N
Nabilone. Cannabinoid antiemetic drug, acting on CB1 and CB2 cannabis receptors, used in chemotherapy-induced nausea and vomiting. Has also been used in palliative care.
Nalbuphine hydrochloride. Opioid analgesic drug and opioid receptor antagonist, synthesised in 1968. Partial agonist at kappa and sigma opioid receptors, and partial antagonist at mu receptors. Used for premedication, anaesthesia and treatment of pain. Active within 2–3 min of iv, or 15 min of im injection. Half-life is about 5 h. Undergoes hepatic metabolism and excreted renally. Side effects such as vomiting are thought to be less than with morphine, although maximal analgesia attainable is also less. Psychomimetic effects are less problematic than with pentazocine. Withdrawn from the UK market in 2003.
Nalmefene hydrochloride. Opioid receptor antagonist, introduced in the USA in 1995. Longer half-life (10.8 h) than naloxone, thus less likely for opioid effects to recur following reversal.
Nalorphine hydrochloride/hydrobromide. Opioid analgesic drug and opioid receptor antagonist, synthesised in 1941. Partial agonist at kappa and sigma opioid receptors, and antagonist at mu receptors. Psychomimetic effects are common at analgesic doses (5–10 mg). No longer available.
Naloxone hydrochloride. Opioid receptor antagonist, synthesised in 1961. N-Allyl derivative of oxymorphone. Although it has a high affinity for the mu receptor, it has no intrinsic activity. Used to reverse unwanted effects of opioid analgesic drugs, e.g. sedation, respiratory depression, spasm of the biliary sphincter. Also reverses opioid-mediated analgesia. Reverses the effects of pentazocine but not buprenorphine. Used to reverse ventilatory depression and pruritus following spinal opioids, without reversing analgesia. Also used in poisoning and overdoses due to other depressant drugs, e.g. alcohols, benzodiazepines, barbiturates, although its efficacy is disputed. Reportedly useful in septic shock, increasing BP and cardiac output; the mechanism is unclear but may involve increase of endogenous catecholamine release or antagonism of increased levels of endorphins that occur in sepsis. Effective within 1–2 min of iv injection, with a half-life of 1–2 h; thus depressant effects of opioid analgesic drugs may recur after a few hours. Metabolised in the liver and excreted mainly renally.
• Dosage:
 opioid poisoning: 0.4–2.0 mg iv/im/sc, repeated after 2–3 min to a total of 10 mg. Administration by infusion (3–10 µg/kg/h) may be required.
opioid poisoning: 0.4–2.0 mg iv/im/sc, repeated after 2–3 min to a total of 10 mg. Administration by infusion (3–10 µg/kg/h) may be required.
• Side effects: hypertension, arrhythmias, pulmonary oedema and cardiac arrest have followed rapid iv injection, possibly due to sudden catecholamine release secondary to reversal of sedation and analgesia.
Acute withdrawal may be precipitated in patients dependent on opioids.
Naltrexone hydrochloride. Opioid receptor antagonist, synthesised in 1965. Derived from naloxone, with similar actions but longer duration (24 h after a single dose). Used in the treatment of opioid and alcohol dependence.
Naproxen. NSAID derived from propionic acid. Has a favourable side-effect profile among NSAIDs (although not as potent as ibuprofen), and can be given just twice daily.
Narcotic drugs. Strictly, drugs which induce sleep, but the term usually refers to morphine-like drugs. Preferred terms include opiates, opioids and opioid analgesic drugs.
Nasal inhalers. Used instead of facemasks for dental anaesthesia. Designed to fit over the nose, leaving the mouth free. During induction of anaesthesia, the patient is instructed to breathe through the nose. During anaesthesia, a mouth pack prevents mouth breathing. Now rarely used.
 Goldman’s: black rubber, with an inflatable rim as for facemasks. Incorporates an adjustable pressure-limiting valve, and attaches to the breathing system over the patient’s forehead. It should be held from behind the patient’s head using both thumbs whilst the other fingers support the jaw. May also be held with a head harness using two studs incorporated into the sides.
Goldman’s: black rubber, with an inflatable rim as for facemasks. Incorporates an adjustable pressure-limiting valve, and attaches to the breathing system over the patient’s forehead. It should be held from behind the patient’s head using both thumbs whilst the other fingers support the jaw. May also be held with a head harness using two studs incorporated into the sides.
 McKesson’s: made of malleable black rubber, thus adjustable. Connected to the breathing system via two tubes which pass around the sides of the head to meet behind, helping to hold the inhaler in place. Incorporates an expiratory valve.
McKesson’s: made of malleable black rubber, thus adjustable. Connected to the breathing system via two tubes which pass around the sides of the head to meet behind, helping to hold the inhaler in place. Incorporates an expiratory valve.
 newer types are made of plastic, and may incorporate unidirectional gas flow, e.g. through inspiratory and expiratory tubes passing around the head. Scavenging of exhaled gases is thus aided.
newer types are made of plastic, and may incorporate unidirectional gas flow, e.g. through inspiratory and expiratory tubes passing around the head. Scavenging of exhaled gases is thus aided.
Nasal positive pressure ventilation, see Non-invasive positive pressure ventilation
Nasogastric intubation. Performed for enteral nutrition, or gastric drainage. Fine-bore tubes are used for the former, usually inserted using a wire stylet, which is removed after placement. Larger tubes (e.g. 10–16 Ch) are used for gastric drainage, e.g. following abdominal surgery or in intestinal obstruction. They may be placed in the awake patient (who aids placement by swallowing or sipping water) or unconscious patient (e.g. after induction of anaesthesia, either before or after tracheal intubation). Placement can often be performed blindly, and may be aided by passage through a plain nasal tracheal tube placed into the pharynx or by prior transient inflation of the upper oesophagus via a tightly fitting facemask. Placement may require direct vision using a laryngoscope and forceps (the oesophagus lies posterior to the larynx and to the left of the midline). Correct placement is confirmed by aspiration of gastric contents (should be tested for acidity), auscultation over the left hypochondrium during injection of air, abdominal X-ray or palpation by the surgeon during surgery. If already in place, withdrawal of the tube before induction of anaesthesia has been suggested, to avoid increasing gastro-oesophageal reflux or rendering cricoid pressure inefficient. However, this is rarely done.
National Confidential Enquiry into Patient Outcome and Death (NCEPOD). Ongoing study originally commissioned by the Association of Anaesthetists of Great Britain and Ireland, together with the Association of Surgeons of Great Britain and Ireland, and published in 1987 as the Confidential Enquiry into Perioperative Deaths (CEPOD). The first UK study to involve both anaesthetists and surgeons, it analysed all 4000 NHS deaths occurring within 30 days of surgery (excluding obstetric and cardiac surgery) in three regions during 1986. The first national Report (NCEPOD; 1989) focused on children under 10 years; subsequent Reports have focused on particular aspects, e.g. deaths following specific surgical or interventional procedures or in specific age groups, out-of-hours operating, acute kidney injury.
• General findings and recommendations:
 most deaths occur in elderly and/or the sickest patients.
most deaths occur in elderly and/or the sickest patients.
 overall care is good but there are identifiable deficiencies:
overall care is good but there are identifiable deficiencies:
– inadequate consultation between trainees and their seniors and between anaesthetists and surgeons.
– inappropriate decisions to operate in futile cases.
– operating outside the surgeon’s subspecialty.
– inadequate prophylaxis against DVT.
– inappropriate operating at night.
– insufficient emergency operating theatre, ICU and HDU facilities.
– lack of involvement of senior staff in emergency lists and paediatric cases.
– non-availability of fibreoptic intubating equipment.
– sending inappropriate staff with critically ill patients during transfer.
– poor quality and unavailability of medical records.
 the need for post-mortem examination, audit and morbidity/mortality assessments has been repeatedly stressed.
the need for post-mortem examination, audit and morbidity/mortality assessments has been repeatedly stressed.
National Institute of Academic Anaesthesia (NIAA). Established in 2008 by the Royal College of Anaesthetists, Association of Anaesthetists of Great Britain and Ireland, British Journal of Anaesthesia and Anaesthesia, to further the academic profile of the specialty of anaesthesia and promote high-quality research. Coordinates the assessment and awarding of anaesthetic research grants within the UK, and houses the Health Services Research Centre (HSRC) which coordinates national, outcome-based projects.
National Patient Safety Agency (NPSA). NHS Special Health Authority, formed in 2001 to coordinate reports of adverse events or ‘near misses’ and their analysis. Operated three main services until its abolishment in 2012:
 National Clinical Assessment Service (NCAS), providing advice and support to the NHS regarding concerns about individual doctors’, dentists’ and pharmacists’ performance. This role transferred to NICE.
National Clinical Assessment Service (NCAS), providing advice and support to the NHS regarding concerns about individual doctors’, dentists’ and pharmacists’ performance. This role transferred to NICE.
See also, Confidential Enquiry into Maternal Deaths; National Confidential Enquiry into Patient Outcome and Death
Natriuretic hormone, see Atrial natriuretic peptide
Nausea, see Postoperative nausea and vomiting; Vomiting
Near-drowning. Defined as initial survival following immersion in liquid, usually water; death at the time of immersion may be due to anoxia (drowning) or cardiac arrest caused by sudden extreme lowering of temperature (immersion syndrome). Secondary drowning refers to death following near-drowning after a period of relative wellbeing and is usually due to ARDS/acute lung injury.
Autopsy following drowning reveals little or no lung water in 15% of cases (dry drowning); laryngospasm following initial laryngeal contamination has been suggested. In 85% of cases, pulmonary aspiration of water occurs (wet drowning); this may involve:
Both types cause pulmonary oedema and hypoxaemia. Haemodynamic changes due to fluid shifts are rare; thus in practice the type of water may have little clinical significance.
Other adverse factors include hypothermia, aspiration of gastric contents and predisposing conditions, e.g. alcoholism or drug abuse, trauma, epilepsy, MI, CVA.
Complications include ARDS, cerebral oedema, acute kidney injury, pneumonia, pancreatitis, acidosis and shock. Sepsis is especially likely if the water is contaminated.
 CPR.
CPR.
 treatment of complications as appropriate.
treatment of complications as appropriate.
 antibiotics as appropriate. Use of corticosteroids is controversial and declining.
antibiotics as appropriate. Use of corticosteroids is controversial and declining.
Szpilman D, Bierens JJLM, Handley AJ, Orlowski JP (2012). N Engl J Med; 366: 2102–10.
Near infrared oximetry/spectroscopy (NIRS). Monitoring technique based on the principle that light in the near infrared spectrum (650–900 nm wavelength) transmits through biological tissues. Increasingly used to image biological events in the cerebral cortex. Photons produced by a laser photodiode are directed into the skull; whilst many are reflected and dispersed, a proportion is transmitted. Coloured compounds within the tissues (chromophores), especially oxyhaemoglobin, deoxyhaemoglobin and oxidised cytochrome oxidase, have characteristic absorption spectra. The emergent light intensity is detected and a computer converts the changes in light intensity into changes in chromophore concentration. Clinical applications include monitoring of cerebral oxygenation and cerebral blood flow and volume, e.g. in neurosurgery, cardiac surgery and head injury.
Murkin JM, Arango M (2009). Br J Anaesth; 103 (suppl 1); i3–13
Nebulisers. Devices used to provide a suspension of droplets in a gas, for administration of inhaled drugs or humidification. Droplets of 5 µm are deposited in the trachea and bronchi; those of 1 µm pass to alveoli and may impair gas exchange. Thus the ideal droplet size is between 1 and 5 µm.
 gas-driven: water is entrained by the gas flow (Venturi principle) and broken into a spray; this may be directed against an anvil which breaks up the drops into smaller droplets. May be combined with a heater.
gas-driven: water is entrained by the gas flow (Venturi principle) and broken into a spray; this may be directed against an anvil which breaks up the drops into smaller droplets. May be combined with a heater.
 mechanical: water is dispersed into a mist by a spinning disc.
mechanical: water is dispersed into a mist by a spinning disc.
Gas-driven devices are used for drug delivery; all types may be used for humidification.
Neck, cross-sectional anatomy. At the level of C6, major anatomical structures within the layer of skin, fat and subcutaneous tissue may be described in terms of fascial layers (Fig. 113):
 superficial fascia: encloses platysma muscle and deep fascial layers.
superficial fascia: encloses platysma muscle and deep fascial layers.
 deep fascia: composed of three layers:
deep fascia: composed of three layers:
– investing fascia: lies posterior to the anterior and external jugular veins. Splits to enclose sternohyoid, sternothyroid, omohyoid, sternomastoid and trapezius muscles.
 pretracheal fascia: contains the trachea, oesophagus and thyroid gland.
pretracheal fascia: contains the trachea, oesophagus and thyroid gland.
