Musculoskeletal Disorders
Perspective
Each year there are more than 11 million pediatric injury-related emergency department (ED) visits, 14.3% of which are due to fractures and dislocations.1
Principles of Disease
Fracture Patterns
• Plastic deformation: Bone is bowed with no obvious cortical disruption.
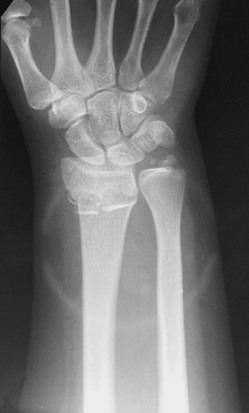
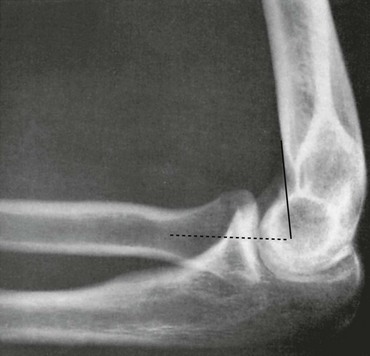
• Torus fracture (buckle fracture): Buckling of bone without cortical disruption tends to occur because of compression failure of the bone at the metaphyseal-diaphyseal junction (Fig. 176-1). These fractures may be immobilized with a splint or cast or, if they involve the distal end of the radius, a Velcro wrist splint2 or backslab.3 The splint or backslab can be removed by the family or primary care provider in 3 to 4 weeks, thereby obviating the need for orthopedic follow-up. Children treated with removable splints also had less pain, improved function, and fewer unscheduled ED visits because of problems with the cast.4 Buckle fractures also may be treated with a soft cast5,6 or a plaster splint.7
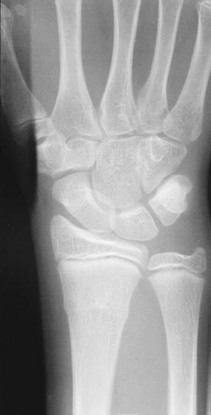
Figure 176-1 Buckle fracture of the distal end of the radius. Treatment consists of immobilization for 3 to 6 weeks.
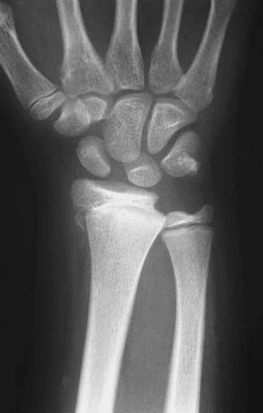
• Greenstick fracture: Bone and one cortex are disrupted; the periosteum on the fracture’s compression side remains intact (Fig. 176-2). Some authors recommend immobilization of minimally angulated (<15 degrees) distal radius greenstick fractures with splints8; others contend that casting is necessary because of continued fracture displacement during the first 2 weeks of healing.9
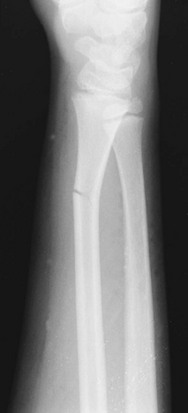
Figure 176-2 Greenstick fracture of the radius. This fracture may need to be completed for an anatomic reduction to be achieved.
• Complete fracture: The fracture propagates completely through the bone; included are transverse (Fig. 176-3), spiral (Fig. 176-4), oblique, and comminuted (Fig. 176-5) fractures.
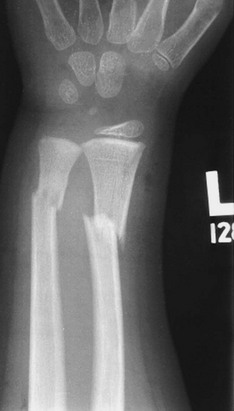
Figure 176-3 Transverse fractures of the radius and ulna.
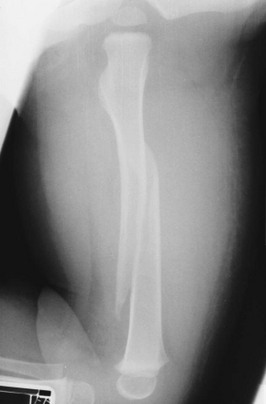
Figure 176-4 Spiral fracture of the femur.
