Myasthenia Gravis
After reading this chapter, will be able to:
• List the anatomic alterations of the lungs associated with myasthenia gravis.
• Describe the causes of myasthenia gravis.
• List the cardiopulmonary clinical manifestations associated with myasthenia gravis.
• Describe the general management of myasthenia gravis.
• Describe the clinical strategies and rationales of the SOAPs presented in the case study.
• Define key terms and complete self-assesment questions at the end of the chapter and on Evolve.
Anatomic Alterations of the Lungs Associated with Myasthenia Gravis
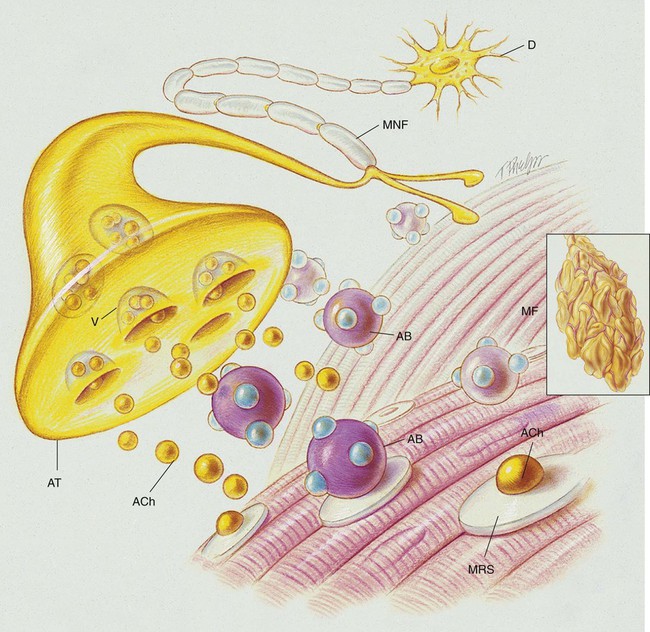
Myasthenia gravis is a chronic disorder of the neuromuscular junction that interferes with the chemical transmission of acetylcholine (ACh) between the axonal terminal and the receptor sites of voluntary muscles (see Figure 29-1). It is characterized by fatigue and weakness, with improvement following rest. Because the disorder affects only the myoneural (motor) junction, sensory function is not lost.
Etiology and Epidemiology
The cause of myasthenia gravis appears to be related to ACh receptor antibodies (the IgG antibodies) that block the nerve impulse transmissions at the neuromuscular junction. It is believed that the IgG antibodies disrupt the chemical transmission of ACh at the neuromuscular junction by (1) blocking the ACh from the receptor sites of the muscular cell, (2) accelerating the breakdown of ACh, and (3) destroying the receptor sites (see Figure 29-1). Receptor-binding antibodies are present in 85% to 90% of persons with myasthenia gravis. Although the specific events that activate the formation of the antibodies remain unclear, the thymus gland is almost always abnormal; it is generally presumed that the antibodies arise within the thymus or in related tissue.
Screening and Diagnosis
Ice Pack Test
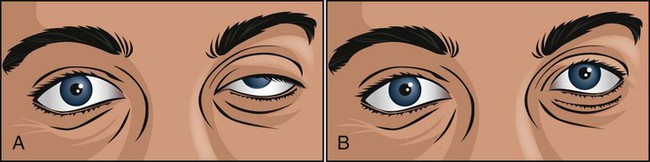
The ice pack test (Figure 29-2) is a very simple, safe, and reliable procedure for diagnosing myasthenia gravis in patients who have ptosis (droopy eye). In addition, the ice pack test does not require special medications or expensive equipment and is free of adverse effects. The test consists of the application of an ice pack to the patient’s symptomatic eye for 3 to 5 minutes. The test is considered positive for myasthenia gravis when there is improvement of the ptosis (an increase of at least 2 mm in the palpebral fissure from before to after the test).
General Management of Myasthenia Gravis
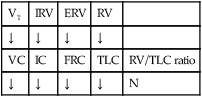
Good clinical indicators of acute ventilatory failure include the following:
• NIF <−25 cm H2O—In other words, the patient is unable to generate a negative inspiratory pressure of 25 cm H2O or more. For example, an NIF of only −15 would confirm severe muscle weakness and, important, that acute ventilatory failure was likely.
Respiratory Care Treatment Protocols
Oxygen Therapy Protocol
Oxygen therapy is used to treat hypoxemia, decrease the work of breathing, and decrease myocardial work. Because of the hypoxemia that may develop in myasthenia gravis, supplemental oxygen may be required. However, because of the alveolar consolidation and atelectasis associated with myasthenia gravis, capillary shunting may be present. Hypoxemia caused by capillary shunting is refractory to oxygen therapy (see Oxygen Therapy Protocol, Protocol 9-1).
Bronchopulmonary Hygiene Therapy Protocol
Because of the excessive mucous production and accumulation associated with myasthenia gravis, a number of bronchial hygiene treatment modalities may be used to enhance the mobilization of bronchial secretions (see Bronchopulmonary Hygiene Therapy Protocol, Protocol 9-2).
Mechanical Ventilation Protocol
Mechanical ventilation may be needed to provide and support alveolar gas exchange and eventually return the patient to spontaneous breathing. Because acute ventilatory failure is often seen in patients with severe myasthenia gravis, continuous mechanical ventilation may be required. Continuous mechanical ventilation is justified when the acute ventilatory failure is thought to be reversible (see Mechanical Ventilation Protocols, Protocol 9-5, Protocol 9-6, and Protocol 9-7).
