Chapter 38 Musculoskeletal Disorders of the Upper Limb
Upper Limb Physical Examination
Shoulder Special Tests
Anterior Apprehension and Relocation Tests
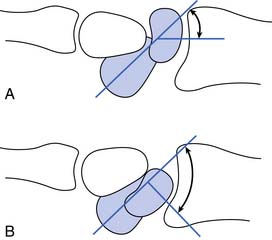
These are tests for anterior glenohumeral joint instability. The patient is placed in the supine position. The examiner abducts the patient’s shoulder 90 degrees and flexes the elbow 90 degrees. The examiner uses one hand to slowly externally rotate the patient’s humerus using the patient’s forearm as the lever. At the same time, the examiner’s other hand is placed posterior to the patient’s proximal humerus and exerts an anteriorly directed force on the humeral head. The test result is considered positive if the patient indicates a feeling of impending anterior dislocation. If the examiner removes the hand from behind the proximal humerus and places it over the anterior proximal humerus and then exerts a posteriorly directed force, and the patient subsequently reports a reduction in apprehension, then a positive relocation test has occurred.101
Posterior Apprehension Test
This test evaluates posterior glenohumeral joint stability. The patient’s affected shoulder is forward flexed to 90 degrees and then maximally internally rotated. A posteriorly directed force is then placed on the patient’s elbow by the examiner. A positive test result causes a 50% or greater posterior translation of the humeral head or a feeling of apprehension in the patient.101
Sulcus Sign
The sulcus sign is used to evaluate inferior glenohumeral joint instability. The patient is seated or standing with the arm relaxed in shoulder adduction. The patient’s forearm is grasped by the examiner, and a distal traction force is placed through the patient’s arm. In the presence of inferior instability, a sulcus will develop between the humeral head and the acromion.101
O’Brien Test
This test evaluates for acromioclavicular (AC) joint and labral abnormalities. The shoulder is flexed to 90 degrees with the elbow fully extended. The arm is then adducted 15 degrees, and the shoulder is internally rotated so that the patient’s thumb is pointing down. The examiner applies a downward force against the arm, which the patient is instructed to resist. The shoulder is then externally rotated so that the patient’s palm is facing up, and the examiner applies a downward force on the patient’s arm, which the patient is instructed to resist. A positive test result is indicated by pain during the first part of the maneuver with the patient’s thumb pointing down, which is then lessened or eliminated when the patient resists a downward force with the palm facing up. Pain in the region of the AC joint indicates AC pathology, whereas pain or painful clicking deep inside the shoulder suggests labral pathology.128
Horizontal Adduction Test
The shoulder is passively flexed to 90 degrees and then horizontally adducted across the chest. Pain located in the region of the AC joint suggests AC joint pathology, whereas posterior shoulder pain suggests posterior capsular tightness.105
Speed’s Test
This test is for biceps tendonitis. The patient’s shoulder is forward flexed to 90 degrees with the elbow fully extended and the palm facing up. The examiner applies a downward force against the patient’s active resistance. Pain in the region of the bicipital groove suggests bicipital tendonitis.101
Yergason’s Test
With the patient’s arm at the side, the elbow is flexed to 90 degrees and the forearm is pronated. The patient then tries to simultaneously supinate the forearm and externally rotate the shoulder against the examiner’s resistance. This test can provoke bicipital region pain in patients with bicipital tendonitis, and a painful “pop” in patients with bicipital tendon instability.101
Neer-Walsh Impingement Test
The patient’s shoulder is internally rotated while at the side. The examiner passively forward flexes the patient’s shoulder to 180 degrees while maintaining internal rotation. Pain in the subacromial area suggests rotator cuff tendonitis.101
Hawkins-Kennedy Impingement Test
The patient’s shoulder and elbow are each passively flexed to 90 degrees, respectively. The examiner then grasps the patient’s forearm, stabilizes the patient’s scapulothoracic joint, and uses the forearm as a lever arm to internally rotate the glenohumeral joint. A positive test result is indicated by pain in the subacromial region occurring with the internal rotation.101
Elbow Special Tests
Cozen’s Test
The patient is asked to fully extend the elbow, pronate the forearm, and make a fist. The examiner then resists the patient’s attempt to extend and radially deviate the wrist. Pain over the lateral epicondyle represents a positive test result and suggests the presence of lateral epicondylitis.99
Ligamentous Instability Test
The examiner flexes the patient’s elbow 20 to 30 degrees and stabilizes the patient’s arm by placing a hand at the elbow and a hand on the distal forearm. Varus and valgus forces are placed across the elbow by the examiner to test the stability of the radial and ulnar collateral ligaments (UCL), respectively.99
Wrist and Hand Special Tests
Finkelstein Test
This test is used to detect tenosynovitis of the extensor pollicis brevis and abductor pollicis longus tendons (de Quervain’s tenosynovitis). The patient makes a fist with the thumb inside the fingers, and the examiner passively deviates the wrist in an ulnar direction. A positive test result causes pain in the affected tendons.100
Watson Test
This test assesses scapholunate stability. The patient’s wrist begins in an ulnarly deviated position. The examiner places a dorsally directed force against the proximal volar pole of the scaphoid. The examiner then radially deviates the wrist while continuing to place the same force against the scaphoid. A “pop” or subluxation of the scaphoid indicates a positive test result.100
Rehabilitation Principles of Upper Limb Injury
Once an accurate diagnosis has been made with a thorough history, physical examination, and appropriate diagnostic testing, an effective treatment program can be developed. Kibler83 has proposed three broad stages of rehabilitation, including the acute stage, recovery stage, and the functional stage. The acute stage of rehabilitation focuses on reducing the patient’s symptoms and facilitating tissue healing. In specific circumstances, immobilization through splinting or casting might be used during the acute stage of rehabilitation.
During the acute phase of rehabilitation, cryotherapy can be used for acute injuries to decrease pain, inflammation, muscle guarding, edema, and local blood flow.70,82 Heat increases blood flow, reduces muscle “spasm,” reduces pain, and can be used in the acute phase of rehabilitation for chronic injuries.82 High-frequency electrical stimulation is often used during the acute phase of rehabilitation to reduce muscle guarding and increase local circulation.189
Opioid and nonopioid analgesics might be required for pain control during the acute phase of rehabilitation. Nonsteroidal antiinflammatory drugs (NSAIDs) are often used for their analgesic and antiinflammatory properties. Randomized, placebo-controlled trials have demonstrated reduced pain, edema, and tenderness, and a faster return to activity in NSAID-treated athletes than in those treated with placebo.4,186 It is important to remember, however, that NSAIDs are not entirely benign and can cause significant gastrointestinal, renal, cardiovascular, hematologic, dermatologic, and neurologic side effects.32,60,175 Because of these concerns, NSAIDs should be used only if local physical modalities and less toxic medications such as acetaminophen are not effective.
Oral and injected corticosteroids have also been used for pain control and reduction of inflammation during the acute phase of rehabilitation. Because of the possibility of significant systemic and localized consequences of corticosteroid use, however, their use should be limited to very select cases.53,156 These side effects include suppression of the hypothalamic–pituitary–adrenal axis, osteoporosis, avascular necrosis, infection, and tendon or ligament rupture.
The patient can advance to the recovery phase of rehabilitation when the pain has been adequately controlled and tissue healing has occurred.83 This is indicated by full pain-free ROM and the ability to participate in strengthening exercises for the injured limb. The emphasis of the recovery phase of rehabilitation involves the restoration of flexibility, strength, and proprioception in the injured limb. Strength and flexibility imbalances and maladaptive movement patterns and muscle substitutions should be corrected in this phase of rehabilitation. Open kinetic chain exercises can be beneficial when correcting strength imbalances, whereas closed chain exercises are frequently used to provide joint stabilization through muscle co-contraction. Cardiovascular and general fitness should be maintained. Progression to functional activities is begun toward the end of this phase.
The patient can begin the functional stage of rehabilitation when the injured limb has regained 75% to 80% of normal strength compared with the uninjured limb, and when there are no strength and flexibility imbalances.83 The patient’s rehabilitation needs to continue to address maladaptive movement patterns and muscle substitutions, and full strength should be obtained. Functional activities should be incorporated into the rehabilitation program with a vocational/avocational-specific progression that eventually leads to a return to normal activities.
Musculoskeletal Problems of the Upper Limb
Conditions of the Shoulder
Sternoclavicular Joint Sprains
Sternoclavicular joint dislocations account for less than 1% of all joint dislocations. Two thirds of sternoclavicular joint dislocations occur anteriorly, whereas one third of dislocations occur posteriorly. The etiology is usually traumatic.38
Ligament injuries are commonly graded on a scale of 1 to 3 (Table 38-1).38 The presence of tenderness at the sternoclavicular joint without subluxation or dislocation indicates a grade 1 injury; tenderness with subluxation indicates a grade 2 injury; and tenderness with associated dislocation indicates a grade 3 injury.190
| Grade | Signs |
|---|---|
| 1 | Tenderness to palpation without joint laxity |
| 2 | Tenderness to palpation with joint laxity but a good endpoint |
| 3 | Tenderness to palpation with significant joint laxity and no endpoint |
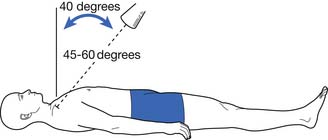
The radiologic evaluation of sternoclavicular joint injuries includes an anteroposterior radiograph of the chest or sternoclavicular joint and a serendipity view, which involves a 40-degree cephalic tilt view of the sternoclavicular joints (Figure 38-1).157 Often standard radiographs leave the diagnosis in question, and computed tomography (CT) scan is frequently used for definitive evaluation of sternoclavicular joint injuries.
Grade 3 sternoclavicular joint sprains frequently can be treated nonoperatively as described for grade 1 and 2 injuries.41 Those that remain unstable postreduction, however, might require surgical intervention.190 Because complications frequently arise with grade 3 posterior dislocations, a thorough evaluation to rule out pulmonary or vascular damage should be undertaken. If mediastinal compression is present, posterior grade 3 dislocations require immediate surgical intervention.190 Because of the significant risk of vascular complications, posterior sternoclavicular joint dislocations should be reduced in the hospital setting with a vascular surgeon present.
Clavicle Fractures
A majority of clavicular fractures occur in childhood and adults less than 25 years of age. Eighty percent of clavicular fractures occur in the middle third of the clavicle, whereas approximately 15% occur in the lateral one third and 5% in the medial one third. Most clavicular fractures occur as a result of a direct blow to the point of the shoulder, but a small percentage occur from a fall onto an outstretched arm.17
Most patients know when they have fractured their clavicle. It is important to rule out associated neurovascular and pulmonary injuries during the physical examination and radiologic evaluation. Routine anteroposterior radiographs of the clavicle are usually adequate for visualization of clavicular middle third fractures. For proximal third fractures, the addition of a serendipity view is often used. When a lateral third fracture is suspected, a 15-degree cephalic tilt anteroposterior view centered on the AC joint using a soft tissue technique (Zanca view) and an axillary lateral view are usually diagnostic when combined with anteroposterior radiographs. Occasionally, CT scanning is required for more definitive evaluation.17
If the fracture has good alignment, partial immobilization is the treatment of choice using an immobilization device such as a sling or figure-of-eight bandage. If 15 to 20 mm of shortening occurs as a result of displacement, surgical intervention should be considered.143 Other common indications for surgical intervention include displaced fractures with tissue interposition between the fracture ends, open fractures, neurovascular compromise, tenting of the skin over the fracture site that might lead to tissue necrosis, and displaced fractures located in the lateral third of the clavicle.17
Acromioclavicular Joint Sprains
AC joint sprains account for only 9% of all shoulder injuries, are most frequent in males in their third decade of life, and are usually partial rather than complete sprains.105 Most injuries occur as a result of direct trauma from a fall or blow to the acromion. Physical examination demonstrates point tenderness, a positive horizontal adduction test, and a positive O’Brien test.
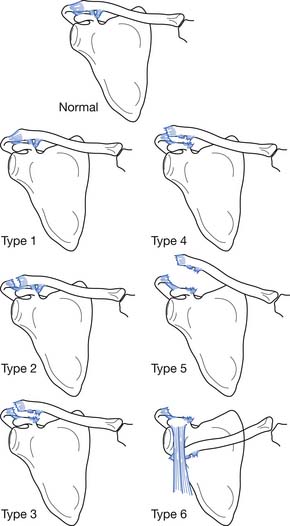
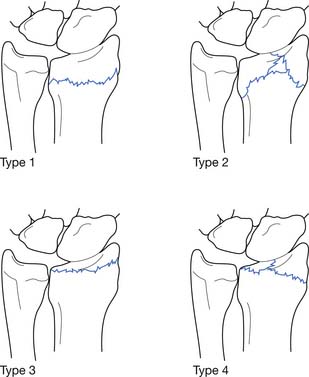
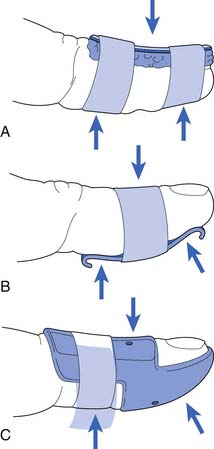
Rockwood158 classified AC joint sprains into six types (Figure 38-2). Type 1 sprains involve a mild injury to the AC ligaments, and radiologic evaluation is normal. Type 2 injuries involve the complete disruption of the AC ligaments but with intact coracoclavicular ligaments. Radiographs might demonstrate clavicular elevation relative to the acromion but less than 25% displacement. Type 3 sprains result in the complete disruption of the AC and coracoclavicular ligaments, but the deltotrapezial fascia remains intact. Radiographs reveal a 25% to 100% increase in the coracoclavicular interspace relative to the normal shoulder. Type 4 sprains involve complete disruption of the coracoclavicular and AC ligaments, with posterior displacement of the distal clavicle into the trapezius muscle. In type 5 sprains, the coracoclavicular and AC ligaments are fully disrupted along with a rupture of the deltotrapezial fascia. This results in an increase in the coracoclavicular interspace to greater than 100% of the normal shoulder. Type 6 sprains involve complete disruption of the coracoclavicular and AC ligaments, as well as the deltotrapezial muscular attachments, with displacement of the distal clavicle below the acromion or the coracoid process.
