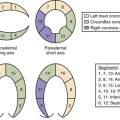
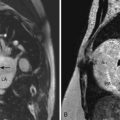
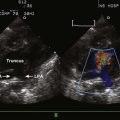
13 Myocardial Pathology
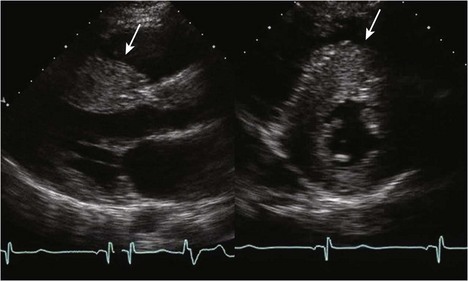
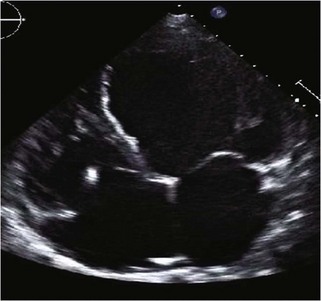
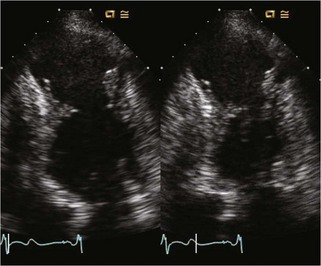
Hypertrophic Cardiomyopathy
Background
TABLE 13-1 FEATURES DISTINGUISHING “ATHLETE’S HEART” FROM HCM IN ADULTS*
| Feature | Athlete’s Heart | HCM |
|---|---|---|
| Maximal wall thickness | ≤16 mm | ≥13 mm |
| Pattern of LVH | Predominantly concentric | Concentric or asymmetrical |
| LV cavity dimension | Often > 55 mm (in endurance athletes) | Usually < 45 mm |
| Diastolic function | Normal | Normal or abnormal |
| Gender | Male > female | Male = female |
| Family history of HCM or SCD | No | Yes or no |
| Delayed enhancement (MRI) | No | Yes or no |
| Exercise capacity | Above normal | Normal to below normal |
| Response to deconditioning | LVH regression | No change in LVH |
* Intended for adults or adult-sized teenagers. Corresponding Z scores can be calculated for children but have not been validated.
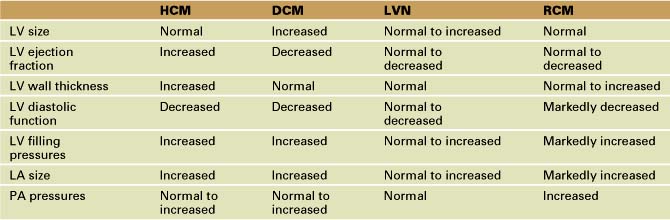
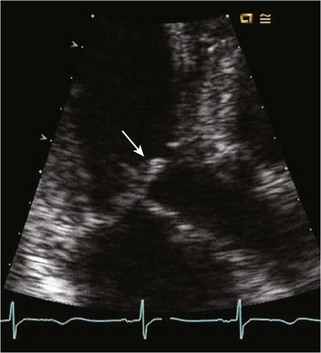
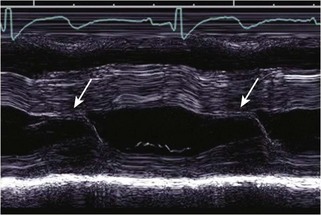
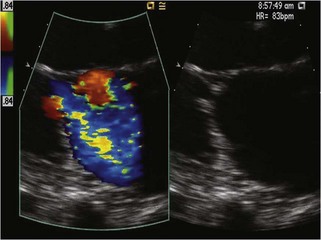
Echocardiographic Approach (Table 13-2)
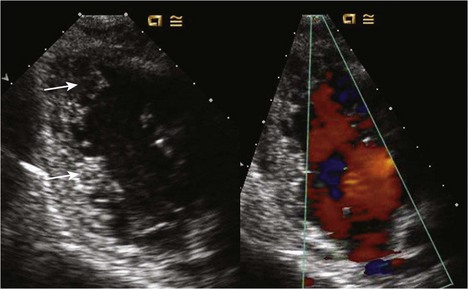
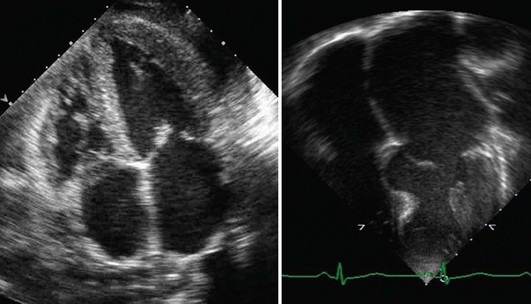
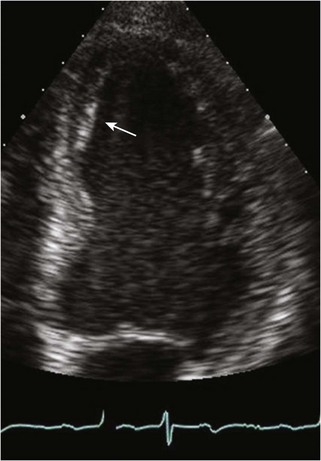
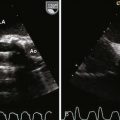
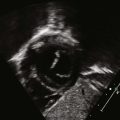
Anatomic Imaging
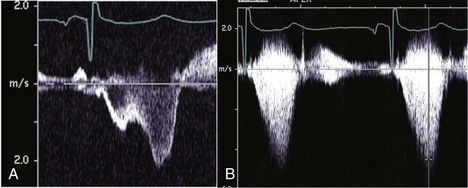
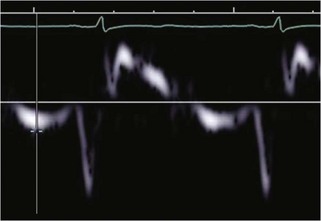
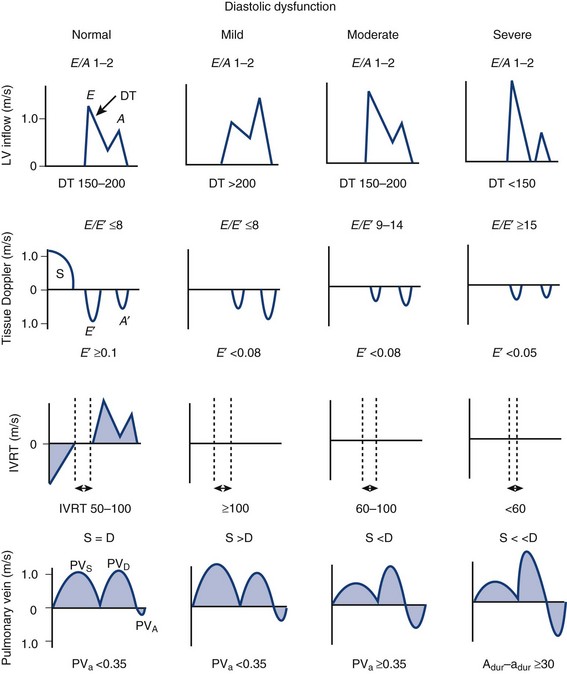