CASE STUDY
Myasthenia Gravis
Admitting History
Respiratory Assessment and Plan
O No spontaneous ventilations; vital signs: BP 132/86, HR 90, RR 10 (controlled), T 38° C (100.5° F); normal breath sounds over right lung; diminished-to-absent breath sounds over left lung; ABGs (on Fio2 = 0.50): pH 7.28, Paco2 64, 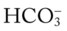 26, Pao2 52; Spo2 80%
26, Pao2 52; Spo2 80%
• Endotracheal tube possibly placed in right main stem bronchi (diminished-to-absent breath sounds over left lung, ABGs)
• Acute ventilatory failure with moderate hypoxemia (ABGs)
• Condition likely caused by misplacement of endotracheal tube
P Notify physician stat. Check CXR. Pull endotracheal tube back until breath sounds can be auscultated over both lungs. Confirm initial placement of the endotracheal tube when x-ray image is available. Mechanical Ventilation Protocol (increase tidal volume and increase Fio2). Monitor and reevaluate immediately.
Respiratory Assessment and Plan
S N/A (patient intubated on ventilator)
O Vital signs: BP 123/75, HR 74, T normal; normal bronchovesicular breath sounds over both lung fields; CXR: #7 endotracheal tube in good position (20 cm @ lip); lungs adequately ventilated; ABGs: pH 7.53, Paco2 27, 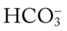 22, Pao2 176; Spo2 98%
22, Pao2 176; Spo2 98%
• Acute ventilator-induced alveolar hyperventilation (respiratory alkalosis) with overly corrected hypoxemia (ABGs)
P Adjust present settings per Mechanical Ventilation Protocol (decreasing tidal volume). Down-regulate Oxygen Therapy Per Protocol (decrease Fio2 to 0.21). Monitor and reevaluate.
Respiratory Assessment and Plan
O No improvement seen in muscular paralysis; skin: pale; vital signs: BP 146/88, HR 92, T 37.9° C (100.2° F); large amounts of thick, yellowish sputum; rhonchi over both lung fields; CXR: pneumonia and atelectasis in right lower lobe; ABGs: pH 7.28, Paco2 36, 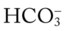 17, Pao2 41; Spo2 69%
17, Pao2 41; Spo2 69%
• Excessive bronchial secretions (rhonchi, sputum)
• Infection likely (yellow sputum, fever, CXR: pneumonia)
• Metabolic acidosis with moderate-to-severe hypoxemia (ABGs)
P Up-regulate Bronchopulmonary Hygiene Therapy Protocol (med. neb. with 0.5 mL albuterol in 2 mL 10% acetylcysteine q4h; therapist to suction patient frequently; sputum culture check in 24 and 48 hours). Initiate Lung Expansion Therapy Protocol (add 10 cm H2O PEEP to ventilator settings). Up-regulate Oxygen Therapy Protocol (increase Fio2 to 0.6). Monitor closely and reevaluate (check ABGs in 30 minutes).
Discussion
The final assessment suggested that the patient had taken another turn for the worse. The sputum was now purulent, rhonchi were heard over both lung fields, and a right lower lobe pneumonia or atelectasis had developed. The patient had an uncompensated metabolic acidemia that required evaluation. The fact that the patient’s Pao2 was only 41 provided a significant clinical indicator that the cause of the metabolic acidosis was “lactic acid” generated from a low tissue oxygen level. It was clearly appropriate for the respiratory care practitioner to focus on the patient’s oxygenation status. This was done by up-regulating the Oxygen Therapy Protocol (Protocol 9-1) (increasing the Fio2 to 0.6) and starting the Lung Expansion Therapy Protocol (Protocol 9-3) (the addition of 10 cm H2O PEEP to ventilator settings).
The therapist should have anticipated this development, obtained appropriate cultures, and, if not done before, prophylactically started the Bronchopulmonary Hygiene Therapy Protocol (Protocol 9-2) and Aerosolized Medication Therapy Protocol (Protocol 9-4)—with frequent suctioning, percussion, postural drainage, and possibly mucolytics. In addition to understanding lactic acidosis, the reader may wish to review other possible causes of metabolic acidemia at this time (e.g., diabetic ketoacidosis, renal failure).


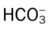
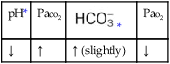
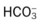 values will be lower than expected for a particular Pa
values will be lower than expected for a particular Pa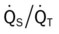
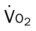
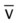 )
)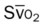

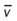 )O2, Arterial-venous oxygen difference; DO2, total oxygen delivery; O2ER, oxygen extraction ratio;
)O2, Arterial-venous oxygen difference; DO2, total oxygen delivery; O2ER, oxygen extraction ratio; 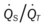 , pulmonary shunt fraction;
, pulmonary shunt fraction; 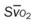 , mixed venous oxygen saturation;
, mixed venous oxygen saturation; 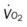 , oxygen consumption.
, oxygen consumption.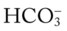 23, and Pa
23, and Pa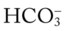 26, and Pa
26, and Pa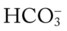 22, and Pa
22, and Pa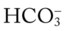 17, and Pa
17, and Pa