Radiographic evaluation of the AC joint should include anteroposterior and lateral views of the AC joint, and a Zanca view. Stress views do not provide additional clinically useful information.105
Type 1, 2, and 3 AC joint sprains are usually treated nonoperatively, using the previously described principles of rehabilitation. A brief period of sling immobilization might be required for pain control. Indications for surgical intervention for type 3 sprains include persistent pain or unsatisfactory cosmetic results. Some authors advocate operative treatment of type 3 sprains in heavy laborers and athletes who participate in sports that place a high demand on the upper limbs,30,89,141 but the current literature favors nonoperative treatment for type 3 sprains because of the similar outcomes between operative and nonoperative management and higher complication rates after operative intervention.171 Type 4, 5, or 6 sprains require surgical treatment.105
Intraarticular AC joint fractures are treated similarly to type 1 through 3 AC joint sprains, unless the injury is open or involves neurovascular compromise. Posttraumatic arthrosis in the AC joint can occur. Persistent pain, despite an appropriate nonoperative treatment program, necessitates surgical resection of the distal clavicle.105
Osteolysis of the Distal Clavicle
Repetitive overload of the distal clavicle can result in osteolysis. Young weight lifters who perform a significant amount of bench press and military press lifts are the most susceptible to this condition.26 The athlete usually presents with gradual onset of AC joint pain that is increased with overhead presses or bench press, particularly when the bar is lowered all the way to the chest because this results in a significant sheer stress to the AC joint. It frequently occurs bilaterally.167
Radiographic evaluation is the same as for AC joint injuries. The pathologic changes found on radiographs include distal clavicular subchondral bone loss and cystic changes. Widening of the AC joint can occur in late stages. Bone scans are positive during active disease and can be used to confirm the diagnosis.105
Nonoperative treatment of distal clavicular osteolysis involves avoidance of the aggravating activities, and application of the previously discussed rehabilitation principles. Occasionally, AC joint intraarticular corticosteroid injections can assist in pain control. For many athletes, however, activity modification is not practical or does not result in significant pain relief. In those athletes who fail to improve with nonoperative measures or are unable to adequately modify their activities, distal clavicular resection is the surgical procedure of choice.167
Scapulothoracic Crepitus
Multiple authors have reported the occurrence of pathologic sounds at the scapulothoracic joint, often referred to as a snapping scapula or scapular crepitus.116,140,144 The three primary types of sound that can occur at this joint include a gentle friction sound, a louder grating sound, and a loud snapping. Although the gentle friction sound is probably physiologic, a loud grating sound suggests soft tissue disease such as bursitis, fibrotic muscle, muscular atrophy, or anomalous muscular insertions.116 Occasionally an excessive thoracic kyphosis or thoracic scoliosis can cause pathologic crepitus at the scapulothoracic articulation. Scapulothoracic dyskinesis or scapular winging can also cause painful friction between the scapula and the thorax.84 A loud snapping sound is frequently caused by bony pathology such as an osteophyte, a rib or scapular osteochondroma, hooked superomedial angle of the scapula, or malunion of rib fractures.117,140,154
Treatment is warranted when the scapular snapping or crepitus is symptomatic. Nonoperative treatment involves correction of biomechanical deficits such as scapulothoracic dyskinesis, strength and flexibility imbalances, and poor posture. Soft tissue mobilization, NSAIDs, and local modalities might assist in pain control. An injection of corticosteroids into the painful area has been advocated by some authors,33,106 but caution must be exercised because complications such as pneumothorax can occur. For those who fail to improve with nonoperative measures, several surgical procedures have been described for treatment of this condition.106
Rotator Cuff Tendonitis and Impingement
Injuries to the rotator cuff are common. Although macrotrauma can cause rotator cuff injuries, repetitive microtrauma and outlet impingement between the acromion and greater tuberosity of the humerus are more common.115 Neer categorized this type of rotator cuff injury into three stages.122 Stage 1 injuries involved inflammation and edema in the rotator cuff. Stage 2 rotator cuff injuries had progressed to fibrosis and tendonitis. When a partial or complete rotator cuff tear occurred, this was considered a stage 3 injury. In the overhead athlete, underlying instability patterns of the glenohumeral joint with associated excessive translation of the humeral head frequently led to outlet impingement and rotator cuff disease.6
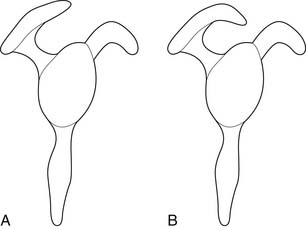
On cadaveric examination, Bigliani21 found a relationship between the acromial shape and the presence of rotator cuff tears. He classified the acromions into three types (Figure 38-3). Type 1 acromions were relatively flat, whereas type 2 acromions demonstrated a curve, and type 3 acromions were hooked. The incidence of rotator cuff tears increased as the acromion progressed from a type 1 to a type 3 shape. This was presumably related to the greater outlet impingement of the rotator cuff caused by an increasing acromial curve.
A phenomenon referred to as internal impingement has recently been reported in young overhead athletes.40,184 When the arm is abducted 90 degrees and maximally externally rotated, there is contact between the undersurface of the rotator cuff and the posterosuperior glenoid rim. This is augmented by anterior glenohumeral joint instability and posterior glenohumeral joint capsular tightness. Internal impingement causes pathologic changes to the undersurface of the rotator cuff.
Impingement can be primary or secondary. Examples of causative factors leading to primary impingement include a hooked acromion or a thick coracoacromial ligament. Secondary impingement has many causes, including glenohumeral joint instability, weak scapular stabilizers, or scapulothoracic dyskinesis and instability.66,67 Lack of adequate scapular control or weakness in the scapular stabilizers can lead to poor scapular positioning during arm elevation. This can result in an acromion that does not retract during overhead activities, creating secondary impingement. Regardless of whether the impingement is primary or secondary, the underlying cause must be determined to formulate an appropriate treatment program.
Rotator cuff microtrauma can also be caused by “eccentric overload” of the shoulder external rotators during the deceleration phase of throwing.
Microvascular studies of the rotator cuff have found a hypovascular zone in the leading edge of the supraspinatus tendon during shoulder adduction compared with shoulder abduction and on the articular compared with bursal surface of the supraspinatus.97,149 These hypovascular areas have been implicated as a cause of rotator cuff degeneration and correlate with the higher incidence of articular-sided partial-thickness tears of the rotator cuff.
Radiographic evaluation of patients with suspected rotator cuff pathology should include anteroposterior, supraspinatus outlet, and axillary radiographs. Anteroposterior radiographs should be performed in the neutral, external, and internal rotation positions to adequately visualize the glenohumeral joint and the greater and lesser tuberosities. Large rotator cuff tears can be indicated by an acromiohumeral distance of less than 7 mm and sclerosis on the undersurface of the acromion. The supraspinatus outlet view allows for categorization of the acromion type and will reveal AC joint osteophytes. Double-contrast arthrograms identify full-thickness rotator cuff tears, partial-thickness articular surface tears, and biceps tendon pathology. Bursal surface and intrasubstance partial-thickness rotator cuff tears are poorly evaluated with this technique. Ultrasound and MRI have high levels of sensitivity and specificity for rotator cuff pathology.13,73
Nonoperative treatment should include application of the previously described rehabilitation principles. Strengthening exercises for the scapular stabilizing muscles rather than the rotator cuff should be emphasized in the acute rehabilitation stage. Specifically, strengthening muscles that retract and depress the scapula (e.g., serratus anterior and inferior trapezius) and stretching muscles that protract and elevate the scapula (e.g., pectoralis minor and upper trapezius) reduce impingement. Posterior glenohumeral joint capsular tightness should be corrected, particularly in patients with internal impingement. It is imperative to reestablish normal scapulothoracic kinematics through neuromuscular retraining. This can begin once shoulder ROM is pain-free. Rotator cuff muscle strengthening should begin with closed chain exercises to promote stability and proprioception. Open chain exercises can be used to correct strength imbalances, such as weakness of the shoulder external rotators relative to the internal rotators. Some patients benefit from a subacromial corticosteroid injection, although studies of corticosteroid injections have shown mixed results.68,112,121 Patients recalcitrant to these measures might benefit from extracorporeal shock-wave therapy or, if calcific tendonitis is present, ultrasound-guided percutaneous lavage and aspiration of the calcification.42,130 If the patient fails to respond to the above measures, surgical evaluation should be considered.
Long Head of the Biceps Tendon Strains
Rupture of the long head of the biceps brachii tendon usually occurs in patients more than 40 years of age with a prolonged history of outlet impingement and rotator cuff disease. The patient frequently experiences a “pop” at the time of the injury, which often occurs during lifting or pulling activities. Some patients, however, just present with a painless retraction of the biceps distally resulting in an exaggeration of the biceps muscle contour. Pain can occur with an acute rupture but is not usually a factor in chronic cases. Because rupture of the long head of the biceps brachii tendon results in a loss of approximately 8% of elbow flexion strength and 21% of supination strength,103 function is not significantly affected in most individuals.
In younger or physically active patients, early surgical intervention might be warranted. Mariani et al.103 reported that 93% of the surgically treated patients and 63% of the nonsurgically treated patients were able to return to full work capacity.
Pectoralis Major Strain
Pectoralis major muscle strains are most commonly seen in athletes who perform forceful shoulder adduction and/or internal rotation against resistance, such as weight lifters and football players.110 Pectoralis muscular strains can be categorized into three grades, with grade 1 being minimal muscle disruption and grade 3 a complete rupture. The strain can be located in the muscle belly, musculotendinous junction, or tendon.
Glenohumeral Joint Instability
The static stabilizers of the glenohumeral joint include the bony congruence between the humeral head and the glenoid fossa, the glenoid labrum, the negative intraarticular pressure, the glenohumeral joint capsule, and the glenohumeral ligaments.1
The dynamic stabilizers of the glenohumeral joint include the scapular stabilizing muscles, the rotator cuff muscles, and the long head of the biceps.90 The importance of optimal scapular function for glenohumeral joint stability cannot be overemphasized. The scapular stabilizing muscles orient the scapula properly in relation to the humerus for optimal static and dynamic stability of the glenohumeral joint and stabilize the scapula during glenohumeral joint movements.51 The primary scapular stabilizing muscles include the serratus anterior, trapezius, pectoralis minor, rhomboideus minor and major, latissimus dorsi, and levator scapulae.51
The rotator cuff muscles include the supraspinatus, infraspinatus, subscapularis, and teres minor. These muscles contribute to dynamic glenohumeral joint stability through a number of mechanisms. Concavity compression, first described by Lippitt et al.,95 refers to the compressive forces placed on the glenohumeral joint during rotator cuff muscle co-contractions. These forces press the humeral head into the glenoid fossa, center the humeral head within the glenoid fossa, and help resist glenohumeral translation. Because the glenohumeral ligaments are lax in the midranges of glenohumeral joint motion, coordinated rotator cuff muscle contraction and concavity compression are particularly important mechanisms for glenohumeral joint stability in these ranges.90
At the distal insertion of the rotator cuff muscles on the humerus, there is an intertwining of the joint capsule with the rotator cuff tendons. With rotator cuff muscle contraction, it is possible that the glenohumeral joint capsule develops tension and increases in stiffness, consequently acting as a dynamic musculoligamentous stabilizing system.90
The rotator cuff muscles also provide glenohumeral joint stability through passive muscle tension and act as barriers to glenohumeral joint translation during active motion.25,31 The subscapularis appears to be an especially important stabilizer for both anterior and posterior glenohumeral joint stability.22,43
Proprioception and neuromuscular control refer to the mechanism by which the position and movements of the shoulder girdle are sensed (proprioception), processed, and result in an appropriate motor response (neuromuscular control).120 Glenohumeral joint instability is often associated with a concomitant decrement in proprioception.93 The abnormal proprioception is restored after surgical correction of the joint instability, suggesting that one of the mechanisms causing proprioceptive deficits in unstable glenohumeral joints is a lack of appropriate capsuloligamentous tension.94
The classification of glenohumeral joint instability includes the degree, frequency, etiology, and direction of instability.14 The degree includes dislocation, subluxation, or microinstability. A dislocation implies that the humeral head is disassociated from the glenoid fossa and often requires manual reduction. A subluxation occurs when the humeral head translates to the edge of the glenoid, beyond normal physiologic limits, followed by self-reduction. Microinstability is due to excessive capsular laxity, is multidirectional, and is frequently associated with internal impingement of the rotator cuff.14
The frequency of instability can be either acute or chronic.14 Acute instability involves a new injury resulting in subluxation or dislocation of the glenohumeral joint. Chronic instability refers to repetitive instability episodes.
