Chapter 39 Myocardial Viability
Comparison with Other Techniques
INTRODUCTION
Coronary artery disease (CAD) is the most common cause of chronic heart failure.1 Over the last decades, significant progress in the treatment of patients with chronic ischemic heart failure has been made. Advances in pharmacologic therapy, the introduction of device therapy, and developments in cardiac surgery have led to improved prognosis in these patients (Table 39-1). Pharmacologic therapy in ischemic left ventricular (LV) dysfunction typically consists of angiotensin-converting enzyme (ACE) inhibitors, β-blockers, and spironolactone and may improve symptoms and clinical outcome. However, pharmacologic therapy is a partial success. In general, patients with chronic ischemic heart failure have a history of myocardial infarction, multivessel CAD, and a variable degree of LV dysfunction. The primary question in these patients is whether they will benefit from surgical revascularization. In patients with a substantial amount of viable myocardium, coronary revascularization may improve heart failure symptoms, left ventricular ejection fraction (LVEF), and long-term prognosis. On the other hand, patients without viable myocardium are unlikely to benefit from surgical revascularization and are at increased risk for perioperative complications.2,3
| Medical Therapy |
| ACE inhibitors |
| Amiodarone |
| AT-II receptor blockers |
| β-blockers |
| Diuretics |
| Digoxin |
| Spironolactone |
| Despite new drugs, survival on medical therapy is poor |
| Device Therapy |
| Biventricular pacing |
| Internal cardiac defibrillator (ICD) |
| Left ventricular assist device (as bridge to transplantation) |
| Heart Transplantation |
| Limited number of donor hearts does not meet large demand |
| Excessive comorbidity in potential recipients |
| Good long-term survival |
| Surgery |
| Revascularization if viable myocardium present, but associated risk is high |
| Additional Surgery |
| Mitral valve repair |
| Left ventricular aneurysmectomy |
| Left ventricular restoration |
DEFINITION OF MYOCARDIAL VIABILITY (See Chapter 36)
Patients with ischemic cardiomyopathy often have a variable degree of coexisting hibernating and stunned myocardium and nonviable scar tissue.4 The term myocardial hibernation was first described by Rahimtoola5 to explain the condition of chronic LV dysfunction attributable to chronically reduced blood flow in patients with CAD in whom coronary revascularization may result in recovery of LV contractility. Myocardial stunning refers to reversible myocardial contractile dysfunction in the presence of normal myocardial blood flow at rest.6 Repetitive stunning is a condition that may be caused by repeated ischemic periods inducing chronic dysfunction in the presence of normal or mildly reduced myocardial blood flow at rest; coronary flow reserve, however, is reduced. It has been suggested that a temporal progression exists from stunning, characterized by normal blood flow at rest, to repetitive stunning with normal or mildly reduced blood flow at rest (but reduced flow reserve), to hibernation with reduced blood flow at rest.
Biopsy samples obtained in patients with ischemic LV dysfunction undergoing coronary revascularization revealed that myocardial stunning and hibernation are not just pathophysiologic concepts. Hibernating myocardium shows signs of energy depletion and down-regulation of energy turnover, which may cause and maintain contractile dysfunction, tissue degeneration, and loss of cardiomyocytes.7 Structural dedifferentiation of cardiomyocytes has been observed in hibernating myocardium, characterized by a loss of contractile filaments, small mitochondria, nuclei with uniformly distributed chromatin, and a nearly absent sarcoplasmic reticulum.8 It appears that cardiomyocytes adapt their activity level to prevailing circumstances. This may explain why in hibernating myocardium some characteristics (e.g., contractile reserve) can be lost, whereas more basal characteristics such as cell membrane integrity and glucose metabolism are still intact. Several noninvasive imaging techniques have been developed to probe the different characteristics of dysfunctional but viable myocardium. Some techniques address rather basic characteristics such as cell membrane integrity and glucose metabolism, and other techniques rely on assessment of contractile reserve; this (in part) explains the differences in diagnostic accuracy of the various techniques (Table 39-2). The general term viability includes both hibernation and repetitive stunning but also includes segments with subendocardial scar tissue. These segments are indeed viable, but they contain a mixture of subendocardial necrosis and normal, viable myocardium in the epicardial layers. Whereas both hibernation and repetitive stunning benefit from revascularization (with functional recovery, and potentially improve long-term outcome), dysfunctional segments with subendocardial scar tissue and normal, viable myocardium do not benefit from revascularization.
Table 39-2 Characteristics of Dysfunctional but Viable Myocardium in Relation to Imaging Modalities
| Imaging Modality | Characteristics of Viability |
|---|---|
| Echocardiography at rest | Wall thickness |
| Echocardiography with dobutamine | Contractile reserve |
| Echocardiography using contrast | Perfusion |
| Magnetic resonance imaging at rest | Wall thickness |
| Magnetic resonance imaging with dobutamine | Contractile reserve |
| Magnetic resonance with intravenous contrast | Scar tissue |
| PET or SPECT with FDG | Glucose utilization |
| SPECT with thallium-201 | Perfusion and cell membrane integrity |
| SPECT with technetium-99m | Perfusion and cell membrane/mitochondrial integrity |
FDG, fluorine-18 2-fluorodeoxyglucose; PET, positron emission tomography; SPECT, single-photon emission computed tomography.
NONINVASIVE IMAGING TECHNIQUES (See Chapters 37 and 38)
Echocardiography
Echocardiography at Rest
A crude differentiation between viable and nonviable myocardium can be made using measurement of end-diastolic wall thickness with echocardiography at rest. Schinkel et al.9 evaluated 150 consecutive patients with ischemic LV dysfunction and demonstrated that the end-diastolic wall thickness was reduced (<6 mm) in 2% of the dysfunctional region and preserved (≥6 mm) in 309 dysfunctional regions. Regions with a reduced end-diastolic wall thickness virtually never exhibited contractile reserve during dobutamine stress echocardiography. In regions with a wall thickness equal to 6 mm, the response to dobutamine varied; approximately 60% of these segments were viable on dobutamine stress echocardiography, and the remaining 40% were nonviable. Conceivably, the regions with an end-diastolic wall thickness less than 6 mm consist of scarred and fibrotic tissue and do not improve in function following revascularization. Assessment of LV end-diastolic wall thickness can be used as an initial evaluation for myocardial viability: Dysfunctional segments with an end-diastolic thickness less than 6 mm are nonviable and may not need further evaluation; segments with a preserved end-diastolic wall thickness (≥6 mm) can be further classified as viable and nonviable myocardium based on the presence/absence of contractile reserve during dobutamine challenge.
Dobutamine Stress Echocardiography
Methodology. Extensive clinical experience has been obtained with dobutamine stress echocardiography to assess myocardial viability.2,3 Dobutamine is a β1-specific agonist that increases myocardial oxygen demand by increasing heart rate, contractility, and arterial blood pressure. The safety of dobutamine stress echocardiography was evaluated by Secknus et al.,10 reporting an incidence of 7.6% side effects in 3011 patients undergoing dobutamine stress echocardiography. β-blockers can be used as antidotes. Infusion of low-dose dobutamine (5 to 10 mcg/kg/min) has been demonstrated to increase contractility (without a substantial increase in heart rate) in dysfunctional but viable myocardium, which has been referred to as contractile reserve. Segments without viable myocardium do not show contractile reserve. The protocol can be extended with high-dose dobutamine infusion (with infusions up to 40 mcg/kg/min with addition of atropine if needed), which allows assessment of myocardial ischemia.12 With the low-high dobutamine dose stress protocol, four response patterns can be observed:
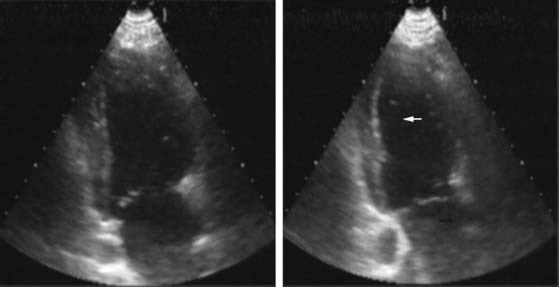
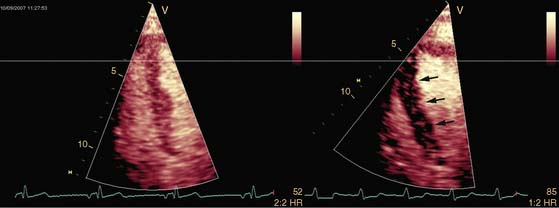
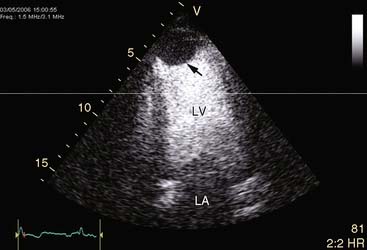
Pattern 1 represents transmural scar, whereas pattern 2 represents severe ischemia in a region subtended by a critically stenosed artery. Pattern 3, on the other hand, is probably related to subendocardial scar tissue, whereas pattern 4 represents viability with superimposed ischemia. An example of a patient with myocardial ischemia during dobutamine stress echocardiography is shown in Figure 39-1.