See also, Carotid arteries; Tracheobronchial tree

Fig. 113 Cross-section of neck at C6
Necrotising enterocolitis (NEC). Necrosis of GIT mucosa (especially terminal ileum, caecum and ascending colon) seen in neonates, usually within the first week of life. Prevalence is up to 8% in premature and low-birth-weight babies; predisposing factors include asphyxia, hypotension and umbilical catheterisation. Mucosal damage follows hypoperfusion and ischaemia, leading to abdominal distension, vomiting and faecal blood and mucus, although the onset may be insidious. Pallor, bradycardia, jaundice, intestinal perforation and DIC may occur. Plain abdominal X-ray shows dilated loops of bowel and intramural gas bubbles.
Management is largely supportive, with iv fluids, IPPV, correction of anaemia, antibacterial drugs (including anaerobic cover), probiotic agents and TPN. Surgery may be required if perforation occurs or there is no improvement despite medical therapy. Quoted mortality ranges from 10% to 50%. Survivors commonly exhibit impaired psychomotor development.
Necrotising fasciitis. Uncommon deep-seated infection of subcutaneous tissue, resulting in destruction of fat and fascia. Predisposing factors include immunosuppression, alcoholism, diabetes, peripheral vascular disease, surgery, penetrating injuries (which may be minor) and varicella infection, although it may affect young healthy individuals. Systemic upset is thought to result from bacterial toxins, endogenous cytokines and other inflammatory mediators. May be rapidly fatal unless aggressively treated. May be caused by:
 Gram-negative bacilli, enterococci and mixed anaerobes. Infection involves fat and fascia, although the skin is usually spared. Includes Fournier’s gangrene of the perineum.
Gram-negative bacilli, enterococci and mixed anaerobes. Infection involves fat and fascia, although the skin is usually spared. Includes Fournier’s gangrene of the perineum.
 group A streptococci. Features include systemic toxicity, pain, necrosis of subcutaneous tissue and skin, gangrene, shock and MODS.
group A streptococci. Features include systemic toxicity, pain, necrosis of subcutaneous tissue and skin, gangrene, shock and MODS.
 prompt diagnosis; made on clinical grounds, although MRI and biopsy may help distinguish it from acute cellulitis.
prompt diagnosis; made on clinical grounds, although MRI and biopsy may help distinguish it from acute cellulitis.
 early surgical debridement (the diagnosis is usually confirmed at surgery), with fasciotomy to prevent compartment syndrome.
early surgical debridement (the diagnosis is usually confirmed at surgery), with fasciotomy to prevent compartment syndrome.
 antibacterial drug therapy and general supportive care; hyperbaric oxygen has been used.
antibacterial drug therapy and general supportive care; hyperbaric oxygen has been used.
Needles. Christopher Wren described injection via a quill and bladder in 1659. Metal tubes and stylets were subsequently used, but the hypodermic cannula and trocar were first described by Rynd in 1845. Different sizes and types are available for different uses, e.g. for iv/hypodermic use, epidural and spinal anaesthesia. Short-bevelled needles are traditionally preferred for regional anaesthesia. Hollow needles are not required for acupuncture or electrical stimulation/recording.
Needle size is described by a wire gauge classification (G; Stubs gauge; Birmingham gauge), which originally referred to the number of times the wire was drawn through the draw plate (Table 30). It differs slightly from the American and Standard wire gauges. Internal diameter varies according to different materials and needle strengths. The system is also used for iv cannulae. For hypodermic needles, colour-coding is mandatory in the UK for certain sizes: 26 G brown; 25 G orange; 23 G blue; 22 G black; 21 G green; 20 G yellow; 19 G cream.
Table 30 Diameter of needles of different gauge number
| Gauge number (G) | Outside diameter (mm) |
| 36 | 0.10 |
| 30 | 0.30 |
| 29 | 0.33 |
| 28 | 0.36 |
| 27 | 0.41 |
| 26 | 0.46 |
| 25 | 0.51 |
| 24 | 0.56 |
| 23 | 0.64 |
| 22 | 0.71 |
| 21 | 0.81 |
| 20 | 0.90 |
| 19 | 1.08 |
| 18 | 1.27 |
| 17 | 1.50 |
| 16 | 1.65 |
| 15 | 1.83 |
| 14 | 2.11 |
| 13 | 2.41 |
| 12 | 2.77 |
| 11 | 3.05 |
| 10 | 3.40 |
| 9 | 3.76 |
| 8 | 4.19 |
| 7 | 4.57 |
| 6 | 5.16 |
| 1 | 7.62 |
Needlestick injury, see Environmental safety of anaesthetists
Nefopam hydrochloride. Centrally acting analgesic drug, unrelated to opioid analgesic drugs and NSAIDs. Inhibits reuptake of 5-HT, noradrenaline and dopamine. Has similar analgesic potency to NSAIDs. Peak action occurs 1–2 h after im injection. Drowsiness and respiratory depression may occur, but less than with opioids.
• Dosage: 30–90 mg orally tds.
• Side effects: nausea, headache, confusion, anticholinergic effects. Should be avoided in patients with epilepsy and those taking monoamine oxidase inhibitors.
Evans MS, Lysakowski C, Tramèr MR (2008). Br J Anaesth; 101: 610–17
Negative end-expiratory pressure (NEEP). Adjunct to IPPV, popular in the 1960s–70s as a method of reducing the adverse cardiovascular effects of IPPV by maintaining a subatmospheric airway pressure at end-expiration. However, NEEP increases airway collapse, alveolar–arterial O2 difference and dead space, whilst reducing FRC. Thus no longer used.
Negligence. Civil charge in which the following must be established:
Neisserial infections. Mostly caused by two species of the Gram-positive cocci genus:
 Neisseria gonorrhoeae: may cause acute endocarditis, urethritis, pelvic inflammatory disease and pelvic abscesses.
Neisseria gonorrhoeae: may cause acute endocarditis, urethritis, pelvic inflammatory disease and pelvic abscesses.
 N. meningitidis (meningococcus): causes meningococcal disease, including meningitis. The organism may be carried in the nasopharynx of about 5% of otherwise healthy subjects, increasing to about 30% during epidemics.
N. meningitidis (meningococcus): causes meningococcal disease, including meningitis. The organism may be carried in the nasopharynx of about 5% of otherwise healthy subjects, increasing to about 30% during epidemics.
Neisserial infection is common in complement deficiency.
Neomycin sulphate. Aminoglycoside and antibacterial drug, used orally for selective decontamination of the digestive tract, specifically in hepatic failure and before bowel surgery. Also used topically for skin or mucous membrane infections.
Neonatal resuscitation, see Cardiopulmonary resuscitation, neonatal
Neonatal Resuscitation Program (NRP). Programme of training in neonatal CPR, established in 1988 in the USA and administered by the American Heart Association and American Academy of Pediatrics. Similar in concept to the ATLS and related courses. Has been referred to as ‘NALS’ (Neonatal Advanced Life Support), but the term is not an official one.
Neonate. Child within 28 days of birth. Normally weighs 3–4 kg, with body surface area approximately 0.19 m2.
• Major changes at birth include the following:
 change from fetal circulation to adult circulation via transitional circulation. The fibrous left ventricle, which is of similar size to the right ventricle at birth, gradually increases in compliance and contractility.
change from fetal circulation to adult circulation via transitional circulation. The fibrous left ventricle, which is of similar size to the right ventricle at birth, gradually increases in compliance and contractility.
Anatomical and physiological features and principles of anaesthesia are as for paediatric anaesthesia. Perioperative risks are higher than for older children, especially in premature neonates; surgery is usually deferred if possible.
See also, Cardiopulmonary resuscitation, neonatal; Fetal haemoglobin; Fetal monitoring; Neurobehavioural testing of neonates; Obstetric analgesia and anaesthesia; Paediatric advanced life support; Paediatric intensive care; Surfactant
Neostigmine methylsulphate/bromide. Acetylcholinesterase inhibitor, first synthesised in 1931. Used to increase acetylcholine concentrations at the neuromuscular junction, e.g. reversal of non-depolarising neuromuscular blockade and myasthenia gravis. Also has a direct stimulatory effect on skeletal muscle acetylcholine receptors; in addition, it is thought to have significant presynaptic action, increasing the amount of acetylcholine released. May cause depolarising neuromuscular blockade in overdosage. Other effects are those of muscarinic stimulation, e.g. bradycardia, increased GIT motility and bladder contractility, sweating, salivation, miosis, bronchospasm. Has been used to treat urinary retention and ileus, e.g. postoperatively. Effects on autonomic ganglia are small, consisting of stimulation at low doses and depression at high doses. A quaternary ammonium compound, it crosses the blood–brain barrier poorly and has few CNS effects. Routinely given with atropine or glycopyrronium when administered iv to prevent muscarinic side effects. Active within 1 min of iv injection, with action lasting 20–30 min. Active for up to 4 h after oral administration. Excreted mainly renally, mostly unchanged. Elimination half-life is 50–90 min. May be administered parenterally (as methylsulphate) or orally (as bromide). Has also been given intrathecally; produces analgesia but with increased nausea and vomiting.
Nephritic syndrome. Acute glomerulonephritis characterised by reduction of glomerular filtration rate, haematuria, proteinuria, salt and water retention, increased intravascular volume and hypertension. Most commonly a post-infectious condition, it is also seen in SLE. Usually mild; severe cases may result in acute kidney injury. Distinction between it and nephrotic syndrome has been overplayed in the past and both share common aetiologies.
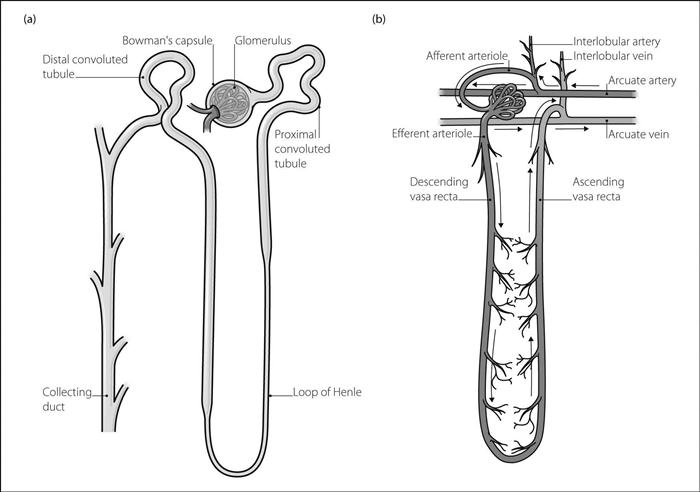
Nephron. Basic renal unit; each kidney contains about 1.3 million.
 glomerulus: formed by a 200 µm diameter invagination of capillaries into the blind end of the nephron (Bowman’s capsule). Water is filtered from the blood across the glomerular membrane, together with substances under 4–8 nm in diameter. GFR equals about 120 ml/min (180 1/day).
glomerulus: formed by a 200 µm diameter invagination of capillaries into the blind end of the nephron (Bowman’s capsule). Water is filtered from the blood across the glomerular membrane, together with substances under 4–8 nm in diameter. GFR equals about 120 ml/min (180 1/day).
 tubule: 45–65 mm long. The site of reabsorption/secretion of substances from/into the filtrate, giving rise to the eventual composition of urine. Consists of:
tubule: 45–65 mm long. The site of reabsorption/secretion of substances from/into the filtrate, giving rise to the eventual composition of urine. Consists of:
– proximal convoluted tubule: 15 mm long. Lies within the renal cortex. Lined by a brush border. Site of active reabsorption of sodium and potassium ions, bicarbonate, phosphate, glucose, uric acid and amino acids. Water moves passively from the tubule by osmosis. Up to 80% of filtered water and solutes is reabsorbed.
– loop of Henle: about 15–25 cm long; length depends on whether the glomerulus lies within the outer or inner renal cortex (short in the former, long in the latter). A further 15% of filtered water is reabsorbed. 15% of loops extend into the medulla, where interstitial osmolarity is very high (up to 1200 mosmol/l). Water moves out of the descending limb, followed by sodium ions along a concentration gradient as the tubular fluid becomes more concentrated. In the ascending limb, which is impermeable to both water and sodium ions, sodium and chloride ions are actively co-transported from the tubule. The fluid thus becomes more dilute as it ascends. Urea is relatively free to pass across the tubular membranes. The solutes remain in the region of the medulla because of the countercurrent multiplier mechanism whereby the blood vessels supplying the loop pass close to those draining it. Solutes pass down concentration gradients from ascending vessels to descending vessels, and thus recirculate at the tip of the loop. Water passes from the descending vessels to the ascending vessels, and is thus removed from the area. This maintains the high osmolarity in the medullary region. The thick ascending segment forms part of the juxtaglomerular apparatus where it passes near the glomerulus.