The etiology of glenohumeral joint instability can be traumatic or atraumatic.14 Unidirectional instability is frequently caused by a traumatic event resulting in disruption of the glenohumeral joint. Atraumatic instability refers to glenohumeral joint instability resulting from congenital capsular laxity or repetitive microtrauma. Atraumatic instability can be subclassified into voluntary and involuntary categories. Voluntary instability refers to an individual who volitionally subluxes or dislocates his or her glenohumeral joint, whereas those with involuntary instability do not. Some patients with voluntary instability have associated psychologic pathology, which often portends a poor outcome if surgical stabilization is performed.161
Glenohumeral joint instability can be unidirectional or multidirectional. Unidirectional instability refers to instability only in one direction. The most frequent type of unidirectional instability is traumatic anterior instability.14 Multidirectional instability is instability in two or more directions and is usually due to congenital capsular laxity or chronic repetitive microtrauma.14
Traumatic anterior glenohumeral dislocation frequently tears the anterior–inferior glenohumeral joint capsule (e.g., the middle glenohumeral ligament and/or anterior band of the inferior glenohumeral ligament [IGHL]) and avulses the anterior–inferior glenoid labrum with or without some underlying bone from the glenoid rim.90 The latter of these two entities is frequently referred to as a Bankart lesion.16 Other anatomic lesions that contribute to anterior glenohumeral joint instability include humeral avulsion of the glenohumeral ligament, superior labral anterior to posterior (SLAP) lesions, injury to the rotator interval, or rotator cuff tears (particularly to the subscapularis muscle).90 Acute anterior glenohumeral joint dislocations are also frequently associated with a compression fracture of the posterolateral aspect of the humeral head, referred to as a Hill-Sachs defect.163
Congenital glenoid hypoplasia or excessive glenoid or humeral retroversion has been reported to contribute to posterior glenohumeral joint instability. The more common lesions that lead to posterior glenohumeral joint instability, however, include excessive capsuloligamentous laxity, or an injury to the rotator interval, SGHL, posterior band of the IGHL, coracohumeral (CHL), or subscapularis muscle.9 A tear of the posterior inferior glenoid labrum causing separation from the glenoid fossa rim, often referred to as a “reverse Bankart lesion,” or a fracture of the posterior inferior glenoid fossa rim can also cause posterior glenohumeral joint instability.9,146 A “reverse Hill-Sachs defect” can also be present, representing an impaction fracture of the anterior humeral head.9,146
Multidirectional instability can be caused by primary or secondary capsuloligamentous laxity. It is frequently seen bilaterally and can be accompanied by generalized ligamentous laxity.14 Recurrent unilateral joint instability occasionally stretches the glenohumeral capsuloligamentous structures to the point that multidirectional instability develops secondarily.14 Another possible cause for secondary multidirectional instability is the presence of an underlying connective tissue disorder such as Marfan or Ehlers-Danlos syndromes.14
Although many patients with glenohumeral joint instability have vague symptoms, common complaints of patients with shoulder instability include pain, popping, catching, locking, an unstable sensation, stiffness, and swelling.18 A history of acute trauma or chronic, repetitive microtrauma should be obtained. Some patients might have a history of glenohumeral joint dislocation, and the examiner should find out the direction of dislocation, the duration of the dislocation, whether it has recurred, and whether it required manual reduction or spontaneously reduced. Subluxation episodes are commonly associated with a burning or aching dead feeling in the arm. Repetitive overhead activities such as baseball pitching can cause enough microtrauma to lead to symptomatic laxity.18 Patients should be asked whether they or their family members have a history of generalized ligamentous laxity or connective tissue disorders.
The most common initial radiographic views for the evaluation of glenohumeral joint instability include the anteroposterior shoulder view, axillary lateral view, and scapular “Y” view.14 The anteroposterior view allows visualization of the osseous structures of the shoulder, including the scapula, clavicle, upper ribs, humeral head, and glenoid rim.163 With internal rotation, the anteroposterior view can also allow visualization of a Hill-Sachs defect.163 The scapular Y view can help in the assessment of glenohumeral joint alignment after acute dislocations.163 The axillary lateral view can assess anterior or posterior subluxation or dislocation, as well as fractures of the anterior or posterior glenoid rim.163 Other specialized views include the Garth view and the West Point view, both of which are useful in the detection of Bankart fractures. The Stryker Notch view can be used for evaluation of Hill-Sachs defects and stress views for the documentation of the degree of glenohumeral joint instability.163Magnetic resonance arthrography provides optimal visualization of the labrum, cartilage, and joint capsule. Imaging of nondisplaced injuries to the IGHL and anteroinferior glenoid labrum is improved by placing the arm in an abducted and externally rotated position.
For patients who have suffered a first-time traumatic anterior glenohumeral joint dislocation, the decision between a trial of nonoperative treatment versus immediate surgical stabilization is more controversial. In the older, less active patient, nonoperative management frequently is successful.3 In the younger, more active patient involved in contact sports, studies have shown a very high redislocation rate in those treated nonoperatively compared with those receiving early operative intervention.11,12,85,173
Regardless of whether a patient opts for early surgical intervention, closed reduction confirmed by radiologic examination should be performed on all patients who sustain an acute glenohumeral joint dislocation that does not spontaneously reduce. Radiologic studies should be performed in two planes, such as anteroposterior with the humerus in internal rotation and axillary lateral views, to confirm relocation and exclude an associated fracture.163 Sensory testing over the deltoid muscle is important to rule out an associated axillary nerve injury.14
Standard sling immobilization can be used for comfort but does not change future redislocation rates.71,72,166 If initiated within the first 24 hours postinjury, 3 weeks of shoulder immobilization with the humerus externally rotated 30 degrees can reduce the risk of recurrent dislocation by nearly 100%, but the benefits of this type of immobilization are not statistically significant if begun after the first day.75–78 The previously described shoulder instability rehabilitation program can be used to treat patients who opt for nonoperative treatment of their shoulder dislocation.
Adhesive Capsulitis
Adhesive capsulitis, or “frozen shoulder,” is characterized by painful, restricted shoulder ROM in patients with normal radiographs.61,123 Adhesive capsulitis occurs in approximately 2% to 5% of the general population, is 2 to 4 times more common in women than men, and is most frequently seen in individuals between 40 and 60 years of age.27,35
Adhesive capsulitis is usually an idiopathic condition but can be associated with diabetes mellitus, inflammatory arthritis, trauma, prolonged immobilization, thyroid disease, cerebrovascular accident, myocardial infarction, or autoimmune disease. Pathologic evaluation can reveal perivascular inflammation, but the predominant abnormality is fibroblastic proliferation with increased collagen and nodular band formation.27
Adhesive capsulitis has been divided into four stages (Table 38-2).64 Stage 1 occurs for the first 1 to 3 months and involves pain with shoulder movements but no significant glenohumeral joint ROM restriction when examined under anesthesia. In stage 2, the “freezing stage,” symptoms have been present for 3 to 9 months and are characterized by pain with shoulder motion and progressive glenohumeral joint ROM restriction in forward flexion, abduction, and internal and external rotation. During stage 3, or the “frozen stage,” symptoms have been present for 9 to 15 months and include a significant reduction in pain but maintenance of the restricted glenohumeral joint ROM. In stage 4, frequently referred to as the “thawing stage,” symptoms have been present for approximately 15 to 24 months and ROM gradually improves.
| Stage | Symptom Duration | Signs and Symptoms |
|---|---|---|
| 1 | 1-3 mo | Painful shoulder movement, minimal restriction in motion |
| 2 | 3-9 mo | Painful shoulder movement, progressive loss of glenohumeral joint motion |
| 3 | 9-15 mo | Reduced pain with shoulder movement, severely restricted glenohumeral joint motion |
| 4 | 15-24 mo | Minimal pain, progressive normalization of glenohumeral joint motion |
Treatment during stages 1 and 2 of adhesive capsulitis includes physical modalities, analgesics, and activity modification to reduce pain and inflammation. Up to three intraarticular corticosteroid injections can be used during stages 1 and 2 of adhesive capsulitis to reduce inflammation and pain, facilitate rehabilitation, and shorten the duration of this condition.64,165 Postural retraining to reduce kyphotic posture and forward humeral positioning should be undertaken. Aggressive ROM exercises should be avoided until the patient’s pain has been adequately controlled.178 Otherwise, the patient’s symptoms can worsen, leading the patient to immobilize his or her shoulder, thus resulting in a further loss of shoulder ROM. Early in the rehabilitation process, however, passive joint glides and nonpainful passive ROM exercises might be beneficial. Early scapular stability exercises and closed chain rotator cuff exercises can be instituted. As the patient’s symptoms improve, active-assisted and active ROM activities can be added, along with open chain and proprioceptive exercises. Most patients will have restoration of normal function over a 12- to 14-month period.63,118 In patients who do not improve after 6 months of nonoperative treatment, more aggressive treatments such as capsular hydrodilatation, manipulation under anesthesia, and arthroscopic lysis of adhesions can be considered.∗
Superior Labral Anterior to Posterior Lesions
Andrews et al.7 were the first to report the importance of the superior labrum and biceps tendon complex as a source of pain and shoulder dysfunction. The term SLAP lesion, which stands for superior labral anterior to posterior injury, has been used to name injuries to the superior labrum and biceps tendon complex.169 The glenoid labrum, which serves to deepen the glenoid socket, increases the contact area of the humeral head by approximately 70%.145 In addition to increasing the static stability of the glenohumeral joint, the superior aspect of the glenoid labrum serves as an attachment point for the long head of the biceps tendon. Research has demonstrated the importance of the long head of the biceps in providing dynamic stability to both anterior–posterior and superior–inferior humeral head translation.135,159
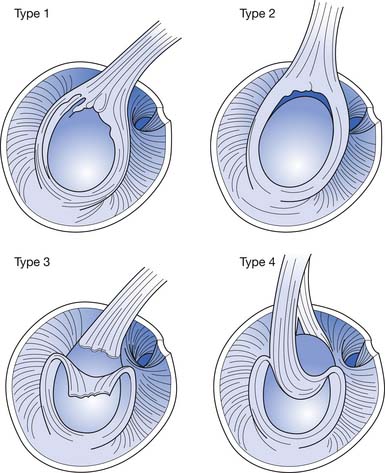
Snyder et al.169 proposed a classification system for SLAP lesions (Figure 38-4). Type 1 lesions involve a fraying injury to the superior labrum without detachment of the biceps tendon. In type 2 lesions, the biceps tendon is detached from the supraglenoid tubercle. Type 3 lesions are characterized by bucket handle tearing of the superior labrum without detachment of the biceps tendon. Type 4 lesions involve a tear of the superior labrum that extends into the biceps tendon.
Mechanisms of injury for SLAP lesions include falling on an outstretched arm, traction injuries, torsional peeling back of the labrum during the late cocking phase of overhead throwing, and traction forces from the long head of the biceps tendon during the deceleration phase of overhead throwing.7,28,98,169,178
Conditions of the Elbow
Lateral Epicondylitis
Lateral epicondylitis is a common tendinopathy of the lateral elbow and is frequently referred to as tennis elbow. This malady dates back to 1883 when it was described as an injury resulting from lawn tennis.38 It is reported to occur in up to 50% of tennis players.126 Any activity that places excessive repetitive stress on the lateral forearm musculature, however, can cause this condition.
Lateral epicondylitis is more common in patients more than 35 years of age and peaks in those between 40 and 50 years old.81,125 It is more common in male than in female tennis players but does not display a gender predilection in the general population.37 When lateral epicondylitis results from a work-related injury, conservative measures are less successful, and surgical intervention occurs more frequently.58
Lateral epicondylitis is a misnomer for this condition because the pathologic changes (i.e., angiofibroblastic hyperplasia) are not inflammatory but rather degenerative.125,151 A more appropriate term for this condition is lateral epicondylosis rather than epicondylitis. The degenerative changes occur most commonly in the origin of the extensor carpi radialis brevis but also involve the extensor digitorum communis origin in 30% of cases.125 The origins of the extensor carpi radialis longus or extensor carpi ulnaris are only rarely involved.
Patients with lateral epicondylosis frequently report a gradual onset of symptoms, which usually occur after specific activities. Traumatic or sudden onset of symptoms can also occur. The backhand swing in tennis frequently exacerbates the symptoms, as does gripping and activities that require repetitive wrist extension and forearm pronation and supination. Physical examination can reveal point tenderness over the lateral epicondyle and a positive Cozen’s test. Entrapment of the posterior interosseous branch of the radial nerve can mimic lateral epicondylosis, but the tenderness associated with this condition is 3 to 4 cm distal to the lateral epicondyle rather than directly over it.187
Although standard anteroposterior and lateral radiographs are usually normal, an oblique view of the lateral epicondyle might reveal punctuate calcifications in the extensor tendon origin.37
Treatment involves discontinuation of provocative activities, oral analgesics, physical modalities, and bracing (e.g., lateral counter-force strap or neutral wrist splint). It is important to correct kinetic chain deficits, training errors, and inappropriate tennis racquet grip size and string tension. Eccentric strengthening of the wrist extensors appears to be the most effective exercise regimen for the treatment of lateral epicondylosis.177 Peritendinous corticosteroid injections are occasionally used, but their long-term efficacy is questionable.5,124 Promising new treatments include ultrasound-guided percutaneous needle tenotomy, autologous blood injections, and platelet-rich plasma injections.48,113,119 Extracorporeal shock-wave therapy for lateral epicondylosis has been reported to be successful in 48% to 73% of cases recalcitrant to other nonoperative measures.130 Recalcitrant cases can also be treated with surgical debridement of the degenerative tissue with approximately 85% of surgically treated patients reporting good to excellent results and only 3% reporting no improvement.47,126
Medial Epicondylitis
Medial epicondylitis, frequently referred to as golfer’s elbow, has the same degenerative pathologic changes as those described in lateral epicondylitis and is more correctly termed medial epicondylosis.132 This condition occurs 3 to 7 times less frequently than lateral epicondylosis, and the degenerative changes are most frequently found in the pronator teres and flexor carpi radialis (FCR) origins.132 Risk factors for medial epicondylosis include training errors, faulty equipment, repetitive activities requiring wrist flexion and forearm pronation, and biomechanical abnormalities (such as poor strength, flexibility imbalances, and joint instability).
Oblique radiographs of the medial epicondyle can reveal punctuate calcifications in the region of the flexor tendon origins.37 It is also important to rule out degenerative changes in the posterior medial aspect of the olecranon because this condition can cause symptoms similar to those seen with medial epicondylosis.