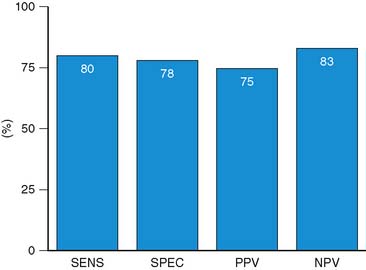
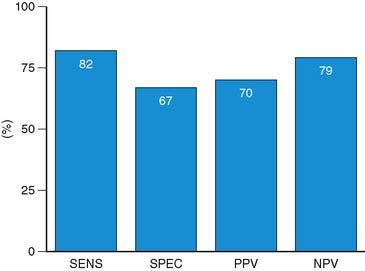
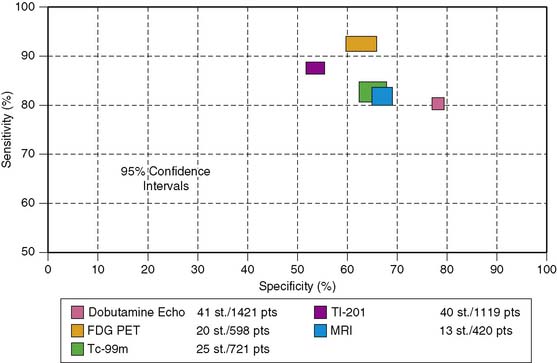
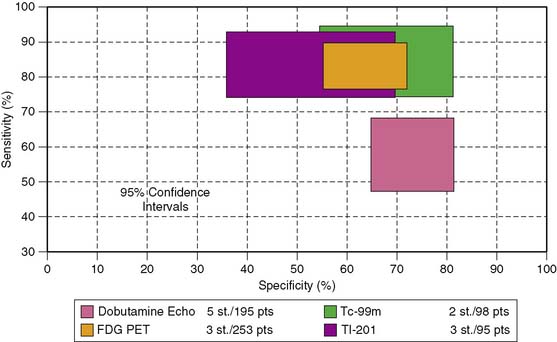
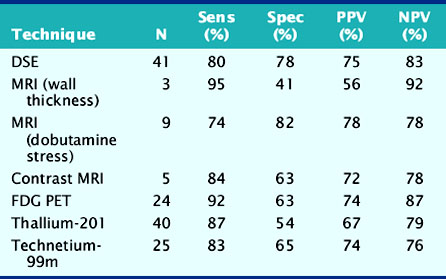
Accuracy. Stress echocardiography has been evaluated extensively for the detection of viable myocardium in patients with chronic ischemic LV dysfunction. Forty-one studies with dobutamine stress echocardiography have reported on the sensitivity and specificity for detection of myocardial viability and prediction of recovery of regional function following revascularization.11–51 A total of 1849 patients were included in these 41 studies. The weighted mean sensitivity and specificity respectively were 80% and 78%, with a positive and negative predictive value of 75% and 83% (Fig. 39-2; Table 39-3).
Table 39-3 Pooled Data from Viability Studies Using Different Techniques to Predict Improvement of Regional Contractility After Revascularization*
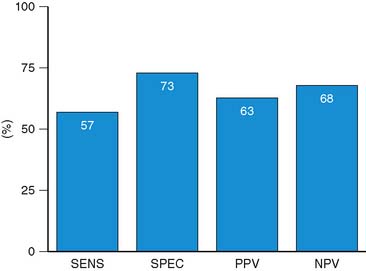
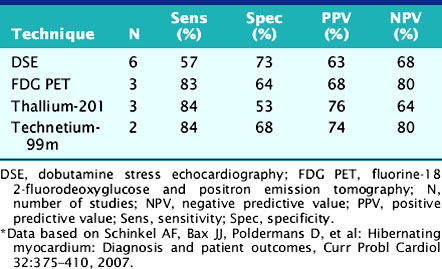
For the prediction of recovery of global LV function, 6 studies including 287 patients are available, using dobutamine stress echocardiography.45–47,50,52,53 Pooled analysis of these six studies revealed a weighted mean sensitivity and specificity of 57% and 73%, respectively with a positive and negative predictive value of 63% and 68% (Fig. 39-3; Table 39-4).
Table 39-4 Pooled Data from Viability Studies Using Different Techniques Predicting Improvement of Global Left Ventricular Function After Revascularization*
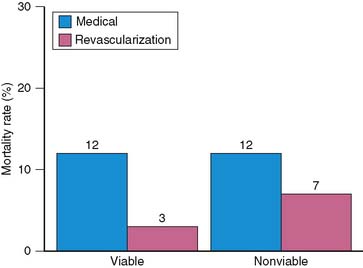
The prognostic value of viability assessment using dobutamine stress echocardiography was addressed in 11 studies that included 1753 patients (5 studies used high-dose dobutamine, 5 low-dose dobutamine, 1 low-high dose dobutamine).54–63 The event rates in relation to viability and therapy are presented in four groups:
Patients with viable myocardium undergoing revascularization had the best survival, while the highest annualized mortality rates were noted in patients receiving medical therapy, either with or without viable myocardium. An intermediate mortality rate was observed in patients without viable myocardium who underwent revascularization (Fig. 39-4).
Novel Technical Developments in Echocardiography and Viability Assessment
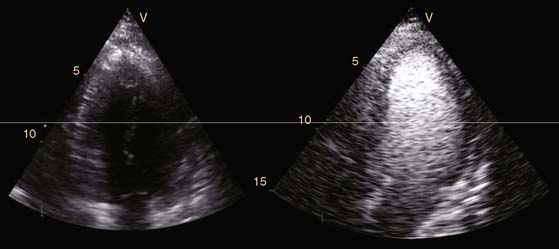
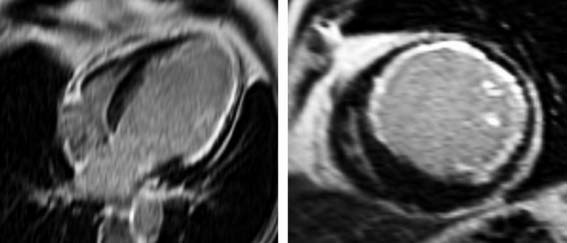
Echocardiography is a widespread imaging modality that has a number of advantages, including bedside availability, real-time imaging, low costs, and absence of radiation. Besides information on systolic and diastolic LV function, information on LV volumes, pericardial pathology, and valvular function can be obtained. However, especially in obese patients and those with chronic obstructive pulmonary disease, the technique may be limited by a poor acoustic window, making precise endocardial border delineation and assessment of regional wall motion difficult. Second harmonic imaging has significantly improved endocardial border delineation.64 Intravenous administration of contrast agents can further improve visualization of the endocardium (Figs. 39-5, 39-6 and 39-7).65 Reilly et al.66 determined the value of contrast echocardiography for assessment of LV wall motion in 70 patients in the intensive care unit, who had a poor acoustic window. Wall motion was evaluated on standard echocardiography, harmonic echocardiography, and after intravenous contrast administration. Uninterpretable wall motion was present in 5.4 segments per patient on standard echocardiography, 4.4 on harmonic echocardiography (P = 0.2), and 1.1 on contrast echocardiography (P < 0.0001). An average of 7.8 segments were read with surety on standard echocardiography, 9.2 on harmonic echocardiography (P = 0.1), and 13.7 on contrast echocardiography (P < 0.0001). The safety of the use of intravenous contrast has recently been questioned, but various studies have clearly demonstrated the safety, both in patients with stable CAD and patients with recent myocardial infarction.67,68 Aggeli et al.67 evaluated 5250 individuals with dobutamine stress echocardiography and intravenous contrast and reported very low adverse event rate, without deaths or infarction and serious cardiac arrhythmias in 0.04%. Therefore, particularly in patients with a poor acoustic window, contrast echocardiography appears to have incremental value for assessment of LV function and wall motion.
