Myomectomy
Abdominal myomectomy is performed as an alternative to hysterectomy. The indications for myomectomy are collateral and consist of the desire to preserve the uterus together with the presence of symptomatic intramural or subserosal myomata uteri. Typical symptoms experienced by women in whom no submucous component exists are pressure on the bladder or bowel, partial obstruction of the ureters, and pain. Although this operation has been performed laparoscopically, most surgeons consider laparotomy to be the route of choice.
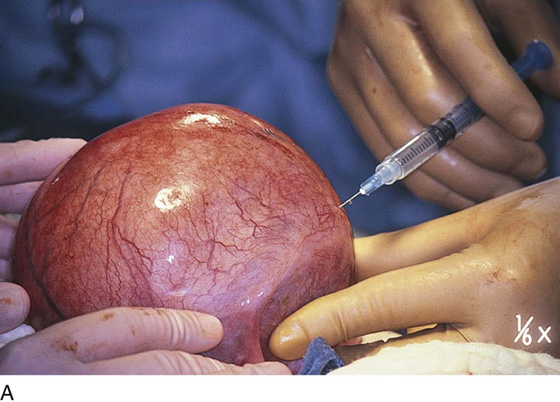
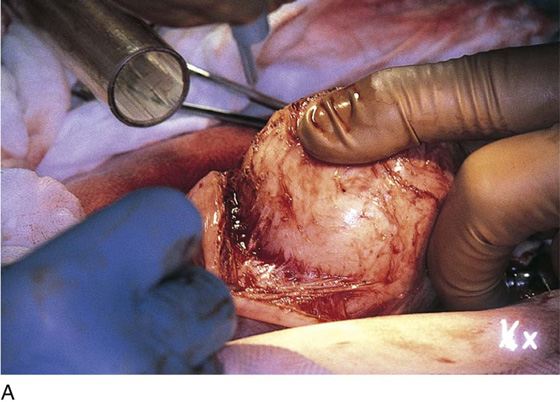
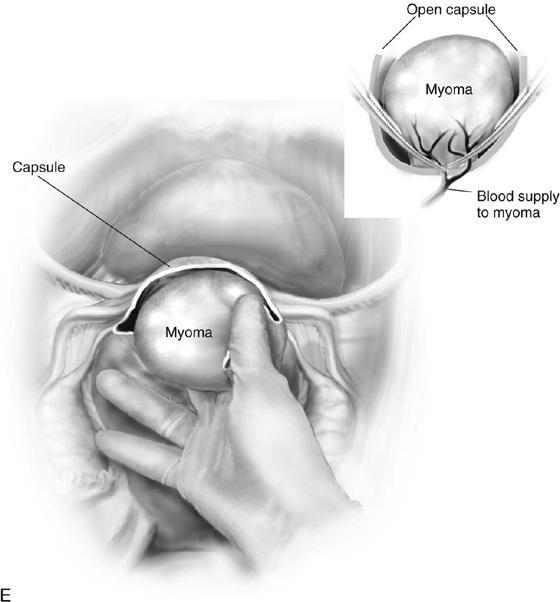
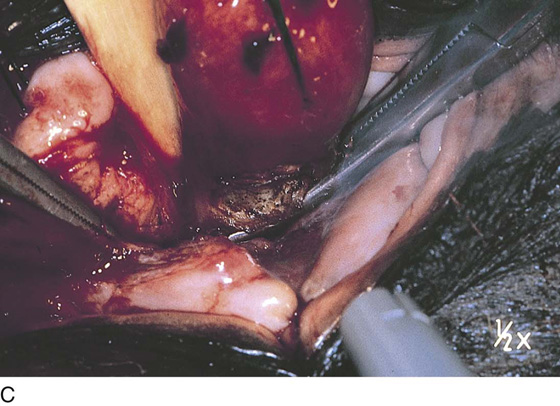
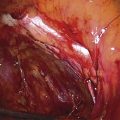
The uterus is typically distorted (Fig. 14–1). Although the arterial supply to myomata is relatively sparse, the venous return is large, thin-walled, and anomalous (Figs. 14–2 through 14–4). The surgeon must cut through the capsule to reach the core of the myoma to remove it, and must traverse tissue planes that contain these venous sinuses. Because of the increased vascularity, many surgeons prefer to use an energy source to diminish bleeding (e.g., carbon dioxide [CO2] laser, electrosurgical needle electrode). The author additionally uses a 1 : 200 solution of vasopressin (20 units). Approximately 20 to 30 mL of this solution is injected just beneath the capsule (Fig. 14–5A). The anesthesiologist should be alerted to monitor the patient’s blood pressure and pulse during injection of vasopressin. Next, an outline is made for the incision. This may be performed with cold steel, CO2 laser, or needle electrode (Fig. 14–5B). The author prefers to limit the posterior extent of the incision to diminish subsequent adhesion formation (Fig. 14–5C). In the case illustrated, a slightly defocused CO2 laser handpiece is utilized with power set at 50W and a laser spot 1.5 to 2.0 mm in diameter (power density 1250–2200 W/cm2) (Fig. 14–6). The edges of the capsule are retracted, and the myoma is dissected peripherally off the capsule (Fig. 14–7). The operator’s index finger can actually be used to separate the myoma from the capsule. The laser, needle electrode, or scissors may be used to cut away adhesions (Fig. 14–8). Care should be taken to carry out the dissection gently and carefully to avoid entry into the uterine cavity and injury to the interstitial portion of the oviduct (Fig. 14–9A–C).