Nonoperative treatment should include discontinuation of aggravating activities and use of analgesic medications, physical modalities, bracing (e.g., medial counter-force strap and neutral wrist splint) for pain control, and correcting kinetic chain deficits and training errors. Eccentric exercises can be used for the treatment of tendinopathy. Corticosteroid injections have been used to treat this condition but can lead to further tendon degeneration and predispose to tendon rupture. Autologous blood injections might be beneficial.176 Medial epicondylosis can also be responsive to extracorporeal shock-wave therapy.130 Operative treatment can be warranted for those who fail to improve with conservative measures.
Distal Biceps Tendonitis and Rupture
Rupture of the distal biceps brachii tendon is also an uncommon injury. This usually occurs in patients between 30 and 50 years of age, with a significant predilection for men and the dominant limb.10,15,44 The injury usually occurs during a heavy lifting activity while the elbow is at 90 degrees of flexion.56 The rupture usually involves the distal biceps brachii tendon at the radial tuberosity insertion site, with variable involvement of the lacertus fibrosus.44 Although prodromal symptoms are usually absent, pathologic examination of the avulsed tendon frequently shows degenerative changes in the distal biceps brachii tendon.147
Treatment of biceps brachii tendon ruptures is surgical, and early intervention is recommended.
Distal Triceps Tendonitis and Rupture
Activities that require repetitive elbow extension such as throwing, weight lifting, or using a hammer can result in distal triceps brachii tendonitis. This condition can be found in conjunction with loose bodies in the posterior compartment of the elbow or with lateral epicondylitis.170
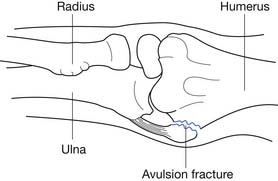
Triceps tendon rupture can occur in individuals who fall onto an outstretched hand or receive a direct blow to the distal triceps brachii tendon. An audible “pop” might occur at the time of the injury. Elbow extension weakness, erythema, ecchymosis, and edema at the area of the tendon rupture are usually present. Minimal trauma might be all that is required for tendon disruption in those who have received previous corticoteroid injections around the distal triceps tendon, or in people who have been treated with chronic oral corticoteroids for other system conditions.170,183 The most common site of disruption is at the distal insertion site on the olecranon, and a small avulsion fracture from the olecranon might be present.
Standard radiographs of the elbow might reveal a small avulsion fracture on the lateral view.52 Ultrasonography or MRI readily demonstrates the pathology.179
Surgical repair of the disrupted triceps tendon is recommended.
Snapping Triceps Tendon
Occasionally a pathologic band over the medial side of the distal triceps can cause a snapping sensation over the medial epicondyle during elbow flexion and extension.46 The astute clinician should try to differentiate this condition from ulnar nerve subluxation, but both conditions can occur at the same time. The patient might report a history of medial elbow popping or snapping during elbow flexion and extension activities. The snapping of the band over the medial epicondyle is frequently palpable on examination. Conservative treatment for this condition includes deep tissue massage, stretching of the triceps muscle, and occasionally local corticosteroid injection. Patients who do not respond to this regimen might require surgical intervention.
Olecranon Bursitis
The olecranon bursa lies subcutaneously over the olecranon process. Olecranon bursitis can be septic or aseptic. Aseptic bursitis is either acute hemorrhagic bursitis resulting from a macrotraumatic insult to the bursa or chronic bursitis caused by repetitive microtrauma. Aseptic bursitis is frequently seen in athletes who participate in football or hockey.87
For those with chronic bursitis, treatment should include ice, intermittent NSAIDs, and protection of the area from further insult. If edema is present, a compressive dressing should be applied. An intrabursal injection of corticosteroid medications can be helpful. For aseptic bursitis that is recalcitrant to standard treatment, sclerotherapy using a tetracycline injection into the bursa has been advocated.65 Surgical excision is also an option for patients who do not obtain relief with the above measures.
Ulnar Collateral Ligament Sprain
Injuries to the UCL of the elbow are a result of valgus stress to the elbow. This can be caused by a single traumatic episode but is frequently seen with the repetitive microtrauma associated with throwing. The late cocking phase of overhead throwing is characterized by a valgus torque of approximately 64 N-m across the elbow.54 During the acceleration phase of overhead throwing, the valgus forces across the elbow are increased, leading to further stress on the UCL.
The UCL is composed of an anterior, posterior, and transverse bundle. It is primarily stabilized by the anterior bundle.180 The anterior bundle has a thin superficial and thick deep layer, and the superficial or deep layer can be torn individually or as a unit.181
Anteroposterior, lateral, and oblique radiographs of the elbow should be obtained. Calcification of the UCL can occur, and avulsions from the humeral or ulnar attachments should be ruled out.164 CT or MRI arthrograms can further define the injury, particularly if the injury is a partial tear of the UCL that only involves the deep fibers.164 MRI has the advantage of excellent soft tissue visualization.
Partial UCL injuries should be initially treated with nonoperative measures. Conservative treatment includes discontinuation of throwing activities for 3 to 6 weeks and initiation of gentle elbow ROM, modalities, and analgesics. Progressive strengthening of the medial forearm musculature should be done because these muscles are dynamic stabilizers of the medial elbow against valgus stress. Occasionally a hinged elbow brace is used for initial support and comfort. Taping the medial elbow to resist valgus stress can be helpful. When the patient has full pain-free ROM and symmetric strength, an interval throwing program can be initiated with the overhead-throwing athlete (Box 38-1). Proper throwing mechanics should be ensured. Patients who fail to improve after conservative management can be candidates for UCL reconstruction.
Valgus Extension Overload of the Elbow
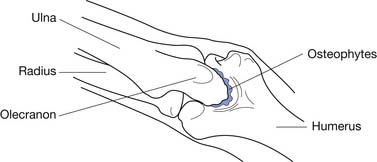
Valgus extension overload (VEO) is a common disorder in overhead-throwing athletes, accounting for approximately 65% of surgeries for medial elbow pain in professional baseball players.8 This disorder is characterized by impingement of the posteromedial olecranon against the medial wall of the olecranon fossa from the valgus elbow stress during overhead throwing. Over time, repetitive impingement at this site can result in olecranon osteophyte formation, intraarticular loose bodies, and deposition of fibrous tissue in the olecranon fossa (Figure 38-5).188
Medial Epicondylar Traction Apophysitis and Stress Fracture
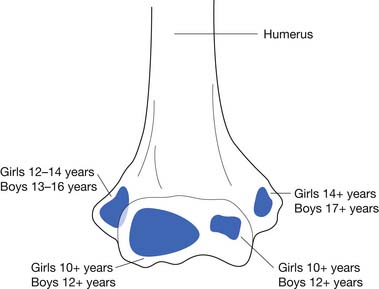
Injuries to the medial elbow in young throwing athletes have been referred to as “little leaguer’s elbow” and include medial epicondylitis, traction apophysitis, stress fractures through the medial epicondylar epiphyses, and avulsion fractures of the medial epicondyle.24 These injuries classically occur in the dominant arm of throwing athletes between the ages of 9 and 12 years.23 Excessive throwing can cause chronic repetitive valgus stress to medial elbow, resulting in a traction injury to the immature medial epicondyle. The medial epicondylar apophysis does not close until 14 years of age in females and 17 years of age in males, and is the last elbow physis to close (Figure 38-6).23 Following the currently recommended number of pitches and rest intervals for young overhead-throwing athletes might prevent this type of injury.111 The currently recommended number of pitches and rest intervals by Little League can be found at: http://www.littleleague.org/Assets/old_assets/media/RS_Pitching_Regulation_Changes_Baseball_2008.pdf.
Radiologic evaluation should include anteroposterior, lateral, axial, and comparison views of the elbow. Common radiologic abnormalities include medial epicondylar enlargement, fragmentation, beaking, and avulsion of the medial epicondyle (Figure 38-7).24 In the past, three-phase bone scan followed by tomograms or CT scan through the areas of increased activity have been used. MRI, however, currently has the advantage of evaluating for both osseous and soft tissue injuries that might not be identified with other techniques.
The initial treatment is 4 to 8 weeks of rest from throwing activities. Ice, analgesics, elbow ROM exercises, and correction of kinetic chain deficits should be instituted early. As symptoms reside, progressive strengthening of the medial forearm musculature should be performed. An interval throwing program can begin when full pain-free ROM has been restored, and there are no strength asymmetries (see Box 38-1). When pitching is resumed, the athlete should follow the currently recommended rest intervals and limitations on the number of pitches to prevent injury recurrence (refer to the earlier mentioned Little League website).111 If pain resumes with throwing activities, several months of rest from throwing activities should be initiated. This should be followed by the same gradually progressive resumption of throwing activities. If after 6 months of treatment the patient is still symptomatic, surgical consultation is warranted.
Osteochondrosis and Osteochondritis Desiccans of the Capitellum
Panner’s disease is an osteochondrosis of the capitellum that occurs in children 7 to 10 years of age. It begins with degeneration or necrosis of the capitellum, followed by regeneration and calcification of this area.139 The cause of Panner’s disease is unknown, but it appears to be related to disordered endochondral ossification in association with trauma or vascular impairment.
Osteochondritis desiccans of the capitellum can also occur and has several unique characteristics that enable clinicians to differentiate it from osteochondrosis of the capitellum. Osteochondritis desiccans occurs in throwing athletes 9 to 15 years of age, whereas osteochondrosis occurs in 7 to 10 year olds.23 Osteochondritis desiccans involves focal lesions to the capitellum, whereas osteochondrosis involves the entire capitellum.23 Osteochondritis desiccans frequently leads to loose body formation. Osteochondrosis is usually self-limited and resolves with rest and time.23
Treatment should begin with rest, ice, and analgesics. Elbow immobilization with a posterior long arm splint might provide symptomatic relief. Gentle progressive ROM and strengthening exercises should begin once pain has resolved, and protection from forceful activities should continue for a minimum of 6 weeks to allow healing. Once full strength and ROM have been restored, the patient can begin a gradual return to functional activities. Serial radiographic evaluation should be performed to ensure adequate healing of the lesion, although in some cases symptomatic improvement can occur despite persistent radiologic abnormalities. In patients with large loose bodies, persistent mechanical symptoms, or pain recalcitrant to nonoperative measures, surgical intervention might be required.
Elbow Dislocations
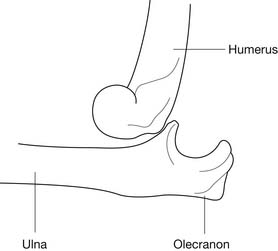
Elbow dislocations involving the proximal ulna and distal humerus most frequently occur in a posterolateral direction and are a result of a fall on an outstretched arm with the elbow in hyperextension (Figure 38-8).129 Anterior, lateral, and posteromedial dislocations also occur but are much less frequent. When a posterolateral elbow dislocation occurs, associated injuries include disruption of the ulnar and radial collateral ligaments of the elbow, and occasionally disruption of the flexor pronator forearm musculature, or periarticular and intraarticular fractures.80,127,182 Injuries to the brachial artery or to the median, ulnar, or radial nerve should be identified and treated. Acute anterior forearm compartment syndrome can also occur with elbow dislocations.
Treatment should begin with reduction of the dislocation. Prereduction and postreduction films should be obtained to ensure proper reduction. If the elbow cannot be reduced in a closed manner, then open reduction should be performed. Postreduction neurovascular examination is very important to rule out new or continued neurovascular compromise. If the flexor–pronator musculature has been disrupted along with the UCL, early surgical intervention is warranted.127 Gross medial elbow instability without flexor–pronator musculature rupture can be treated nonoperatively if the patient does not participate in overhead-throwing sports. Overhead-throwing athletes with complete disruption of the UCL, however, frequently require surgical intervention to reduce their postinjury morbidity.
Conservative treatment of elbow dislocations includes a sling or posterior long arm splint for 2 to 3 days followed by progressive ROM exercises. Prolonged immobilization should be avoided because flexion contractures are a frequent complication of this injury. A hinged elbow brace that allows flexion and extension of the elbow can be used to facilitate early ROM activities. Ice and analgesics can assist in patient comfort. Progressive strengthening activities should begin when the patient’s pain level allows, and full return to activities should be expected in approximately 8 weeks. A majority of patients have had 90% restoration of function by 3 months postinjury.79,155 In addition to the surgical indications previously mentioned, surgical intervention can be warranted in patients who experience chronic recurrent elbow instability.
Conditions of the Forearm and Wrist
Flexor Carpi Ulnaris Tendonitis
Flexor carpi ulnaris (FCU) tendonitis occurs as a result of repetitive microtrauma from activities requiring wrist flexion and ulnar deviation such as racquet sports or golf.69 Because the pisiform bone is embedded in the FCU tendon, pisotriquetral compression syndrome with eventual pisotriquetral osteoarthritis can be associated with this condition.153
Patients with FCU tendonitis typically report the insidious onset of volar ulnar wrist pain. Activities requiring repetitive wrist flexion and ulnar deviation frequently aggravate this condition. The patient might also experience crepitus in the region of the pisotriquetral joint during wrist movements. Physical examination can show localized edema and tenderness to palpation over the distal FCU tendon. Compression of the pisiform against the triquetrum can cause pain if osteoarthritis is present in this joint. Lateral wrist radiographs with slight supination and extension can also demonstrate pisotriquetral joint osteoarthritis.153
Wrist hand orthoses with the wrist in 25 degrees of flexion can assist in symptom control.153
Patients who do not respond to a comprehensive nonoperative treatment program can consider surgical resection of the pisiform bone with or without Z-plasty lengthening of the FCU tendon.138
Flexor Carpi Radialis Tendonitis
The FCR tendon passes through a tunnel formed by the transverse carpal ligament, trapezial ridge, and scaphoid tuberosity before inserting on the second metacarpal base.153 Although tendonitis in the FCR is relatively uncommon, activities that require repetitive gripping with wrist flexion and radial deviation predispose to this condition.45
If splinting is required, a wrist hand orthosis with 25 degrees of wrist flexion is appropriate.153
De Quervain’s Syndrome
The first dorsal compartment of the wrist contains the abductor pollicis longus and extensor pollicis brevis tendons. These tendons run beneath a sheath over the dorsal aspect of the radial styloid process, along the inferior portion of the anatomic snuff box. Shear and repetitive microtrauma in this area can result in a stenosing tenosynovitis referred to as de Quervain’s syndrome.153 De Quervain’s syndrome is the most common tendonitis of the wrist and is most frequently seen in patients who perform activities requiring forceful gripping with ulnar deviation of the wrist or repetitive use of the thumb.34,96
Patients with this condition present with insidious onset of pain over the dorsal radial aspect of the wrist aggravated by activities such as racquet sports, golf, or fly fishing. Patients can also report a sensation of wrist crepitus. Physical examination frequently demonstrates mild edema localized to the dorsal radial wrist and tenderness to palpation over the first dorsal compartment. Finkelstein’s test is pathognomonic for the diagnosis.153 Finkelstein’s test is performed by placing the patient’s elbow in extension, with the forearm in neutral rotation and the wrist in radial deviation. The patient should be asked to place the thumb in the palm and grip the thumb with the fingers. The examiner should then passively bring the wrist into ulnar deviation. A positive test results in pain provocation in the first dorsal wrist compartment tendons.
