66 Myasthenia gravis
Instruction
This patient complains of drooping of the eyelids in the evenings; examine this patient.
Salient features
History
• Weakness in muscles is more marked in the evening
• Muscle weakness which increases with exercise (remember that fatigability is the hallmark of myasthenia gravis) and is painless
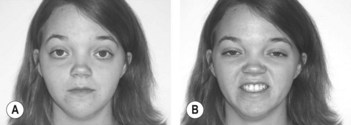
• Muscle weakness affects smiling (Fig. 66.1), chewing, speaking, muscles of the neck, walking, breathing, movements at the elbow and hand movements
• Obtain history of thyrotoxicosis, diabetes mellitus, rheumatoid arthritis, SLE and thymoma
• Ask about d-pencillamine treatment for rheumatoid arthritis (myasthenia gravis is sometimes caused by d-pencillamine).
Examination
• The patient may have obvious ptosis
• Check for worsening of ptosis after sustained upward gaze for at least 45 s
• Check extraocular movements for diplopia and variable squint
• Comment on snarling face when the patient attempts to smile
• Weakness without loss of reflexes or alteration of sensation or coordination. The weakness may be generalized; it may affect the limb muscles, often proximal in distribution, as well as the diaphragm and neck extensors
• Muscle wasting is rare and when present inidicates it is late in the disease
Questions
What investigations would you like to do in this patient?
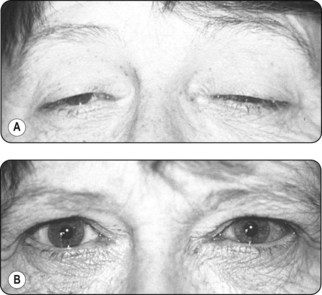
• Edrophonium (Tensilon) test. Edrophonium is a short-acting acetylcholinesterase inhibitor that gives an immediate increase in muscle strength (Fig. 66.2)
• Imaging of the mediastinum: chest radiography, CT or MRI of the chest
• Serum acetylcholine receptor antibodies (present in >80% of cases) (J Neurol Neurosurg Psychiatry 1985;48:1246–52). Remember that the basic deficit is a deficiency of acetylcholine receptors at the neuromuscular junction (Science 1973; 182:293–5); 50% have antibodies directed against muscle-specific kinase receptors
• Plasma thyroxine (to rule out an associated thyroid disorder)
• Anti-striated muscle antibody (seen in association with thymoma)
• Anti-nuclear antibody, rheumatoid factor and anti-thyroid antibodies may be positive
• Tuberculin test if immunosuppressive therapy is contemplated
• Electromyography (EMG): abnormalities include a decremental response to tetanic train stimulation at 5–10 Hz, and evidence of neuromuscular blockade in the form of jitter and blocking of motor action potentials in single-fibre EMG (Muscle Nerve 1992;15:720–4).
What are the treatment modalities available?
• Symptomatic treatment entails administration of an anticholinesterase drug (e.g. pyridostigmine) given up to five times per day.
• Definitive treatment entails immunosuppression, e.g. steroids, azathioprine, cylosporin, mycophenolate mofetil, rituximab, plasmapheresis, intravenous immunoglobulin, thymectomy.
Advanced-level questions
How is the myasthenia graded?
The Osserman grading has four stages:
I: involves focal disease (e.g. restricted to ocular muscles, ocular myasthenia)
IIa: mild generalized myasthenia with slow progression, no crises and drug responsive
IIb: moderate generalized myasthenia with severe skeletal and bulbar involvement but no crises; drug response is less satisfactory
III: acute fulminating myasthenia with rapid progression of severe symptoms of respiratory crises and poor drug response; high incidence of thymoma and high mortality rate
IV: late severe myasthenia; same as grade III but takes 2 years to progress from class I to II; crises; high mortality rate with life-threatening respiratory impairment.
The Newsom-Davies clinical grading has three subgroups:
1 Patients with thymoma, equal sex incidence, peak age of onset 30–50 years, no HLA association and poor response to thymectomy.
2 Young onset (<40 years), typically female, thymic medullary germinal centres present, strong association with HLA-B8 and HLA-DR3 and usually a good response to thymectomy.
3 Older onset (>40 years), more common in males, thymic involution, an association with HLA-B7 and HLA-DR9 and doubtful response to thymectomy.
What is the role of immunomodulation in myasthenia gravis?
Intravenous immunoglobulin seems as efficacious as plasma exchange.
LM Eaton (1905–1958), Professor of Neurology at the Mayo Clinic, Rochester, Minnesota.
EH Lambert (b. 1915), Professor of Physiology, University of Minnesota.
John Newsom-Davies, FRS, contemporary Professor of Clinical Neurology, Oxford.
Alastair Composton, PhD, FRCP, contemporary Professor of Neurology, Cambridge.