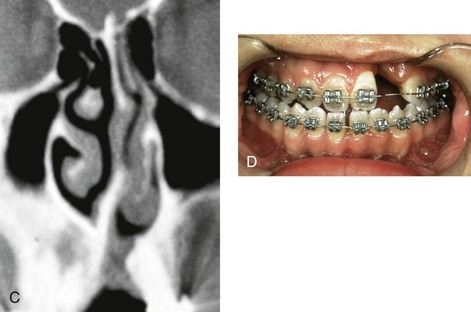
Figure 10-7 A and B, A teenage girl with unilateral cleft lip and palate shows multiple causes of nasal obstruction. These include—as shown in C and D—septal deviations, inferior turbinate hypertrophy, nasal aperture narrowing, and nasal floor deformities, including a residual unrepaired alveolar cleft.
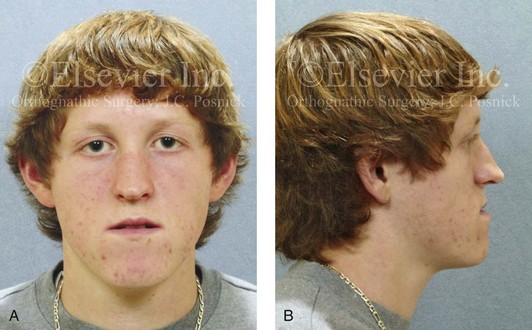
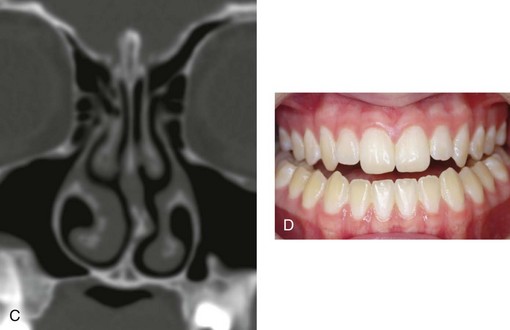
Figure 10-8 A and B, A teenager with a maxillary deficiency and a relative mandibular excess growth pattern. C and D, The physical findings of septal deviations, inferior turbinate hypertrophy, and a narrow (tight) nasal aperture are consistent with the patient’s history of nasal airway obstruction.
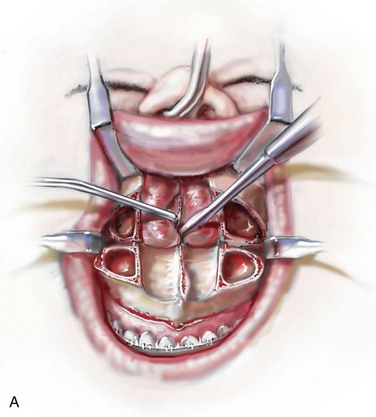
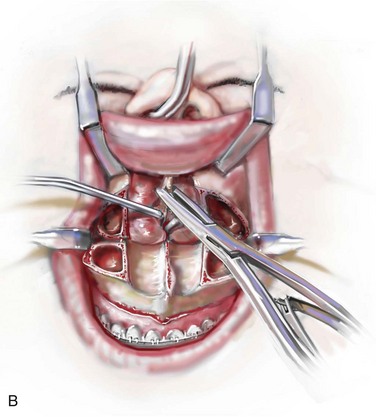
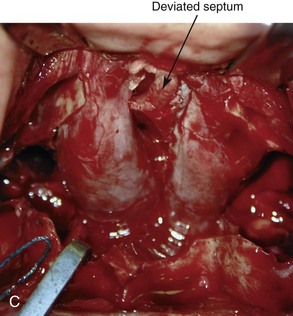
Figure 10-9 Illustrations and intraoperative view of a deformed nasal septum seen through a Le Fort I down-fracture. This is a demonstration of the technique used to carry out the submucous resection of septal bone and cartilage. When indicated on the basis of the patient’s history and clinical examination, attention is turned to the removal of the deviated portions of the septum of the nose. A, With an elevator, the submucosal dissection of the cartilaginous (quadrangular cartilage) and bony (vomer and perpendicular plate of ethmoid) septum is carried out. B, The deviated and thickened aspects of the cartilaginous and bony septum are resected with a rongeur. The more anterior components of the cartilaginous septum that provide needed support to the nasal dorsum and tip are not disturbed. This includes at least 1 cm of the dorsal and caudal cartilage. C, An intraoperative view through a Le Fort down-fracture shows a deviated septum.
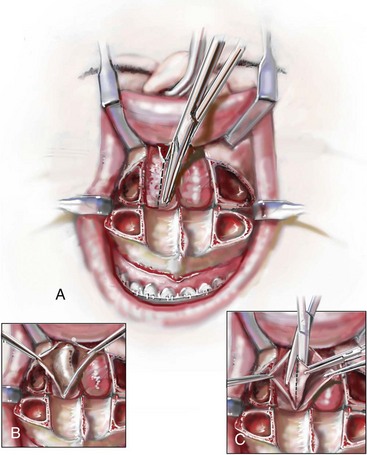
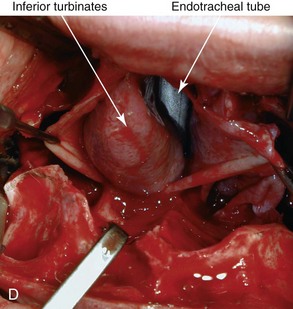
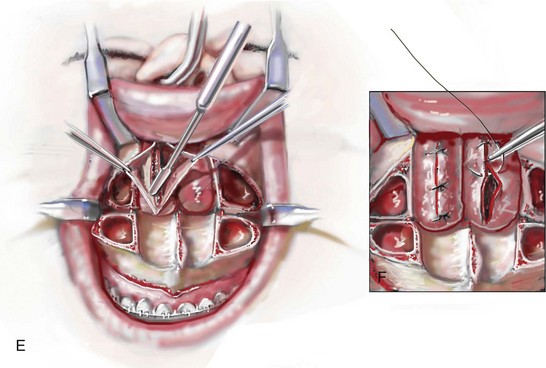
Figure 10-10 Illustrations and intraoperative view of inferior turbinates seen through a Le Fort I down-fracture. This is a demonstration of the technique used to carry out a partial turbinate reduction. A, An incision parallel and just below each inferior turbinates is completed through the nasal mucosa. B, The elevated nasal mucosa flaps expose the enlarged inferior turbinate. C, The resection of the inferior aspect of the hypertrophic inferior turbinate is completed with the use of a straight (Mayo) scissors. D, An intraoperative view of the elevated nasal flaps. The endotracheal tube is also visualized. E, After the partial resection of each inferior turbinate, the raw surfaces are cauterized. F, The nasal mucosa flaps are then closed with interrupted resorbable sutures.