– distal convoluted tubule: 5 mm long. A further 5% of filtered water is reabsorbed. Sodium ions are reabsorbed in exchange for potassium or hydrogen ions, under the influence of aldosterone.
 collecting ducts: 20 mm long. Each receives several tubules. Pass through the cortex and medulla, opening into the renal pelvis at the medullary pyramids. Some sodium/potassium/hydrogen ion exchange occurs at the cortical part. Water is reabsorbed depending on the amount of vasopressin present, which increases tubular permeability to water and thus increases urine concentration.
collecting ducts: 20 mm long. Each receives several tubules. Pass through the cortex and medulla, opening into the renal pelvis at the medullary pyramids. Some sodium/potassium/hydrogen ion exchange occurs at the cortical part. Water is reabsorbed depending on the amount of vasopressin present, which increases tubular permeability to water and thus increases urine concentration.
 afferent and efferent arterioles supply and drain the capillaries to the glomerulus respectively.
afferent and efferent arterioles supply and drain the capillaries to the glomerulus respectively.
 peritubular capillaries and ascending vasa recta drain into interlobular veins.
peritubular capillaries and ascending vasa recta drain into interlobular veins.
See also, Acid–base balance; Clearance; Diuretics; Renin/angiotensin system
Nephrotic syndrome. Defined by daily urinary protein excretion exceeding 3.5 g/1.73 m2 body surface area. May be caused by primary or secondary (e.g. to diabetes mellitus, pre-eclampsia, connective tissue disease, post-viral hepatitis or streptococcal infection, drugs such as NSAIDs or captopril) glomerular disease. Features include generalised oedema, susceptibility to infection and thromboembolism (especially renal vein thrombosis and DVT) and hyperlipidaemia. Hypoalbuminaemia may lead to altered drug binding.
Treatment includes a low-sodium diet and diuretic therapy to reduce oedema, and a low-protein diet and angiotensin converting enzyme inhibitors to reduce proteinuria. Other treatment is directed against the cause, e.g. corticosteroids in glomerulonephritis.
Nernst equation. Equation for calculating the membrane potential at which an individual ion is at equilibrium across the membrane (assuming complete permeability to that ion). For ion X:
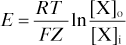
where E = equilibrium potential
F = Faraday constant (coulombs per mole of charge)
[X]o = extracellular concentration of X
[X]i = intracellular concentration of X.
For chloride, potassium and sodium, E = −70 mV, −94 mV and +60 mV respectively. Since the normal resting membrane potential is about −70 mV, other factors must affect potassium and especially sodium distribution (e.g. relative permeability and the sodium/potassium pump).
[Hermann W Nernst (1864–1941), German physicist; Michael Faraday (1791–1867), English scientist]
Nerve. Excitable tissue whose function is the transmission of nerve impulses. Typical peripheral nerves consist of several groups of fascicles. Each fascicle is surrounded by the perineurium and contains a group of neurones, the axons of which are encased in the endoneurium (Fig. 115).
Peripheral nerves originate in the spinal cord, and may be sensory, motor or mixed. Some also carry autonomic nervous system fibres.
See also, Motor pathways; Sensory pathways
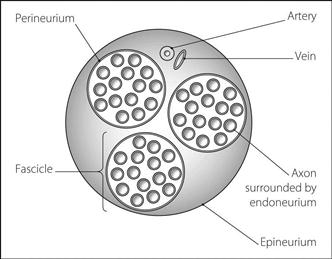
Fig. 115 Cross-section of a typical nerve
Nerve conduction. Passage of an action potential along neurones; involves waves of depolarisation and repolarisation that move longitudinally across the nerve membrane.
In unmyelinated nerves, impulses spread at up to 2 m/s. Positive charge flows into the depolarised area from the membrane just distally, altering the distal permeability to ions (especially sodium and potassium) as for action potential generation. When the threshold potential is reached, depolarisation occurs. Retrograde conduction is prevented by the refractory period of the membrane proximally.
The myelin sheath of myelinated nerves acts as an insulator that prevents the flow of ions across the nerve membrane. Breaks in the myelin (nodes of Ranvier), approximately 1 mm apart, allow ions to flow freely between the neurone and the ECF at these points. Depolarisation ‘jumps’ from node to node (saltatory conduction), a process that increases conduction velocity (up to 120 m/s) and conserves energy.
[Louis A Ranvier (1835–1922), French pathologist and physician]
Nerve growth factor (NGF). Protein produced by many cell types; taken up by small sensory and sympathetic nerve fibres via specific receptors and retrogradely transported to the cell body. Required for growth and survival of neurones in the fetus and neonate; released from connective tissue and inflammatory cells following tissue injury in response to cytokine stimulation. Causes hyperalgesia via both central and peripheral effects; thus thought to be important in acute and chronic pain states.
Sofroniew MV, Howe CL, Mobley WC (2001). Ann Rev Neurosci; 24: 1217–81
Nerve injury during anaesthesia. May occur during general, local or regional anaesthesia.
• Causes of neuronal injury include:
– poor positioning of the patient; thought to cause local nerve ischaemia.
– ischaemia caused by hypotension or use of tourniquets.
– hypothermia.
– extravasation of drugs into perineural tissue.
– toxicity of degradation products of anaesthetic agents, classically trichloroethylene with soda lime.
 local/regional anaesthesia: positioning/ischaemia/hypothermia as above plus:
local/regional anaesthesia: positioning/ischaemia/hypothermia as above plus:
– direct trauma from a needle or catheter.
– intraneural injection of local anaesthetic agent.
– cauda equina syndrome following use of spinal catheters for continuous spinal anaesthesia.
– chemical contamination of local anaesthetic, or injection of the wrong solution.
– poor positioning of the anaesthetised limb with ischaemia as above.
• Classic division of nerve injuries:
 axonotmesis: axonal and myelin loss within the intact connective tissue sheath. Typically there is complete motor and sensory loss, with slow recovery due to nerve regeneration from proximal to distal nerve.
axonotmesis: axonal and myelin loss within the intact connective tissue sheath. Typically there is complete motor and sensory loss, with slow recovery due to nerve regeneration from proximal to distal nerve.
 neurotmesis: partial or complete severance. Recovery is rare.
neurotmesis: partial or complete severance. Recovery is rare.
• Many specific neuropathies have been described, including lesions of the following:
 brachial plexus: usually stretched, typically by shoulder abduction and extension, with supination. Stretch is exacerbated by bilateral abduction. Upper roots are usually affected; weakness lasts up to several months, although recovery usually occurs within 2–3 months. Lower roots may be damaged during sternal retraction in cardiac surgery. Compression may be caused by shoulder rests in the steep head-down position, resulting in temporary palsy.
brachial plexus: usually stretched, typically by shoulder abduction and extension, with supination. Stretch is exacerbated by bilateral abduction. Upper roots are usually affected; weakness lasts up to several months, although recovery usually occurs within 2–3 months. Lower roots may be damaged during sternal retraction in cardiac surgery. Compression may be caused by shoulder rests in the steep head-down position, resulting in temporary palsy.
 ulnar nerve (most common nerve injury reported): may be compressed between the humeral epicondyle and the operating table or arm supports, or injured by poles during transfer of the patient.
ulnar nerve (most common nerve injury reported): may be compressed between the humeral epicondyle and the operating table or arm supports, or injured by poles during transfer of the patient.
 radial nerve: caused by the patient’s arm hanging over the side of the operating table.
radial nerve: caused by the patient’s arm hanging over the side of the operating table.
 median nerve: may be damaged by direct needle trauma, or drug extravasation in the antecubital fossa.
median nerve: may be damaged by direct needle trauma, or drug extravasation in the antecubital fossa.
 abducens nerve: temporary lesions may follow spinal or epidural anaesthesia.
abducens nerve: temporary lesions may follow spinal or epidural anaesthesia.
 trigeminal nerve: typically damaged by the trichloroethylene/soda lime interaction.
trigeminal nerve: typically damaged by the trichloroethylene/soda lime interaction.
 supraorbital nerve: compressed by the tracheal tube connector, catheter mount, head harness or ventilator tubing.
supraorbital nerve: compressed by the tracheal tube connector, catheter mount, head harness or ventilator tubing.
 common peroneal nerve: compressed between lithotomy pole and fibular head.
common peroneal nerve: compressed between lithotomy pole and fibular head.
 saphenous nerve: compressed between lithotomy pole and medial tibial condyle.
saphenous nerve: compressed between lithotomy pole and medial tibial condyle.
 pudendal nerve: compressed between a poorly padded perineal post and the ischial tuberosity.
pudendal nerve: compressed between a poorly padded perineal post and the ischial tuberosity.
Nerve injury may also be caused by surgical trauma/compression.
Similar concerns exist for patients undergoing prolonged treatment on ICU.
Nerve stimulator, see Neuromuscular blockade monitoring; Regional anaesthesia; Transcutaneous electrical nerve stimulation
Netilmicin. Aminoglycoside and antibacterial drug with similar activity to gentamicin but less active against pseudomonas. Less ototoxic than gentamicin.
Neuralgia. Pain in the distribution of a defined nerve or group of nerves.
Neuritis. Inflammation of nerve(s).
Neurobehavioural testing of neonates. Investigation of the effects of obstetric analgesia and anaesthesia on the neonate is difficult because of many variables, e.g. obstetric details, fetal distress, method of delivery, type and route of drugs administered, methods of analysis of data. In many early studies, aortocaval compression was not avoided.
 pethidine: reduces alertness and responsiveness before respiratory depression is evident. Greatest effect is at 2 days. Rapid placental transfer follows maternal iv injection.
pethidine: reduces alertness and responsiveness before respiratory depression is evident. Greatest effect is at 2 days. Rapid placental transfer follows maternal iv injection.
 anaesthetic agents: thiopental causes more neonatal depression than ketamine (but tone is increased by ketamine, giving higher scores). Low concentrations of volatile inhalational anaesthetic agents produce little, if any, effects. Regional techniques consistently produce higher scores.
anaesthetic agents: thiopental causes more neonatal depression than ketamine (but tone is increased by ketamine, giving higher scores). Low concentrations of volatile inhalational anaesthetic agents produce little, if any, effects. Regional techniques consistently produce higher scores.
 local anaesthetic agents: initial fears of hypotonia following lidocaine have now been dispelled. All local anaesthetic drugs have similar effects, lowering scores only when very sensitive testing is employed. The significance of this is unknown.
local anaesthetic agents: initial fears of hypotonia following lidocaine have now been dispelled. All local anaesthetic drugs have similar effects, lowering scores only when very sensitive testing is employed. The significance of this is unknown.
Neurofibromatosis. Group of neurocutaneous diseases characterised by multiple tumours derived from the neurilemma sheath of cranial and peripheral nerves/nerve roots.
 neurofibromatosis 1 (NF-1; von Recklinghausen’s disease). Autosomal dominant disease with gene locus at chromosome 17, with an incidence of 1:3000. Flat, brown ‘café au lait’ spots occur in all sufferers, six or more spots larger than 1.5 cm being diagnostic. Neurofibromata may be subcutaneous/cutaneous, or may occur in deeper peripheral nerves or autonomic nerves supplying viscera. They may also occur at the foramen magnum or within the theca, causing nerve root or spinal cord compression. Pulmonary fibrosis occurs in 20% of cases; hypertension is present in 6% of patients and may be associated with phaeochromocytoma (in 1%) or renal artery stenosis. Intracranial tumours occur in 5–10% of cases, and skeletal abnormalities (including kyphoscoliosis) in 10%. Potential anaesthetic problems result from the distribution of neurofibromata and may include difficulty with tracheal intubation or regional blocks. Despite earlier reports, patients exhibit normal sensitivity to neuromuscular blocking drugs.
neurofibromatosis 1 (NF-1; von Recklinghausen’s disease). Autosomal dominant disease with gene locus at chromosome 17, with an incidence of 1:3000. Flat, brown ‘café au lait’ spots occur in all sufferers, six or more spots larger than 1.5 cm being diagnostic. Neurofibromata may be subcutaneous/cutaneous, or may occur in deeper peripheral nerves or autonomic nerves supplying viscera. They may also occur at the foramen magnum or within the theca, causing nerve root or spinal cord compression. Pulmonary fibrosis occurs in 20% of cases; hypertension is present in 6% of patients and may be associated with phaeochromocytoma (in 1%) or renal artery stenosis. Intracranial tumours occur in 5–10% of cases, and skeletal abnormalities (including kyphoscoliosis) in 10%. Potential anaesthetic problems result from the distribution of neurofibromata and may include difficulty with tracheal intubation or regional blocks. Despite earlier reports, patients exhibit normal sensitivity to neuromuscular blocking drugs.
[Friedrich D von Recklinghausen (1833–1910), German pathologist]
Hirsch NP, Murphy A, Radcliffe JJ (2001). Br J Anaesth; 86: 555–64
Neurokinin-1 receptor antagonists. Antiemetic drugs, acting via inhibition at neurokinin-1 (NK1) receptors present in the GIT and CNS. Aprepitant is currently licensed for nausea and vomiting induced by cancer chemotherapy. Have also been studied in PONV.