Treatment for de Quervain’s syndrome includes rest, modalities, analgesics, and a thumb spica splint. First dorsal compartment peritendinous corticosteroid injection reduces symptoms in 62% to 100% of cases.86,191
Intersection Syndrome
The abductor pollicis longus and extensor pollicis brevis tendons cross the extensor carpi radialis longus and brevis tendons 4 to 6 cm proximal to Lister’s tubercle. An inflammatory condition can develop at this crossing as a result of the friction from the tendons repetitively rubbing against each other. This condition is called intersection syndrome and is most frequently seen in oarsmen, weight lifters, and racquet sport athletes.36,134,153
Intersection syndrome presents with the insidious onset of dorsoradial distal forearm pain aggravated by activities requiring repetitive wrist extension.153 Physical examination reveals mild edema and acute tenderness 4 to 6 cm proximal to Lister’s tubercle, frequently associated with crepitation during wrist flexion and extension.
Extensor Carpi Ulnaris Tendonitis and Subluxation
The second most frequent tendonitis of the wrist is extensor carpi ulnaris (ECU) tendonitis. Repetitive wrist extension with ulnar deviation, such as occurs in the nondominant hand of the tennis two-handed backhand, predisposes to this condition.153 Rupture or attenuation of the overlying tendon sheath can result in subluxation of the ECU tendon.162
ECU tendonitis usually presents with insidious onset of dorsoulnar wrist pain that occurs during activities requiring forceful or repetitive wrist extension and ulnar deviation. Patients with tendon subluxation often report a history of a “pop” after a forceful volar wrist flexion and ulnar deviation with subsequent pain.162 Physical examination reveals tenderness to palpation over the ECU tendon and pain with resisted wrist extension with ulnar deviation. Subluxation can be induced by having the patient perform active ulnar wrist deviation while the forearm is fully supinated. The ECU tendon might palpably and/or visually sublux ulnarly over the ulnar styloid process.153
Acute ECU tendon subluxations can be treated with cast immobilization of the wrist in a pronated and dorsiflexed position for 6 weeks.29 Early surgical repair for acute injuries, however, has also been advocated to improve predictability of outcome.162 In injuries characterized by chronic recurrent subluxation, surgical reconstruction of the subsheath should be performed.
Scapholunate Instability
Scapholunate instability resulting from ligamentous injury is the most common type of wrist ligament injury.104 This can occur when a person falls on a pronated outstretched hand with the wrist in extension and ulnar deviation. After scapholunate ligament disruption, the scaphoid moves into a flexed position, whereas the lunate and triquetrum become extended. This pattern is referred to as dorsal intercalated segmental instability, or a “DISI” pattern (Figure 38-9).92 If this injury is not diagnosed early and treated properly, joint stress will ultimately lead to progressive wrist arthrosis and scapholunate advanced collapse.152
Patients who sustain a scapholunate injury typically report falling on their outstretched hand with subsequent wrist edema, ecchymosis, and restricted ROM. Physical examination shows tenderness over the scapholunate joint, particularly on the dorsum of the wrist. The patient typically has a positive result on the Watson test.100 To perform the Watson test, the patient’s wrist should be placed passively into ulnar deviation. The examiner should place his or her thumb over the scaphoid tubercle, located on the volar surface of the wrist. The patient’s wrist should be brought passively from ulnar to radial deviation while the examiner places a dorsally directed force against the scaphoid tubercle with his or her thumb. A positive test result is indicated by a painful click and dorsal shift of the scaphoid bone relative to the lunate bone during this movement.
Radiographic evaluation should include anteroposterior, anteroposterior with a clenched fist, lateral, and oblique radiographs.152 The presence of associated fractures should be assessed. The lateral radiograph can reveal the presence of a DISI pattern injury (see Figure 38-9). This is characterized by a scapholunate angle of more than 60 degrees.152 A gap of more than 3 mm between the scaphoid and lunate on anteroposterior radiographs is also diagnostic for scapholunate instability (Figure 38-10).152 The clenched fist view exaggerates this finding. Radiographic evaluation should include comparison studies with the opposite wrist.
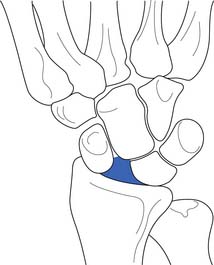
FIGURE 38-10 A gap between the scaphoid and lunate on anteroposterior radiographs indicates scapholunate dissociation.
If standard radiographs are not diagnostic, further studies including arthrography or MRI can be obtained. The sensitivity and specificity of these studies, however, are variable.152 Wrist arthroscopy has become the gold standard for diagnosing this entity.
Acute scapholunate ligament injuries should be treated surgically. Chronic scapholunate injuries are more difficult to treat but also require surgical intervention. Partial wrist arthrodesis can improve chronic scapholunate instability, and a proximal row carpectomy is frequently used to treat advanced scapholunate collapse.152
Scaphoid Fracture
Approximately 6% of all fractures involve the carpal bones, and 70% of carpal fractures involve the scaphoid.49 The scaphoid is positioned distal to the radius and is part of the proximal row of carpals. The scaphoid is the primary restraint to excessive wrist extension and is therefore prone to injury. The scaphoid fractures in the middle third 80% of the time, the proximal third 15% of the time, and the distal third 4% of the time. Only 1% of scaphoid fractures occur through the distal tubercle.49
The scaphoid receives its blood supply from a branch of the radial artery that enters the scaphoid through its distal pole. Consequently, proximal or middle third fractures of the scaphoid are prone to avascular necrosis and nonunion as a result of a disruption in the blood supply.49
Radiologic evaluation of the wrist should include anteroposterior, lateral, right and left oblique, and anteroposterior clenched fist views.49 Scaphoid fractures can be very subtle and not readily apparent on radiographs. Associated carpal instability patterns should be assessed radiographically with particular attention paid to scapholunate dissociation, which is suggested by a 3-mm or larger gap between the scaphoid and lunate during clenched fist anteroposterior views, or with a DISI pattern on lateral radiographs.49
Because minimal edema occurs with this fracture, early cast immobilization can be used. For nondisplaced middle or proximal third fractures, the first 6 weeks of immobilization should use a long arm–thumb spica cast with the elbow in 90 degrees of flexion and the wrist in neutral flexion–extension and neutral deviation incorporating the first metacarpophalangeal joint with the thumb in slight extension and abduction.49
If the fracture occurs in the distal third of the scaphoid, or if there is a high index of suspicion for a scaphoid fracture that was not seen on radiographs, a short arm–thumb spica cast with the wrist and thumb positioned the same as described previously can be used.49
Patients with nondisplaced middle or proximal third fractures can be switched from the long arm–thumb spica cast to a short arm–thumb spica cast after 6 weeks, with the total duration of immobilization being for 12 to 20 weeks depending on when radiographic union occurs.49 Patients with nondisplaced distal third fractures typically require between 10 and 12 weeks of immobilization in a short arm–thumb spica cast.49 Radiographs should be repeated every 2 to 3 weeks until radiographic union is documented.49
During cast immobilization, the patient should be encouraged to perform ROM and strengthening exercises of the nonimmobilized upper limb regions. After immobilization, the patient should begin an exercise program to restore full pain-free ROM of the immobilized joints and strengthening the immobilized musculature.
Distal Radial Fractures
The distal radius is one of the most frequently fractured areas of the body. Postmenopausal women and children appear to be particularly susceptible to this fracture.50 The distal radius has three articulations, including the distal radioulnar joint, the radioscaphoid joint, and the radiolunate joint. The distal radius normally displays a volar tilt of approximately 11 degrees, a radial inclination along the articular surface as viewed on the anterior–posterior radiograph that is 23 degrees, and a 12-mm distance between the tip of the radial styloid process to a line drawn perpendicular to the distal ulnar articular surface (this can be measured on a standard anteroposterior radiographic image) (Figure 38-11).50
Frykman proposed a classification system for distal radial fractures in which types 1 and 2 are extraarticular fractures and types 3 and 4 are intraarticular fractures involving the radiocarpal joint.50 Type 5 and 6 fractures are intraarticular fractures involving the radioulnar joint, whereas types 7 and 8 are intraarticular fractures that involve both the radioulnar and radiocarpal joints (Figure 38-12). The even-numbered fractures indicate the presence of an associated ulnar styloid fracture. The potential for adverse outcome increases as the Frykman classification number increases.
Distal radial fractures can be assessed using anteroposterior, lateral, and oblique radiographs.50 The anteroposterior view allows measurements of the radial inclination and length, whereas the lateral view allows evaluation of the volar tilt.
The most common distal radius fracture is a Frykman type 1 fracture called the Colles fracture.50 This fracture is characterized by a fracture line 2 cm proximal to the distal radius with dorsal angulation of the distal fragment and radial shortening. Minimally displaced Frykman type 1 or 2 fractures can be managed with closed reduction and immobilization with a double sugar-tong splint with the wrist in slight flexion and ulnar deviation.50 The forearm should be in a neutral position, and the elbow should be flexed 90 degrees.50 Satisfactory reduction requires that there is less than 5 mm of radial shortening, no dorsal tilt of the distal radius, and less than 2 mm of displacement of fracture fragments. The patient should be evaluated again in 3 days for repeat radiographs.50
By approximately 3 days postinjury, patients with a nondisplaced fracture can be placed in a short arm cast with the wrist in a neutral position for approximately 6 weeks, with repeat radiographs every 2 weeks for the first 6 weeks.50 Patients with a minimally displaced fracture who are status postreduction should receive repeat radiographs at the 3-day follow-up evaluation to ensure maintenance of the reduction, and should be placed in a long arm cast with the wrist in slight flexion and ulnar deviation, the forearm in a neutral position, and the elbow flexed to 90 degrees for 3 to 4 weeks.50 This can then be switched to a short arm cast with the wrist in a neutral position for 3 to 4 more weeks until healing has occurred.50 Patients with minimally displaced fractures status postreduction should receive repeat radiographs weekly for the first 3 weeks and then every 2 weeks until healing is complete.50
Kienbock’s Disease
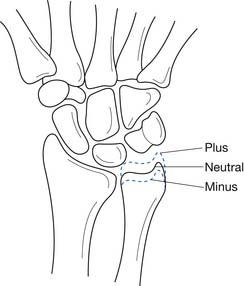
Kienbock first described a condition characterized by progressive collapse of the lunate in 1910.107 This entity, now commonly referred to as Kienbock’s disease, is hypothesized to result from repetitive compressive forces to the wrist causing microfractures in the lunate leading to vascular compromise, avascular necrosis, and eventual collapse of the lunate.57,107 This condition occurs more frequently in patients who have ulnar-minus variant wrists (Figure 38-13).57 Kienbock’s disease presents with pain and stiffness in the wrist. Physical examination can reveal limited wrist ROM and tenderness to palpation over the lunate on the dorsum of the wrist.
Radiographic evaluation typically reveals an ulnar-minus variant in the wrist.57 The lunate can appear normal in early cases but frequently becomes sclerotic with cystic changes, followed by fragmentation and collapse.107 Bone scans might demonstrate increased uptake even when radiographs are still negative.107 MRI can also be helpful in diagnosing Kienbock’s disease.
In early cases of Kienbock’s disease, the wrist should also be immobilized in an attempt to allow revascularization.172 If the patient has an ulnar-minus wrist variant, an attempt at relieving lunate trauma through an ulnar lengthening procedure or radial shortening procedure can be performed.107 For more advanced cases of Kienbock’s disease, partial wrist arthrodesis or lunate excision with soft tissue or silicone replacement might be required.107
Triangular Fibrocartilage Complex Injuries
The triangular fibrocartilage complex (TFCC) is composed of an avascular central articular disc and vascular dorsal and palmar radioulnar ligaments.152 The TFCC is the primary stabilizer of the distal radioulnar joint and can be injured in an acute traumatic event such as falling on an outstretched hand or through repetitive microtrauma such as in gymnastics. Axially loading the wrist results in 18% of the load being born through the TFCC and the remaining 82% through the radiocarpal joint.137 A positive ulnar variance results in an increase in the load-bearing function of the TFCC, which results in a higher incidence of TFCC injuries (see Figure 38-13).137
Patients who sustain a TFCC injury might report either an insidious onset or a single traumatic event. Traumatic tears occur more frequently in young athletes, whereas degenerative tears are more common in older patients. Patients with acute injuries often report an axial load to the wrist associated with rotational stress.152 The patient can also report wrist catching and locking. The physical examination can show tenderness to palpation in the hollow between the FCU tendon and the extensor carpi ulnaris tendon, just distal to the ulnar styloid process.152
Radiographic evaluation of the wrist can reveal an ulnar-plus variant on the anteroposterior view. Tricompartment wrist arthrogram, MRI, and MRI arthrogram can provide more specific information regarding TFCC pathology.152
When the central articular disc of the TFCC has been acutely injured, surgical debridement is the treatment of choice. A 90% good-to-excellent result with this treatment was reported by Bednar and Osterman.19 Peripheral tears of the TFCC also respond well to surgical intervention, but the postoperative recovery process is slower than for central articular disc injuries.152 Patients with degenerative tears of the TFCC should be evaluated for an ulnar-plus variant, and if this is present, an ulnar shortening procedure should be considered along with surgical debridement of the TFCC.152
Conditions of the Hand and Fingers
Extensor Tendon Central Slip Disruption
A rupture of the central slip of the extensor tendon at the base of the middle phalanx results in a boutonnière injury (Figure 38-14). A boutonnière injury is characterized by the inability to actively extend the proximal interphalangeal (PIP) joint, but patients with this injury are able to maintain full PIP joint extension if they are passively placed in this position.59 The reason the PIP joint is held in flexion is due to the migration of the lateral bands of the extensor mechanism volarly below the axis of rotation in the PIP joint resulting in a flexion force at this joint.59 The lateral bands, however, continue to exert an extension force on the distal interphalangeal (DIP) joint.