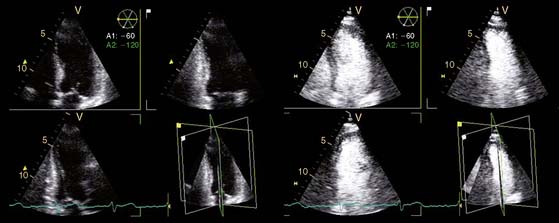
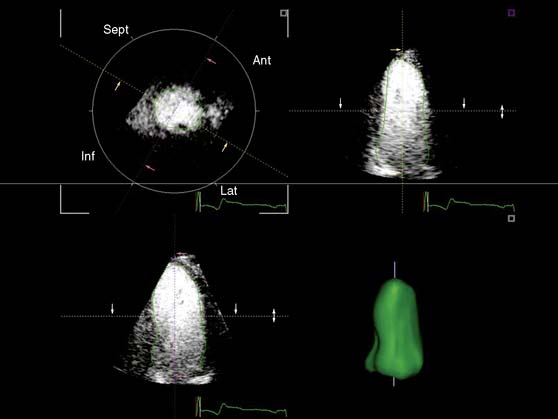
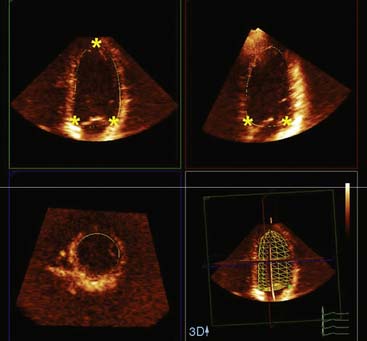
In addition, the combination of intravenous contrast and three-dimensional (3D) echocardiography may even further increase accuracy. This technique has resulted in superior visualization of the endocardial walls and excellent assessment of LV volumes and LVEF (see Figs. 39-6 and 39-7). Traditionally, analysis of the dobutamine stress echo data was performed visually, whereas nuclear imaging permitted quantitative (objective) analysis. Tissue Doppler imaging, however, allows quantification of systolic (and diastolic) velocities of the myocardium (Fig. 39-8).69 Rambaldi and colleagues70 have evaluated 40 patients with chronic CAD and LV dysfunction (mean ejection fraction 33% ± 11%); all underwent metabolic imaging with fluorine-18-fluorodeoxyglucose (FDG) SPECT (as the gold standard for viability) and dobutamine stress echocardiography. The authors used tissue Doppler imaging to quantify the peak systolic velocity of dysfunctional segments, both at rest and during dobutamine infusion. The peak systolic velocity at rest was not different between viable and nonviable segments (according to findings on metabolic imaging), but the velocities during low-dose dobutamine (8.6 ± 2.9 cm/sec versus 6.0 ± 1.8 cm/sec, P < 0.05) and high-dose dobutamine (9.3 ± 3.1 cm/sec versus 6.2 ± 2.1 cm/sec, P < 0.05) were significantly higher in viable segments compared to nonviable segments.
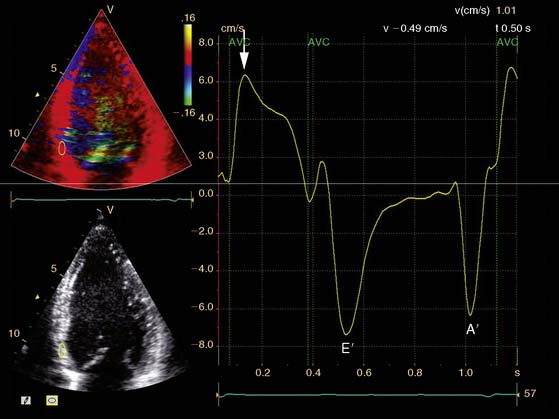
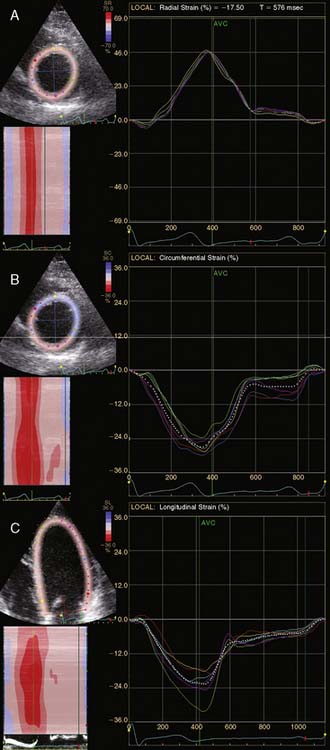
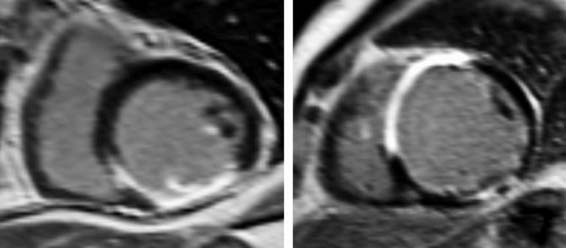
A further improvement in quantification of data is the use of strain. This approach has the advantage over velocity imaging in that it can differentiate between passive motion and active deformation. In theory, strain imaging may therefore provide perfect information on viability and may prevent the well-known problem of tethering in stress echocardiography. Recently, speckle-tracking strain imaging was introduced as a novel echocardiographic technique that enables the assessment of multidirectional myocardial strain. Based on the detection of natural acoustic markers (so-called speckles) equally distributed in the myocardial wall, speckle-tracking imaging quantifies myocardial deformation in the radial, circumferential, and longitudinal directions, with independence of the angle insonation of the ultrasound beam (which is not the case with Doppler-derived strain imaging).69 An example of these different strains in a normal individual is shown in Figure 39-9. Even the resting strain of dysfunctional myocardium is already an indicator for viability. Becker and coworkers71 evaluated 47 patients with chronic ischemic LV dysfunction and reported that radial strain and strain rate decreased in parallel to an increasing extent of scar tissue as assessed with contrast-enhanced MRI. Radial strain allowed differentiation between nontransmural and transmural infarction with a sensitivity of 70.0% and a specificity of 71.2%, when a cutoff value for radial strain of 16.5% was used. In a subsequent study, 53 patients underwent two-dimensional (2D) radial strain imaging to assess viability; these patients underwent revascularization with assessment of functional recovery at 9 ± 2 months of follow-up.72 Segments with recovery of function had more preserved radial strain (22.6% ± 6.3% versus 15.2% ± 7.5%, P < 0.001); using a cutoff of 17.2% for peak systolic radial strain, functional recovery could be predicted with a sensitivity of 70.2% and a specificity of 85.1%). The quantification of strain during low-dose dobutamine stress to assess viability has also been explored. In 55 patients undergoing revascularization, the quantification of strain during low-dose dobutamine stress echocardiography yielded a sensitivity ranging from 74% to 80%, with a specificity around 77%, to predict improvement of function after revascularization.73 Finally, postsystolic thickening can be derived from tissue Doppler imaging and has been proposed as a marker for viability.74 Some groups argued that postsystolic thickening is an accurate marker of viability and demonstrated good agreement with viability on metabolic imaging with FDG,75 whereas others suggested that postsystolic shortening represents scar tissue. Lim et al.76 evaluated 25 patients with chronic LV dysfunction (mean ejection fraction 32% ± 10%) with tissue Doppler imaging (to assess postsystolic thickening) and contrast-enhanced MRI. The authors demonstrated that postsystolic thickening was most often observed in segments with transmural scar formation on MRI.
Echocardiography with Intravenous Contrast
Methodology. The improved technical properties of the myocardial contrast agents now allow for assessment of myocardial perfusion (Fig. 39-10). In early studies, intracoronary injection of contrast was still needed, but with the newer generation of contrast agents, intravenous administration is possible. The recent contrast agents are composed of high-molecular-weight inert gases. The microbubbles stay in the vascular space and do not enter the extravascular space. Within the vascular space, microbubbles behave like red cells in terms of rheology and can be used in combination with echocardiography to directly visualize the myocardial perfusion. Since myocardial perfusion is a prerequisite for myocardial viability, myocardial contrast echocardiography has been used to assess myocardial viability. It has been shown that echo contrast parameters of myocardial perfusion correlate positively with the microvascular density and the capillary area and inversely with the extent of fibrosis.77 In the clinical setting, myocardial perfusion by myocardial contrast echocardiography is evaluated qualitatively, and segments are visually classified as being viable with normal or patchy perfusion, and nonviable when perfusion is absent (see Fig. 39-10).