Diemunsch P, Joshi GP, Brichant J-F (2009). Br J Anaesth; 103: 7–13
Neurolepsis, see Neuroleptanaesthesia and analgesia
Neuroleptanaesthesia and analgesia. Use of very potent opioid analgesic drugs (e.g. fentanyl and phenoperidine) combined with butyrophenones (e.g. droperidol and haloperidol) to produce a state of reduced motor activity and passivity (neurolepsis). Introduced in 1959. The term neuroleptanaesthesia is usually restricted to the combination of opioid, butyrophenone and N2O. Characterised by profound analgesia, sedation and antiemesis, with cardiovascular stability (although mild hypotension may occur). Has been used for premedication, sedation and as the sole anaesthetic technique, with/without neuromuscular blocking drugs, for surgical procedures (now rarely employed for the latter use because of prolonged recovery).
Neuroleptic malignant syndrome (NMS). Rare condition first described in 1960, characterised by altered consciousness, hyperthermia, autonomic dysfunction and muscle rigidity. Usually triggered by drugs (e.g. butyrophenones, phenothiazines, metoclopramide, lithium, reserpine), although it has been reported during withdrawal of L-dopa in patients with Parkinson’s disease. The mechanism is thought to involve dopamine receptor blockade in the basal ganglia and hypothalamus. Occurs mostly in young males. Incidence is increased with dehydration, CNS disease and exhaustion.
• Features develop over 1–3 days:
 extrapyramidal dysfunction: rigidity, dystonia, tremor.
extrapyramidal dysfunction: rigidity, dystonia, tremor.
 autonomic dysfunction: labile BP, sweating, salivation, urinary incontinence.
autonomic dysfunction: labile BP, sweating, salivation, urinary incontinence.
 increased creatine kinase (> 1000 units/l) and white cell count.
increased creatine kinase (> 1000 units/l) and white cell count.
Differential diagnosis is as for hyperthermia (in particular MH), Parkinson’s disease, catatonia, central anticholinergic syndrome, monoamine oxidase inhibitor reaction and infection, e.g. tetanus. Although similar to MH, NMS is generally considered an entirely separate entity.
 supportive: O2, cooling, hydration, DVT prophylaxis.
supportive: O2, cooling, hydration, DVT prophylaxis.
 increased central dopaminergic activity, e.g. with bromocriptine (dopamine agonist) 2.5–20 mg tds (orally only). Amantidine and L-dopa have also been used.
increased central dopaminergic activity, e.g. with bromocriptine (dopamine agonist) 2.5–20 mg tds (orally only). Amantidine and L-dopa have also been used.
 dantrolene and non-depolarising neuromuscular blocking drugs have been used to treat the peripheral muscle effects, reducing fever, rigidity and tachycardia. The latter drugs are effective in NMS, in contrast to MH.
dantrolene and non-depolarising neuromuscular blocking drugs have been used to treat the peripheral muscle effects, reducing fever, rigidity and tachycardia. The latter drugs are effective in NMS, in contrast to MH.
 anticholinergic drugs have also been used.
anticholinergic drugs have also been used.
Mortality is 20–30%, from renal failure, arrhythmias, PE or aspiration pneumonitis.
Adnet P, Lestovel P, Krivosic-Horber R (2000). Br J Anaesth; 85: 129–35
Neuromuscular blockade monitoring. Ideally, this should be undertaken whenever non-depolarising neuromuscular blocking drugs are used since residual block is common in the recovery room, even after the use of intermediate-acting drugs such as atracurium and vecuronium. A nerve stimulator is used to stimulate a peripheral nerve via surface or needle electrodes; the muscle response is then assessed.
 mechanical: reflects both neuromuscular transmission and muscle contractility. Assessed by:
mechanical: reflects both neuromuscular transmission and muscle contractility. Assessed by:
– measurement of tension developed in a muscle with a strain gauge or pressure transducer.
 electrical: registers the EMG response via two surface/needle electrodes. Only monitors transmission across the neuromuscular junction, and thus is more specific than mechanical assessment.
electrical: registers the EMG response via two surface/needle electrodes. Only monitors transmission across the neuromuscular junction, and thus is more specific than mechanical assessment.
 unipolar square waveform lasting 0.2–0.3 ms (ensures constant current during stimulation).
unipolar square waveform lasting 0.2–0.3 ms (ensures constant current during stimulation).
– post-tetanic stimulation using single pulses.
– double-burst stimulation: used to assess recovery from non-depolarising blockade. Two short tetanic stimulations (e.g. 50 Hz for 60 ms) are applied 750 ms apart. The second response is weaker than the first in non-depolarising blockade. More sensitive at detecting fade than TOF.
 normal neuromuscular function:
normal neuromuscular function:
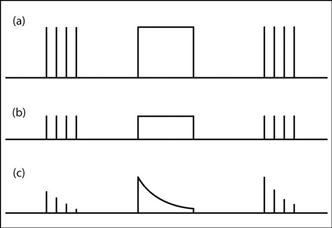
– equal twitches in response to single pulses (Fig. 116a).
– sustained tetanic contraction, with post-tetanic potentiation (PTP) revealed by mechanical assessment only.
 depolarising neuromuscular blockade:
depolarising neuromuscular blockade:
– equal but reduced twitches in response to single pulses and TOF (Fig. 116b). TOF ratio thus equals unity.
– sustained but reduced tetanic contraction, with neither fade nor PTP.
– dual block may supervene if large amounts of suxamethonium are administered.
 non-depolarising neuromuscular blockade.
non-depolarising neuromuscular blockade.
– progressively decreasing twitches in response to single pulses (Fig. 116c), with eventual disappearance.
– tetanic contraction exhibits fade and PTP.
– TOF count of 1 for tracheal intubation.
– TOF count of 3–4 before attempting reversal of blockade, especially with long-acting drugs.
– TOF ratio (at the thumb) of 0.9 for adequate maintenance of spontaneous ventilation.
Fuchs-Buder T, Schreiber JU, Meistelman C (2009). Anaesthesia; 64 (Suppl 1): 82–9
Neuromuscular blocking drugs. Drugs used to impair neuromuscular transmission and provide skeletal muscle relaxation during anaesthesia or critical care.
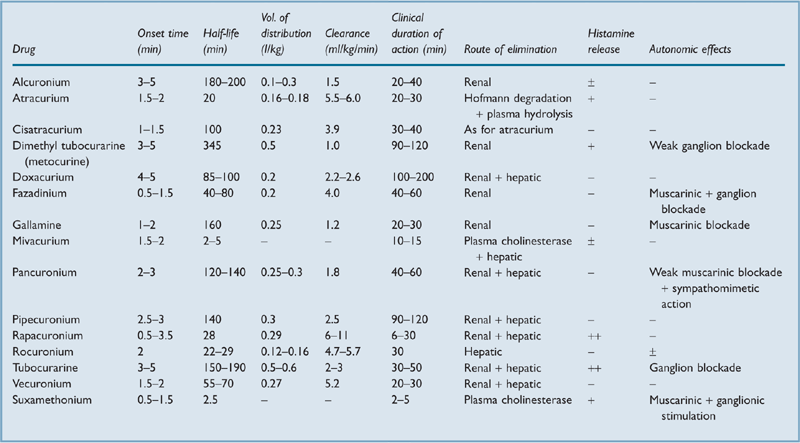
 non-depolarising: include tubocurarine (first used as curare in 1912), gallamine (1948), dimethyl tubocurarine (1948), alcuronium (1961), pancuronium (1967), fazadinium (1972), atracurium (1980), vecuronium (1983), pipecuronium (1990), doxacurium (1991), mivacurium (1993), rocuronium (1994), cisatracurium (1995), and rapacuronium (1999). Non-depolarising agents are competitive antagonists at postsynaptic acetylcholine (ACh) receptors of the neuromuscular junction. They are highly ionised at body pH, containing two quaternary ammonium groups (tubocurarine and vecuronium contain one each, but acquire a second following injection). Poorly lipid-soluble with variable protein binding. Following injection, the drugs are rapidly redistributed from blood to the ECF and other tissues, e.g. kidney, liver. The clinical effect depends on individual drug characteristics and drug concentration at the neuromuscular junction, which depends on the drug’s pharmacokinetics.
non-depolarising: include tubocurarine (first used as curare in 1912), gallamine (1948), dimethyl tubocurarine (1948), alcuronium (1961), pancuronium (1967), fazadinium (1972), atracurium (1980), vecuronium (1983), pipecuronium (1990), doxacurium (1991), mivacurium (1993), rocuronium (1994), cisatracurium (1995), and rapacuronium (1999). Non-depolarising agents are competitive antagonists at postsynaptic acetylcholine (ACh) receptors of the neuromuscular junction. They are highly ionised at body pH, containing two quaternary ammonium groups (tubocurarine and vecuronium contain one each, but acquire a second following injection). Poorly lipid-soluble with variable protein binding. Following injection, the drugs are rapidly redistributed from blood to the ECF and other tissues, e.g. kidney, liver. The clinical effect depends on individual drug characteristics and drug concentration at the neuromuscular junction, which depends on the drug’s pharmacokinetics.
 depolarising: cause depolarisation by mimicking the action of ACh at ACh receptors, but without rapid hydrolysis by acetylcholinesterase. An area of depolarisation around the ACh receptor–drug complex results in local currents which open sodium channels before the continuing current flow inactivates them. Propagation of an action potential is prevented by the area of inexcitability that develops around the ACh receptors. Thus fasciculations occur before paralysis. Examples are suxamethonium (1951) and decamethonium (1948); only the former is available for clinical use in the UK.
depolarising: cause depolarisation by mimicking the action of ACh at ACh receptors, but without rapid hydrolysis by acetylcholinesterase. An area of depolarisation around the ACh receptor–drug complex results in local currents which open sodium channels before the continuing current flow inactivates them. Propagation of an action potential is prevented by the area of inexcitability that develops around the ACh receptors. Thus fasciculations occur before paralysis. Examples are suxamethonium (1951) and decamethonium (1948); only the former is available for clinical use in the UK.
Apart from the presence or absence of fasciculation, non-depolarising and depolarising neuromuscular blockade may be distinguished by neuromuscular blockade monitoring.
In general, suxamethonium is used for paralysis of rapid onset and short duration, e.g. to allow rapid tracheal intubation. The slower-acting non-depolarising drugs were traditionally used for prolonged paralysis when rapid intubation was not required, although rocuronium, atracurium and vecuronium have bridged the gap between these drugs and suxamethonium (Table 31).
See also, Interonium distance; Nicotine and nicotinic receptors
Neuromuscular junction. Synapse between the presynaptic motor neurone and the postsynaptic muscle membrane. On approaching the junction, the axon divides into terminal buttons that invaginate into the muscle fibre. The synaptic cleft is 50–70 nm wide and filled with ECF and a basement membrane containing high concentrations of acetylcholinesterase. The muscle membrane is folded into longitudinal gutters, whose ridges conceal orifices to secondary clefts. The orifices lie opposite the release points for acetylcholine (ACh) (Fig. 117).
• Three types of acetylcholine receptor have been identified at the neuromuscular junction:
 postjunctional: involved in traditional neuromuscular transmission. Following activation of both α subunits, sodium and calcium move into the myocyte and potassium exits through specific ion channels (see also Fig. 2b; Acetylcholine receptors).
postjunctional: involved in traditional neuromuscular transmission. Following activation of both α subunits, sodium and calcium move into the myocyte and potassium exits through specific ion channels (see also Fig. 2b; Acetylcholine receptors).
 prejunctional: control an ion channel specific for sodium and respond to released ACh by mobilising further ACh storage vesicles to the active zone of the junction, ready for release. Blockade of these receptors is thought to underlie the phenomenon of fade in non-depolarising neuromuscular blockade; activation during tetanic stimulation results in post-tetanic potentiation.
prejunctional: control an ion channel specific for sodium and respond to released ACh by mobilising further ACh storage vesicles to the active zone of the junction, ready for release. Blockade of these receptors is thought to underlie the phenomenon of fade in non-depolarising neuromuscular blockade; activation during tetanic stimulation results in post-tetanic potentiation.
 extrajunctional: normally present in small numbers, but proliferate over the muscle membrane in denervation hypersensitivity, burns and certain muscle diseases.
extrajunctional: normally present in small numbers, but proliferate over the muscle membrane in denervation hypersensitivity, burns and certain muscle diseases.
