Myocardial infarction
Pathology
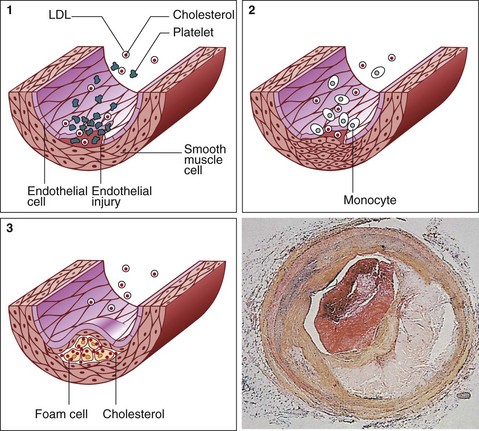
The underlying pathology in MI is atherosclerosis, an inflammatory process located within the arterial wall in the form of atheromatous plaques (Fig 27.1). These cause narrowing of the arterial lumen, resulting in reduced coronary perfusion, the clinical manifestation of which is chest pain (angina pectoris). If an unstable plaque ruptures, the released contents precipitate the formation of a clot. This process, known as thrombosis, may result in sudden complete occlusion of the affected artery and infarction of the area of myocardium it supplies.
Definitions
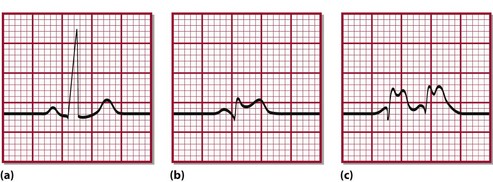
The term acute coronary syndrome (ACS) refers to chest pain and other related symptoms attributed to impaired blood supply to the heart. It encompasses ST-segment elevation myocardial infarction (STEMI), non–ST-segment elevation myocardial infarction (NSTEMI) and unstable angina. The ST segment refers to part of the electrical tracing of the heart beat recorded on the electrocardiogram or ECG (Fig 27.2). Pathologically, it is almost always associated with rupture of an atherosclerotic plaque and partial or complete thrombosis of a coronary artery. In some instances ACS may occur from increased demands on the heart, e.g. with severe blood loss, anaemia, tachycardia or severe infections.
Myocardial infarction
The universal definition of acute myocardial infarction (AMI) has undergone changes in recent years in tandem with newer developments in the assays for diagnostic biomarkers. Table 27.1 briefly outlines the Experts Consensus Document on behalf of the Joint Task Force of European and American Cardiology Societies for the Redefinition of Myocardial Infarction.
Table 27.1
Definition of myocardial infarction (MI)
Criteria for acute myocardial infarction
The term myocardial infarction should be used when there is evidence of myocardial necrosis in a clinical setting consistent with myocardial ischaemia. Under these conditions the following meets the diagnosis for myocardial infarction:
 Detection of rise and/or fall of cardiac biomarkers (preferably troponin) with at least one value above the 99th percentile of the upper reference limit, together with evidence of myocardial ischaemia with at least one of the following:
Detection of rise and/or fall of cardiac biomarkers (preferably troponin) with at least one value above the 99th percentile of the upper reference limit, together with evidence of myocardial ischaemia with at least one of the following:
Criteria for prior myocardial infarction
Any one of the following criteria meets the diagnosis for prior myocardial infarction:
 Development of new pathological Q waves* with or without symptoms;
Development of new pathological Q waves* with or without symptoms;
 Imaging evidence of a region of viable myocardium that is thinned and fails to contract, in the absence of a non-ischaemic cause;
Imaging evidence of a region of viable myocardium that is thinned and fails to contract, in the absence of a non-ischaemic cause;
 Pathological findings of a healed or healing myocardial infarction.
Pathological findings of a healed or healing myocardial infarction.
*Q waves signify that the area of myocardial necrosis extends through the full thickness of the heart muscle (usually the ventricular wall).
Diagnosis
The essential components of diagnosis are the history, the characteristic ECG changes and the detection in blood of biochemical markers of myocardial injury. Patients experiencing MI classically complain of severe crushing central chest pain. However, such a characteristic history is not always obtained, and a minority of patients may even have a ‘silent’ MI. When present, the characteristic ECG changes (Fig 27.2) are specific to MI, but they are equivocal or absent in up to 30% of patients. It is in this group of patients that cardiac markers are most useful.
Cardiac biomarkers
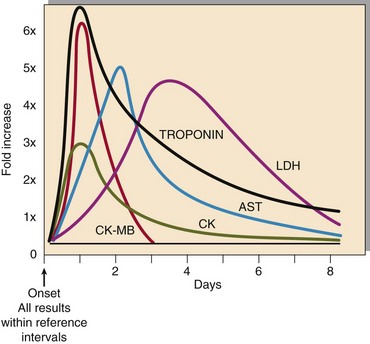
When myocardial cells die, they break up and release their contents. This is the basis for the role of cardiac biomarkers in MI diagnosis. Historically, various ‘cardiac enzymes’ have been used (Fig 27.3); however, at present, cardiac troponins are used in the diagnosis of MI. Troponin is a complex contractile protein comprising of three subunits: C, T and I. Troponin T and I are cardiospecific (therefore used in MI diagnosis; Tables 27.2 and 27.3), whereas C is also present in skeletal muscle. Troponins rise within a few hours on onset of symptoms and remain elevated for 1–2 weeks. This property enables early as well as late diagnosis. The diagnostic sensitivity of troponin reaches 100% 12 hours after onset of symptoms, i.e. MI can be excluded with confidence with a negative (undetectable) troponin if the blood sample is collected 12 hours or more after the onset of chest pain. Point-of-care devices are also available, which allow on-site measurement of troponin, but these tests are still relatively expensive.
Table 27.2
Potential roles for troponin measurement
 Diagnosis of acute myocardial infarction (MI)
Diagnosis of acute myocardial infarction (MI)
 Prognosis in acute coronary syndrome
Prognosis in acute coronary syndrome
 Diagnosis of perioperative MI (where there is coexistent skeletal muscle damage)
Diagnosis of perioperative MI (where there is coexistent skeletal muscle damage)
 Monitoring thrombolytic therapy
Monitoring thrombolytic therapy
 Identification of patients who will respond to interventions, e.g. low-molecular-weight heparins, platelet glycoprotein IIb/IIIa antagonists, angioplasty
Identification of patients who will respond to interventions, e.g. low-molecular-weight heparins, platelet glycoprotein IIb/IIIa antagonists, angioplasty
Table 27.3
| Troponin T | Troponin I | |
| Molecular weight | 37 kDa | 22.5 kDa |
| Nature of protein | Structural | Catalytic |
| Kinetics of release | Biphasic | Only a single peak |
| Duration of elevation | Up to 14 days | 5–7 days |
| Number of assays | One | Several |
| Ontogeny | May be expressed in skeletal muscle in utero | Only ever expressed in myocardium |