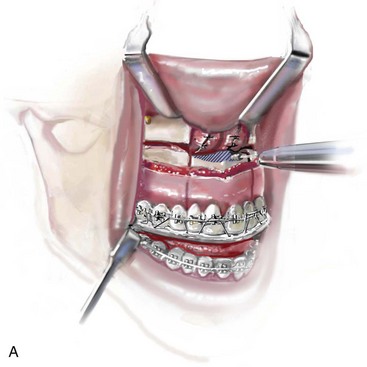
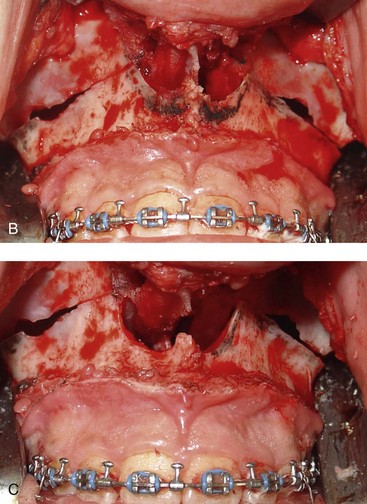
Figure 10-11 Illustration and intraoperative views of the nasal rims and floor seen through a Le Fort I down-fracture to demonstrate the techniques of rim, nasal floor, and anterior nasal spine recontouring with the use of a bur on a rotary drill. A, Illustration of recontouring with rotary drill. B and C, Intraoperative views before and after recontouring, respectively.
Maxillofacial literature has traditionally addressed aspects of nasal obstruction, nasal anatomy, and sinus drainage in the patient who is undergoing orthognathic surgery, but often only in subjective and limited ways.8,18,24,27,32,33,36,38,55,56,71,73,74,76,103,104,107 Authors traditionally discuss the effects of Le Fort I osteotomies on nasal morphology (i.e., aesthetics) and occasionally recommend adjunctive soft-tissue techniques in the hopes of limiting suboptimal aesthetics (e.g., the alar cinch stitch).37 Little consideration is given to either baseline upper airway findings or how the maxillary surgery may affect long-term nasal breathing. Warren and colleagues discusses issues of nasal airway changes after Le Fort I osteotomy with maxillary impaction, advancement, and transverse widening.108 They used a pressure flow meter to assess nasal airway breathing before and after maxillary osteotomies.21,29 Turvey and colleagues recognized preexisting nasal obstruction in many of their orthognathic patients.103 They suggested methods of avoiding adverse effects on nasal airway resistance that may result from Le Fort I maxillary osteotomy procedures.104 Moses and coworkers reported about a series of patients who were treated for nasal obstruction and sinus drainage difficulties after Le Fort I maxillary procedures were carried out for long face growth patterns (e.g., vertical maxillary excess with anterior open bite).71 They concluded that Le Fort I impaction frequently aggravates preexisting nasal airway obstruction and sinus disease. Williams and colleagues examined the nasal airway function (breathing) in a consecutive series of subjects (n = 50) before and 5 months after undergoing (1) Le Fort I osteotomy with advancement and vertical lengthening (2) maxillary segmentation with transverse expansion (14 of 50 subjects, 28%); and (3) recontouring of the nasal floor and pyriform rims with a rotary drill. Despite these favorable nasal airway surgical maneuvers, when ignoring potential pathology of the septum (e.g., buckling and deviation) and the inferior turbinates (e.g., hypertrophy/enlargement), a full 20% of their study subjects experienced a worsening of their nasal airway function after surgery.113a
Unfortunately, in the individual with a dentofacial deformity, a methodic approach to the evaluation and management of chronic nasal airway obstruction at the time of orthognathic surgery is often not undertaken. We suggest that any upper airway obstructions be assessed by the orthognathic surgeon with the same vigor as the effects of the presenting jaw deformities on malocclusion are evaluated (Figs. 10-12 and 10-13).51,83–85,87


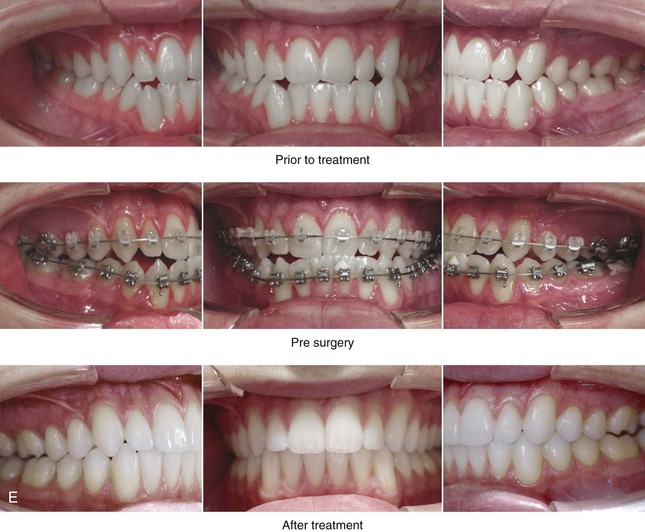
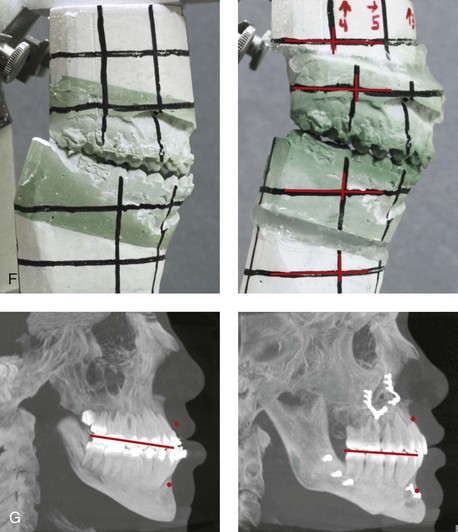
Figure 10-12 A 20-year-old man with a combined long face growth pattern (environmental) and a right side hemimandibular elongation (hereditary). There is an asymmetrical Class III negative overjet malocclusion. The patient had a lifelong history of obstructed nasal breathing with documented septal deviation, hypertrophic inferior turbinates, and an elevated nasal floor. He was referred to this surgeon for evaluation, and he agreed to a comprehensive orthodontic and orthognathic approach. With the relief of dental compensation, the patient’s surgery included a maxillary Le Fort I osteotomy (horizontal advancement and vertical shortening); bilateral sagittal split ramus osteotomies (mandibular straightening and asymmetrical correction); osseous genioplasty (vertical shortening); and septoplasty, inferior turbinate reduction, and nasal floor recontouring. A, Frontal views in repose before and after reconstruction. B, Frontal views with smile before and after reconstruction. C, Oblique facial views before and after reconstruction. D, Profile views before and after reconstruction. E, Occlusal views before retreatment, after orthodontic decompensation, and after treatment. F, Articulated dental casts that indicate analytic model planning. G, Lateral cephalometric radiographs before and after reconstruction.