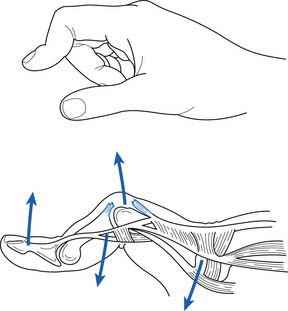
FIGURE 38-14 A boutonnière deformity caused by rupture of the central slip and volar migration of the lateral bands.
This injury can result from a rupture of the central slip tendon itself or an avulsion fracture at the central slip’s insertion on the dorsal proximal aspect of the middle phalanx. Mechanisms of injury include a crush injury, forced flexion of the interphalangeal joints, or lateral volar PIP joint dislocation.153
Standard radiographic evaluation of the PIP joint should be performed to check for an associated avulsion fracture of the dorsal proximal aspect of the middle phalanx. If the injury is evaluated within approximately 6 weeks, and the patient has full passive extension of the PIP joint, then treatment should involve continuous extension splinting of the PIP joint for 5 to 6 weeks (Figure 38-15).59 The DIP joint can be left unsplinted, and flexion exercises of the DIP joint should be initiated early in the treatment program. It is important to educate the patient that even a single episode of PIP joint flexion over this time period can prevent successful treatment of this condition, as compliance is critical.
In patients who have a chronic boutonnière deformity, or if the PIP joint is not able to be passively extended, an aggressive rehabilitation program involving serial casting or splinting to restore full passive ROM should be implemented.153 If adequate passive joint ROM is obtained and symptoms are minimal, no further treatment is required. If the patient is not able to regain significant PIP joint extension ROM, however, then surgical intervention to repair the extensor mechanism can be warranted. Surgical results are variable, however, and a trial of nonoperative treatment should be used in all patients before considering surgical intervention.59
If a large displaced avulsion fracture from the dorsal proximal aspect of the middle phalanx accompanies the boutonnière deformity, early surgical intervention is the treatment of choice.153
Terminal Extensor Tendon Disruption
Disruption of the distal extensor tendon at its insertion on the dorsal proximal aspect of the distal phalanx results in an injury called a mallet finger (Figure 38-16).59 This can be caused by a tendon rupture or an avulsion fracture of the dorsal proximal distal phalanx. This injury usually occurs as a result of a hyperflexion force to an extended DIP joint. A zone of hypovascularity within the distal extensor tendon predisposes to this injury.185
FIGURE 38-16 A, An extensor tendon rupture (top) or avulsion fracture (bottom) resulting in a mallet finger deformity (B).
The treatment for this injury is usually nonoperative unless a large avulsion fracture is present, in which case surgical intervention can be required.59 Nonoperative treatment involves splinting the DIP joint in extension 24 hr/day for 6 to 8 weeks (Figure 38-17).59 The patient should be reminded of the importance of never allowing DIP flexion during this period because any flexion can prevent adequate healing and predispose to a permanent extension lag. After an adequate period of splinting, the patient should be placed in a rehabilitation program to restore full joint ROM and to ensure adequate strength, flexibility, and endurance in the forearm and hand musculature.
Flexor Digitorum Profundus Rupture
Distal disruption of the flexor digitorum profundus (FDP) tendon can occur with vigorous gripping activities, such as when a football player is making a tackle by gripping the opponent’s jersey. Because of this mechanism of injury, distal FDP tendon disruptions are frequently referred to as a “jersey finger.”88 Biomechanical studies have revealed that the flexor digitorum tendon to the ring finger has a lower breaking strength than the other FDP tendons, which might explain the increased incidence of disruption in the tendon to this digit compared with other digits.102
The history of patients with a jersey finger usually includes a vigorous gripping activity resulting in a sudden severe pain, frequently associated with a “pop.” The patient will subsequently be unable to actively flex the affected DIP joint. Physical examination shows an inability to actively flex the DIP joint, which becomes apparent when the patient is asked to make a fist (Figure 38-18). The retracted tendon mass can be palpable in the palm of the hand.
The primary treatment for this injury is surgical repair. If the tendon has retracted to the palm, then the repair should be performed within 7 to 10 days of the injury to prevent adhesion formation.88 If the tendon does not retract below the PIP joint, however, then delayed repair can be performed within 6 to 8 weeks of the injury.88 Associated fractures should be addressed at the time of surgery.
Proximal Interphalangeal Joint Dislocations
The PIP joint is the most frequently dislocated joint in the hand.59 Dislocations can occur in dorsal, volar, and lateral directions, with dorsal dislocations being the most frequent.153 Dorsal dislocations occur as a result of an axial load to the joint combined with hyperextension.153 Dorsal dislocations result in various levels of injuries to the volar plate of the PIP joint and radial and UCLs and can also be accompanied by a fracture.
Volar PIP joint dislocations are usually due to a varus or valgus force across the joint combined with a volar force to the middle phalynx.153 Associated injuries with volar PIP joint dislocations include radial and UCL sprains, central slip injuries, partial volar plate injuries, and fractures.
Radiologic evaluation should include standard radiographs of the PIP joint to assess for associated fractures. Avulsion fractures from the volar proximal aspect of the middle phalanx are commonly seen with dorsal dislocations. Postreduction films should also be performed to ensure that adequate reduction has been achieved. After a digital block for local anesthesia, the joint’s stability can be assessed by having the patient actively flex and extend the PIP joint under fluoroscopy.59
After relocation, treatment for dorsal dislocations of the PIP joint includes a 45-degree extension block splint of the PIP joint to allow for healing of the volar plate injury and associated collateral ligament injuries (Figure 38-19).59 The extension block splint angle can be reduced by 10 degrees each week, with a return to full extension in the fifth week. When the splinting is discontinued, buddy taping the injured finger to the adjacent finger for a few weeks can provide some stability for the joint as tissue healing continues and ROM exercises are instituted.
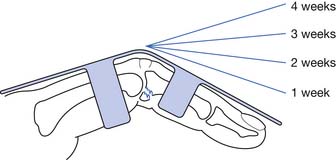
FIGURE 38-19 A dorsal extension splint with a reduction in extension block by 10 degrees each week for 4 weeks.
If stable closed reduction of a volar PIP joint dislocation can be attained, then nonoperative treatment can proceed with splinting of the PIP joint in full extension for 4 to 5 weeks (see Figure 38-15).59 This can be followed by buddy taping the injured finger to an adjacent digit for approximately 2 weeks and resumption of normal activities as tolerated.
Surgical indications for PIP joint injuries include radiologic or gross instability after reduction, fractures involving greater than 50% of the articular surface, inability to reduce the dislocation, pilon fractures (i.e., fractures that extend from the volar to dorsal aspects of the phalanx, include intraarticular involvement, and are usually comminuted), or recurrent instability.59
First Metacarpophalangeal Joint Ulnar Collateral Ligament Sprain
Radially directed forces across the first metacarpophalangeal (MCP) joint can result in an UCL injury. This injury, often referred to as “gamekeeper’s thumb,” is frequently seen in skiers and athletes who participate in sports such as basketball and football.153 UCL injuries to the first MCP joint can be categorized into a three-grade severity scale of ligament sprains (see Table 38-1).
When a grade 3 UCL sprain of the first MCP joint occurs as a result of avulsion of the distal end of the ligament from the base of the first proximal phalanx, there is the possibility of interposition of the adductor pollicis aponeurosis between the base of the first proximal phalanx and the ruptured end of the UCL.174 This is called a Stener lesion and can prevent adequate healing of this injury, leading to chronic joint pain and instability.
Patients who sustain first MCP joint UCL injuries report a radially directed force across the first MCP joint. They might report an associated “pop” and a feeling of instability in the joint. Physical examination reveals tenderness to palpation over the UCL. If a Stener lesion is present, a palpable mass on the ulnar side of the first MCP joint might be present, representing the avulsed UCL.2
UCL stress examination should be performed after local anesthesia via a wrist block, although an initial examination without local anesthesia can be attempted. The stress examination should be performed with the joint in both full extension and 30 degrees of flexion. A complete tear is indicated by an angular difference between the injured and uninjured first MCP joint during stress examination of greater than 15 to 30 degrees.74,136 A lack of an endpoint during stress examination also suggests complete UCL disruption.
Treatment of partial tears involves modalities, analgesics, and immobilization in a thumb spica cast for 10 to 14 days, followed by a wrist–hand–thumb spica orthosis for 2 weeks and a hand-based thumb spica orthosis for 2 to 4 more weeks.59 Patients who participate in contact sports should continue to wear a thumb spica splint during competition for the remainder of the season. Local taping for stability during activity can be used after the period of splinting is completed. Gentle progressive ROM exercises should begin after cast immobilization by removing the splint twice daily, and activity should be progressed as tolerated.
Early surgical repair has been advocated for complete ruptures of the UCL, particularly if a Stener lesion is present.55,91,108,133,168 Surgery is also indicated for individuals with an avulsion fracture of the base of the proximal phalanx with angulation and displacement greater than 3 mm, or with chronic recurrent instability.114
Collateral Ligament Injuries of the Second to Fifth Digits Proximal and Distal Interphalangeal Joints
Radial and UCL injuries to the PIP and DIP joints can be associated with multiple ligament injuries and dislocation, but they can also occur in isolation. Collateral ligament injuries of the interphalangeal joints can be classified according to the standard three-grade ligamentous sprain scale (see Table 38-1).
Partial tears of the radial or UCLs of the PIP or DIP joints can be treated with modalities, analgesics, and buddy taping the digit to an adjacent digit for 3 to 6 weeks. Activities can be allowed as tolerated. If there is a complete disruption of the ligament with significant instability, an attempt at nonoperative treatment can be performed, but some authors advocate early open repair of this injury.109,150,160
1. Abboud J., Soslowsky L.J. Interplay of the static and dynamic restrains in glenohumeral instability. Clin Orthop. 2002;400:48-57.
2. Abrahamsson S., Sollerman C., Lundborg G., et al. Diagnosis of displaced ulnar collateral ligament of the metacarpophalangeal joint of the thumb. J Hand Surg Am. 1990;15:457-460.
3. Abrams J., Savoie F.H.3rd, Tauro J.C., et al. Recent advances in the evaluation and treatment of shoulder instability: anterior, posterior, and multidirectional. Arthroscopy. 2002;18:1-13.
4. Almekinders L. The efficacy of nonsteroidal anti-inflammatory drugs in the treatment of ligament injuries. Sports Med Arthrosc Rev. 1990;9:137-142.
5. Altay T., Gunal I., Ozturk H. Local injection treatment for lateral epicondylitis. Clin Orthop. 2002;398:127-130.
6. Altchek D., Dines D.M. Shoulder injuries in the throwing athlete. J Am Acad Orthop Surg. 1995;3:159-165.
7. Andrews J., Carson W.G., McLeod W.D. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13:337-341.
8. Andrews J., Timmerman L.A. Outcome of elbow surgery in professional baseball players. Am J Sports Med. 1995;23:404-413.
9. Antoniou J., Harryman D.T. Posterior instability. Orthop Clin North Am. 2001;32:463-473.
10. Anzel S., Covey K.W., Weiner A.D., et al. Disruption of muscles and tendons: an analysis of 1,014 cases. Surgery. 1959;45:406.
11. Arciero R., Wheeler J.H., Ryan J.B., et al. Arthroscopic Bankart repair versus nonoperative treatment for acute, initial anterior shoulder dislocations. Am J Sports Med. 1994;22:589-594.
12. Aronen J., Regan K. Decreasing the incidence of recurrence of first time anterior shoulder dislocations with rehabilitation. Am J Sports Med. 1984;12:283-291.
13. Awerbuch M. The clinical utility of ultrasonography for rotator cuff disease, shoulder impingement syndrome and subacromial bursitis. Med J Aust. 2008;188:50-53.
14. Backer M., Warren R.F. Glenohumeral instabilities. In: DeLee J., Drez D., Miller M.D., editors. DeLee and Drez’s orthopaedic sports medicine principles and practice. Philadelphia: Saunders, 2003.
15. Baker B., Bierwagen D. Rupture of the distal tendon of the biceps brachii: Operative versus non-operative treatment. J Bone Joint Surg Am. 1985;67:414-417.
16. Bankart A. The pathology and treatment of recurrent dislocation of the shoulder joint. Br J Surg. 1938;26:23-29.
17. Basamania C. Clavicle Fractures in Adult Athletes. In: Jesse C., DeLee D.D.J., editors. Orthopaedic sports medicine principles and practice. Philadelphia: Saunders, 2003.
18. Beasley L., Faryniarsz D.A., Hannafin J.A. Multidirectional instability of the shoulder in femal athletes. Clin Sports Med. 2000;19:331-349.