Accuracy. The number of studies using contrast echocardiography for the prediction of functional recovery is limited. A small direct comparison between dobutamine stress echocardiography, thallium-201 imaging, and contrast echocardiography was performed in 18 patients undergoing revascularization.41 Both thallium-201 imaging and contrast echocardiography had a high sensitivity with a relatively low specificity, whereas dobutamine stress echocardiography had a lower sensitivity with a relatively high specificity for the prediction of improvement of regional function after revascularization. In that particular study, contrast echocardiography had a sensitivity of 89% with a specificity of 51%. Two other studies using contrast echocardiography confirmed this observation and showed a high sensitivity with a lower specificity.78,79 The lower specificity is related to the inability to identify subendocardial scar, resulting in “over-prediction of functional recovery”: Segments that contain subendocardial scar are indeed partially viable, but will not improve in function after revascularization. These segments consist of a mixture of scar and viable but normal myocardium, and since jeopardized but viable myocardium is not present, recovery of function will not occur. One study demonstrated that patients with three or more viable segments on contrast echocardiography had a high likelihood of improvement in global LV function post revascularization.41 At present, no studies with contrast echocardiography are available on the improvement in symptoms or long-term outcome.
Many more studies using echo contrast for viability assessment have been performed in patients with acute myocardial infarction. It has been shown that the extent and severity of perfusion defects after acute infarction correlated (inversely) with the likelihood of functional recovery at follow-up. Pooled analyses of 23 studies (including > 1,100 patients) revealed high sensitivity (approximately 85%) but lower specificity (approximately 74%) to predict recovery of regional and/or global function after acute infarction.80 The lack of viability after acute infarction on contrast echocardiography was also related to development of LV remodeling at longer follow-up. Galiuto and colleagues81 demonstrated in the Acute Myocardial Infarction Contrast Imaging (AMICI) study that 27% of 110 patients developed LV dilation at 6 months follow-up, and this was related mainly to no-reflow and absence of viability on contrast echocardiography. Finally, the prognostic value of contrast echocardiography after acute infarction has been demonstrated. Dwivedi et al.82 evaluated 95 patients with contrast echocardiography after acute infarction and demonstrated that among the clinical, biochemical, electrocardiographic, echocardiographic, and coronary angiographic markers of prognosis, the absence of viability on contrast echocardiography was independently predictive of cardiac death or acute infarction.
Magnetic Resonance Imaging
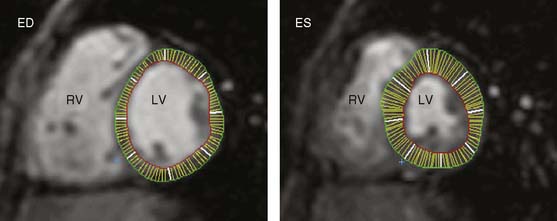
Accuracy. The described MRI techniques have been evaluated for assessment of myocardial viability in patients with chronic ischemic LV dysfunction (Fig. 39-16). For the prediction of recovery of regional contractile function following revascularization, 3 studies including 100 patients used end-diastolic wall thickness assessment by MRI.87–89 Pooled analysis resulted in a weighted mean sensitivity and specificity of 95% and 41%, respectively, whereas the positive predictive value and negative predictive value were 56% and 92%, respectively. Nine studies87,88,90–96 including 272 patients, employed dobutamine stress MRI. In these studies, the mean sensitivity and specificity to predict recovery of function post revascularization were 74% and 82%, respectively; positive predictive value and negative predictive value were both 78%. Contrast-enhanced MRI was used in 5 studies,95–99 including 178 patients, resulting in a weighted mean sensitivity and specificity of 84% and 63%, respectively, with positive and negative predictive values of 72% and 78%, respectively. No studies are currently available using MRI for the prediction of recovery of global LV function or for evaluation of prognosis following revascularization.
Additional Information Provided by Echocardiography and MRI
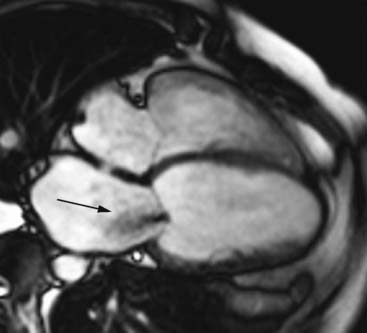
In patients with severe heart failure, the left ventricle dilates and becomes less elliptical and more spherical, a process referred to as LV remodeling. LV volumes will increase and LVEF will decline. LV volumes are important to determining the likelihood of functional improvement after revascularization. It has been shown previously that patients with severely dilated left ventricles have a low likelihood of improved function, despite the presence of viable tissue. For example, among 61 patients with substantial viability (= 4 viable segments), the likelihood of improvement in LVEF was minimal when the LV end-systolic volume exceeded 153 mL.100 Accordingly, precise information on LV volumes and size, and LVEF is needed. This can be obtained by both echocardiography (using 3D approaches with or without intravenous contrast to improve endocardial border opacification) and MRI (see Figs. 39-7, 39-11, and Fig. 39-17). Surgical resection of dysfunctional myocardium and/or scar tissue is increasingly used in patients with ischemic cardiomyopathy. It has been demonstrated that these procedures can reduce LV size and restore the normal geometry of the left ventricle. In the RESTORE study, surgical ventricular restoration was applied in 1198 patients with a previous infarction.101 A clear reduction in LV volumes with an improvement in LV shape was obtained. The LV end-systolic volume index decreased from 80.4 ± 51.4 mL/m2 before surgery to 56.6 ± 34.3 mL/m2 after surgery (P < 0.001); in these patients, the 5-year survival was 68.6% ± 2.8%. Prior to these surgical procedures, precise information on the presence, location, and extension of LV aneurysms is needed. This can be obtained by transthoracic echocardiography, preferably in combination with intravenous. In addition, MRI is extremely suited to depict LV aneurysms. The excellent spatial resolution of MRI permits measurement of precise wall thickness at the aneurismal regions; the use of contrast-enhanced MRI permits detection of scar tissue in these regions.
Another area of importance concerns the presence of ischemic mitral regurgitation, which is frequently observed in patients with ischemic heart failure, as a consequence of mitral annular dilation and systolic retraction of the mitral leaflets. Chronic ischemic mitral regurgitation causes volume overload, resulting in further LV dilation and progressive mitral regurgitation, which is associated with poor long-term outcome. It has also been shown that surgical correction of severe mitral regurgitation (mitral valve repair) at the time of revascularization resulted in reverse LV remodeling, a reduction in left atrial size, and good outcome.102 Accordingly, information on the presence, severity, and etiology of mitral regurgitation in patients with ischemic cardiomyopathy is needed. Transthoracic echocardiography is the technique of choice and permits quantification of regurgitant volume. Transesophageal echocardiography may be preferred for determination of etiology of regurgitation and to determine the feasibility of surgical mitral valve repair. Eventually, MRI may provide superior information on valve anatomy (to determine precise etiology of mitral regurgitation) and regurgitant volume (Fig. 39-18), but, at present, availability and post processing of data hamper routine use of MRI to evaluate ischemic mitral regurgitation.
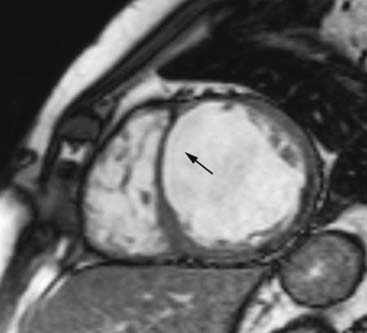
Finally, information on the presence, location, shape, and size of LV thrombi is important. This information can be provided either with echocardiography (using intravenous contrast, Fig. 39-19) or (contrast-enhanced) MRI.
COMPARISON OF THE DIFFERENT TECHNIQUES
It is important to realize that the different viability techniques detect different aspects of myocardial viability (see Table 39-2). Nuclear imaging with technetium-99m-labeled agents or thallium-201 detects cell membrane integrity, intact mitochondria, or preserved perfusion, whereas imaging with FDG detects preserved glucose metabolism. Dobutamine stress echocardiography can detect contractile reserve. Previous comparative studies have shown that severely damaged myocardium may still have preserved perfusion or glucose utilization, or intact cell membranes/mitochondria, but may no longer have contractile reserve, secondary to the severely damaged contractile apparatus. Indeed, head-to-head comparisons between nuclear imaging with thallium-201 or FDG and dobutamine stress echocardiography demonstrated that about 50% of the dysfunctional segments considered viable on nuclear imaging did not have contractile reserve. For example, 114 patients with ischemic cardiomyopathy underwent perfusion imaging with technetium-99m-tetrofosmin and dobutamine stress echocardiography.103 Of the 1336 dysfunctional segments, perfusion was preserved in 51% of segments and contractile reserve in 31% (P < 0.05); 47% of the segments with perfusion did not exhibit contractile reserve.