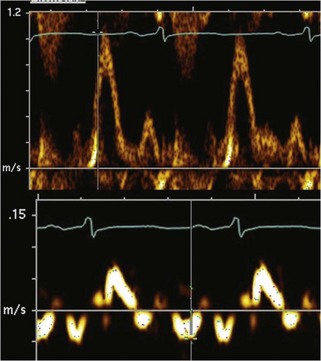
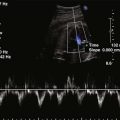
Physiologic Data
Alternate Approaches
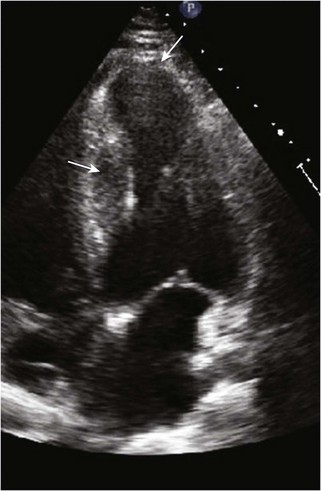
Dilated Cardiomyopathy
TABLE 13-3 CONGENITAL AND ACQUIRED CAUSES OF DCM PRESENTING IN CHILDREN AND ADULTS
| Children | Adults |
|---|---|
Echocardiographic Approach (See Table 13-2)
Anatomic Imaging
Physiologic Data
Key Points
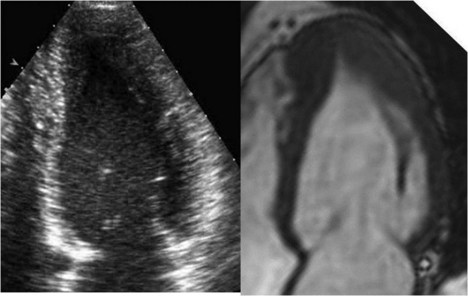
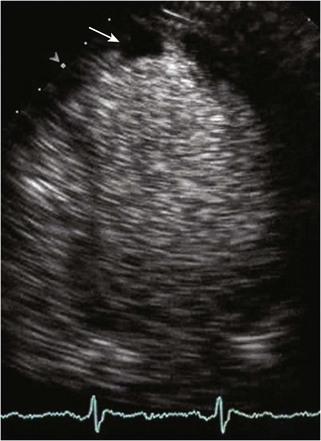
Left Ventricular Noncompaction
Background
Echocardiographic Approach (See Table 13-2)
Alternate Approaches
Restrictive Cardiomyopathy
Background
Echocardiographic Approach (See Table 13-2)
Anatomic Imaging
Physiologic Data
TABLE 13-4 ECHOCARDIOGRAPHIC PARAMETERS IN RCM AND CONSTRICTIVE PERICARDITIS
| RCM | Constrictive Pericarditis | |
|---|---|---|
| LV size | Normal | Normal |
| LV systolic function | Normal | Normal |
| LV wall thickness | Normal to increased | Normal |
| LV filling pressures | Markedly increased | Increased |
| LA size | Markedly increased | Increased |
| PA pressures | Markedly increased | Increased |
| Pericardium | Normal | Echobright |
| Septal shifting with respiration | Absent | Present |
| Variability in MV inflow | Absent | Present |
| MV-TV inflow velocities | Concordant | Discordant |
Alternate Approaches
Key Points
1 Woo A, Wigle ED, Rakowski H. Echocardiography in the evaluation and management of patients with hypertrophic cardiomyopathy. In: Otto CM, editor. The Practice of Clinical Echocardiography. Philadelphia: Elsevier/Saunders; 2007:653-709.
2 Nagueh SF, Mahmarian JJ. Noninvasive cardiac imaging in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2006;48(12):2410-2422.