Martyn JAJ, Jonsson Fagerlund M, Eriksson LI (2009). Anaesthesia; 64 (Suppl 1): 1–9
See also, Neuromuscular blocking drugs
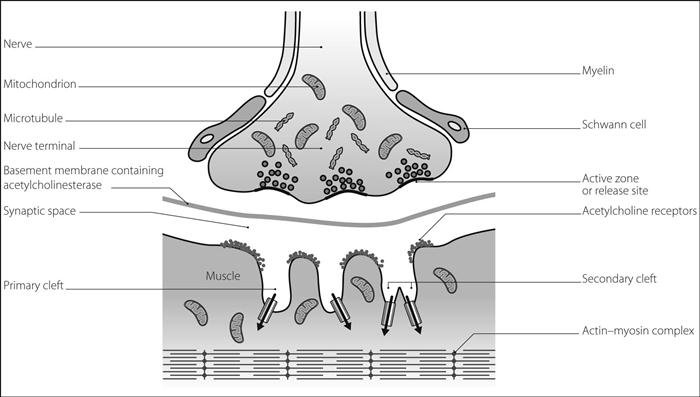
Fig. 117 Structure of neuromuscular junction
Neuromuscular transmission. Stages of transmission:
 depolarisation of the motor nerve leading to action potential propagation to the nerve endings at the neuromuscular junction.
depolarisation of the motor nerve leading to action potential propagation to the nerve endings at the neuromuscular junction.
 opening of presynaptic voltage-gated calcium channels. Resultant increase in intracellular calcium causes mobilisation of acetylcholine (ACh) vesicles to the active zone and subsequent release into the synapse.
opening of presynaptic voltage-gated calcium channels. Resultant increase in intracellular calcium causes mobilisation of acetylcholine (ACh) vesicles to the active zone and subsequent release into the synapse.
 binding of ACh to postsynaptic nicotinic ACh receptors, allowing sodium and calcium ion influx and causing an end-plate potential. If the latter is large enough, depolarisation of the muscle membrane occurs.
binding of ACh to postsynaptic nicotinic ACh receptors, allowing sodium and calcium ion influx and causing an end-plate potential. If the latter is large enough, depolarisation of the muscle membrane occurs.
 resultant action potential causing muscle contraction.
resultant action potential causing muscle contraction.
 hydrolysis of ACh by acetylcholinesterase within 1 ms.
hydrolysis of ACh by acetylcholinesterase within 1 ms.
• Transmission may be impaired by:
 inhibition of ACh synthesis, storage or release, e.g. by hemicholinium, β-bungarotoxin and botulinum toxins respectively. Aminoglycosides are also thought to impair ACh release, as does the myasthenic syndrome.
inhibition of ACh synthesis, storage or release, e.g. by hemicholinium, β-bungarotoxin and botulinum toxins respectively. Aminoglycosides are also thought to impair ACh release, as does the myasthenic syndrome.
 blockade of ACh receptors, e.g. by neuromuscular blocking drugs, α-bungarotoxin, receptor destruction in myasthenia gravis.
blockade of ACh receptors, e.g. by neuromuscular blocking drugs, α-bungarotoxin, receptor destruction in myasthenia gravis.
Naguib M, Flood P, McArdle JJ, Brenner HR (2002). Anesthesiology; 96: 202–31
Neurone. Basic unit of the nervous system. Consists of:
 cell body: contains the nucleus and most of the cytoplasm. Usually at the dendritic end of the neurone. The dendritic zone is the site of integration of incoming impulses via dendrites, and of initiation of the action potential.
cell body: contains the nucleus and most of the cytoplasm. Usually at the dendritic end of the neurone. The dendritic zone is the site of integration of incoming impulses via dendrites, and of initiation of the action potential.
 axon: may exceed 1 metre in length. May be myelinated or unmyelinated (see Myelin; Nerve conduction). Anterograde and retrograde flow of organelles and proteins occurs along the axon.
axon: may exceed 1 metre in length. May be myelinated or unmyelinated (see Myelin; Nerve conduction). Anterograde and retrograde flow of organelles and proteins occurs along the axon.
 terminal buttons (nerve endings): situated near the cell body or dendrites of other neurones and contain neurotransmitters.
terminal buttons (nerve endings): situated near the cell body or dendrites of other neurones and contain neurotransmitters.
 A: 1–20 µm diameter myelinated fibres. Subdivided into:
A: 1–20 µm diameter myelinated fibres. Subdivided into:
– α: 70–120 m/s conduction; somatic motor and proprioception sensation.
– β: 50–70 m/s; touch and pressure sensation.
– γ: 30–50 m/s; motor fibres to muscle spindles.
– δ: < 30 m/s; pain, cold, touch sensation.
 B: 1–3 µm diameter; < 15 m/s conduction: myelinated preganglionic autonomic fibres.
B: 1–3 µm diameter; < 15 m/s conduction: myelinated preganglionic autonomic fibres.
Local anaesthetic agents block C fibres first, then B, then A fibres. Pressure blocks A, B and C fibres in order, and hypoxia B, A and C fibres.
Neuropathy of critical illness, see Critical illness polyneuropathy
Neuroradiology. Most neuroradiological procedures are painless and do not require anaesthetic intervention; sedation or anaesthesia may be required in children, uncooperative or neurologically impaired patients or for prolonged procedures. Principles are as for radiology and neurosurgery.
 myelography: injection of contrast into the thecal sac to examine the spinal cord. Usually performed via lumbar puncture but occasionally via a cervical approach. Steep tilting is often required to aid spread of the contrast. Complications include headache, convulsions and arachnoiditis.
myelography: injection of contrast into the thecal sac to examine the spinal cord. Usually performed via lumbar puncture but occasionally via a cervical approach. Steep tilting is often required to aid spread of the contrast. Complications include headache, convulsions and arachnoiditis.
 cerebral angiography: injection of radiological contrast media via femoral or carotid puncture. Hyperventilation improves the arteriogram quality by increasing cerebrovascular resistance. Complications include CVA (1% of patients), haemorrhage, haematoma, thrombosis, arterial spasm and bradycardia (especially during vertebral angiography).
cerebral angiography: injection of radiological contrast media via femoral or carotid puncture. Hyperventilation improves the arteriogram quality by increasing cerebrovascular resistance. Complications include CVA (1% of patients), haemorrhage, haematoma, thrombosis, arterial spasm and bradycardia (especially during vertebral angiography).
 ventriculography and pneumoencephalography: injection of gas (usually air) into the ventricular system, with imaging in different positions. N2O is usually avoided. Bradycardia may occur. Rarely performed now.
ventriculography and pneumoencephalography: injection of gas (usually air) into the ventricular system, with imaging in different positions. N2O is usually avoided. Bradycardia may occur. Rarely performed now.
– balloon angioplasty and stenting: e.g. of occlusive cerebral disease and vasospasm secondary to subarachnoid haemorrhage. Deliberate hypertension may be required to maintain cerebral perfusion pressure and avoid ischaemia.
– carotid artery stenting for stenosis.
– thrombolysis of acute thromboembolic stroke. Cerebral haemorrhage may occur postoperatively.
Schulenburg E, Matta B (2011). Curr Opin Anesthesiol; 24: 426–32
Neurosurgery. Encompasses procedures involving the cranium, brain, meninges, cranial nerves, spinal cord and vertebral column, and those performed for pain management. Basic principles for intracranial surgery are related to maintenance of normal cerebral perfusion pressure and cerebral blood flow, with avoidance of cerebral ischaemia, cerebral steal and increased ICP. Cerebral protection has been employed.
– preoperative assessment of neurological status, hydrocephalus, etc. Endocrine abnormalities may be present (e.g. pituitary gland surgery).
– fluid and electrolyte imbalance may be present, especially if associated with reduced oral intake and vomiting.
– hypertension may be present, especially in association with subarachnoid haemorrhage.
– drug therapy may include anticonvulsant drugs and corticosteroids.
– other injuries may accompany head injury.
– sedative premedication is usually avoided because of possible perioperative respiratory depression and decreased conscious level.
– iv induction of anaesthesia is usual; most iv anaesthetic agents are suitable apart from ketamine. Smooth induction avoiding hypoxaemia, hypercapnia, hypertension and tachycardia is required. Hyperkalaemia has followed suxamethonium in certain upper and lower motor neurone lesions. β-Adrenergic receptor antagonists may be given to reduce the hypertensive response to laryngoscopy, whilst lidocaine 0.5–1.5 mg/kg may be given iv to reduce the increase in ICP. Adequate time should be allowed for full paralysis before tracheal intubation is attempted. Lidocaine spray may be employed during laryngoscopy. Use of a reinforced tracheal tube is usual, with thorough fixation. The eyes and face should be protected with padding.
– a large-bore iv cannula is necessary, since blood loss may be considerable. CVP measurement may be required, especially if the patient is to be positioned sitting. Arterial cannulation is usual. End-tidal CO2 measurement, pulse oximetry, ECG and temperature measurement are mandatory. Neuromuscular blockade monitoring is especially useful, since inadequate paralysis may have disastrous results. ICP monitoring, evoked potentials and EEG derivatives are sometimes employed.
– permissive hypothermia (to 35°C) is increasingly popular in an attempt to reduce cerebral metabolism.
– perioperative problems include:
– those related to positioning of the patient. The supine position is common; others include:
– lateral/prone: vena caval obstruction and damage to the face, eyes, etc., may occur.
– sitting (for posterior fossa lesions): air embolism, hypotension and obstruction of neck veins may occur. The first two may be reduced by the antigravity suit, PEEP and administration of iv fluids.
– inaccessibility of the airway.
– those of prolonged surgery, e.g. heat loss, fluid balance.
– arrhythmias and cardiovascular instability during manipulation of brainstem structures (posterior fossa lesions).
– maintenance is usually with air/O2 mixture with a volatile inhalational anaesthetic agent (e.g. isoflurane or sevoflurane) with or without a short-acting opioid, e.g. fentanyl, remifentanil. N2O is usually avoided because it increases cerebral blood flow and ICP and because of the risk of expansion of a pneumoencephalocoele. TIVA is also used. An arterial PCO2 of 4.5–5.0 (35–40 mmHg) is considered optimal.
– hypotensive anaesthesia is sometimes employed, especially for vascular lesions.
– bradycardia may follow application of suction to intracranial and extracranial drains.
– some procedures involving CT scanning (e.g. stereotactic surgery) require moving the anaesthetised patient between operating and imaging rooms.
– tracheal extubation is usually possible at the end of surgery and is performed under deep anaesthesia; coughing or straining should be avoided. Elective IPPV may be required, e.g. following prolonged operations and when ICP is critically raised. Airway obstruction caused by acute swelling of the tongue has been reported following posterior fossa surgery.
– close observation is required, in case of bleeding, vasospasm, increased ICP, convulsions, hypotension or hypertension. The Glasgow coma scale is employed for monitoring progress. ICP monitoring may be used.
– the patient should be kept normothermic to prevent postoperative shivering.
– morphine is a suitable choice for postoperative analgesia.
– diabetes insipidus or the syndrome of inappropriate antidiuretic hormone secretion may occur.
– increased risk of DVT has been associated with neurosurgery. In the immediate postoperative period mechanical methods of prophylaxis are usually preferred to heparin due to the potentially catastrophic effects of postoperative bleeding.
Neurotransmitters. Substances secreted from presynaptic nerve endings, which act at the postsynaptic membrane to cause excitatory or inhibitory effects. Act via specific receptors that elicit intracellular effects (e.g. by opening membrane ion channels, activating intracellular enzymes or altering DNA transcription). The same neurotransmitter may be excitatory at one synapse, and inhibitory at another.
 amines, e.g. noradrenaline, adrenaline, dopamine, 5-HT, histamine.
amines, e.g. noradrenaline, adrenaline, dopamine, 5-HT, histamine.
 amino acids, e.g. glycine, glutamate, GABA, aspartate.
amino acids, e.g. glycine, glutamate, GABA, aspartate.
 polypeptides, e.g. substance P, enkephalins. Substances active as circulating hormones may also function as neurotransmitters, e.g. vasopressin, oxytocin, vasoactive intestinal peptide, glucagon, somatostatin.
polypeptides, e.g. substance P, enkephalins. Substances active as circulating hormones may also function as neurotransmitters, e.g. vasopressin, oxytocin, vasoactive intestinal peptide, glucagon, somatostatin.
See also, Neuromuscular junction; Receptor theory; Synaptic transmission
Neutral thermal range, see Thermoneutral range
New injury severity score, see Injury severity score
New York Heart Association classification. Method of assessment of cardiac disease (originally cardiac failure), e.g. in preoperative assessment:
NGF, see Nerve growth factor
Nicardipine hydrochloride. Calcium channel blocking drug. Used in the UK for treatment of hypertension and ischaemic heart disease; also available parenterally in the USA for short-term reduction of BP, e.g. perioperatively. IV preparation is incompatible with bicarbonate and Hartmann’s solutions.
NiCO. Commercial non-invasive cardiac output measurement system that employs partial CO2 rebreathing and the Fick principle to estimate cardiac output. A small rebreathing loop is inserted into the patient’s breathing circuit and intermittently increases the volume of the circuit. Concentration and flow of CO2 are measured by a sensor placed between the patient and the rebreathing loop. The change in cardiac output is proportional to the ratio of the change in CO2 elimination and the resulting change in end-expiratory CO2.
Nicorandil. Potassium channel activator with a nitrate component, used to prevent and treat angina. Causes arterial and venous vasodilatation. Peak plasma levels occur within 30–60 min of administration. Only slightly protein-bound.