When the base of the myoma is reached, the arterial pedicle should be clamped and suture-ligated (Fig. 14–9D, E). The specimen is then removed. Typically, the author cuts the myoma to determine whether there is any gross suspicion of sarcoma or infection. A pulpous, rotting interior suggests the need for a frozen section or at least a careful postoperative histologic assessment. Some excess capsule may be trimmed away (Fig. 14–9F). The uterus is reconstructed by bringing muscle to muscle together with interrupted 0 Vicryl (Fig. 14–10A, B). This may require a two-layered closure. Next, the serosa is closed with running or interrupted 2-0 or 3-0 Vicryl sutures. At the completion of closure, the author prefers to cover the exposed suture line with a parietal peritoneal graft or a patch of Interceed absorbable adhesion barrier or other suitable material. Typically, the surgeon measures and cuts the specimen (Fig. 14–11A, B). Submucous myomata are responsible for 90% of the bleeding associated with these common tumors and should be treated hysteroscopically. If the myoma is too large for hysteroscopic extirpation, even after 3 to 4 months of gonadotropin-releasing hormone (GnRH) agonist suppression, the patient should undergo a hysterectomy (Fig. 14–12).
Occasionally, a myomectomy is performed and no suspicion of malignancy is evidenced (Fig. 14–13A–D), but it is surprising to note that the permanent histopathologic sections reveal leiomyosarcoma (Fig. 14–14A, B). In this circumstance, the patient must be promptly notified of these findings and strongly advised to undergo total abdominal hysterectomy (Fig. 14–15A, B).
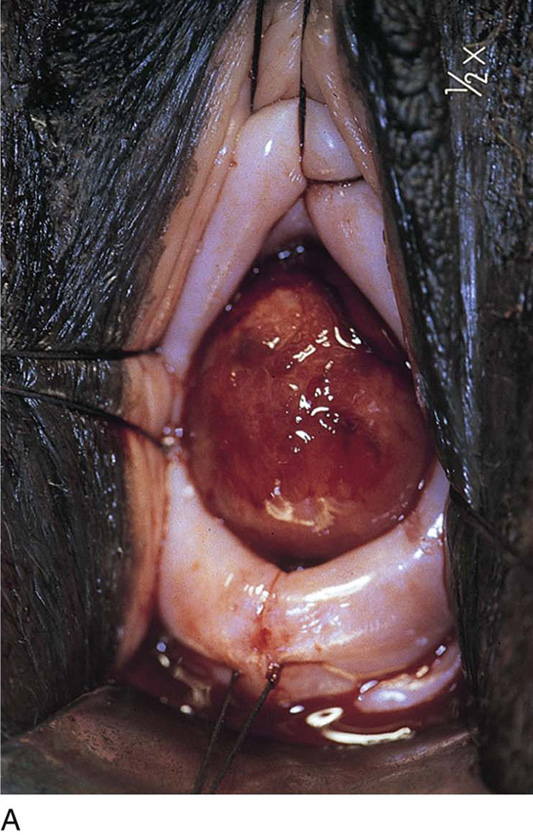
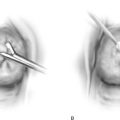
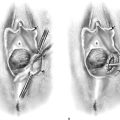
Cervical myomata may be excised via the vaginal route with the use of a microscope-mounted CO2 laser. Essentially, the anterior or posterior wall or both walls of the cervix are divided to afford exposure. The myoma is then cut out from the respective wall to which it is attached. The cervix is repaired in layers with 2-0 or 3-0 Vicryl. The split walls are closed with 3-0 Vicryl (Fig. 14–16A–E). Occasionally, a cervical myoma is so large and so vascular that an abdominal hysterectomy is indicated. In this circumstance, the size of the cervical myoma would make the abdominal route a safer choice than vaginal hysterectomy (Fig. 14–17A–C).