3 Ho CY, Sweitzer NK, McDonough B, et al. Assessment of diastolic function with Doppler tissue imaging to predict genotype in preclinical hypertrophic cardiomyopathy. Circulation. 2002;105(25):2992-2997.
4 Geske JB, Sorajja P, Nishimura RA, Ommen SR. Evaluation of left ventricular filling pressures by Doppler echocardiography in patients with hypertrophic cardiomyopathy: correlation with direct left atrial pressure measurement at cardiac catheterization. Circulation. 2007;116(23):2702-2708.
5 St. John Sutton M. Doppler echocardiography in heart failure and cardiac resynchronization. In: Otto CM, editor. The Practice of Clinical Echocardiography. Philadelphia: Elsevier/Saunders; 2007:629-652.
6 Kirkpatrick JN, Vannan MA, Narula J, Lang RM. Echocardiography in heart failure: applications, utility, and new horizons. J Am Coll Cardiol. 2007;50(5):381-396.
7 Frischknecht BS, Attenhofer Jost CH, Oechslin EN, et al. Validation of noncompaction criteria in dilated cardiomyopathy, and valvular and hypertensive heart disease. J Am Soc Echocardiogr. 2005;18(8):865-872.
8 Eidem BW. Noninvasive evaluation of left ventricular noncompaction: what’s new in 2009? Pediatr Cardiol. 2009;30(5):682-689.
9 Naqvi T. Restrictive cardiomyopathy: diagnosis and prognostic implications. In: Otto CM, editor. The Practice of Clinical Echocardiography. Philadelphia: Elsevier/Saunders; 2007:679-711.
10 Pieroni M, Chimenti C, De Cobelli F, et al. Fabry’s disease cardiomyopathy: echocardiographic detection of endomyocardial glycosphingolipid compartmentalization. J Am Coll Cardiol. 2006;47(8):1663-1671.