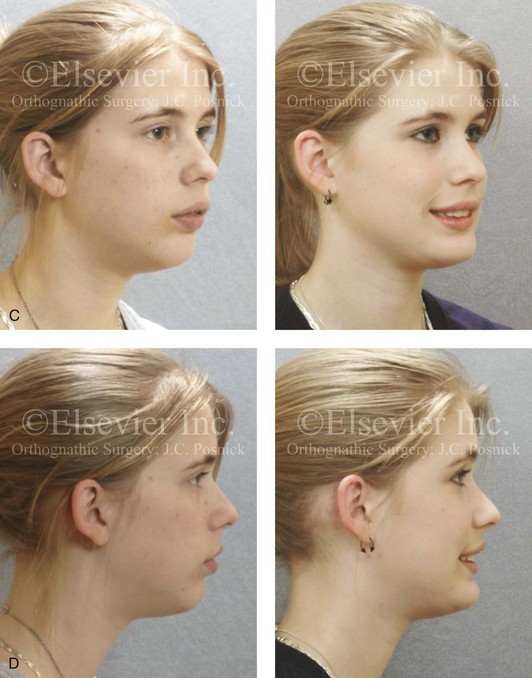
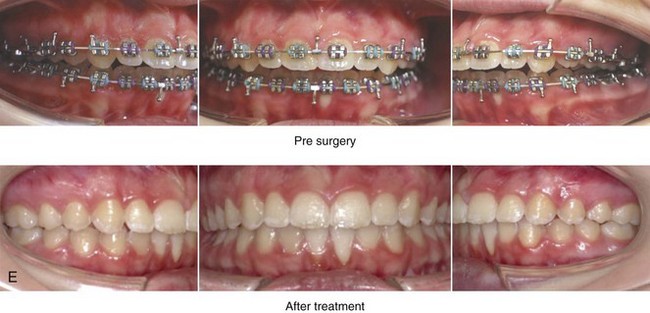
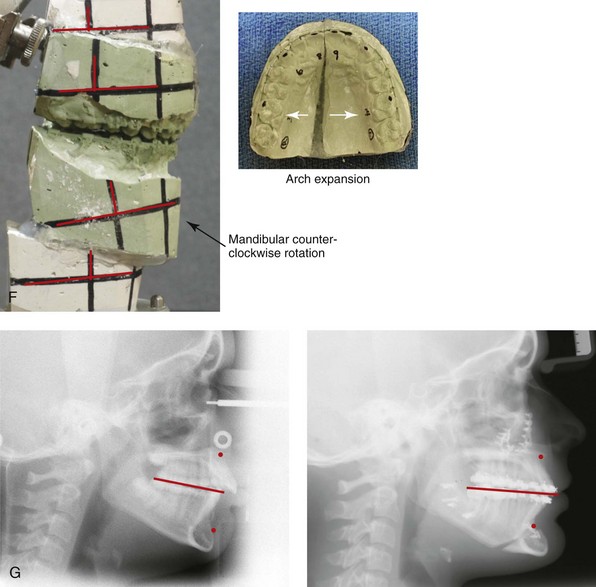
Figure 10-13 A teenage girl with a long face growth pattern is treated with a comprehensive orthodontic and surgical approach. After orthodontic (dental) decompensation, her surgical procedures included a maxillary Le Fort I osteotomy in segments (vertical intrusion, horizontal advancement, and arch expansion) bilateral sagittal split osteotomies of the mandible (horizontal advancement and counterclockwise rotation); oblique osteotomy of the chin (vertical shortening and horizontal advancement); and septoplasty, inferior turbinate reduction, and recontouring of the nasal floor. A, Frontal views in repose before and after treatment. B, Frontal views with smile before and after treatment. C, Oblique facial views before and after treatment. D, Profile views before and after treatment. E, Occlusal views with orthodontics in progress and then after surgery. F, Articulated dental casts that indicate analytic model planning. G, Lateral cephalometric views before and after treatment.