Nicotine. Toxic alkaloid derived from tobacco, mimics certain actions of acetylcholine, and was used to investigate the physiology of the autonomic nervous system. At low doses, it stimulates postsynaptic nicotinic acetylcholine receptors of the neuromuscular junction, autonomic ganglia and adrenal medulla; at high doses, it blocks them. Also causes CNS stimulation, followed by depression.
Nifedipine. Dihydropyridine calcium channel blocking drug, affecting coronary and peripheral vascular smooth muscle more than myocardial muscle. Negative inotropic effect is usually insignificant because of baroreceptor-mediated tachycardia.
Has no antiarrhythmic action. Used in hypertension, ischaemic heart disease and Raynaud’s phenomenon. Active within 20–30 min of oral administration, but a faster response follows sublingual retention of the capsule’s contents (though not licensed for sublingual use). May thus be administered sublingually during anaesthesia. 95% protein-bound. Half-life is 3–5 h. Metabolised in the liver and excreted renally.
Nimodipine. Dihydropyridine calcium channel blocking drug, preferentially affecting cerebral vascular smooth muscle. Increases cerebral blood flow, especially to poorly perfused areas, e.g. those affected by arterial spasm following subarachnoid haemorrhage (SAH).
Nitrazepam. Benzodiazepine widely used as a hypnotic drug. Has also been used as an anticonvulsant in childhood myoclonic epilepsy. Onset of sleep occurs within an hour; duration of action is 4–8 h. Extensively protein-bound; elimination half-life is up to 30 h, resulting in hangover effects during the day.
Nitric oxide (NO). Oxide of nitrogen, active as a biological mediator throughout the body but especially in:
 vascular endothelium: responsible for vascular relaxation. Reduced production has been implicated in vasospasm associated with various disease states, e.g. diabetes mellitus, hypertension and following subarachnoid haemorrhage. NO is thought to be the effector molecule for all nitrate vasodilator drugs.
vascular endothelium: responsible for vascular relaxation. Reduced production has been implicated in vasospasm associated with various disease states, e.g. diabetes mellitus, hypertension and following subarachnoid haemorrhage. NO is thought to be the effector molecule for all nitrate vasodilator drugs.
 brain tissue: acts as a neurotransmitter.
brain tissue: acts as a neurotransmitter.
 macrophages: involved in the response to infection.
macrophages: involved in the response to infection.
 platelets: involved in aggregation and adhesion.
platelets: involved in aggregation and adhesion.
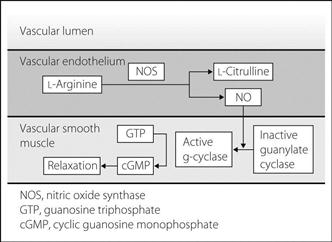
Synthesised in endothelial cells during the oxidation of L-arginine to L-citrulline, the reaction being catalysed by NO synthase (NOS). The NO thus produced diffuses into vascular smooth muscle and converts inactive guanylate cyclase into the active form; the latter converts guanosine triphosphate into cyclic guanosine monophosphate, which causes vascular relaxation (Fig. 118). Two forms of NOS exist: the constitutive (endothelial) form, present in vascular and brain tissue, which produces small quantities of NO continuously (eNOS); and the inducible form, present in macrophages (iNOS).
In sepsis, NO production is thought to be increased by endotoxin, the action of cytokines, e.g. tumour necrosis factor and certain interleukins. The amount of the inducible form of NOS increases, resulting in overproduction of NO with resultant excessive vasodilatation. NOS inhibitors have been investigated experimentally in the treatment of sepsis.
In neonatal, paediatric or adult pulmonary hypertension, inhaled NO (1–150 ppm) has been used to produce selective pulmonary vasodilatation without systemic effects. A clear effect on outcome in ARDS has not been conclusively demonstrated.
NO has a biological half-life of < 5 s, its action being terminated by combining with haemoglobin to form methaemoglobin.
Nitrogen. Non-metallic element existing in the atmosphere as a colourless, odourless ‘inert’ gas (isolated in 1772). Forms 78.03% of atmospheric air. Atomic weight is 14; boiling point is −195°C. Obtained by fractional distillation of air. Reacts poorly with other substances. Blood/gas solubility coefficient is 0.014. Has anaesthetic properties at hyperbaric pressures (see Inert gas narcosis). Converted into organic compounds by nitrifying bacteria and plants, and present throughout the body in amino acids and proteins.
Nitrogen balance. Difference between the amount of nitrogen ingested (as amino acids or proteins) and the amount of nitrogen excreted (mainly urinary). Usually measured within a 24-h period. Negative if losses exceed intake, e.g. catabolism, starvation; positive if intake exceeds losses, e.g. during recovery from severe illness.
 intake = the nitrogen content of all foods/fluids taken.
intake = the nitrogen content of all foods/fluids taken.
 output = the sum of nitrogen losses calculated from the following three components:
output = the sum of nitrogen losses calculated from the following three components:
– from urinary urea: nitrogen (g/24 h) = urea (mmol/24 h) × 6/5 because 1/6 is excreted as substances other than urea
× 1/1000 to convert mmol to mol
× 60 to convert mol urea to g
× 28/60 to convert g urea to g nitrogen
i.e. urea (mmol/24 h) × 0.0336.
– from blood urea: nitrogen (g/24 h) = change in urea (mmol/l/24 h) × 1/1000
× 60
× 28/60 as above
× 60% × body weight (kg) since urea is distributed amongst total body water
i.e. change in urea (mmol/l/24 h) × 0.0168 × body weight
– from other routes of loss, e.g. proteinuria: nitrogen loss (g/24 h) = protein loss (g/24 h)
× 1/6.25 since 6.25 g protein contains 1 g nitrogen.
Other losses occur from sweat and faeces (e.g. 2–4 g per l GIT fistula fluid lost per 24 h).
Nitrogen, higher oxides of. Nitric oxide (NO), nitrogen dioxide (NO2) and nitrogen trioxide (N2O3); the latter decomposes to form NO and NO2. NO reacts with O2, forming NO2, which dissolves in water to form nitrous and nitric acids. The gases are produced during some fires, during manufacture of N2O, and in the metal industry. Irritant if inhaled, they cause mild upper airway symptoms initially but pulmonary oedema several hours after initial recovery. Severe pulmonary fibrotic destruction may follow 2–3 weeks later. Formation of nitrates in the body may result in vasodilatation and hypotension, and cause methaemoglobinaemia. Treatment is supportive. Contamination of some N2O cylinders in 1967 in the UK led to their widespread recall. May be tested for using moistened starch iodide paper, which turns blue on exposure. NO is involved in intercellular communication and control of vascular tone.
Nitrogen narcosis, see Inert gas narcosis
Nitrogen washout. Elimination of nitrogen from the lungs whilst breathing non-nitrogen-containing gas. During successive breaths, the concentration of nitrogen exhaled falls as an exponential process, falling to about 2.5% after 7 min in normal patients. During anaesthesia using circle systems, 7–10 min high fresh gas flow is required to remove most body nitrogen. Elimination is prolonged if ventilation is distributed unevenly (see below).
• Tests employing nitrogen washout:
 single-breath nitrogen washout (Fowler’s method).
single-breath nitrogen washout (Fowler’s method).
 multiple-breath nitrogen washout: the patient breathes 100% O2, with nitrogen measurement at the lips. Log nitrogen concentration is plotted against number of breaths. If lung ventilation is uniform, expired nitrogen concentration decreases by the same fraction with each breath, as demonstrated by a straight line on the graph. A curved line is obtained if ventilation is uneven, as nitrogen is quickly washed out from well-ventilated alveoli but only slowly from poorly ventilated ones (Fig. 119).
multiple-breath nitrogen washout: the patient breathes 100% O2, with nitrogen measurement at the lips. Log nitrogen concentration is plotted against number of breaths. If lung ventilation is uniform, expired nitrogen concentration decreases by the same fraction with each breath, as demonstrated by a straight line on the graph. A curved line is obtained if ventilation is uneven, as nitrogen is quickly washed out from well-ventilated alveoli but only slowly from poorly ventilated ones (Fig. 119).
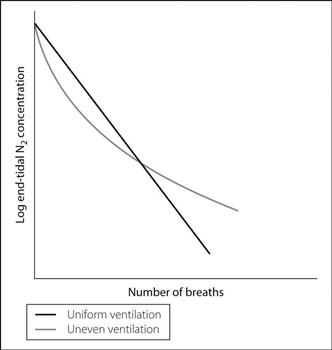
Fig. 119 Multiple-breath nitrogen washout
Nitroglycerin, see Glyceryl trinitrate
Nitroprusside, see Sodium nitroprusside
Nitrous oxide (N2O). Inhalational anaesthetic agent, first isolated by Priestley in 1772. Suggested as being a useful analgesic by Davy in 1799; first used for dental extraction by Wells in 1844 but superseded by diethyl ether. Reintroduced by Colton in 1863.
 colourless, slightly sweet-smelling gas, 1.53 times denser than air.
colourless, slightly sweet-smelling gas, 1.53 times denser than air.
 critical temperature 36.5°C.
critical temperature 36.5°C.
 MAC 105%.
MAC 105%.
 non-flammable but supports combustion, breaking down to O2 and nitrogen at high temperatures.
non-flammable but supports combustion, breaking down to O2 and nitrogen at high temperatures.
 supplied as a liquid/gas in French blue cylinders with pin index positions 3 and 5: pressure is 40 bar at 15°C and 54 bar at room temperature. Ice often forms on the cylinder during use because of latent heat of vaporisation. Also supplied as gaseous Entonox.
supplied as a liquid/gas in French blue cylinders with pin index positions 3 and 5: pressure is 40 bar at 15°C and 54 bar at room temperature. Ice often forms on the cylinder during use because of latent heat of vaporisation. Also supplied as gaseous Entonox.
• Effects:
– fast onset and recovery; strongly analgesic but weakly anaesthetic.
– increases cerebral metabolism, cerebral blood flow and ICP slightly.
– has inhibitory effects on NMDA receptors; stimulatory on opioid and adrenergic receptors.
– non-irritant. Depresses respiration slightly.
– may cause diffusion hypoxia (Fink effect) at the end of surgery.
 GIT: associated with PONV; possible causes include expansion of gas-containing bowel or inner ear cavities or a direct central effect (possibly via opioid receptors).
GIT: associated with PONV; possible causes include expansion of gas-containing bowel or inner ear cavities or a direct central effect (possibly via opioid receptors).
– does not affect hepatic or renal function, nor uterine or skeletal muscle tone.
– interacts with methionine synthase; prolonged use may cause bone marrow depression, megaloblastic anaemia and peripheral neuropathy. Implicated in causing fetal abnormalities and spontaneous abortion, but no direct evidence exists. Generally considered as being safe during pregnancy.
– expands air-filled cavities because it is over 40 times as soluble as nitrogen; thus passes from the blood into the cavity faster than the nitrogen can diffuse out. Can double the size of a pneumothorax in 10 min at 70%. Also expands air embolism and may cause pneumoencephalocoele following neurosurgery.
Excreted unchanged from the lungs; a small amount diffuses through the skin.
See also, Environmental safety of anaesthetists; Nitrogen, higher oxides of; Pollution; Relative analgesia
Nizatidine. H2 receptor antagonist used in peptic ulcer disease; similar to cimetidine but does not cause enzyme inhibition. Well absorbed orally, it is 40% protein-bound, with 90% excreted by the kidneys.
NMDA receptors, see N-Methyl-D-aspartate receptors
NO, see Nitric oxide
No reflow phenomenon. Reduction in organ blood flow following a period of ischaemia or infarction, without mechanical vessel obstruction. Has been observed affecting the heart and brain, e.g. after MI/myocardial ischaemia and CVA/cerebral ischaemia respectively. The aetiology is unclear but small vessel vasospasm, endothelial oedema or extrinsic compression by tissue oedema, increased blood viscosity, platelet aggregation and venous congestion have all been suggested. Treatment has been aimed at all these factors, with varying degrees of success.
Nociceptin, see Orphanin FQ
Nociception. Sensation of noxious stimuli, i.e. associated with injury or threatened injury.
• Occurs via specialised nerve endings (nociceptors) of certain neurones:
 C-fibres: respond to heat, mechanical and chemical stimuli, giving rise to pain. Action potentials generated by these stimuli are thought to be generated via the transient receptor potential vanilloid receptor-1 (TRPV1) receptor channels.
C-fibres: respond to heat, mechanical and chemical stimuli, giving rise to pain. Action potentials generated by these stimuli are thought to be generated via the transient receptor potential vanilloid receptor-1 (TRPV1) receptor channels.
They also respond to endogenous pain-producing substances, e.g. bradykinin, histamine and potassium ions. Because of their responsiveness to many stimuli, they are also known as ‘polymodal nociceptors’.