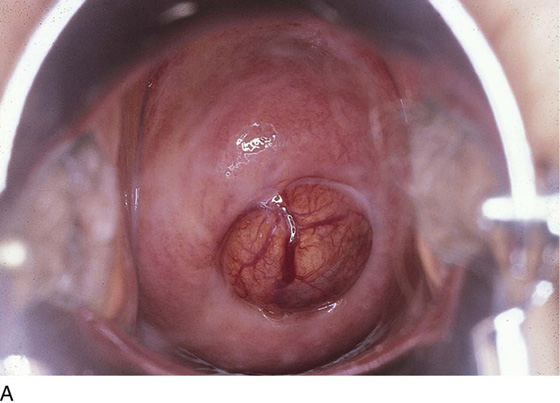
FIGURE 14–1 The uterus is lifted out of the pelvis so any anatomic alterations created by the myomatous mass can be identified.
FIGURE 14–2 The myoma is a large 6- to 8-cm solitary lesion.
FIGURE 14–3 Venous return from the myomatous uterus is large and anomalous. Large, sinusoidal vessels are seen in the subserous location.
FIGURE 14–4 The course of the large vessels should be carefully identified before a uterine incision is made.
FIGURE 14–5 A. A 1 : 200 solution of vasopressin is injected into the uterus for hemostasis. Care should be taken to avoid intravascular injection. B. The injection is performed with the use of a 10-mL triple-ring syringe and a 1½-inch, 25-gauge needle. The subserosa is first injected. The tissue immediately blanches white. The needle is advanced into the substance of the myoma, and the solution is injected. Typically, 20 to 25 mL is injected. C. A transverse or vertical incision is made into the myoma. If possible, an anterior or anterofundal cut is made.
FIGURE 14–6 Alternatively, a carbon dioxide (CO2) laser (handpiece delivery system) may be used to open the uterus. The laser is a precise energy device that offers additional hemostasis.
FIGURE 14–7 When the capsule of the myoma is reached, deeper incising should cease. The myoma is now dissected peripherally.
FIGURE 14–8 Small adhesions may be encountered between the uterine wall and the myoma capsule. These adhesions should be cut sharply.
FIGURE 14–9 A and B. With a combination of sharp and blunt dissection, the myoma is separated from the normal myometrium. C and D. The dissection is carried to its basal attachment (the tumor) to the uterine wall. E. The feeding artery is clamped and suture-ligated with 0 Vicryl. F. Excess uterine serosa is trimmed away. The anterior wall (A) will be closed to approximate and overlap the posterior wall (B).
FIGURE 14–10 A. Closure is implemented by suturing the anterior margin over the posterior margin to strengthen the wound integrity. This illustrates a transverse closure. B. The interrupted-suture technique for vertical incision closure will be completed by tacking peritoneum or Interceed over the incision.
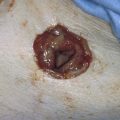
FIGURE 14–11 A. The extracted myoma is grossly studied and measured. B. The myoma is bisected to allow examination of its interior. The specimen is then placed in formalin and sent to pathology. If the myoma has undergone purulent degeneration, a culture should be taken before the tumor is placed in fixative. If sarcoma is suspected, a sample on the interior may be sent for frozen section analysis.