The Effects of Respiratory Pattern and Jaw Posture on Maxillofacial Growth
The link between an open mouth or oral breathing pattern and maxillofacial growth has been documented in the literature.4,5,11,17,24,25,28,31,41,43,45–48,59–66,96,97,105,106,109,110 There has been both animal experimental and human clinical research to support a link between oral respiration, open mouth posture, and the development of maxillomandibular disharmonies. With the use of rhesus monkeys as an experimental model, Harvold and colleagues completely blocked the nares of newborn monkeys.45–48 The researchers observed consistent facial morphologic changes that gradually occurred with growth in the presence of a constant open mouth and tongue protrusive mandibular posture. The research of this group documented that a constantly open mouth (i.e., clockwise rotation of the mandible) in a growing rhesus monkey was followed by the vertical lengthening of the maxilla. The mandibular inferior border then gradually steepens, with a resulting obtuse gonial angle. Through years of primate laboratory research, Harvold and coworkers confirmed that there are significant correlations between alterations in respiratory patterns (i.e., forced mouth breathing), mandibular (open mouth) posture, tongue protrusion, and characteristic maxillomandibular dysmorphic growth (i.e., long face growth pattern).45–48
In human children who have significant nasal obstruction and a resulting open mouth posture, clinical observational studies document a similar downward and backward rotation of the mandible with hypereruption of the maxillary dentition.59–65 Over several years, the affected children develop what we now recognize as a long face growth pattern. This is documented as a vertically long and horizontally retrusive maxillomandibular complex. The term adenoid facies was coined to describe the child who presents with a long, narrow, flat face; protruding teeth; and lips that are widely separated at rest. These features are often accompanied by an anterior open-bite malocclusion and lip strain when attempting mouth closure. To better understand the concept, Linder-Aronson and Woodside completed a study in which they analyzed the faces of children with chronic nasal obstruction who were scheduled to undergo tonsillectomies and adenoidectomies at a pediatric hospital in Stockholm, Sweden.63 They documented a tendency toward a long lower anterior facial height in this group of children. Interestingly, they found that, after the adenoids were removed and with enough time, there was at least some improvement in the children’s facial morphology. Unfortunately, the structure of these long faces did not return to normal. With or without adenoidectomy being carried out during childhood, many of these children reach adolescence with persistent nasal airway obstruction in combination with significant jaw dysmorphology and malocclusion (i.e., the long face growth pattern). It is our recommendation that, by adolescence, all aspects (i.e., airway obstruction, skeletal dysmorphology, and malocclusion) be simultaneously addressed to improve function in the head and neck (e.g., breathing, swallowing, speech, chewing, lip closure) and to enhance facial aesthetics.83–85
Simultaneous Management of Chronic Obstructed Nasal Breathing and the Presenting Dentofacial Deformity
Posnick and colleagues conducted a clinical study with the use of the Nasal Obstruction Symptom Evaluation (NOSE) survey questionnaire to prospectively assess a consecutive series of patients (N = 43) who reported both chronic nasal obstruction and the presence of a significant dentofacial deformity with malocclusion that involved at least the maxilla.83 The NOSE questionnaire is considered to be a useful marker of symptomatic nasal airway obstruction.42,98–101 Each study subject was documented to have septal deviation and inferior turbinate enlargement that were consistent with airway symptoms. Any presenting physical findings of a tight nasal inlet or an elevated nasal floor were not specifically studied but were clinically addressed in each patient, when indicated. Each study patient simultaneously underwent septoplasty, the reduction of the inferior turbinates, and the surgical correction of the presenting jaw deformity that included, at minimum, a Le Fort I osteotomy. Septoplasty and inferior turbinate reduction were performed at the time of Le Fort I osteotomy through exposure provided by the down-fracture. The consecutive series of patients (N = 43) who met the inclusion criteria were given the NOSE survey questionnaire before they underwent surgery. The NOSE questionnaire was completed in each patient 3 months after surgery and again at least 6 months after surgery.
Approaching the Nasal Airway Through Le Fort I Down-Fracture ( Video 1 and 2)
Video 1 and 2)
A Le Fort I osteotomy provides excellent access to the nasal septum, the inferior turbinates, the pyriform rims, the nasal floor, the anterior nasal spine, the maxillary sinus cavities, and the ostium, thereby allowing for the correction of any presenting nasal airway obstruction or maxillary sinus pathology at the time of down-fracture (see Figs. 10-9, 10-10, and 10-11).82,83 Interestingly, most otolaryngologists/head and neck surgeons are not used to viewing these structures from this perspective (i.e., through the down-fracture), whereas orthognathic surgeons are. There are several technical details to consider when completing septoplasty or inferior turbinate reduction through the exposure provided by the Le Fort I osteotomy. As always, care is taken to maintain the integrity of the nasal mucosa when down-fracturing the maxilla. Nevertheless, tears in the mucosa are common. After the down-fracture and disimpaction of the maxilla, an elevator is used to achieve the submucosal exposure of all buckled and deviated portions of the cartilaginous and bony septum. With the use of a rongeur, the buckled or deviated portions of the cartilaginous and bony septum are removed (i.e., submucosal resection); however, adequate dorsal and caudal components of the cartilaginous septum are preserved for structural support. If a cosmetic rhinoplasty is a future consideration, then the preservation of as much useful septal cartilage as possible for later tip grafting is important. Next, an incision that is parallel and just below each inferior turbinate is completed through the nasal mucosa. If the nasal mucosa was torn during the down-fracture, then the exposure provided is used and extended as needed for the additional visualization of each inferior turbinate. With the use of a scissors (e.g., Mayo scissors), the inferior aspect of each enlarged inferior turbinate can be excised via partial resection. Other surgical techniques to reduce enlarged inferior turbinates are described, such as intramural ablation. The direct visualization of the more cephalic positioned nasotracheal tube within the surgical field limits the chance of injury during the turbinate reduction procedure. There is sufficient room for the surgeon to work around the endotracheal tube. With no oxygen leaks in the ventilation system, the cauterization of the exposed raw surface of each inferior turbinate is completed. Suture repair of the nasal floor mucosa is then accomplished. No nasal packs or splints are required for postoperative management. With this approach, the author has not experienced significant intraoperative bleeding or postoperative airway compromise.
Management of Residual Postoperative Nasal Obstruction
When the baseline presurgical nasal airway obstruction is clarified and then managed simultaneously through the Le Fort I down-fracture, residual nasal obstruction is uncommon. When a degree of postsurgical nasal obstruction persists, it is generally the result of an incomplete presurgical diagnosis or the inadequate operative correction of all of the aspects that are causing the blockage (e.g., constricted aperture, elevated floor, enlarged inferior turbinates, septal deviations). After septoplasty and inferior turbinate procedures and after routine postoperative intranasal swelling subsides, continued nasal obstruction may also occur as a result of scar formation.67,79,83 Should a degree of nasal obstruction persist, it is important to confirm that residual septal deviation, turbinate hypertrophy, and nasal valve (internal and external) stenosis are not factors. If these causes are ruled out and if postsurgical obstruction remains, scar bands of varied thickness are likely to be the cause. If so, the intranasal synechiae can extend from the operated septum to the inferior turbinates or to the healing areas along the nasal mucosal floor. The resulting scar bands are usually small to moderate in size, and they can often be corrected in the surgeon’s office with the use of local spray anesthesia and scissors transaction under endoscopic visualization. Occasionally, dense, thick synechiae occur; these may contract a straightened septum into a lateralized position. For more extensive scar bands and when further septoplasty or turbinate reduction is required, management in the operating room via an intranasal approach (with the placement of a separating splint between the septum and the lateral nose to prevent the adjacent fresh mucosal edges from re-creating the scar contracture) is preferred.