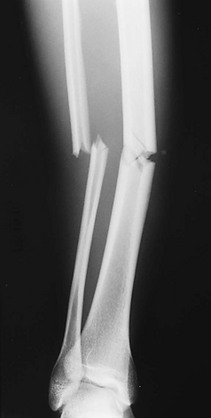
Figure 176-5 Comminuted fractures of the tibia and fibula.
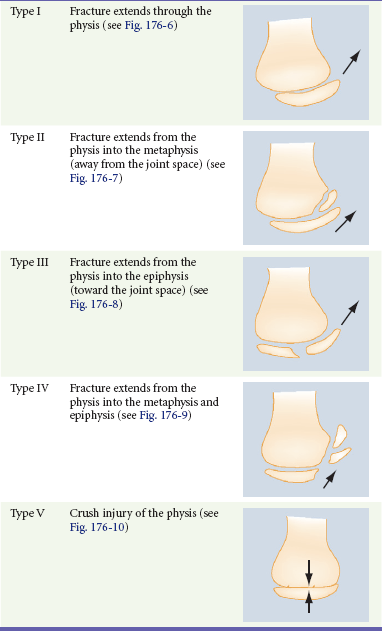
The relatively weak cartilaginous physis in growing bone makes physeal injury common in the skeletally immature. Although injuries to the physis can occur at any age, they are more common during rapid skeletal growth. The Salter-Harris classification system is the most frequently used tool to describe physeal injuries. This classification system is based on the extent of involvement of the physis, epiphysis, and joint (Table 176-1). Types I and II Salter-Harris injuries (Figs. 176-6 and 176-7) do not involve the germinal layer of the growth plate; therefore, the risk for growth arrest is small. In general, the higher the Salter-Harris classification (Salter-Harris types III to V), the greater the damage to the growth plate and the greater the likelihood of growth arrest or limb-length abnormalities (Figs. 176-8 to 176-10). Type II fractures are the most common Salter-Harris injury and account for approximately three fourths of all growth plate injuries.
Specific Disorders
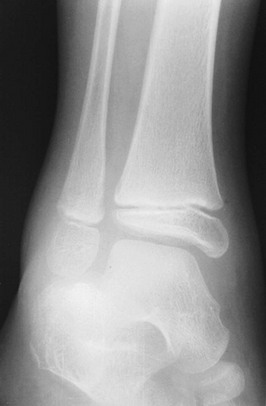
An anteroposterior (AP) radiograph of the clavicle (Fig. 176-11) confirms the diagnosis. If clinical suspicion is high and the AP view does not reveal a fracture, a 30-degree cephalic view can be helpful. Specialized imaging studies are rarely needed, although computed tomography (CT) and duplex ultrasonography may be considered with proximal, posteriorly displaced fractures or dislocations to evaluate for the aforementioned complications.10
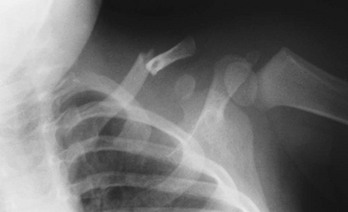
Figure 176-11 Fracture of the middle third of the clavicle.
Supracondylar Fractures of the Humerus
Supracondylar fractures are classified as flexion or extension according to mechanism of injury. The extension type of fracture constitutes 95% of all supracondylar fractures and typically results from a hyperextension injury to the elbow incurred in a fall onto the outstretched arm. In this injury, the olecranon is forcefully driven into the olecranon fossa, and the forces are concentrated in the supracondylar area. This mechanism results in failure of the anterior cortex and displacement of the distal fragment posteriorly. With extension-type supracondylar fractures, the degree of displacement and continuity of the cortex are further defined by the Gartland classification11 (Table 176-2). In the less common flexion type of supracondylar fracture, the elbow is flexed when it hits the ground, and energy is transferred from the posterior aspect of the proximal end of the ulna to the distal end of the humerus. This mechanism results in a supracondylar fracture with anterior displacement of the distal fragment and failure of the cortex posteriorly.