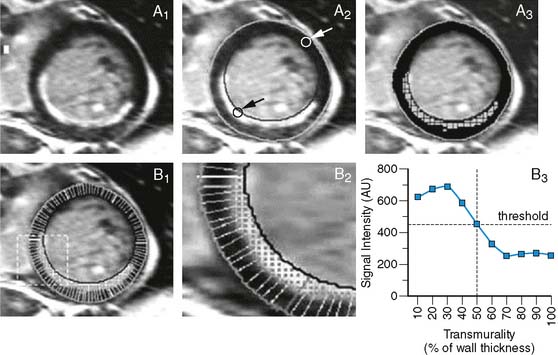
Contrast-enhanced MRI detects scar tissue; with great precision, the extent and location of scar tissue is delineated. The remaining myocardium, however, is alive but it cannot be derived whether this is normal myocardium or ischemically jeopardized myocardium (chronic stunning, hibernation). Indeed, in direct comparisons between nuclear imaging and contrast-enhanced MRI, it was observed that great agreement existed between the two techniques for assessment of scar tissue.104–106 Ibrahim and coworkers105 compared contrast-enhanced MRI with technetium-99m-sestamibi SPECT in 78 patients imaged within 7 days after acute infarction. The authors demonstrated that contrast-enhanced MRI was superior to SPECT to detect small regions of scar tissue; particularly in non-Q-wave infarctions, contrast-enhanced MRI was more sensitive to detect scar formation.
Klein and colleagues106 evaluated 31 patients with chronic ischemic LV dysfunction using both FDG PET and contrast-enhanced MRI. The authors demonstrated that the sensitivity to detect FDG-perfusion matches (indicating scar tissue) was excellent (96% on a patient level and 100% on a segment level). However, Roes et al.104 recently demonstrated in another head-to-head comparison between FDG SPECT and contrast-enhanced MRI that in the non-scarred myocardium on contrast-enhanced MRI, FDG imaging showed a mixture of normal, chronically stunned, and hibernating myocardium. To enhance the detection of viability with MRI (and better identify jeopardized but viable myocardium and discriminate this from normal, viable myocardium), the combined assessment of contrast-enhanced MRI and low-dose dobutamine MRI has been proposed. While contrast-enhanced MRI provides the information on the scar tissue, the addition of low-dose dobutamine MRI helps to differentiate in the non scarred myocardium among normal, chronically stunned, and hibernating myocardium.107
The diagnostic accuracies of the noninvasive imaging modalities for the prediction of recovery of regional dysfunction after revascularization (Fig. 39-20) are summarized in Table 39-3. In general, FDG PET had the highest sensitivity (92%, P < 0.05 versus other techniques) and is therefore often considered the reference technique. The specificities of all imaging techniques are lower compared to the sensitivities. Dobutamine stress echocardiography has the highest specificity for the prediction of recovery of regional dysfunction (78%, P < 0.05 versus other techniques).
For the prediction of improvement of global LV function after revascularization (Fig. 39-21), the diagnostic accuracy of the techniques is summarized in Table 39-4. Currently, no studies are available using MRI for the prediction of recovery of global LV function. Pooling of the data showed that all techniques have a relatively good sensitivity, whereas dobutamine stress echocardiography has a somewhat lower sensitivity. On the other hand, this technique appeared to have a higher specificity for the prediction of improvement of global function, although the differences compared to the other techniques were not statistically significant.
Accordingly, noninvasive imaging generally provides a high sensitivity for the prediction of improvement of function post revascularization. The specificity for all techniques is lower, however, which means that some segments that are classified as viable do not improve in function after revascularization. A variety of reasons can be considered to understand this phenomenon. These are summarized in Table 39-5 and can be divided into factors influencing specificity before, during, and after revascularization.
Table 39-5 Influences on the Specificity of Noninvasive Imaging Techniques for Predicting Functional Recovery After Revascularization
| Factors Before Revascularization |
| Imperfect assessment of jeopardized but viable myocardium (partial scar/fibrosis: subendocardial scar) |
| Ischemic events in the time interval between viability assessment and revascularization |
| Duration of hibernation before revascularization |
| Too extensive left ventricular remodeling |
| Presence of large scar adjacent to area of viable myocardium |
| Factors During Revascularization |
| Incomplete revascularization |
| Perioperative ischemic events |
| Factors After Revascularization |
| Too short follow-up for functional recovery assessment after revascularization (≤6 months) |
| Ischemic events during follow-up |
| Bypass graft occlusion |
Factors before revascularization include the imperfect assessment of jeopardized but viable myocardium; a substantial number of dysfunctional segments are classified as viable but contain only a mixture of subendocardial scar and normal (viable) myocardium, with jeopardized (viable) myocardium. These segments are indeed viable, but they cannot improve in function after revascularization. It has been discussed, however, whether revascularization of these segments may have other beneficial effects (instead of improvement in function)—for example, prevention of ventricular arrhythmias and prevention of LV remodeling. This remains to be determined. In addition, often ischemic events occur after imaging and before revascularization. Accordingly, viable segments may become damaged and progress to scar formation prior to revascularization, thereby preventing recovery of function post revascularization. The duration of hibernation before revascularization is also important. Various studies have demonstrated that hibernation is an unstable substrate, and delay in revascularization may result in progress from hibernation to scar tissue, without recovery of function post revascularization.108,109 The extent of LV dilation is also important. It has been shown that too-severely dilated ventricles will not improve in function post revascularization, despite the presence of substantial viable tissue. During revascularization, prolonged ischemia and incomplete revascularization may also prevent recovery of function after revascularization. The severity of CAD and coronary anatomy may hamper complete revascularization. Finally, after revascularization, the most important factor is that almost every study has performed assessment of functional recovery within 6 months post revascularization. It has been shown, however, that more severely damaged myocardium needs a longer time to improve in function. Bax et al.109 demonstrated that hibernating myocardium may need up to 1 year to improve in function after revascularization, whereas chronically stunned myocardium (which is less damaged myocardium) may improve within 3 months post revascularization. In addition, the natural progression of CAD may result in ischemic events during follow-up, preventing dysfunctional but viable myocardium from recovering after revascularization, or inducing new dysfunctional areas. In a similar way, early or late bypass graft occlusion may prevent dysfunctional but viable myocardium from recovering. All these different factors may have an impact on the recovery of regional and global contractile dysfunction and as a result, reduce the specificity of the noninvasive imaging techniques and, more important, may influence patient outcome.
1. Gheorghiade M., Bonow R.O. Chronic heart failure in the United States. A manifestation of coronary artery disease. Circulation. 1998;97:282-289.
2. Bax J.J., Poldermans D., Elhendy A., et al. Sensitivity, specificity, and predictive accuracies of various noninvasive techniques for detecting hibernating myocardium. Curr Probl Cardiol. 2001;26:142-186.
3. Schinkel A.F., Bax J.J., Poldermans D., et al. Hibernating myocardium: Diagnosis and patient outcomes. Curr Probl Cardiol. 2007;32:375-410.
4. Schinkel A.F., Bax J.J., van Domburg R., et al. Dobutamine-induced contractile reserve in stunned, hibernating, and scarred myocardium in patients with ischemic cardiomyopathy. J Nucl Med. 2003;44:127-133.
5. Rahimtoola S.H. The hibernating myocardium. Am Heart J. 1989;117:211-221.
6. Braunwald E., Kloner R.A. The stunned myocardium: prolonged, postischemic ventricular dysfunction. Circulation. 1982;66:1146-1149.
7. Elsasser A., Muller K.D., Skwara W., Bode C., Kubler W., Vogt A.M. Severe energy deprivation of human hibernating myocardium as possible common pathomechanism of contractile dysfunction, structural degeneration and cell death. J Am Coll Cardiol. 2002;39:1189-1198.
8. Depre C., Vanoverschelde J., Gerber B., et al. Correlation of functional recovery with myocardial blood flow, glucose uptake, and morphologic features in patients with chronic left ventricular ischemic dysfunction undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 1997;113:371-378.
9. Schinkel A.F., Bax J.J., Boersma E., et al. Assessment of residual myocardial viability in regions with chronic electrocardiographic Q-wave infarction. Am Heart J. 2002;144:865-869.
10. Secknus M., Marwick T.H. Evolution of dobutamine echocardiography protocols and indications: Safety and side effects in 3011 studies over 5 years. J Am Coll Cardiol. 1997;29:1234-1240.
11. Gerber B.L., Vanoverschelde J-LJ., Bol A., Michel C., Labar D., Wijns W., et al. Myocardial blood flow, glucose uptake and recruitment of inotropic reserve in chronic left ventricular ischemic dysfunction. Implications for the pathophysiology of chronic hibernation. Circulation. 1996;94:651-659.
12. Charney R., Schwinger M.E., Chun J., Cohen M.V., Nanna M., Menegus M.A., et al. Dobutamine echocardiography and resting-redistribution thallium-201 scintigraphy predicts recovery of hibernating myocardium after coronary revascularization. Am Heart J. 1994;128:864-869.