Effects of Maxillary Arch Expansion and Nasal Floor Recontouring on the Airway
Nasal Floor Recontouring: Effects on the Airway ( Video 3 and 4)
Video 3 and 4)
The presence of excess vertical alveolar height of the anterior maxilla above the roots of the teeth results in a high nasal floor that constricts the nasal passageways, thereby causing increased nasal airway resistance.24,113 This is a frequent occurrence in patients with a long face growth pattern (see Chapter 21). The need for vertical intrusion (i.e., superior repositioning) of the anterior maxilla to reduce the gummy smile, to improve lip closure, and to reduce mentalis strain is generally required as part of the long face correction (see Figs. 10-5, 10-11, and 10-13). As a result of maxillary intrusion, any baseline nasal obstruction caused by a high nasal floor will worsen unless it is simultaneously addressed. The need to recontour the nasal floor, the pyriform rims, and the anterior nasal spine region is also frequent in patients with cleft lips and palates (e.g., unilateral or bilateral cleft lip and palate) that present with a maxillary deformity. Recontouring to lower and level the nasal floor, to widen the pyriform rims, and to reduce and reshape the anterior nasal spine can simultaneously be performed through the Le Fort I down-fracture. This will both enhance upper lip and nose aesthetics and reduce nasal airway resistance. The recontouring procedure is carried out with a watermelon-shaped bur on a low-speed rotary drill and a rongeur under direct visualization either before or after the maxilla is secured (i.e., fixed with plates and screws) in its new location (see Chapter 15 and Fig. 10-11).
Maxillary Arch Expansion: Effects on the Airway
Studies predominantly show that maxillary arch expansion will improve a tight nasal inlet and thereby decrease nasal airway resistance.15,22,68 The documented improved nasal breathing is believed to result from a widened airway space, primarily at the level of the internal nasal valve brought about by maxillary arch expansion.* Seeberger and colleagues92 and Wreidt and colleagues114 examined patients via acoustic rhinometry before and after surgically assisted rapid maxillary expansion and recorded profound increases in total nasal volumes. They also reported that their patients experience better nasal airflow with a distinct subjective improvement in nasal breathing. Maxillary widening may be accomplished with a number of techniques (e.g., rapid palatal expansion, surgically assisted rapid palatal expansion, segmental Le Fort I osteotomy). The method selected is dependent on the age of the patient and the combination of airway, occlusal, and facial aesthetic needs. The frequent need for arch expansion in the individual with a long face growth pattern is well recognized (see Fig. 10-4), and it is also common with other dentofacial deformities (e.g., maxillary deficiency with relative mandibular excess, primary mandibular deficiency, bimaxillary dental protrusion, and cleft maxillary deformities). Expiratory pressure, expiratory flow values, and inspiratory flow values have all been documented to improve immediately after lateral nasal wall widening. These benefits should remain long term unless lateral wall relapse occurs.
Comprehensive Approach to Chronic Nasal Airway Obstruction
The fact that Posnick and colleagues showed a more significant symptomatic improvement in nasal breathing in their study patients as compared with that found by Stewart and colleagues when septoplasty only was carried out is not surprising.100 This likely resulted from the strict patient selection process and the other procedures that were simultaneously carried out (e.g., inferior turbinate reduction, transverse widening of the maxilla, horizontal maxillary advancement, lowering the nasal floor by recontouring).
An interesting observation emerged from the COG-ENT chronic rhinosinusitis study that was completed in 2001.98–101 The COG-ENT researchers found that patients who were undergoing functional endoscopic sinus surgery in combination with septoplasty and inferior turbinate reduction showed even greater clinical improvement in nasal breathing as compared with those undergoing functional endoscopic sinus surgery alone. This confirms the added value to the airway (i.e., the improved breathing) related to carefully selected diagnosis-driven procedures being simultaneously carried out.19,35,50,54,70,72,78
References
1. Angell, EC. Treatment of irregularities of the permanent or adult teeth. Dent Cosmos. 1860; 540–544.
2. Angle, EH. Malocclusion of teeth, ed 7. Philadelphia: SS White Dental Mfg Co; 1907.
3. Arunachalam, PS, Kitcher, E, Gray, J, et al. Nasal septal surgery: evaluation of symptomatic and general health outcomes. Clin Otolaryngol Allied Sci. 2001; 26:367–370.
4. Bacon, WH, Turlet, JC, Krieger, J, Stierle, JL. Craniofacial characteristics in patients with obstructive sleep apnea syndrome. Cleft Palate J. 1988; 25:374–378.
5. Baik, UB, Suzuki, M, Ikeda, K, et al. Relationship between cephalometric characteristics and obstructive sites in obstructive sleep apnea syndrome. Angle Orthod. 2002; 72(2):124–134.
6. Basciftci, FA, Mutlu, N, Karaman, AI, et al. Does the timing and method of rapid maxillary expansion have an effect on the changes in nasal dimensions? Angle Orthod. 2002; 72(2):118–123.
7. Bateman, ND, Woolford, TJ. Informed consent for septal surgery: the evidence-base. J Laryngol Otol. 2003; 117(3):186–189.
8. Bell, WH, Sinn, D. Turbinectomy to facilitate superior movement of the maxilla by Le Fort I osteotomy. J Oral Surg. 1979; 37:129–130.
9. Bicakci, AA, Agar, U, Sokucu, O, et al. Nasal airway changes due to rapid maxillary expansion timing. Angle Orthod. 2005; 75:1–6.
10. Blair, VP. Surgery of the mouth and jaws. St. Louis: Mosby; 1914.
11. Bresolin, D, Shapiro, PA, Shapiro, GG, et al. Mouth breathing in allergic children: its relationship to dentofacial development. Am J Orthod. 1983; 84:334.
12. Brown, GVI. The application of orthodontia principles to the prevention of nasal disease. Dental Cosmos. 1903; 45:765–775.
13. Brown, GVI. The surgical and therapeutic aspect of maxillary readjustment with special reference to nasal stenosis, hare lip, cleft palate and speech. Dent Cosmos. 1909; 51:7–17.
14. Brown, GVI. The surgery of oral and facial diseases and malformations, ed 4. London: Kimpton; 1938.
15. Ceroni Compadretti, G, Tasca, I, Alessandri-Boneti, G, et al. Acoustic rhinomanometric measurements in children undergoing rapid maxillary expansion. Int J Pediatr Otorhinolaryngol. 2006; 70(1):27–34.