Table 176-2
Gartland Classification of Extension-Type Supracondylar Fractures
| Type I | Nondisplaced fracture |
| Type II | Displaced fracture with intact posterior cortex |
| Type III | Displaced fracture with no cortical contact |
| A: Posteromedial rotation of the distal fragment | |
| B: Posterolateral rotation of the distal fragment |
Modified from Gartland JJ: Management of supracondylar fractures of the humerus in children. Surg Gynecol Obstet 109:145, 1959.
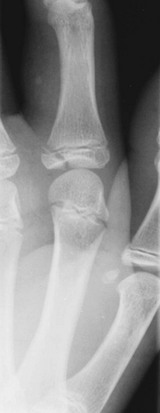
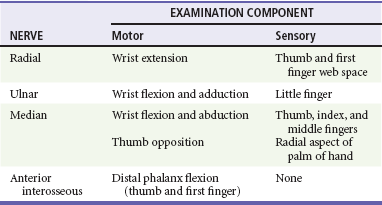
Children with supracondylar humerus fractures may present with anything from mild swelling and elbow pain to a grossly displaced humerus. Gentle palpation is useful to determine the site of injury; however, manipulation should be avoided because movement may cause further neurovascular damage. Children with extension-type supracondylar fractures hold the affected arm in extension with an S-shaped configuration of the elbow and exhibit a prominence at the olecranon. Children with flexion-type supracondylar fractures hold the arm in flexion and exhibit an empty space where the olecranon should be. In all cases it is important to carefully assess distal neurovascular status. Motor and sensory function should be evaluated by assessment of the radial, ulnar, and median nerves (Table 176-3). Two-point discrimination on the fingers provides a sensitive means of assessing sensory status: an abnormal value (>5 mm) indicates a deficit. Vascular assessment should include evaluation of radial and brachial pulses and capillary refill of the hand. Patients with displaced fractures should have ongoing assessment for the development of compartment syndrome of the forearm. With pain on flexion or extension of the fingers, forearm swelling, tenseness or tenderness, or pain that is disproportionate to the injury, compartment pressures should be measured immediately. Unrecognized ischemic injury can result in Volkmann’s ischemic contracture, which is characterized by fixed elbow flexion, forearm pronation, wrist flexion, metacarpophalangeal joint extension, and interphalangeal flexion.
Radiographic evaluation of any elbow injury should include an AP view of the extended elbow and a lateral view of the flexed elbow. If these views do not show a fracture but clinical suspicion is high, oblique views are helpful. Even with proper radiographs, diagnosis of a pediatric elbow fracture can be difficult. The elbow is largely cartilaginous during early childhood, and the six secondary centers of ossification around the elbow can camouflage or be mistaken for fractures (Fig. 176-12). These ossification centers can be remembered by the mnemonic CRITOE: capitellum, radius, internal (medial) epicondyle, trochlea, olecranon, and external (lateral) epicondyle. The approximate ages at which these sites ossify may be estimated at 1, 3, 5, 7, 9, and 11 years, respectively (Table 176-4).
Table 176-4
Sequence of Ossification around the Elbow: CRITOE
| OSSIFICATION CENTER | AGE AT APPEARANCE | AGE AT CLOSURE |
| Capitellum | 6-12 months | 14 years |
| Radial head | 4-5 years | 16 years |
| Medial (Internal) epicondyle | 5-7 years | 15 years |
| Trochlea | 8-10 years | 14 years |
| Olecranon | 8-9 years | 14 years |
| Lateral (External) epicondyle | 9-13 years | 16 years |
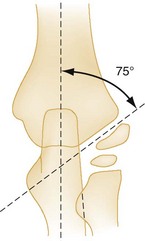
Bone relationships are helpful in evaluation of a radiograph for a supracondylar fracture (Fig. 176-13). A true lateral view should demonstrate a figure-of-eight appearance of the distal humerus with bisection of the capitellum by the anterior humeral line. If the capitellum falls posterior to this line, an extension-type supracondylar fracture is likely. In all views, the proximal end of the radius and radial neck should point to the capitellum. Baumann’s angle also is helpful in diagnosis of subtle supracondylar fractures12 (Fig. 176-14). This angle is formed by a line drawn to follow the growth plate of the capitellum transected with a line that runs perpendicular to the axis of the humerus. The angle should be approximately 75 to 80 degrees. Baumann’s angle should be the same in both elbows, and differences between elbows can be used to detect subtle supracondylar fractures. In children younger than 3 years, difficult-to-distinguish bone landmarks limit the utility of Baumann’s angle. Postreduction alterations in Baumann’s angle reliably predict the final carrying angle.13
Fat pads also provide a means for detection of occult supracondylar fractures. A lateral radiograph with the elbow flexed at 90 degrees may show an anterior fat pad protruding from the coronoid fossa. This finding is normal unless the pad is bulging or in the shape of a ship’s sail. This “sail sign” may indicate fluid in the joint, although alone it may not be a reliable predictor of a fracture. The posterior fat pad, however, sits snugly within the olecranon fossa and should never be seen unless there is a fracture around the elbow. In this case, blood pushes the fat pad laterally, thereby making it visible on a lateral radiograph of the elbow. Accordingly, visualization of a posterior fat pad suggests the presence of an occult fracture around the elbow. The presence of a posterior fat pad without an obvious fracture warrants oblique views of the elbow (Fig. 176-15), splinting, and follow-up.