13. Arnese M., Cornel J.H., Salustri A., Maat A.P.W.M., Elhendy A., Reijs A.E.M., et al. Prediction of improvement of regional left ventricular function after surgical revascularization: a comparison of low-dose dobutamine echocardiography with 201-TL SPECT. Circulation. 1995;91:2748-2752.
14. Perrone-Filardi P., Pace L., Prastaro M., Squame F., Betocchi S., Soricelli A., et al. Assessment of myocardial viability in patients with chronic coronary artery disease. Rest-4-hour-24-hour 201Tl tomography versus dobutamine echocardiography. Circulation. 1996;94:2712-2719.
15. Vanoverschelde J-LJ., D’Hondt A.-M., Marwick T., Gerber B.L., De Kock M., Dion R., et al. Head-to-head comparison of exercise-redistribution-reinjection thallium single-photon emission computed tomography and low dose dobutamine echocardiography for prediction of reversibility of chronic left ventricular ischemic dysfunction. J Am Coll Cardiol. 1996;28:432-442.
16. Marzullo P., Parodi O., Reisenhofer B., Sambuceti G., Picano E., Distante A., et al. Value of rest thallium-201/technetium-99m sestamibi and dobutamine echocardiography for detecting myocardial viability. Am J Cardiol. 1993;71:166-172.
17. Bax J.J., Cornel J.H., Visser F.C., Fioretti P.M., van Lingen A., Reijs A.E.M., et al. Prediction of recovery of myocardial dysfunction following revascularization: comparison of F18-fluorodeoxyglucose/thallium-201 single photon emission computed tomography, thallium-201 stress-reinjection single photon emission computed tomography and dobutamine echocardiography. J Am Coll Cardiol. 1996;28:558-564.
18. Senior R., Glenville B., Basu S., Sridhara B.S., Anagnostou E., Stanbridge R., et al. Dobutamine echocardiography and thallium-201 imaging predict functional improvement after revascularization in severe ischaemic left ventricular dysfunction. Br Heart J. 1995;74:358-364.
19. Perrone-Filardi P., Pace L., Prastaro M., Piscione F., Betocchi S., Squame F., et al. Dobutamine echocardiography predicts improvement of hypoperfused dysfunctional myocardium after revascularization in patients with coronary artery disease. Circulation. 1995;91:2556-2565.
20. Alfieri O., La Canna G., Giubinni R., Pardini A., Zogno M., Fucci C. Recovery of myocardial function. Eur J Cardiothorac Surg. 1993;7:325-330.
21. La Canna G., Alfieri O., Giubbini R., Gargano M., Ferrari R., Visioli O. Echocardiography during infusion of dobutamine for identification of reversible dysfunction in patients with chronic coronary artery disease. J Am Coll Cardiol. 1994;23:617-626.
22. Haque T., Furukawa T., Takahashi M., Kinoshita M. Identification of hibernating myocardium by dobutamine stress echocardiography: comparison with thallium-201 reinjection imaging. Am Heart J. 1995;130:553-563.
23. Baer F.M., Voth E., Deutsch H.J., Schneider C.A., Horst M., de Vivie E.R., et al. Predictive value of low dose dobutamine transesophageal echocardiography and fluorine-18 fluorodeoxyglucose positron emission tomography for recovery of regional left ventricular function after successful revascularization. J Am Coll Cardiol. 1996;28:60-69.
24. DeFilippi C.R., Willett D.W.L., Irani W.N., Eichhorn E.J., Velasco C.E., Grayburn P.A. Comparison of myocardial contrast echocardiography and low-dose dobutamine stress echocardiography in predicting recovery of left ventricular function after coronary revascularization in chronic ischemic heart disease. Circulation. 1995;92:2863-2868.
25. Elhendy A., Cornel J.H., Roelandt J.R.T.C., van Domburg R.T., Fioretti P.M. Akinesis becoming dyskinesis during dobutamine stress echocardiography. A predictor of poor functional recovery after revascularization. Chest. 1996;110:155-158.
26. Kostopoulos K.G., Kranidis A.I., Bouki K.P., Antonellis J.P., Kappos K.G., Rodogianni F.E., et al. Detection of myocardial viability in the prediction of improvement in left ventricular function after successful coronary revascularization by using the dobutamine stress echocardiography and quantitative SPECT rest-redistribution-reinjection 201Tl imaging after dipyridamole infusion. Angiology. 1996;47:1039-1046.
27. Cornel J.H., Bax J.J., Fioretti P.M., Visser F.C., Maat A.P.W.M., Boersma E., et al. Prediction of improvement of left ventricular function after revascularization. 18F-fluorodeoxyglucose SPECT vs low-dose dobutamine echocardiography. Eur Heart J. 1997;18:941-948.
28. Scognamiglio R., Fasoli G., Casarotto D., Miorelli M., Nistri S., Palisi M., et al. Postextrasystolic potentiation and dobutamine echocardiography in predicting recovery of myocardial function after coronary bypass revascularization. Circulation. 1997;96:816-820.
29. Pagano D., Bonser R.S., Townend J.N., Ordoubadi F., Lorenzoni R., Camici P.G. Predictive value of dobutamine echocardiography and positron emission tomography in identifying hibernating myocardium in patients with postischaemic heart failure. Heart. 1998;79:281-288.
30. Baer F.M., Theissen P., Schneider C.A., Voth E., Sechtem U., Schicha H., et al. Dobutamine magnetic resonance imaging predicts contractile recovery of chronically dysfunctional myocardium after successful revascularization. J Am Coll Cardiol. 1998;31:1040-1048.
31. Voci P., Bilotta F., Caretta Q., Mercanti C., Marino B. Low-dose dobutamine echocardiography predicts the early response of dysfunctioning myocardial segments to coronary artery bypass grafting. Am Heart J. 1995;129:521-526.
32. Picano E., Ostojic M., Varga A., Sicari R., Djordjevic-Dikic A., Nedeljkovic I., et al. Combined low dose dipyridamole-dobutamine stress echocardiography to identify myocardial viability. J Am Coll Cardiol. 1996;27:1422-1428.
33. Elhendy A., Cornel J.H., Roelandt J.R.T.C., Nierop P.R., Van Domburg R.T., Geleijnse M.L., et al. Impact of severity of coronary artery stenosis and the collateral circulation on the functional outcome of dyssynergic myocardium after revascularization in patients with healed myocardial infarction and chronic left ventricular dysfunction. Am J Cardiol. 1997;79:883-888.
34. Cornel J.H., Bax J.J., Elhendy A., Poldermans D., Vanoverschelde J.L.J., Fioretti P.M. Predictive accuracy of echocardiographic response of mildly dyssynergic myocardial segments to low-dose dobutamine. Am J Cardiol. 1997;80:1481-1484.
35. Pace L., Perrone-Filardi P., Mainenti P., Prastaro M., Vezzuto P., Varrone A., et al. Combined evaluation of rest-redistribution thallium-201 tomography and low-dose dobutamine echocardiography enhances the identification of viable myocardium in patients with chronic coronary artery disease. Eur J Nucl Med. 1998;25:744-750.
36. Sayad D.E., Willett D.L., Hundley G., Grayburn P.A., Peshock R.M. Dobutamine magnetic resonance imaging with myocardial tagging quantitatively predicts improvement in regional function after revascularization. Am J Cardiol. 1998;82:1149-1151.
37. Sicari R., Varga A., Picano E., Borges A.C., Gimelli A., Marzullo P. Comparison of combination of dipyridamole and dobutamine during echocardiography with thallium scintigraphy to improve viability detection. Am J Cardiol. 1999;83:6-10.
38. Gunning M.G., Anagnostopoulos C., Knight C.J., Pepper J., Burman E.D., Davies G., et al. Comparison of 201Tl, 99mTc-tetrofosmin, and dobutamine magnetic resonance imaging for identifying hibernating myocardium. Circulation. 1998;98:1869-1874.
39. Afridi I., Kleiman N.S., Raizner A.E., Zoghbi W.A. Dobutamine echocardiography in myocardial hibernation. Optimal dose and accuracy in predicting recovery of ventricular function after coronary angioplasty. Circulation. 1995;91:663-670.
40. Qureshi U., Nagueh S.F., Afridi I., Vaduganathan P., Blaustein A., Verani M.S., et al. Dobutamine echocardiography and quantitative rest-redistribution 201Tl tomography in myocardial hibernation. Relation of contractile reserve to 201Tl uptake and comparative prediction of recovery of function. Circulation. 1997;95:626-635.
41. Nagueh S.F., Vaduganathan P., Ali N., Blaustein A., Verani M.S., Winters W.L., et al. Identification of hibernating myocardium: comparative accuracy of myocardial contrast echocardiography, rest-redistribution thallium-201 tomography and dobutamine echocardiography. J Am Coll Cardiol. 1997;29:985-993.