16. Cistulli, PA, Palmisano, RG, Poole, MD. Treatment of obstructive sleep apnea syndrome by rapid maxillary expansion. Sleep. 1998; 21(8):831–835.
17. D’Ascanio, L, Lancione, C, Pompa, G, et al. Craniofacial growth in children with nasal septum deviation: a cephalometric comparative study. Int J Pediatr Otorhinolaryngol. 2010; 74:1180.
18. De Mol van Otterloo, JJ, Leezenberg, JA, Tuinzing, DB, van der Kwast, WA. The influence of the Le Fort I osteotomy on nasal airway resistance. Rhinology. 1990; 28(2):107–112.
19. De Vito, A, Berrettini, S, Carabelli, A, et al. The importance of nasal resistance in obstructive sleep apnea syndrome: a study with positional rhinomanometry. Sleep Breath. 2001; 5(1):3–11.
20. Dinis, PB, Haider, H. Septoplasty: long-term evaluation of results. Am J Otolaryngol. 2002; 23:85–90.
21. Djupesland, PG, Rotnes, JS. Accuracy of acoustic rhinometry. Rhinology. 2001; 39(1):23–27.
22. Doruk, C, Sokucu, O, Sezer, H, Canbay, EI. Evaluation of nasal airway resistance during rapid maxillary expansion using acoustic rhinometry. Eur J Orthod. 2004; 26:397–401.
23. Egeli, E, Demirci, L, Yazycy, B, Harputluoglu, U. Evaluation of the inferior turbinate in patients with deviated nasal septum by using computed tomography. Laryngoscope. 2004; 114(1):113–117.
24. Ellis, E. The nature of vertical maxillary deformities: implications for surgical intervention. J Oral Maxillofac Surg. 1985; 43:756–762.
25. Enache, AM, Nimigean, VR, Mihaltan, F, et al. Assessment of sagittal and vertical skeletal patterns in Romanian patients with obstructive sleep apnea. Rom J Morphol Embryol. 2010; 51:505–508.
26. Enoki, C, Valera, FC, Lessa, FC, et al. Effect of rapid maxillary expansion on the dimension of the nasal cavity and on nasal air resistance. Int J Pediatr Otorhinolaryngol. 2006; 70(7):1225–1230.
27. Erbe, M, Lehotay, M, Gode, U, et al. Nasal airway changes after Le Fort I–impaction and advancement: anatomical and functional findings. Int J Oral Maxillofac Surg. 2001; 30(2):123–129.
28. Fields, DH. Relationship between vertical dentofacial morphology and respiration in adolescents. Am J Orthod. 1991; 99:147–154.
29. Foy, R. Contribution rhinométrique à l’étude de la respiration nasale. Annals des maladies de l’oreille, du larynx, du nez et du pharynx. T XXXVI. 1910; 130–149.
30. Freng, A, Kvam, E. Facial sagittal growth following partial basal resection of the nasal septum: a retrospective study in man. Eur J Orthod. 1979; 1:89–96.
31. Freng, A. Dentofacial development in the long-lasting nasal stenosis. Scand J Dent Res. 1979; 89:260–267.
32. Gotzfried, HF, Hellmut, M. On the improvement of nasal breathing following mid-face osteotomies, and possible reasons for the phenomenon. J Oral Maxillofac Surg. 1984; 12:29.
33. Gotzfried, HF, Masing, H. Improvement of nasal breathing in cleft patients following midface osteotomy. Int J Oral Maxillofac Surg. 1988; 17:41–44.
34. Gray, LP. Results of 310 cases of rapid maxillary expansion selected for medical reasons. J Laryngol Otol. 1975; 89(6):601–614.
35. Gryczynska, D, Powajbo, K, Zakrzewska, A. The influence of tonsillectomy on obstructive sleep apnea children with malocclusion. Int J Pediatr Otorhinolaryngol. 1995; 32(Suppl):S225–S228.
36. Guenther, TA, Sather, AH, Kern, EB. The effect of Le Fort I maxillary impaction on nasal airway resistance. Am J Orthod. 1984; 85(4):308–315.
37. Guymon, M, Crosby, DR, Wolford, LM. The alar base cinch suture to control nasal width in maxillary osteotomies. Int J Adult Orthodon Orthognath Surg. 1988; 2:89–95.
38. Haarmann, S, Budihardja, AS, Wolff, KD, Wargerin, K. Changes in acoustic airway profiles and nasal airway resistance after Le Fort I osteotomy and functional rhinosurgery: a prospective study. Int J Oral Maxillofac Surg. 2009; 38:321–325.
39. Haas, J. The treatment of maxillary deficiency by opening the midpalatal suture. Angle Orthod. 1965; 35(3):200–217.
40. Haas, AJ. Palatal expansion: just the beginning of dento-facial orthopedics. Am J Orthod. 1970; 57(3):219–225.
41. Handelman, CS, Osborne, G. Growth based on nasopharynx and adenoid development from one to eighteen years. Angle Orthod. 1976; 46:243–258.
42. Hannley, M, Stewart, MG, Witsell, D. A nose for evidence: report of the Nasal Obstruction and Septoplasty Effectiveness (NOSE) study. AAO-HNS Bulletin. 2003; 9:42–43.
43. Hannuksela, A. The effects of moderate and severe atopy on the facial skeleton. Eur J Orthod. 1981; 3:187–193.
44. Hartgerink, DV, Vig, PS, Abbot, DW. The effect of rapid maxillary expansion on nasal airway resistance. Am J Orthod. 1987; 92:381–389.
45. Harvold, EP. The role of function in the etiology and treatment of malocclusion. Am J Orthod. 1968; 54:883–898.
46. Harvold, EP, Chierici, G, Varervik, K. Experiments on the development of dental malocclusion. Am J Orthod. 1972; 61:38–44.
47. Harvold, EP, Vargervik, K, Chiericu, G. Primate experiments on oral sensation and dental malocclusion. Am J Orthod. 1973; 63:494–508.
48. Harvold, EP, Tomer, BS, Chierici, G, Vargervik, K. Primate experiments in oral respiration. Am J Orthod. 1981; 79:359–372.
49. Hershey, HG, Stewart, BL, Warren, DW. Changes in nasal airway resistance associated with rapid maxillary expansion. Am J Orthod. 1976; 69:274–284.