19. Bednar J., Osterman A.L. The role of arthroscopy in the treatment of traumatic triangular fibrocartilage injuries. Hand Clin. 1994;10:605-614.
20. Bell S., Coghlan J., Richardson M. Hydrodilatation in the management of shoulder capsulitis. Australas Radiol. 2003;47:247-251.
21. Bigliani L., Morrison D.S., April E.W. The morphology of the acromion and its relationship to rotator cuff tears. Orthop Trans. 1986;10:228.
22. Blasier R., Soslowsky L., Malicky D., et al. Posterior glenohumeral subluxation: active and passive stabilization in a biomechanical model. J Bone Joint Surg Am. 1997;79:433-440.
23. Bradley J., Petrie R.S. Elbow injuries in children and adolescents. In: DeLee J., Drez D., Miller M.D., editors. Orthopaedic sports medicine principles and practice. Philadelphia: Saunders, 2003.
24. Brogdon B., Crowe N.E. Little leaguer’s elbow. Am J Roentgenol. 1960;83:671-675.
25. Browne A., Hoffmeyer P., Tanaka S., et al. Glenohumeral elevation studies in three dimensions. J Bone Joint Surg Br. 1990;72:843.
26. Brunet M., Reynolds M., Cook S. Atraumatic osteolysis of the distal clavicle: histological evidence of synovial pathogenesis. Orthopedics. 1986;9:557-559.
27. Bunker R., Anthony P.P. The pathology of frozen shoulder: a Dupuytren-like disease. J Bone Joint Surg Br. 1995;77:677-683.
28. Burkhart S., Morgan C.D. The peel-back mechanism: Its role in producing and extending posterior type II SLAP lesions and its effect on SLAP repair rehabilitation. Arthroscopy. 1998;14:637-640.
29. Burkhart S., Wood M., Linscheid R.L. Post-traumatic recurrent subluxation of the extensor carpi ulnaris tendon. J Hand Surg. 1982;7:1.
30. Clark H., McCann P. Acromioclavicular joint injuries. Orthop Clin North Am. 2000;31:177-187.
31. Cleland. Notes on raising the arm. J Anat Physiol. 1884;18:275.
32. Clive D., Stoff J.S. Renal syndromes associated with nonsteroidal anti-inflammatory drug-induced gastroduodenal ulceration. N Engl J Med. 1984;327:1575-1580.
33. Cobey M. The rolling scapula. Clin Orthop Rel Res. 1968;60:193-194.
34. Conklin J., White W. Stenosing tenosynovitis and its possible relation to the carpal tunnel syndrome. Surg Clin North Am. 1960;40:531-540.
35. Connolly J. Unfreezing the frozen shoulder. J Musculoskel Med. November, 1998:47-58.
36. Cooney W. Sports injuries to the upper extremity. Postgrad Med. 1984;76:45-50.
37. Coonrad R., Hooper W.R. Tennis elbow: Its course, natural history, conservative and surgical management. J Bone Joint Surg Am. 1973;55:1177-1182.
38. Cope R., Riddervold H.O., Shore J.L., et al. Dislocations of the sternoclavicular joint: anatomic basis, etiologies, and radiologic diagnosis. J Orthop Trauma. 1991;5:379-384.
39. Cyriax H. The pathology and treatment of tennis elbow. J Bone Joint Surg Am. 1936;18:921-940.
40. Davidson P., ElAttrache N.S., Jobe C.M., et al. Rotator cuff and postero-superior glenoid labrum injury associated with increased glenohumeral motion: a new site of impingement. J Shoulder Elbow Surg. 1995;4:384-390.
41. deJong K. Sukui DM: Anterior sternoclavicular dislocation: a long-term follow-up study. J Orthop Trauma. 1990;4:420-423.
42. del Cura J., Torre I., Zabala R., et al. Sonographically guided percutaneous needle lavage of calcific tendinitis of the shoulder: short- and long-term results. Am J Roentgenol. 2007;189:W128-W134.
43. DePalma A., Coker A.J., Probhaker M. The role of the subscapularis in recurrent anterior dislocation of the shoulder. Clin Orthop. 1969;54:35.
44. Dobbie R. Avulsion of the lower biceps brachii tendon: analysis of 51 previously reported cases. Am J Surg. 1941;51:661.
45. Dobyns J., Sim F.H., Linscheid R.L. Sports stress syndromes of the hand and wrist. Am J Sports Med. 1978;6:236.
46. Dugas J., Anderews J.R. Throwing injuries in the adult. In: DeLee J., Drez D., Miller M.D., editors. Orthopedic sports medicine principles and practice. Philadelphia: Saunders, 2003.
47. Dunn J., Kim J.J., Davis L., et al. Ten to 14-year follow-up of the Nirschl surgical technique for lateral epicondylitis. Am J Sports Med. 2008;36:261-266.
48. Edwards S., Calandruccio J.H. Autologous blood injections for refractory lateral epicondylitis. J Hand Surg Am. 2003;28:272-278.
49. Eiff M., Hatch R.L., Calmbach W.L. Carpal fractures. In: Eiff M., Hatch R.L., Calmbach W.L., editors. Fracture management for primary care. Philadelphia: WB Saunders, 1998.
50. Eiff M., Hatch R.L., Calmbach W.L. Radius and ulna fractures. In: Eiff M., Hatch R.L., Calmbach W.L., editors. Fracture management for primary care. Philadelphia: WB Saunders, 1998.
51. Ellen M., Gilhool J.J., Rogers D. Scapular instability: the scapulothoracic joint. Phys Med Rehabil Clin North Am. 2000;11:755-770.
52. Farrar E., Lippert F.G. Avulsion of the triceps tendon. Clin Orthop. 1981;161:242-246.
53. Fitzgerald R. Intrasynovial injection of steroids. Mayo Clin Proc. 1976;51:655-659.
54. Fleisig G., Andrews J.R., Dillman C.J., et al. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23:233-239.
55. Frank W., Dobyns J. Surgical pathology of collateral ligamentous injuries of the thumb. Clin Orthop. 1972;83:102-114.
56. Friedmann E. Rupture of the distal biceps brachii tendon. JAMA. 1963;184:60-63.
57. Gelberman R., Salamon P.B., Jurist J.M., et al. Ulnar variance in Kienbock’s disease. J Bone Joint Surg Am. 1975;57:674-676.
58. Gellman H. Tennis elbow (lateral epicondylitis). Orthop Clin North Am. 1992;23:75-82.
59. Graham T., Mullen D.J. Athletic injuries of the adult hand. In: DeLee J., Drez D., Miller M.D., editors. Orthopaedic sports medicine principles and practice. Philadelphia: Saunders, 2003.
60. Greene J., Winickoff R.N. Cost-conscious prescribing of nonsteroidal anti-inflammatory drugs for adults with arthritis. Arch Intern Med. 1992;152:1995-2002.
61. Griffin L. The female athlete. In: DeLee J., Drez D., Miller M.D., editors. Orthopaedic sports medicine principles and practice. Philadelphia: Saunders, 2003.
62. Hamdan T., Al-Essa K.A. Manipulation under anaesthesia for the treatment of frozen shoulder. Int Orthop. 2003;27:107-109.
63. Hand C., Clipsham K., Rees J.L., et al. Long-term outcome of frozen shoulder. J Shoulder Elbow Surg. 2008;17:231-236.
64. Hannafin J., Chiaia T.A. Adhesive capsulitis. Clin Orthop. 2000;372:95-109.
65. Hassell A., Fowler P.D., Dawes P.T. Intra-bursal tetracycline in the treatment of olecranon bursitis in patients with rheumatoid arthritis. Br J Rheumatol. 1994;33:859-860.
66. Hawkins R., Abrams J.S. Impingement syndrome in the absence of rotator cuff tear (stages 1 and 2). Orthop Clin North Am. 1987;18:373-382.
67. Hawkins R., Kennedy J.C. Impingement syndrome in athletes. Am J Sports Med. 1980;8:151-158.
68. Hay E., Thomas E., Paterson S.M., et al. A pragmatic randomised controlled trial of local corticosteroid injection and physiotherapy for the treatment of new episodes of unilateral shoulder pain in primary care. Ann Rheum Dis. 2003;62:394-399.
69. Helal B. Racquet player’s pisiform. Hand Clin. 1978;10:87-90.
70. Herring S. Rehabilitation of muscle injuries. Med Sci Sports Exerc. 1990;22:453.
71. Hovelius L. Anterior dislocation of the shoulder in teenagers and young adults: five year prognosis. J Bone Joint Surg Am. 1987;69:393-399.
72. Hovelius L., Eriksson K., Fredin H., et al. Recurrences after initial dislocation of the shoulder: results of a prospective study of treatment. J Bone Joint Surg. 1983;65:343.
73. Iannotti J., Zlatkin M.B., Esterhai J.L., et al. Magnetic resonance imaging of the shoulder: sensitivity, specificity and predictive value. J Bone Joint Surg Am. 1991;73:17.
74. Isani A., Melone C.P. Ligamentous injuries of the hand in athletes. Clin Sports Med. 1986;5:757-772.
75. Itoi E., Hatakeyama Y., Kido T., et al. Immobilization in external rotation after dislocation reduces the risk of recurrence: a randomized controlled trial. J Bone Joint Surg Am. 2007;89:2124-2131.
76. Itoi E., Hatakeyama Y., Kido T., et al. A new method of immobilization after traumatic anterior dislocation of the shoulder: a preliminary study. J Shoulder Elbow Surg. 2003;12:413-415.
77. Itoi E., Hatakeyama Y., Urayama M., et al. Position of immobilization after dislocation of the shoulder. J Bone Joint Surg Am. 1999;81:385-390.
78. Itoi E., Sashi R., Minagawa H., et al. Position of immobilization after dislocation of the glenohumeral joint. J Bone Joint Surg Am. 2001;83:661-667.
79. Josefsson P., Johnell O., Gentz C.F. Long-term sequelae of simple dislocation of the elbow. J Bone Joint Surg Am. 1984;66:927-930.
80. Jossefsson P., Johnell O., Wendeberg B. Ligamentous injuries in dislocations of the elbow joint. Clin Orthop. 1987;21:221-225.
81. Kamien M. A rational management of tennis elbow. J Sports Med. 1990;9:173-191.
82. Kaul M., Herring S.A. Superficial heat and cold. Phys Sports Med. 1994;22:65.
83. Kibler W. A framework for sports medicine. Phys Med Rehabil Clin North Am. 1994;5:1.
84. Kibler W. Role of the scapula in the overhead throwing motion. Contemp Orthop. 1991;22:525-532.
85. Kirkley A., Griffin S., Richards C., et al. Prospective randomized clinical trial comparing the effectiveness of immediate arthroscopic stabilization versus immobilization and rehabilitation in first traumatic anterior dislocations of the shoulder. Arthroscopy. 1999;15:507-514.
86. Lapidus P., Guidotti F.P. Stenosing tenovaginitis of the wrist and fingers. Clin Orthop. 1972;83:87-90.
87. Larson R., Osternig L.R. Traumatic bursitis and artificial turf. J Sports Med. 1974;2:183-188.
88. Leddy J., Packer J.W. Avulsion of the profundus tendon insertion in athletes. J Hand Surg. 1977;2:66.
89. Lemos M. The evaluation and treatment of the injured acromioclavicular joint in athletes. Am J Sports Med. 1998;26:137-144.
90. Levine W., Flatow E.L. The pathophysiology of shoulder instability. Am J Sports Med. 2000;28:910-917.
91. Linscheid R., Grainger R.W., Johnson E.W. The thumb metacarpophalangeal joint injuries. Minn Med. 1972;55:1037-1040.
92. Linscheid R.L., Dobyns J.H., Beabout J.W., et al. Traumatic instability of the wrist. Diagnosis, classification, and pathomechanics. J Bone Joint Surg Am. 1972;54:1612-1632.
93. Liphart S., Henry T.J. The physiological basis for open and closed kinetic chain rehabilitation for the upper extremity. J Sport Rehabil. 1996;5:71-87.
94. Liphart S., Warner J.P., Borsa P.A., et al. Proprioception of the shoulder joint in healthy, unstable, and surgically repaired shoulders. J Shoulder Elbow Surg. 1994;3:371-380.
95. Lippitt S., Vanderhooft E., Harris S.L., et al. Glenohumeral stability from concavity-compression: a quantitative analysis. J Shoulder Elbow Surg. 1993;2:27-35.
96. Lipscomb P. Stenosing tenosynovitis at the radial styloid process (deQuervain’s disease). Am J Surg. 1951;134:110-115.
97. Lohr J., Uhthoff H.K. The microvascular pattern of the supraspinatous tendon. Clin Orthop. 1990;254:35.
98. Maffet M., Gartsman G.M., Moseley B. Superior labrum-biceps tendon complex lesions of the shoulder. Am J Sports Med. 1995;23:93-98.
99. Magee D. Elbow Joints. In: Magee D., editor. Orthopedic physical assessment. Philadelphia: WB Saunders, 1992.
100. Magee D. Forearm, wrist, and hand. In: Magee D., editor. Orthopedic physical assessment. Philadelphia: WB Saunders, 1992.
101. Magee D. Shoulder. In: Magee D., editor. Orthopedic physical assessment. Philadelphia: WB Saunders, 1992.
102. Manske P., Lesker P.A. Avulsion of the ring finger flexor digitorum profundus tendon: an experimental study. Hand Clin. 1978;10:52-55.
103. Mariani E., Cofield R.H., Askew L.J., et al. Rupture of the tendon of the long head of the biceps brachii: a surgical versus nonsurgical treatment. Clin Orthop Relat Res. 1988;228:233-239.
104. Mayfield J., Johnson R.P., Kilcoyne R.K. Carpal dislocations: pathomechanics and progressive perilunar instability. J Hand Surg Am. 1980;5:226-241.
