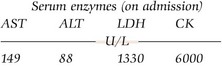Myopathy
Muscle weakness
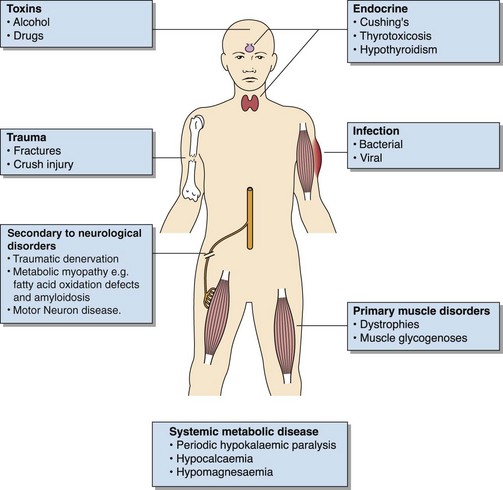
Muscle weakness, which may or may not progress to rhabdomyolysis, has many causes (Fig 73.1). Diagnosis of the condition will depend on the clinical picture and will include investigation of genetic disorders by enzymic or chromosomal analysis, endocrine investigations and the search for drug effects. Infective causes may be diagnosed by isolation of the relevant organism or its related antibody, but often no organism is detected. These cases, known as myalgic encephalitis (ME), post-viral syndrome or chronic fatigue syndrome, are relatively common and are now regarded as true diseases, whereas formally they were thought to be psychosomatic.
Investigation
In all cases of muscle weakness, serum electrolytes should be checked along with creatine kinase (CK). A full drug history should be taken to exclude pharmacological and toxicological causes, and a history of alcohol abuse should be excluded. Neuromuscular electrophysiological studies should be performed to detect neuropathies. Where a genetic or metabolic cause is suspected (Table 73.1), specialist laboratories should be involved in the investigations at an early stage. Investigations include measurement of plasma (and CSF) lactate and specialist metabolic tests in blood, CSF and urine; muscle biopsy for histopatological studies and measurement of muscle enzymes may also be indicated. In contrast to rhabdomyolysis, serum CK may sometimes be normal in myopathic disorders, especially in the chronic setting, and if muscle mass decreases.
Rhabdomyolysis
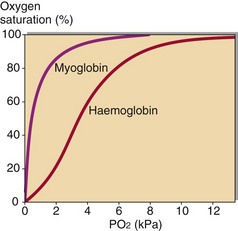
The damaged muscle cells will also leak myoglobin. This compound stores oxygen in the muscle cells for release under conditions of hypoxia, as occurs during severe exercise. The dissociation curve of myoglobin is compared with haemoglobin in Figure 73.2. It delivers up its oxygen only when the PO2 falls to around 3 kPa. When muscle cells become anoxic or are damaged by trauma, myoglobin is released into the plasma. It is filtered at the glomerulus and excreted in the urine, which appears orange or brown coloured; on urine dipstick testing myoglobin gives a false positive reaction for the presence of blood, which can lead to the mistaken diagnosis of haematuria. The damaged muscle cells also release large amounts of potassium and phosphate ions giving rise to hyperkalaemia and hyperphosphataemia; potentially serious hypocalcaemia may develop due to the binding of calcium by released intracellular organic and fatty acids.
Investigation and treatment
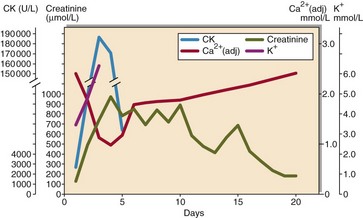
The biochemistry laboratory has a major role to play in the diagnosis and investigation of rhabdomyolysis (Fig 73.3). This includes:
 serum total creatine kinase, which allows the diagnosis to be made, and levels monitored to assess recovery and prognosis
serum total creatine kinase, which allows the diagnosis to be made, and levels monitored to assess recovery and prognosis
 urea and electrolytes, to look for evidence of resulting renal impairment
urea and electrolytes, to look for evidence of resulting renal impairment
 alcohol and drugs of abuse screen, to look for specific causes.
alcohol and drugs of abuse screen, to look for specific causes.
Haemodialysis may be necessary where renal function is severely compromised.