50. Holmberg, H, Linder-Aronson, S. Cephalometric radiographs as a means of evaluating the capacity of the nasal and nasopharyngeal airway. Am J Orthod. 1979; 76:479–490.
51. Hooper, RG. Forced inspiratory nasal flow-volume curves: a simple test of nasal airflow. Mayo Clin Proc. 2001; 76(10):990–994.
52. Jessen, M, Malm, L. The importance of nasal airway resistance and nasal symptoms in the selection of patients for septoplasty. Rhinology. 1984; 22:157–164.
53. Jensen, M, Ivarsson, A, Malm, L. Nasal airway resistance and symptoms after functional septoplasty: comparison of findings at 9 months and 9 years. Clin Otolaryngol. 1989; 14:231–234.
54. Kerr, P, Millar, T, Buckle, P, Kryger, M. The importance of nasal resistance in obstructive sleep apnea syndrome. J Otolaryngol. 1992; 21(3):189–195.
55. Kirk, WS, Bolz, EA. Pre-orthognathic surgery evaluation of nasal obstruction. Int J Adult Orthodon Orthognath Surg. 1988; 3:109–115.
56. Kunkel, M, Hochban, W. The influence of maxillary osteotomy on nasal airway patency and geometry. Mund Kiefer Gesichtschir. 1997; 1:194–198.
57. Lee, BJ, Chung, YS, Jang, YJ. Overcorrected septum as a complication of septoplasty. Am J Rhinol. 2004; 18(6):393–396.
58. Lee, KS, Yang, CC, Huang, JK, et al. Congenital pyriform aperture stenosis: surgery and evaluation with three-dimensional computed tomography. Laryngoscope. 2002; 112(5):918–921.
59. Linder-Aronson, S, Backstrom, A. A comparison between mouth and nose breathers with respect to occlusion and facial dimension. Odontol Rev. 1960; 11:343–376.
60. Linder-Aronson, S, Aschan, G. Nasal resistance to breathing and palatal height before and after expansion of the median palatine suture. Odontol Rev. 1963; 14:254–270.
61. Linder-Aronson, S. Adenoids: their effect on mode of breathing and nasal airflow and their relationship to characteristics of the facial skeleton and the dentition. A biometric, rhino-manometric and cephalometro-radiographic study on children with and without adenoids. Acta Otolaryngol Suppl. 1970; 265:1–132.
62. Linder-Aronson, S, Lindgren, J. The skeletal and dental effects of rapid maxillary expansion. Br J Orthod. 1979; 6:25–29.
63. Linder-Aronson, S, Woodside, DG. Excess face height malocclusion: etiology, diagnosis and treatment. Carol Stream, IL: Quintessence Publishing; 2000.
64. Lundstrom, A. The importance of genetic and non-genetic factors in the facial skeleton studies in 100 pairs if twins. Trans Eur Orthod Soc. 1954.
65. Lundstrom, A, Woodside, DG. Individual variation in growth directions expressed at the chin and the midface. Eur J Orthod. 1980; 2:65–79.
66. Lundstrom, A. Nature versus nature in dento-facial variation. Eur J Orthod. 1984; 6(2):77–91.
67. McKee, GJ, O’Neill, G, Roberts, C, et al. Nasal airflow after septorhinoplasty. Clin Otolaryngol. 1994; 19:254–257.
68. Mitsuda, ST, Pereira, MD, Passos, AP, et al. Effects of surgically assisted rapid maxillary expansion on nasal dimensions using acoustic rhinometry. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010; 109:191–196.
69. Monini, S. Rapid maxillary expansion for the treatment of nasal obstruction in children younger than 12 years. Arch Otolaryngol Head Neck Surg. 2009; 135(1):22–27.
70. Montgomery, W, Vig, PS, Staab, EV, Matteson, SR. Computed tomography: a three-dimension study of the nasal airway. Am J Orthod. 1979; 6:363–375.
71. Moses, JJ, Lange, CR, Arredondo, A. Endoscopic treatment of sinonasal disease in patients who have had orthognathic surgery. Br J Oral Maxillofac Surg. 2000; 38(3):177–184.
72. Nimubona, L, Jokic, M, Moreau, S, et al. Obstructive sleep apnea syndrome and hypertrophic tonsils in infants. Arch Pediatr. 2000; 7(9):961–964.
73. Nustad, RA, Fonseca, RJ, Zeitler, DZ. Evaluation of maxillary sinus disease in maxillary orthognathic surgery patients. Int J Adult Orthodon Orthognath Surg. 1986; 1(3):195–202.
74. O’Ryan, F, Carlotti, A. Nasal anatomy and maxillary surgery. III. Surgical techniques for correction of nasal deformities in patients undergoing maxillary surgery. Int J Adult Orthodon Orthognath Surg. 1989; 4:157–174.
75. Onerci, TM, Ayhan, K, Ogretmenoglu, O. Two consecutive cases of cerebrospinal fluid rhinorrhea after septoplasty operation. Am J Otolaryngol. 2004; 25(5):354–356.
76. Panula, K, Finne, K, Oikarinen, K. Incidence of complications and problems related to orthognathic surgery: a review of 655 patients. J Oral Maxillofac Surg. 2001; 59:1128–1136.
77. Passali, D, Anselmi, M, Lauriello, M, et al. Treatment of hypertrophy of the inferior turbinate: long-term results in 382 patients randomly assigned to therapy. Ann Otol Rhinol Laryngol. 1999; 108:569–575.
78. Perkins, JA, Sie, KCY, Milczuk, H, et al. Airway management in children with craniofacial anomalies. Cleft Palate Craniofac J. 1997; 34:135.
79. Phagoo, SB, Watson, RA, Pride, NB. Use of nasal peak flow to assess nasal patency. Allergy. 1997; 52(9):901–908.
80. Pirila, T, Tikanto, J. Unilateral and bilateral effects of nasal septum surgery demonstrated with acoustic rhinometry, rhinomanometry and subjective assessment. Am J Rhinol. 2001; 15:127–133.
81. Posnick, JC. Maxillary excess with or without mandibular deficiency. In: Posnick JC, ed. Craniofacial and maxillofacial surgery in children and young adults. Philadelphia: W. B. Saunders Company; 2000:1025–1056.
82. Posnick, JC, Le Fort I osteotomy, sagittal splitting of the mandible, and genioplasty: historical perspective and step-by-step approach. Craniofacial and maxillofacial surgery in children and young adults. Posnick, JC, eds. Craniofacial and maxillofacial surgery in children and young adults; vol 2. W. B. Saunders Company, Philadelphia, 2000:1082–1102.