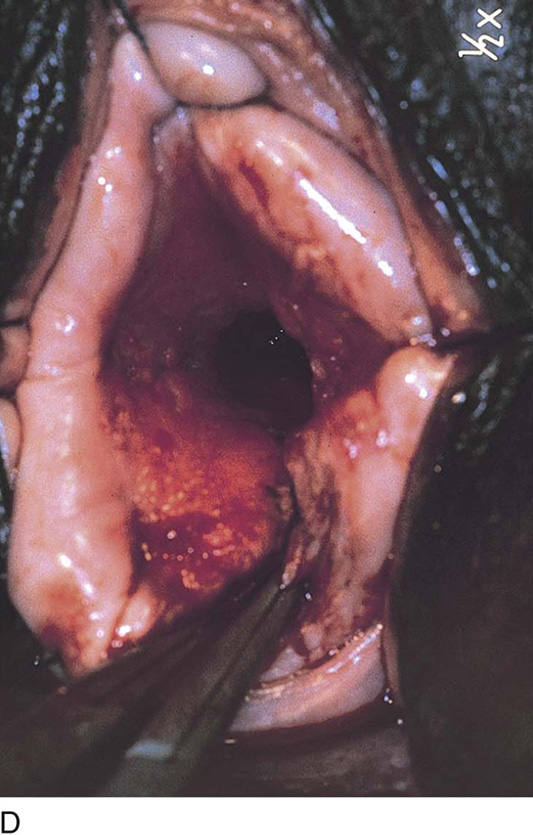
FIGURE 14–12 Bleeding symptoms and anemia suggest the presence of a submucous myoma. The submucous myoma should be treated by hysteroscopy or hysterectomy.
FIGURE 14–13 A. This large myomatous uterus has been injected with a 1 : 200 vasopressin solution. B. An anterior transverse incision is made into the uterus above the urinary bladder peritoneal reflection. C. The myoma is dissected free from the uterine wall; unfortunately, the uterine cavity is entered. D. After removal of the myomatous mass, the uterus is closed in layers with 0 Vicryl. The serosa is closed with 2-0 Vicryl. Note that the uterus has been reduced to a normal size.
FIGURE 14–14 A. Histologic section from the uterus pictured in Figure 10–13A–C. The hematoxylin and eosin (H&E)-stained section (×10) shows increased cellularity, nuclear pleomorphism, and hyperchromatism. B. Histologic section (×20) clearly confirms the diagnosis of leiomyosarcoma. Four mitotic figures are seen in this single field. The stromal (muscle) cells are clearly malignant. (From Baggish MS, Barbot J, Valle V: In Diagnostic and Operative Hysteroscopy, 2nd ed. Mosby, St Louis, 1999.)
FIGURE 14–15 A. Hysterectomy specimen from the patient depicted in Figures 14–13 and 14–14. The normal-sized uterus has been removed. B. The cut wall of the uterus appears to be normal. Microscopic sections were benign (i.e., no residual sarcoma).
FIGURE 14–16 A. A cervical myoma is present, but the pedicle location is not visible. Traction sutures have been placed anteriorly and laterally. B. The posterior lip of the cervix has been injected with a 1 : 100 solution of vasopressin. The carbon dioxide (CO2) laser beam (microscope-coupled) has begun to cut the cervix posteriorly to gain access to the myoma pedicle. Titanium hooks place traction on either side of the incision. The red helium-neon aiming beam is visible. C. The pedicle has been clamped with a Kelly clamp. A moist tongue depressor has been placed between the myoma and the interior of the cervix. The laser beam has partially cut through the base of the myoma. D. The myoma has been removed. The stump of the pedicle will be sutured with 3-0 Vicryl. E. The surgery has been completed. The cervix has been sutured with 3-0 Vicryl. (From Baggish MS, Barbot J, Valle V: In Diagnostic and Operative Hysteroscopy, 2nd ed. Mosby, St Louis, 1999.)
FIGURE 14–17 A. This patient has a large cervical myoma. The thin-walled vessels are subject to rupture. These vessels will not retract and will bleed heavily for a prolonged period. B. The patient opted for hysterectomy rather than myomectomy. C. The total volume of this tumor was much greater than anticipated, in fact, even bigger than the body of the uterus. In this case, hysterectomy was the best choice because the large size of the myoma would preclude use of the vaginal-cervical route of removal.