105. Mazzocca A., Sellards R., Garretson R., et al. Injuries to the acromioclavicular joint in adults and children. In: DeLee J., Drez D., Miller M.D., editors. Orthopedic sports medicine principles and practice. Philadelphia: Saunders, 2003.
106. McCluskey G.I., Bigliani L.U. Surgical management of refractory scapulothoracic bursitis. Orthop Trans. 1991;15:801.
107. McCue F., Bruce J.F., Koman J.D. Wrist and hand. In: DeLee J., Drez D., Miller M.D., editors. Orthopaedic sports medicine principles and practice. Philadelphia: Saunders, 2003.
108. McCue F., Hakala M.W., Andrews J.R., et al. Ulnar collateral ligament injuries of the thumb in athletes. J Sports Med. 1975;2:70-80.
109. McCue F., Honner R., Johnson M.E., et al. Athletic injuries of the proximal interphalangeal joint requiring surgical treatment. J Bone Joint Surg Am. 1970;52:937-956.
110. McEntire J., Hess W.E., Coleman S. Rupture of the pectoralis major muscle. J Bone Joint Surg Am. 1972;54:1040-1046.
111. McFarland E., Ireland M.L. Rehabilitation programs and prevention strategies in adolescent throwing athletes. Instr Course Lect. 2003;52:37-42.
112. McInerney J., Dias J., Durham S., et al. Randomised controlled trial of single, subacromial injection of methylprednisolone in patients with persistent, post-traumatic impingement of the shoulder. Emerg Med J. 2003;20:218-221.
113. McShane J., Shah V.N., Nazarian L.N. Sonographically guided percutaneous needle tenotomy for treatment of common extensor tendinosis in the elbow: is corticosteroid necessary. J Ultrasound Med. 2008;27:1137-1144.
114. Melone C., Beldner S., Basuk R.S. Thumb collateral ligament injuries: an anatomic basis for treatment. Hand Clin. 2000;16:345-357.
115. Meyer A. Chronic functional lesions of the shoulder. Arch Surg. 1937;35:646-674.
116. Milch H. Snapping scapula. Clin Orthop Relat Res. 1961;20:139-150.
117. Milch H., Burman M.S. Snapping scapula and humerus varus: report of six cases. Arch Surg. 1933;26:570-588.
118. Miller M., Wirth M.A., Rockwood C.A.Jr. Thawing the frozen shoulder, the “patient” patient. Orthopedics. 1996;19:849-853.
119. Mishra A., Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med. 2006;34:1774-1778.
120. Myers J., Lephart S.M. Sensorimotor deficits contributing to glenohumeral instability. Clin Orthop. 2002;400:98-104.
121. Naredo E., Cabero F., Beneyto P., et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rhematol. 2004;31:308-314.
122. Neer C.I. Impingement lesions. Clin Orthop. 1983;173:70-77.
123. Neviaser J. Adhesive capsulitis of the shoulder. J Bone Joint Surg Am. 1945;27:211-222.
124. Newcomer K., Laskowski E.R., Idank D.M., et al. Corticosteroid injection in early treatment of lateral epicondylitis. Clin J Sport Med. 2001;11:214-222.
125. Nirschl R. The etiology and treatment of tennis elbow. Am J Sports Med. 2, 1974.
126. Nirschl R., Petrone F.A. Tennis elbow: The surgical treatment of lateral epicondylitis. J Bone Joint Surg Am. 1979;61:832-839.
127. Norwood L., Shook J.A., Andrews J.R. Acute medial elbow ruptures. Am J Sports Med. 1981;9:16-19.
128. O’Brien S., Pagnani M.J., Fealy S., et al. The active compression test: a new and effective test for diagnosing labral tears and acromioclavicular joint abnormality. Am J Sports Med. 1998;26:610-613.
129. O’Driscoll S., Morrey B.F., An K.N. Elbow dislocation and subluxation: a spectrum of instability. Clin Orthop. 1992;280:186-197.
130. Ogden J., Alvarez R.G., Levitt R., et al. Shock wave therapy (Orthotripsy) in musculoskeletal disorders. Clin Orthop. 2001;387:22-40.
131. Ogilvie-Harris D., Biggs D.J., Fitsialos D.P., et al. The resistant frozen shoulder: Manipulation vs arthroscopic release. Clin Orthop. 1995;319:238-248.
132. Olliviere C., Nirschl R.P., Pettrone F.A. Resection and repair for medial tennis elbow. Am J Sports Med. 1995;23:214-221.
133. Osterman A., Hayken G.D., Bora F.W. A quantitative evaluation of thumb function after ulnar collateral repair and reconstruction. J Trauma. 1981;21:854-861.
134. Osterman A., Moskow L., Low D.W. Soft tissue injuries of the hand and wrist in racquet sports. Clin Sports Med. 1988;7:329-348.
135. Pagnani M., Speer K.P., Altchek D.W., et al. Arthroscopic fixation of superior labral lesions using a biodegradable implant: a preliminary report. Arthroscopy. 1995;11:194-198.
136. Palmer A., Louis D.S. Assessing ulnar instability of the metacarpophalangeal joint of the thumb. J Hand Surg Am. 1978;3:542-546.
137. Palmer A., Werner F.W. Biomechanics of the distal radioulnar joint. Clin Orthop. 1984;187:26-35.
138. Palmieri T. Pisiform area pain treatment by pisiform excision. J Hand Surg Am. 1982;7:477-480.
139. Panner H. A peculiar affectation of the capitellum humeri resembling Calvē-Perthes’ disease of the hip. Acta Radiol. 1929;10:234.
140. Parsons T. The snapping scapula and subscapular exostoses. J Bone Joint Surg Br. 1973;55:345-349.
141. Payvandi S., Jeong J., Seitz W.H.Jr. Treatment of complete acromioclavicular separations with a modified Weaver and Dunn technique. Tech Hand Up Extrem Surg. 2008;12:59-64.
142. Pearsall A., Speer K.P. Frozen shoulder syndrome: Diagnostic and treatment strategies in the primary care setting. Med Sci Sports Exerc. 1998;30(suppl 4):S33-S39.
143. Pecci M., Kreher J.B. Clavicle fractures. Am Fam Physician. 2008;77:65-70.
144. Percy E., Birbrager D., Pitt M.J. A review of the literature and presentation of 14 patients. Can J Surg. 1988;31:248-250.
145. Perry J. Anatomy and biomechanics of the shoulder in throwing, swimming, gymnastics, and tennis. Clin Sports Med. 1983;2:247-251.
146. Petersen S. Posterior shoulder instability. Orthop Clin North Am. 2000;31:263-283.
147. Postacchini F., Pudda G. Subcutaneous rupture of the distal biceps brachii tendon. J Sports Med Phys Fitness. 1975;15:84-90.
148. Quraishi N., Johnston P., Bayer J., et al. Thawing the frozen shoulder: a randomised trial comparing manipulation under anesthesia with hydrodilatation. J Bone Joint Surg Br. 2007;89:1197-2000.
149. Rathbun J., Macnab I. The microvascular pattern of the rotator cuff. J Bone Joint Surg Br. 1970;52:540.
150. Redler I., Williams J.T. Rupture of a collateral ligament of the proximal interphalangeal joint of the finger: analysis of 18 cases. J Bone Joint Surg Am. 1967;49:332.
151. Regan W., Wold L., Coonrad R., et al. Microscopic pathology of lateral epicondylitis. Am J Sports Med. 1992;20:746.
152. Rettig A. Athletic injuries of the wrist and hand. I. Traumatic injuries of the wrist. Am J Sports Med. 2003;31:1038-1048.
153. Rettig A. Athletic injuries of the wrist and hand. II. Overuse injuries of the wrist and traumatic injuries to the hand. Am J Sports Med. 2004;32:262-273.
154. Richards R., McKee M.D. Treatment of painful scapulothoracic crepitus by resection of the superomedial angle of the scapula. Clin Orthop Relat Res. 1989;247:111-116.
155. Roberts P. Dislocations of the elbow. Br J Surg. 1969;56:806-815.
156. Robinson J., Brown P.B. Medications in low back pain. Phys Med Rehabil Clin North Am. 1991;2:97-126.
157. Rockwood C.J. Disorders of the sternoclavicular joint. In: Rockwood C.J., Matsen F.A., editors. The shoulder. Philadelphia: WB Saunders, 1990.
158. Rockwood C.J. Injuries to the acromioclavicular joint. In: Rockwood C.J., editor. Fractures in adults. Philadelphia: JB Lippincott., 1984.
159. Rodosky M., Harner C.D., Fu F.H. The role of the long head of the biceps muscle and superior glenoid labrum in anterior stability of the shoulder. Am J Sports Med. 1994;22:121-130.
160. Rodriguez A. Injuries to the collateral ligaments of the proximal interphalangeal joints. Hand Clin. 1973;55:55.
161. Rowe C., Pierce D.S., Clark J.G. Voluntary dislocation of the shoulder: a preliminary report on a clinical, electromyographic and psychiatric study of twenty-six patients. J Bone Joint Surg Am. 1973;62:897-908.
162. Rowland S. Acute traumatic subluxation of the extensor carpi ulnaris tendon at the wrist. J Hand Surg Am. 1986;11:809.
163. Sanders T., Morrison W.B., Miller M.D. Imaging techniques for the evaluation of glenohumeral instability. Am J Sports Med. 2000;28:414-433.
164. Schwartz M., Al-Zahrani S.A. Diagnostic imaging of elbow injuries in the throwing athlete. Oper Techn Sports Med. 1996;4:84-90.
165. Shah N., Lewis M. Shoulder adhesive capsulitis: systematic review of randomised trials using multiple corticosteroid injections. Br J Gen Pract. 2007;57:662-667.
166. Simonet W., Cofield R. Prognosis in anterior shoulder dislocation. Am J Sports Med. 1984;12:19.
167. Slawski D., Cahill B. Atraumatic osteolysis of the distal clavicle: results of open surgical excision. Am J Sports Med. 1994;22:267-271.
168. Smith R. Post-traumatic instability of the metacarpophalangeal joint of the thumb. J Bone Joint Surg Am. 1977;59:14-21.
169. Snyder S., Karzel R.P., Del Pizzo W., et al. SLAP lesions of the shoulder. Arthroscopy. 1990;6:274-279.
170. Sollender J., Rayan G.M., Barden G.A. Triceps tendon rupture in weight lifters. J Shoulder Elbow Surg. 1998;7:151-153.
171. Spencer E. Treatment of grade III acromioclavicular joint injuries: a systematic review. Clin Orthop. 2007;455:38-44.
172. Stahl F. On Lunatomalacia (Kienbock’s disease), a clinical and roentgenological study, especially on its pathogenesis and the late results of immobilization treatment. Acta Chir Scand. 1947;126(suppl):1-133.
173. Stein D., Jazrawi L., Bartolozzi A.R. Arthroscopic stabilization of anterior shoulder instability: a review of the literature. Arthroscopy. 2002;18:912-924.
174. Stener B. Displacement of the ruptured ulnar collateral ligament of the metacarpophalangeal joint of the thumb. J Bone Joint Surg Br. 1962;44:869-879.
175. Stollberger C., Finsterer J. Nonsteroidal anti-inflammatory drugs in patients with cardio- or cerebrovascular disorders. Zeitschrift Kardiologie. 2003;92:721-729.
176. Suresh S., Ali K.E., Jones H., et al. Medial epicondylitis: is ultrasound guided autologous blood injection an effective treatment? Br J Sports Med. 2006;40:935-939.
177. Svernlov B., Adolfsson L. Non-operative treatment regime including eccentric training for lateral humeral epicondylalgia. Scand J Med Sci Sports. 2001;11:328-334.
178. Tasto J., Elias D.W. Adhesive capsulitis. Sports Med Arthrosc Rev. 2007;15:216-221.
179. Tiger E., Mayer D.P., Glazer R. Complete avulsion of the triceps tendon: MRI diagnosis. Comput Med Imaging Graph. 1993;17:51-54.
180. Timmerman L., Andrews J.R. The histologic and arthroscopic anatomy of the ulnar collateral ligament of the elbow. Am J Sports Med. 1994;22:667-673.
181. Timmerman L., Andrews J.R. Undersurface tear of the ulnar collateral ligament in baseball players: A newly described lesion. Am J Sports Med. 1994;22:33-36.
182. Timmerman L., McBride D.G. Elbow dislocations in sports. Sports Med Arthrosc Rev. 1995;3:210-218.
183. Twinning R., Marcus W.Y., Garey J.L. Tendon ruptures in systemic lupus erythematosus. JAMA. 1964;187:123-124.
184. Walch G., Liotard J.P., Boileau P., et al. Postero-superior glenoid impingement: another impingement of the shoulder. J Radiol. 1993;74:47-50.
185. Warren R., Kay N.R., Norris S.H. The microvascular anatomy of the distal digital extensor tendon. J Hand Surg Br. 1988;13:161-163.
186. Weiler J. Medical modifiers of sports injury. The use of nonsteroidal anti-inflammatory drugs (NSAIDs) in sports soft-tissue injury. Clin Sports Med. 1992;11:625-644.
187. Werner C. Lateral elbow pain and posterior interosseous nerve entrapment. Acta Orthop Scand. 1979;174 (suppl):1-62.
188. Wilson F., Andrews J.R., Blackburn T.A., et al. Valgus extension overload in the pitching elbow. Am J Sports Med. 1983;11:83-88.
189. Windsor R., Lester J.P., Herring S.A. Electrical stimulation in clinical practice. Phys Sports Med. 1993;21:85.
190. Wirth M., Rockwood C.A.Jr. Injuries to the sternoclavicular joint in the adult and child. In: DeLee J., Drez D., Miller M.D., editors. Orthopedic sports medicine. Philadelphia: Saunders, 2003.
191. Wood M., Dobyns J. Sports-related extra-articular wrist syndromes. Clin Orthop. 1986;202:93-102.