83. Posnick, JC, Fantuzzo, JJ, Troost, T. Simultaneous intranasal procedures to improve chronic obstructive nasal breathing in patients undergoing maxillary (Le Fort I) osteotomy. J Oral Maxillofac Surg. 2007; 65:2273–2281.
84. Posnick, JC, Agnihotri, N. Consequences and management of nasal airway obstruction in the dentofacial deformity patient. Curr Opin Otolaryngol Head Neck Surg. 2010; 18:323–331.
85. Posnick, JC, Agnihotri, N. Managing chronic nasal airway obstruction at the time of orthognathic surgery: a twofer. J Oral Maxillofac Surg. 2011; 69:695–701.
86. Rhee, JS, Poetker, DM, Smith, TL, et al. Nasal valve surgery improves disease-specific quality of life. Laryngoscope. 2005; 115(3):437–440.
87. Ricketts, RM. Respiratory obstruction syndrome. Am J Orthod. 1968; 54:495–507.
88. Samad, I, Stevens, HE, Maloney, A. The efficacy of nasal septal surgery. J Otolaryngol. 1992; 21:88–91.
89. Sandham, A, Murray, JAM. Nasal septal deformity in unilateral cleft lip and palate. Cleft Palate Craniofac J. 1993; 30:222.
90. Santiago-Diez de Bonilla, J, McCaffrey, TV, Kern, EB. The nasal valve: a rhinomanometric evaluation of maximum nasal inspiratory flow and pressure curves. Ann Otol Rhinol Laryngol. 1986; 95(3 Pt 1):229–232.
91. Schwab, JA, Pirsig, W. Complications of septoplasty. Facial Plastic Surg. 1997; 13(1):3–14.
92. Seeberger, R, Kater, W, Davids, R, Thiele, OC. Long term effects of surgically assisted rapid maxillary expansion without performing osteotomy of the pterygoid plates. J Craniomaxillofac Surg. 2010; 38:175–178.
93. Sessions, RB, Troost, T. The nasal septum. In: Cummings CW, ed. Otolaryngology: head and neck surgery. St. Louis: Mosby–Year Book; 1999:786–806.
94. Siegel, NS, Gliklich, RE, Taghizadeh, F, et al. Outcomes of septoplasty. Otolaryngol Head Neck Surg. 2000; 122:228–232.
95. Sillman, JH. Dimensional changes in the dental arches: longitudinal study from birth to 25 years. Am J Orthod Dentofacial Orthop. 1964; 50:824.
96. Solow, B, Nielsen, SS, Greve, E. Airway adequacy, head posture, and craniofacial morphology. Am J Orthodont Dentofacial Orthoped. 1984; 87:214–223.
97. Sousa, JB, Anselmo-Lima, WT, Valera, FC, et al. Cephalometric assessment of the mandibular growth pattern in mouth-breathing children. Int J Pediatr Otorhinolaryngol. 2005; 69:311.
98. Stewart, EJ, Robinson, K, Wilson, JA. Assessment of patient’s benefit from rhinoplasty. Rhinology. 1996; 34:57–59.
99. Stewart, MG, Witsell, DL, Smith, TL, et al. Development and validation of the Nasal Obstruction Symptom Evaluation (NOSE) scale. Otolaryngol Head Neck Surg. 2004; 130:157–163.
100. Stewart, MG, Smith, TL, Weaver, EM, et al. Outcomes after nasal septoplasty: results from the Nasal Obstruction Septoplasty Effectiveness (NOSE) study. Otolaryngol Head Neck Surg. 2004; 130:283–290.
101. Stewart, MG, Smith, TL. Objective versus subjective outcomes assessment in rhinology. Am J Rhinol. 2005; 19(5):529–535.
102. Timms, DJ. The effect of rapid maxillary expansion on nasal airway resistance. Br J Orthod. 1986; 13(4):221–228.
103. Turvey, TA. Management of the nasal apparatus in maxillary surgery. J Oral Surg. 1980; 38:331–335.
104. Turvey, TA. Alteration in nasal airway resistance following superior repositioning of the maxilla. Am J Orthod. 1984; 99:109–114.
105. Tweed, CH. Clinical orthodontics. St. Louis: Mosby; 1966.
106. Valera, FC, Travitzki, LV, Mattar, SE, et al. Muscular, functional and orthodontic changes in preschool children with enlarged adenoids and tonsils. Int J Pediatr Otorhinolaryngol. 2003; 67:761.
107. Vargervik, K, Mill, AJ, Chierici, G, et al. Morphologic response to changes in neuromuscular patterns experimentally induced by altered mode of respiration. Am J Orthod. 1984; 85:115–124.
108. Walker, D, Turvey, TA, Warren, D. Alterations in nasal respiration and nasal airway size following superior repositioning of the maxilla. J Oral Maxillofac Surg. 1988; 46:276–281.
109. Warren, DW, Hershey, HG, Turvey, TA, et al. The nasal airway following maxillary expansion. Am J Orthod. 1987; 91:111–116.
110. Weber, ZJ, Preston, CB, Wright, PG. Resistance to nasal air flow related to changes in head posture. Am J Orthodon Dentofacial Orthop. 1981; 82:536–545.
111. Wenzel, A, Henriksen, J, Melsen, B. Nasal respiratory resistance and head posture: effect of intra-nasal corticosteroid (Budesonide) in children with asthma and perennial rhinitis. Am J Orthod. 1983; 84:422–426.
112. Wertz, RA. Changes in nasal air flow incident to rapid maxillary expansion. Angle Orthod. 1968; 38(1):1–11.
113. West, RA, McNeill, RW. Maxillary alveolar hyperplasia, diagnosis and treatment planning. J Maxillofac Surg. 1975; 3:239.
113a. Williams, BJD, Isom, A, Laureano Filho, JR, O’Ryan, FS. Nasal airway function after maxillary surgery: A prospective cohort study using the nasal obstruction symptom evaluation scale. J Oral Maxillofac Surg. 2013; 71:343–350.
114. Wriedt, S, Kunkel, W, Zentner, A, Wahlmann, U. Surgically assisted rapid palatal expansion. An acoustic rhinometric, morphometric and sonographic investigation. J Orthofac Orthop. 2001; 62:107–115.