Nodal arrhythmias, see Junctional arrhythmias
Non-depolarising neuromuscular blockade. Caused by competitive antagonism of acetylcholine (ACh) by non-depolarising neuromuscular blocking drugs at the ACh receptors of the neuromuscular junction. The end-plate potential produced by ACh diminishes as receptor occupancy by the neuromuscular blocking drug increases; when it fails to reach the threshold for depolarisation, neuromuscular transmission fails. This only occurs when >80–90% of ACh receptors are blocked, demonstrating the wide margin of safety of neuromuscular transmission.
 absence of fasciculation following administration of drug.
absence of fasciculation following administration of drug.
 exhibits fade and post-tetanic potentiation.
exhibits fade and post-tetanic potentiation.
 antagonised by acetylcholinesterase inhibitors.
antagonised by acetylcholinesterase inhibitors.
 potentiated by aminoglycosides, volatile inhalational anaesthetic agents, acidosis, electrolyte disturbances (especially hypokalaemia, hypermagnesaemia, hypocalcaemia), myasthenia gravis, myasthenic syndrome.
potentiated by aminoglycosides, volatile inhalational anaesthetic agents, acidosis, electrolyte disturbances (especially hypokalaemia, hypermagnesaemia, hypocalcaemia), myasthenia gravis, myasthenic syndrome.
See also, Neuromuscular blockade monitoring; Priming principle
Non-invasive positive pressure ventilation. An alternative to IPPV via tracheal tube, positive pressure ventilation may be applied via a tightly fitting nasal mask, facial mask, nasal ‘pillows’ that fit into the nostrils, or a ‘helmet’ that encloses the whole head. Ventilators may deliver a set volume or, more commonly, a set pressure. Uses include: ventilatory support in acute respiratory failure (especially exacerbations of COPD and cardiogenic pulmonary oedema); facilitation of extubation/weaning from ventilators; support for patients requiring nocturnal IPPV (e.g. central sleep apnoea, neuromuscular disorders); and palliation in end-stage respiratory disease.
• Indications for use in the acute setting:
 increased dyspnoea, tachypnoea and work of breathing.
increased dyspnoea, tachypnoea and work of breathing.
 hypoxaemia despite conventional oxygen therapy.
hypoxaemia despite conventional oxygen therapy.
 inadequate mask fit, uncooperative patient.
inadequate mask fit, uncooperative patient.
 haemodynamic instability, ongoing myocardial ischaemia.
haemodynamic instability, ongoing myocardial ischaemia.
 inability to protect airway (e.g. reduced conscious level, bulbar failure).
inability to protect airway (e.g. reduced conscious level, bulbar failure).
BIPAP (bi-level positive airway pressure) refers to a technique in which two levels of positive pressure are provided during inspiration and expiration. Airflow in the patient circuit is sensed by a transducer and augmented with a preset level of positive pressure. Cycling between inspiratory and expiratory modes may be triggered by the patient’s spontaneous breaths or according to a preset rate (cycling either fully automatically or only if the patient fails to take a spontaneous breath within a certain time period). CPAP may also be delivered in either mode. BIPAP may also be administered via an oral mask.
Disadvantages and complications include discomfort, gastric distension, vomiting and aspiration of gastric contents, and pressure necrosis, e.g. to the bridge of the nose.
Non-parametric tests, see Data; Statistical tests
Non-rebreathing valves. Prevent exhaled gas from passing upstream from the patient in anaesthetic breathing systems, thus almost eliminating rebreathing (but reducing efficiency because dead space gas is wasted). Most commonly used in draw-over techniques and for CPR with self-inflating bags. Also used in demand valves. For use with a fixed fresh gas supply, a reservoir bag is required unless fresh gas flow rate exceeds peak inspiratory flow rate. Should be placed as near to the patient as possible, e.g. attached directly to the facemask/tracheal tube.
 Ambu-E valve (Fig. 120a): contains silicone rubber flaps (mushroom valves) within a clear plastic housing. Those designed for CPR contain one mushroom valve; those for anaesthetic use contain a second distal one to prevent in-drawing of room air.
Ambu-E valve (Fig. 120a): contains silicone rubber flaps (mushroom valves) within a clear plastic housing. Those designed for CPR contain one mushroom valve; those for anaesthetic use contain a second distal one to prevent in-drawing of room air.
Fig. 120 Examples of non-rebreathing valves: (a) Ambu-E; (b) Laerdal; (c) Ruben. FGF, fresh gas flow
 Laerdal valve (Fig. 120b): contains a circular silicone rubber internal valve and a ring-shaped rubber expiratory valve.
Laerdal valve (Fig. 120b): contains a circular silicone rubber internal valve and a ring-shaped rubber expiratory valve.
 Ruben valve (Fig. 120c): contains a bobbin which is held against the upstream port by a spring at rest and moved downstream by gas flow during inspiration.
Ruben valve (Fig. 120c): contains a bobbin which is held against the upstream port by a spring at rest and moved downstream by gas flow during inspiration.
Malfunction (e.g. due to condensation of water vapour) may cause sticking of the valve or rebreathing. Barotrauma may occur if high internal pressure holds the expiratory port closed, e.g. during apnoea with high fresh gas flow.
Non-steroidal anti-inflammatory drugs (NSAIDs). Group of chemically dissimilar compounds with anti-inflammatory, antipyretic and analgesic actions. Widely used for mild pain (e.g. musculoskeletal disease, headache, dysmenorrhoea), inflammatory disorders (especially musculoskeletal) and postoperative analgesia. Individual responses to NSAIDs are variable and many drugs may have to be tried before achieving optimal benefit.
 salicylic acids: e.g. aspirin, benorilate, diflunisal.
salicylic acids: e.g. aspirin, benorilate, diflunisal.
 propionic acids: e.g. fenbufen, ibuprofen, naproxen.
propionic acids: e.g. fenbufen, ibuprofen, naproxen.
 acetic acids: e.g. diclofenac, indometacin.
acetic acids: e.g. diclofenac, indometacin.
 fenamates: mefenamic acid, flufenamic acid.
fenamates: mefenamic acid, flufenamic acid.
 pyrazolones: e.g. phenylbutazone, azapropazone.
pyrazolones: e.g. phenylbutazone, azapropazone.
Effects are via inhibition of cyclo-oxygenase, resulting in reduced prostaglandin, prostacyclin and thromboxane production (see Fig. 15; Arachidonic acid). Inhibitors of cyclo-oxygenase-2 (COX-2) (e.g. parecoxib, celecoxib) have been produced for their relative lack of GIT side effects.
 renal impairment (may occur with both selective and non-selective NSAIDs); may arise from:
renal impairment (may occur with both selective and non-selective NSAIDs); may arise from:
– renal hypoperfusion: especially common in patients with sodium depletion (e.g. those taking diuretics), hypovolaemia or pre-existing renal disease. Results from inhibition of protective prostaglandin-mediated renal vasodilatation; usually occurs soon after administration of the NSAID, in most cases with recovery following discontinuation. May progress to acute tubular necrosis.
– acute interstitial nephritis: typically occurs after chronic administration, with slow recovery in most cases after discontinuation. Has also been reported after acute perioperative use. Acute kidney injury may result, usually associated with severe proteinuria. Corticosteroids have been suggested as being helpful.
– systemic vasculitis (rare) leading to glomerulonephritis and papillary necrosis.
 decreased platelet function and impaired coagulation. Although platelet dysfunction has been shown after perioperative use, bleeding problems are rare, although care is required if other drugs with anticoagulant actions are co-prescribed, e.g. prophylactic heparin. The selective COX-2 inhibitor rofecoxib was withdrawn in 2004 because it was associated with an increased incidence of cardiovascular side effects, particularly MI, that was originally attributed in early studies to a cardioprotective effect of naproxen in the control group. The mechanism is thought to be unequal inhibition of prostacyclin and thromboxane synthesis.
decreased platelet function and impaired coagulation. Although platelet dysfunction has been shown after perioperative use, bleeding problems are rare, although care is required if other drugs with anticoagulant actions are co-prescribed, e.g. prophylactic heparin. The selective COX-2 inhibitor rofecoxib was withdrawn in 2004 because it was associated with an increased incidence of cardiovascular side effects, particularly MI, that was originally attributed in early studies to a cardioprotective effect of naproxen in the control group. The mechanism is thought to be unequal inhibition of prostacyclin and thromboxane synthesis.
 adverse drug reactions are common (cross-sensitivity may occur between different drugs).
adverse drug reactions are common (cross-sensitivity may occur between different drugs).
 others, e.g. fluid retention, hyperkalaemia and metabolic acidosis (via inhibition of renin secretion with resultant hypoaldosteronism), rarely hepatotoxicity. NSAIDs have been implicated in reducing bone healing after fractures, leading some orthopaedic surgeons to suggest they should be avoided perioperatively, but the evidence is weak and they continue to be used widely.
others, e.g. fluid retention, hyperkalaemia and metabolic acidosis (via inhibition of renin secretion with resultant hypoaldosteronism), rarely hepatotoxicity. NSAIDs have been implicated in reducing bone healing after fractures, leading some orthopaedic surgeons to suggest they should be avoided perioperatively, but the evidence is weak and they continue to be used widely.
Noradrenaline (Norepinephrine). Catecholamine, the immediate precursor of adrenaline (differing by one methyl group on the terminal amine). A neurotransmitter in the sympathetic nervous system, ascending reticular activating system and hypothalamus. Also a hormone, forming about 20% of the catecholamines released from the adrenal medulla.
Predominantly stimulates α-adrenergic receptors (non-selectively), although with some β1-receptor stimulation. After secretion, 80% is taken up by postganglionic sympathetic nerve endings for reuse (uptake1); the remainder is metabolised by catechol-O-methyltransferase and monoamine oxidase or taken up by other cells, e.g. vascular smooth muscle (uptake2).
Used as an inotropic drug when SVR is low, e.g. in sepsis. An extremely potent vasopressor drug, it increases both systolic and diastolic arterial BP via arterial and venous vasoconstriction. There may be compensatory bradycardia caused by baroreceptor reflex activation. Coronary perfusion is increased but with increased myocardial O2 demand. Cardiac output may increase or decrease depending on clinical circumstances. Cerebral blood flow and O2 demand may fall. Although hypotension may be corrected, renal and mesenteric vasoconstriction may reduce renal blood flow.
Supplied commercially as noradrenaline tartrate.
Tachyphylaxis may occur. Tissue necrosis may follow extravasation.
Norepinephrine, see Noradrenaline
Normal distribution, see Statistical frequency distributions
Normal solution. One containing 1 gram equivalent weight of substance per litre. So-called ‘normal’ saline solution (0.9%) is incorrectly described, being less than 1/6 normal.
Noscapine, see Papaveretum
Nose. Entrance to the pharynx and thence larynx and lungs. Apart from its olfactory role, it filters, humidifies and warms inspired air with its extensive vascular surfaces (turbinates and septum). Filtering relies on the mucous lining which traps particles larger than 4–6 µm, sweeping them back to the pharynx. Sneezing also rids the nose of irritants.
– bones:
– nasal part of frontal bones.
– frontal process of maxillae.
– cartilages (lower part and septum).
– floor: composed of the palatine process of the maxilla and horizontal plate of the palatine bone. A tissue flap (soft palate) extends into the nasopharynx, closing off the nasal passages during swallowing.
– lateral wall: ethmoidal labyrinth, nasal surface of the maxilla and perpendicular plate of the palatine bone. The three scroll-like conchae hang down over the nasal meatus. The olfactory organ of the first cranial nerve lies above and beside the upper concha. The orifices of the maxillary, sphenoid, frontal and ethmoidal sinuses open on to the lateral nasal wall.
 upper: anterior and posterior ethmoidal branches of the ophthalmic artery.
upper: anterior and posterior ethmoidal branches of the ophthalmic artery.
 lower: sphenopalatine branch of the maxillary artery.
lower: sphenopalatine branch of the maxillary artery.
 anteroinferior septum: septal branch of the superior labial branch of the facial artery.
anteroinferior septum: septal branch of the superior labial branch of the facial artery.
– supratrochlear branch of the frontal nerve (V1).
– anterior ethmoidal branch of the nasociliary nerve (V1).
 maxillary antrum: V2 via sphenopalatine ganglion.
maxillary antrum: V2 via sphenopalatine ganglion.
 frontal sinus: frontal nerve (V1).
frontal sinus: frontal nerve (V1).
 ethmoid region: anterior and posterior branches of the nasociliary nerve (V1).
ethmoid region: anterior and posterior branches of the nasociliary nerve (V1).
– anterior: anterior ethmoidal branch of the nasociliary nerve (V1).
Trauma to the nose may result from passage of a tracheal or nasogastric tube, or nasal airway. Resultant epistaxis may be severe, and may be reduced by prior administration of cocaine spray or paste, or other vasopressor solutions, e.g. xylometazoline 0.1%.
 solution consists of 2 ml 8% cocaine, 2 ml 1% sodium bicarbonate and 1 ml 1:1000 adrenaline.
solution consists of 2 ml 8% cocaine, 2 ml 1% sodium bicarbonate and 1 ml 1:1000 adrenaline.
Maxillary and ophthalmic nerve blocks may be used for operations on or around the nose.