42. Cornel J.H., Bax J.J., Elhendy A., Maat A.P.W.M., Kimman G.J.P., Geleijnse M.L., et al. Biphasic response to dobutamine predicts improvement of global left ventricular function after surgical revascularization in patients with stable coronary artery disease. Implications of time course of recovery on diagnostic accuracy. J Am Coll Cardiol. 1998;31:1002-1010.
43. Dellegrottaglie S., Perrone-Filardi P., Pace L., et al. Prediction of long-term effects of revascularization on regional and global left ventricular function by dobutamine echocardiography and rest Tl-201 imaging alone and in combination in patients with chronic coronary artery disease. J Nucl Cardiol. 2002;9:174-182.
44. Leoncini M., Sciagra R., Bellandi F., et al. Low-dose dobutamine nitrate-enhanced technetium 99m sestamibi gated SPECT versus low-dose dobutamine echocardiography for detecting reversible dysfunction in ischemic cardiomyopathy. J Nucl Cardiol. 2002;9:402-406.
45. Piscione F., De Luca G., Perrone-Filardi P., et al. Relationship between contractile reserve, Tl-201 uptake, and collateral angiographic circulation in collateral-dependent myocardium: implications regarding the evaluation of myocardial viability. J Nucl Cardiol. 2003;10:17-27.
46. Piscione F., Perrone-Filardi P., De Luca G., et al. Low dose dobutamine echocardiography for predicting functional recovery after coronary revascularisation. Heart. 2001;86:679-686.
47. Pace L., Filardi P.P., Cuocolo A., et al. Diagnostic accuracy of low-dose dobutamine echocardiography in predicting post-revascularisation recovery of function in patients with chronic coronary artery disease: relationship to thallium-201 uptake. Eur J Nucl Med. 2001;28:1616-1623.
48. Cwajg J.M., Cwajg E., Nagueh S.F., et al. End-diastolic wall thickness as a predictor of recovery of function in myocardial hibernation: relation to rest-redistribution T1–201 tomography and dobutamine stress echocardiography. J Am Coll Cardiol. 2000;35:1152-1161.
49. Ling L.H., Christian T.F., Mulvagh S.L., et al. Determining myocardial viability in chronic ischemic left ventricular dysfunction: a prospective comparison of rest-redistribution thallium 201 single-photon emission computed tomography, nitroglycerin-dobutamine echocardiography, and intracoronary myocardial contrast echocardiography. Am Heart J. 2006;151:882-889.
50. Hanekom L., Jenkins C., Jeffries L., et al. Incremental value of strain rate analysis as an adjunct to wall-motion scoring for assessment of myocardial viability by dobutamine echocardiography: a follow-up study after revascularization. Circulation. 2005;112:3892-3900.
51. Zaglavara T., Karvounis H.I., Haaverstad R., et al. Dobutamine stress echocardiography is highly accurate for the prediction of contractile reserve in the early postoperative period, but may underestimate late recovery in contractile reserve after revascularization of the hibernating myocardium. J Am Soc Echocardiogr. 2006;19:300-306.
52. Wiggers H., Egeblad H., Nielsen T.T., Botker H.E. Prediction of reversible myocardial dysfunction by positron emission tomography, low-dose dobutamine echocardiography, resting ECG, and exercise testing. Cardiology. 2001;96:32-37.
53. Carluccio E., Biagioli P., Alunni G., et al. Patients with hibernating myocardium show altered left ventricular volumes and shape, which revert after revascularization: evidence that dyssynergy might directly induce cardiac remodeling. J Am Coll Cardiol. 2006;47:969-977.
54. Bax J.J., Poldermans D., Elhendy A., et al. Improvement of left ventricular ejection fraction, heart failure symptoms and prognosis after revascularization in patients with chronic coronary artery disease and viable myocardium detected by dobutamine stress echocardiography. J Am Coll Cardiol. 1999;34:163-169.
55. Meluzin J., Cerny J., Frelich M., et al. Prognostic value of the amount of dysfunctional but viable myocardium in revascularized patients with coronary artery disease and left ventricular dysfunction. Investigators of this Multicenter Study. J Am Coll Cardiol. 1998;32:912-920.
56. Afridi I., Grayburn P.A., Panza J.A., et al. Myocardial viability during dobutamine echocardiography predicts survival in patients with coronary artery disease and severe left ventricular systolic dysfunction. J Am Coll Cardiol. 1998;32:921-926.
57. Anselmi M., Golia G., Cicoira M., et al. Prognostic value of detection of myocardial viability using low-dose dobutamine echocardiography in infarcted patients. Am J Cardiol. 1998;81:21G-38G.
58. Senior R., Kaul S., Lahiri A. Myocardial viability on echocardiography predicts long-term survival after revascularization in patients with ischemic congestive heart failure. J Am Coll Cardiol. 1999;33:1848-1854.
59. Chaudhry F.A., Tauke J.T., Alessandrini R.S., et al. Prognostic implications of myocardial contractile reserve in patients with coronary artery disease and left ventricular dysfunction. J Am Coll Cardiol. 1999;34:730-738.
60. Meluzin J., Cerny J., Spinarova L., et al. Prognosis of patients with chronic coronary artery disease and severe left ventricular dysfunction. The importance of myocardial viability. Eur J Heart Fail. 2003;5:85-93.
61. Sicari R., Picano E., Cortigiani L., et alVIDA (Viability Identification with Dobutamine Administration) Study Group. Prognostic value of myocardial viability recognized by low-dose dobutamine echocardiography in chronic ischemic left ventricular dysfunction. Am J Cardiol. 2003;92:1263-1266.
62. Rambaldi R., Bax J.J., Rizzello V., et al. Post-systolic shortening during dobutamine stress echocardiography predicts cardiac survival in patients with severe left ventricular dysfunction. Coron Artery Dis. 2005;16:141-145.
63. Rizzello V., Poldermans D., Schinkel A.F.L., et al. Long term prognostic value of myocardial viability and ischaemia during dobutamine stress echocardiography in patients with ischaemic cardiomyopathy undergoing coronary revascularisation. Heart. 2006;92:239-244.
64. Sozzi F.B., Poldermans D., Bax J.J., et al. Second harmonic imaging improves sensitivity of dobutamine stress echocardiography for the diagnosis of coronary artery disease. Am Heart J. 2001;142:153-159.
65. Grayburn P.A., Mulvagh S., Crouse L. Left ventricular opacification at rest and during stress. Am J Cardiol. 2002;90:21J-27J.
66. Reilly J.P., Tunick P.A., Timmermans R.J., et al. Contrast echocardiography clarifies uninterpretable wall motion in intensive care unit patients. J Am Coll Cardiol. 2000;35:485-490.
67. Aggeli C., Giannopoulos G., Roussakis G., et al. Safety of myocardial flash-contrast echocardiography in combination with dobutamine stress testing for the detection of ischaemia in 5250 studies. Heart. 2008;94:1571-1577.
68. Nucifora G., Marsan N.A., Siebelink H.M., et al. Safety of contrast-enhanced echocardiography within 24 h after acute myocardial infarction. Eur J Echocardiogr. 2008;9:816-818.
69. Marwick T.H. Quantitative techniques for stress echocardiography: dream or reality? Eur J Echocardiogr. 2002;3:171-176.
70. Rambaldi R., Poldermans D., Bax J.J., et al. Doppler tissue velocity sampling improves diagnostic accuracy during dobutamine stress echocardiography for the assessment of viable myocardium in patients with severe left ventricular dysfunction. Eur Heart J. 2001;21:1091-1098.
71. Becker M., Hoffmann R., Kuhl H.P., et al. Analysis of myocardial deformation based on ultrasonic pixel tracking to determine transmurality in chronic myocardial infarction. Eur Heart J. 2006;27:2560-2566.
72. Becker M., Lenzen A., Ocklenburg C., et al. Myocardial deformation imaging based on ultrasonic pixel tracking to identify reversible myocardial dysfunction. J Am Coll Cardiol. 2008;51(15):1473-1481.
73. Hanekom L., Jenkins C., Jeffries L., et al. Incremental value of strain rate analysis as an adjunct to wall-motion scoring for assessment of myocardial viability by dobutamine echocardiography: a follow-up study after revascularization. Circulation. 2005;112:3892-3900.
74. Brown M.A., Norris R.M., Takayama M., White H.D. Post-systolic shortening: a marker of potential for early recovery of acutely ischaemic myocardium in the dog. Cardiovasc Res. 1987;21:703-716.
75. Rambaldi R., Bax J.J., Rizzello V., et al. Post-systolic shortening during dobutamine stress echocardiography predicts cardiac survival in patients with severe left ventricular dysfunction. Coron Artery Dis. 2005;16:141-145.