Nosocomial infection. Infection acquired as a result of a patient’s admission to hospital. Occurs in up to 10% of patients, with mortality of up to 5%. More common in the acutely ill (e.g. on ICU).
• Aetiology may be related to:
 patient factors: increasingly elderly population with reduced resistance, immunodeficiency states, diabetes, smoking, malnutrition, alcoholism, trauma and drug therapy.
patient factors: increasingly elderly population with reduced resistance, immunodeficiency states, diabetes, smoking, malnutrition, alcoholism, trauma and drug therapy.
 interventions: surgery, tracheal intubation, catheter-related sepsis, bladder catheterisation, use of broad-spectrum antibacterial drugs resulting in resistant organisms, blood transfusion, TPN or stress ulcer prophylaxis.
interventions: surgery, tracheal intubation, catheter-related sepsis, bladder catheterisation, use of broad-spectrum antibacterial drugs resulting in resistant organisms, blood transfusion, TPN or stress ulcer prophylaxis.
Sites of infection in order of decreasing frequency: urinary tract infection, surgical wound infection, nosocomial pneumonia and bacteraemia. Organisms most commonly involved in order of decreasing frequency: Staphylococcus aureus, pseudomonas, coagulase-negative staphylococci, candida species and Escherichia coli. Fungal infection is especially common in immunosuppressed patients.
– effective infection control. Use of care bundles.
– selective decontamination of the digestive tract may be appropriate in certain circumstances.
 source control (e.g. debridement of infected wounds, removal of infected catheters).
source control (e.g. debridement of infected wounds, removal of infected catheters).
 treatment with appropriate anti-infective agents by the use of antimicrobial stewardship programmes.
treatment with appropriate anti-infective agents by the use of antimicrobial stewardship programmes.
Nosocomial pneumonia. Hospital-acquired chest infection occurring > 48 h after hospital admission; excludes infections incubating on admission. May occur in up to 25% of ICU patients and increases hospital mortality, length of stay and costs.
 micro-aspiration of oropharyngeal flora.
micro-aspiration of oropharyngeal flora.
 environment (air, water, food, etc.).
environment (air, water, food, etc.).
 equipment (tracheal tubes, suction catheters, bronchoscopes, etc.).
equipment (tracheal tubes, suction catheters, bronchoscopes, etc.).
 hospital staff (inadequate hygiene).
hospital staff (inadequate hygiene).
• Other contributing factors include:
 patient’s age and general medical condition.
patient’s age and general medical condition.
 drugs (e.g. antacids, corticosteroids, H2 receptor antagonists, sedatives, broad-spectrum antibiotics).
drugs (e.g. antacids, corticosteroids, H2 receptor antagonists, sedatives, broad-spectrum antibiotics).
Often more than one organism is involved.
Approaches to clinical diagnosis vary but criteria include new, progressive or persistent CXR abnormalities, with evidence of infection (e.g. purulent sputum, hypo- or hyperthermia, and a low [< 5000/mm3] or raised [> 10 000/mm3] white cell count). Isolation of the responsible organism(s) is best undertaken using bronchoalveolar lavage, either blind or via fibreoptic bronchoscopy. Therapy should be organism-specific when possible, but broad-spectrum antibiotics are frequently necessary until the organism is identified.
 vaccination, e.g. against Haemophilus influenzae, pneumococcus and influenza virus.
vaccination, e.g. against Haemophilus influenzae, pneumococcus and influenza virus.
 hygiene, e.g. hand-washing policies, removal of wrist-watches, use of gloves.
hygiene, e.g. hand-washing policies, removal of wrist-watches, use of gloves.
 control of gastric pH (avoidance of alkaline pH reduces bacterial overgrowth).
control of gastric pH (avoidance of alkaline pH reduces bacterial overgrowth).
 control of gastric volume and motility (distension risks reflux and aspiration).
control of gastric volume and motility (distension risks reflux and aspiration).
 good airway protocols, e.g. avoiding nasal intubation (risks sinusitis) and the supine position (patients should be nursed 30° head-up), regular aspiration of subglottic secretions.
good airway protocols, e.g. avoiding nasal intubation (risks sinusitis) and the supine position (patients should be nursed 30° head-up), regular aspiration of subglottic secretions.
 selective decontamination of the digestive tract, although this remains controversial except in trauma.
selective decontamination of the digestive tract, although this remains controversial except in trauma.
Torres A, Ferrer M, Badia JR (2010). Clin Infect Dis; 51 Suppl 1:S48–53
See also Infection control; Nosocomial infection; Sepsis; Ventilator-associated pneumonia
Notifiable diseases. Diseases that an attending doctor is required by law to report to the local authority proper officers, usually via the consultant in communicable disease or chief environmental health officer. The scheme allows epidemiological surveillance and early identification of potential epidemics (Table 32). Failure to send details of the case on a certificate may result in conviction and a fine.
Table 32 Notifiable diseases in the UK
| Acute encephalitis Acute poliomyelitis Anthrax Cholera Diphtheria Dysentery Food poisoning Leprosy Leptospirosis Malaria |
Measles Meningitis Meningococcal septicaemia Mumps Ophthalmia neonatorum Paratyphoid fever Plague Rabies Relapsing fever Rubella |
Scarlet fever Smallpox TB Tetanus Typhoid fever Typhus Viral haemorrhagic fever Viral hepatitis Whooping cough Yellow fever |
Novoseven, see Eptacog alfa
NSTEMI. non-S–T segment elevation myocardial infarction, see Acute coronary syndromes; Myocardial infarction
Nuclear cardiology. Assessment of cardiac function using gamma cameras to trace radioisotopes. Technetium-99m-labelled blood may be followed through the heart during first pass of a bolus, or over many cardiac cycles linked to the ECG (multigated acquisition imaging; MUGA). Individual chamber movement and valve function may be observed, and ejection fraction calculated by recording the number of counts in systole and diastole. Alternatively, the uptake of thallium-201 by cardiac tissue may be observed (uptake by normal myocardium is proportional to blood flow). Thallium scanning may be enhanced by giving intravenous dipyridamole to precipitate ischaemia by causing coronary steal. Recently infarcted myocardium may be labelled with technetium-99m pyrophosphate. Other tracers used to identify areas of infarction, necrosis or inflammation include indium-111, gallium-67 citrate, and radiolabelled myosin-specific antibodies.
Nuclear magnetic resonance, see Magnetic resonance imaging
Null hypothesis. In statistics, the assumption that the observed frequency of an event equals the expected frequency. It may state that any observations are due to chance alone, or that the groups studied come from the same population; statistical tests aid the acceptance or rejection of this hypothesis (and whether an alternative hypothesis, e.g. that observed differences are caused by treatment, can be accepted). In traditional ‘hypothesis testing’, results are expressed in terms of the probability that the null hypothesis is true for the case concerned.
Number needed to treat (NNT). Indicator of treatment effect in clinical trials. The inverse of absolute risk reduction, it gives the number of patients that need to be treated in order to prevent a specified undesirable outcome (e.g. PONV). For example, for an antiemetic with NNT for PONV of 5, one needs to treat five patients with the drug in order to prevent one patient suffering PONV. Combines both the efficacy of the drug and the incidence of the condition treated; for example, an antiemetic that is effective in 100% of patients will have a NNT of 5 if the incidence of PONV is 1:5, but if it is only 50% effective (or the incidence of PONV falls to 1:10) the NNT will be 10. Number needed to harm (NNH) is a similar concept, indicating the number of patients needed to receive a drug before one suffers a complication.
See also, Meta-analysis; Odds ratio; Relative risk reduction
Nutrition. An adequately balanced daily supply of carbohydrates, fats, proteins, vitamins, electrolytes, trace elements and water is essential to maintain normal health.
• Average normal adult daily requirements:
 energy: 30–40 Cal/kg.
energy: 30–40 Cal/kg.
– sodium: 1 mmol/kg.
– potassium: 1 mmol/kg.
– calcium: 0.1–0.2 mmol/kg.
– magnesium: 0.1–0.2 mmol/kg.
– selenium: ~ 1 µg/kg.
 vitamins: vary from under 0.1 g/kg to 1.0 mg/kg (see Table 43; Vitamins).
vitamins: vary from under 0.1 g/kg to 1.0 mg/kg (see Table 43; Vitamins).
Energy requirements depend on the particular circumstances for each individual, e.g. they increase after trauma and burns (see Catabolism), and with pyrexia (by about 10% for every °C above normal). Patients should be fed via the oral route if possible, preferably with normal food.
For critically ill patients, more precise estimation of energy balance is necessary, whatever the route of administration. Once energy requirements have been determined, it is divided into carbohydrate (4 Cal/g) and fat (9 Cal/g) components to accompany nitrogen (150–200 Cal/g nitrogen). Carbohydrate should comprise 40–50% of energy requirements. Appropriate enteral or parenteral solutions are then selected from commercially available products (some pharmacies make up their own solutions), satisfying requirements for energy, fluid and electrolytes. Vitamins may be added as required.
Bistrian BR, McCowen KC (2006). Crit Care Med; 34: 1525–31
See also, Malnutrition; Metabolism; Nitrogen balance; Nutrition, enteral; Nutrition, total parenteral
Nutrition, enteral. Feeding via the GIT. Ideal route is by mouth using normal or liquidised food and calorific/protein supplements, if necessary. Commonly performed via fine-bore nasogastric tubes on ICU. Alternatives include a nasojejunal feeding tube (using a weighted tube or passed via endoscopy), percutaneous endoscopic gastrostomy or a jejunostomy tube placed at the time of surgery.
In patients with adequate GIT function, it is preferable to TPN as it is more physiological, provides protection against stress ulcers, maintains intestinal barrier integrity (thus reducing bacterial translocation) and promotes biliary flow, preventing the cholestasis commonly seen with TPN.
Principles are those of nutrition generally. Carbohydrate is the usual energy source in most enteral feeds, but high concentrations increase osmolality, causing diarrhoea. The protein source is usually whole protein, although preparations containing oligopeptides or amino acids are useful in pancreatic disease and malabsorption syndromes. Medium chain triglycerides are the usual source of fat. Most feeds also contain fibre.
 mechanical, e.g. tube blockage, passage of the tube into the trachea, regurgitation.
mechanical, e.g. tube blockage, passage of the tube into the trachea, regurgitation.
 nausea and vomiting: occur in 10% of cases; may require antiemetic drugs or prokinetic drugs.
nausea and vomiting: occur in 10% of cases; may require antiemetic drugs or prokinetic drugs.
 diarrhoea: occurs in up to 60% of cases; may be caused by intolerance of high osmotic load, underlying bowel disorder, contaminated feed or concurrent antibacterial drug therapy. Administration of pre- and probiotic supplements may be beneficial.
diarrhoea: occurs in up to 60% of cases; may be caused by intolerance of high osmotic load, underlying bowel disorder, contaminated feed or concurrent antibacterial drug therapy. Administration of pre- and probiotic supplements may be beneficial.
Nutrition, total parenteral (TPN). Administration of total nutritional requirements by iv infusion; it may be required in patients who are hypercatabolic and/or have an abnormal GIT. Commonly required in critically ill patients on ICU with abdominal pathology or multiorgan failure. Only indicated if enteral nutrition fails to deliver requirements or is impossible to use. May also be used to support enteral nutrition.
Principles are those of nutrition generally, i.e. calculation of nitrogen balance, energy and fluid requirements. Nitrogen and the energy source should be given together, preferably continuously. 5–6 mmol potassium and 1–2 mmol magnesium are required per gram of nitrogen.
The nitrogen component is given as mixtures of essential and non-essential amino acids (the nitrogen content varies considerably between different solutions). Some amino acid solutions contain electrolytes and most are hypertonic. Some contain energy sources, e.g. glucose and fructose. Carbohydrate is usually given as glucose 10–50%, and requires central venous infusion to avoid venous thrombosis. Other energy sources have also been used, e.g. sorbitol, xylitol and ethanol. Insulin is usually required to control hyperglycaemia associated with glucose-rich infusions. ‘Tight’ glycaemic control is no longer recommended as it is associated with greater morbidity secondary to hypoglycaemia. Fat is usually administered as 10 or 20% soya bean oil emulsions; allergic reactions may occur rarely with the 20% preparation. Trace elements and vitamins must be added.
The patient should be encouraged to mobilise to prevent muscle breakdown.
 those associated with central venous cannulation.
those associated with central venous cannulation.
– refeeding syndrome with hypophosphataemia, hypokalaemia and hypomagnesaemia.
– trace element or vitamin deficiency.
 cholestasis resulting in acute cholecystitis.
cholestasis resulting in acute cholecystitis.
• Routine monitoring should include:
Blackburn GL, Wollner S, Bistrian BR (2010). Arch Surg; 145: 533–8
Nystatin. Polyene antifungal drug, principally used for treatment of Candida albicans infections of skin, mucous membranes and GIT. Not absorbed when administered by mouth and too toxic for parenteral use. Available as tablets, oral suspension, cream or pessaries.