76. Lim P., Pasquet A., Gerber B., et al. Is postsystolic shortening a marker of viability in chronic left ventricular ischemic dysfunction? Comparison with late enhancement contrast magnetic resonance imaging. J Am Soc Echocardiogr. 2008;21:452-457.
77. Shimoni S., Frangogiannis N.G., Aggeli C.J., et al. Microvascular structural correlates of myocardial contrast echocardiography in patients with coronary artery disease and left ventricular dysfunction: Implications for the assessment of myocardial hibernation. Circulation. 2002;106:950-956.
78. DeFilippi C.R., Willett D.L., Irani W.N., Eichhorn E.J., Velasco C.E., Grayburn P.A. Comparison of myocardial contrast echocardiography and low-dose dobutamine stress echocardiography in predicting recovery of left ventricular function after coronary revascularization in chronic ischemic heart disease. Circulation. 1995;92:2863-2868.
79. Shimoni S., Frangogiannis N.G., Aggeli C.J., et al. Identification of hibernating myocardium with quantitative intravenous myocardial contrast echocardiography: comparison with dobutamine echocardiography and thallium-201 scintigraphy. Circulation. 2003;107:538-544.
80. Hayat S.A., Senior R. Myocardial contrast echocardiography in ST elevation myocardial infarction: ready for prime time? Eur Heart J. 2008;29:299-314.
81. Galiuto L., Garramone B., Scara A., et al. The extent of microvascular damage during myocardial contrast echocardiography is superior to other known indexes of post-infarct reperfusion in predicting left ventricular remodeling: results of the multicenter AMICI study. J Am Coll Cardiol. 2008;51:552-559.
82. Dwivedi G., Janardhanan R., Hayat S.A., et al. Prognostic value of myocardial viability detected by myocardial contrast echocardiography early after acute myocardial infarction. J Am Coll Cardiol. 2007;50:327-334.
83. Fieno D.S., Kim R.J., Chen E.L., Lomasney J.W., Klocke F.J., Judd R.M. Contrast-enhanced magnetic resonance imaging of myocardium at risk: distinction between reversible and irreversible injury throughout infarct healing. J Am Coll Cardiol. 2000;36:1985-1991.
84. Wu E., Judd R.M., Vargas J.D., Klocke F.J., Bonow R.O., Kim R.J. Visualisation of presence, location, and transmural extent of healed Q-wave and non-Q-wave myocardial infarction. Lancet. 2001;357:21-28.
85. Schuijf J.D., Kaandorp T.A., Lamb H.J., et al. Quantification of myocardial infarct size and transmurality by contrast-enhanced magnetic resonance imaging in men. Am J Cardiol. 2004;94:284-288.
86. Gerber B.L., Belge B., Legros G.J., et al. Characterization of acute and chronic myocardial infarcts by multidetector computed tomography: comparison with contrast-enhanced magnetic resonance. Circulation. 2006;113:823-833.
87. Schmidt M., Voth E., Schneider C.A., et al. F-18-FDG uptake is a reliable predictor of functional recovery of akinetic but viable infarct regions as defined by magnetic resonance imaging before and after revascularization. Magn Reson Imaging. 2004;22:229-236.
88. Baer F.M., Theissen P., Schneider C.A., et al. Dobutamine magnetic resonance imaging predicts contractile recovery of chronically dysfunctional myocardium after successful revascularization. J Am Coll Cardiol. 1998;31:1040-1048.
89. Klow N.E., Smith H.J., Gullestad L., Seem E., Endresen K. Outcome of bypass surgery in patients with chronic ischemic left ventricular dysfunction. Predictive value of MR imaging. Acta Radiol. 1997;38:76-82.
90. Baer F.M., Theissen P., Crnac J., et al. Head to head comparison of dobutamine-transoesophageal echocardiography and dobutamine-magnetic resonance imaging for the prediction of left ventricular functional recovery in patients with chronic coronary artery disease. Eur Heart J. 2000;21:981-991.
91. Gunning M.G., Anagnostopoulos C., Knight C.J., et al. Comparison of 201Tl, 99mTc-tetrofosmin, and dobutamine magnetic resonance imaging for identifying hibernating myocardium. Circulation. 1998;98:1869-1874.
92. Sayad D.E., Willett D.L., Hundley W.G., Grayburn P.A., Peshock R.M. Dobutamine magnetic resonance imaging with myocardial tagging quantitatively predicts improvement in regional function after revascularization. Am J Cardiol. 1998;82:1149-1151.
93. Sandstede J.J., Bertsch G., Beer M., et al. Detection of myocardial viability by low-dose dobutamine Cine MR imaging. Magn Reson Imaging. 1999;17:1437-1443.
94. Trent R.J., Waiter G.D., Hillis G.S., McKiddie F.I., Redpath T.W., Walton S. Dobutamine magnetic resonance imaging as a predictor of myocardial functional recovery after revascularisation. Heart. 2000;83:40-46.
95. Wellnhofer E., Olariu A., Klein C., et al. Magnetic resonance low-dose dobutamine test is superior to SCAR quantification for the prediction of functional recovery. Circulation. 2004;109:2172-2174.
96. Van Hoe L., Vanderheyden M. Ischemic cardiomyopathy: value of different MRI techniques for prediction of functional recovery after revascularization. AJR Am J Roentgenol. 2004;182:95-100.
97. Kim R.J., Wu E., Rafael A., et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445-1453.
98. Selvanayagam J.B., Kardos A., Francis J.M., et al. Value of delayed-enhancement cardiovascular magnetic resonance imaging in predicting myocardial viability after surgical revascularization. Circulation. 2004;110:1535-1541.
99. Kuhl H.P., Lipke C.S., Krombach G.A., et al. Assessment of reversible myocardial dysfunction in chronic ischaemic heart disease: comparison of contrast-enhanced cardiovascular magnetic resonance and a combined positron emission tomography-single photon emission computed tomography imaging protocol. Eur Heart J. 2006;27:846-853.
100. Schinkel A.F., Poldermans D., Rizzello V., Vanoverschelde J.L., Elhendy A., Boersma E., et al. Why do patients with ischemic cardiomyopathy and a substantial amount of viable myocardium not always recover in function after revascularization? J Thorac Cardiovasc Surg. 2004;127:385-390.
101. Athanasuleas C.L., Buckberg G.D., Stanley A.W., et alRESTORE group. Surgical ventricular restoration in the treatment of congestive heart failure due to post-infarction ventricular dilation. J Am Coll Cardiol. 2004;44:1439-1445.
102. Bax J.J., Braun J., Somer S., et al. Restrictive annuloplasty and coronary revascularization in ischemic mitral regurgitation results in reverse left ventricular remodeling. Circulation. 2004;110(11 Suppl 1):II-103–II-108.
103. Bax J.J., Poldermans D., Schinkel A.F., et al. Perfusion and contractile reserve in chronic dysfunctional myocardium: relation to functional outcome after surgical revascularization. Circulation. 2002;106(12 Suppl 1):I14-I18.
104. Roes S.D., Kaandorp T.A., Ajmone Marsan N., et al. Agreement and disagreement between contrast-enhanced magnetic resonance imaging and nuclear imaging for assessment of myocardial viability. Eur J Nucl Med Mol Imaging. 2009;36:594-601.
105. Ibrahim T., Bülow H.P., Hackl T., et al. Diagnostic value of contrast-enhanced magnetic resonance imaging and single-photon emission computed tomography for detection of myocardial necrosis early after acute myocardial infarction. J Am Coll Cardiol. 2007;49:208-216.
106. Klein C., Nekolla S.G., Bengel F.M., et al. Assessment of myocardial viability with contrast-enhanced magnetic resonance imaging: Comparison with positron emission tomography. Circulation. 2002;105:162-167.
107. Kaandorp T.A., Bax J.J., Schuijf J.D., et al. Head-to-head comparison between contrast-enhanced magnetic resonance imaging and dobutamine magnetic resonance imaging in men with ischemic cardiomyopathy. Am J Cardiol. 2004;93:1461-1464.
108. Beanlands R.S., Hendry P.J., Masters R.G., deKemp R.A., Woodend K., Ruddy T.D. Delay in revascularization is associated with increased mortality rate in patients with severe left ventricular dysfunction and viable myocardium on fluorine 18-fluorodeoxyglucose positron emission tomography imaging. Circulation. 1998;98(Suppl 19):II51-II56.
109. Bax J.J., Schinkel A.F., Boersma E., et al. Early versus delayed revascularization in patients with ischemic cardiomyopathy and substantial viability: impact on outcome. Circulation. 2003;108(Suppl 1):II39-II42.