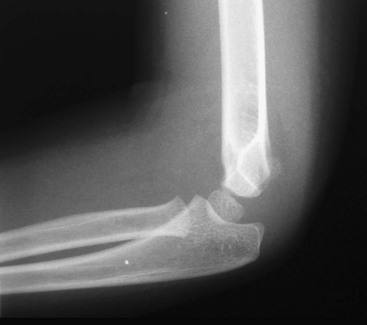
Figure 176-15 Lateral radiograph of a supracondylar fracture with an anterior fat pad “sail sign” and a posterior fat pad.
Plain radiographs usually are sufficient for diagnosis of supracondylar fractures. However, if the diagnosis remains in question after AP, lateral, and oblique radiographs are obtained, ultrasound imaging may be useful in infants,14 and magnetic resonance imaging (MRI) may be useful in older children.15
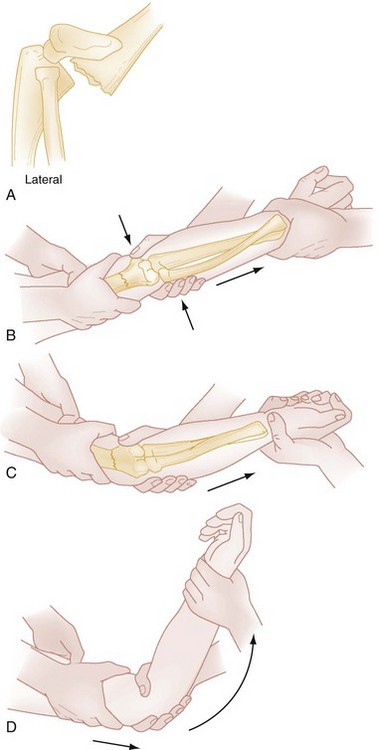
ED treatment of supracondylar humeral fractures is determined by displacement and neurovascular status. A pale, pulseless cold hand mandates emergency consultation with an orthopedic surgeon. If an orthopedic surgeon is unavailable and the vascular supply has not been restored, reduction should be attempted (Fig. 176-16). If necessary, reduction can be performed by a single operator. With the patient supine, the shoulder held in 90 degrees of forward flexion, and the elbow slightly flexed, both hands are placed on the arm proximal to the fracture, and both thumbs are placed on the posterior aspect of the fracture fragment. Then, while the thumbs are directed distally, the fragment is lifted onto the distal metaphysis. The return of blood supply is marked by the hand’s becoming warm and pink. If perfusion does not improve, another reduction may be attempted, with care taken to not entrap the brachial artery and median nerve. Multiple attempts at reduction increase the likelihood of neurovascular injury and swelling; therefore, no more than two reductions should be attempted. If perfusion is not reestablished, the child requires urgent operative intervention. A supracondylar fracture with a pulseless hand that is warm and pink does not need to be reduced immediately and should be splinted as it lies so that vascular status is not further compromised. The elbow should be splinted in relative extension because too much flexion in conjunction with swelling may obstruct the brachial artery and contribute to limb ischemia.
The primary complications of supracondylar fractures are related to neurovascular injury. Type III fractures are at greatest risk, with neurovascular compromise occurring in as many as 49% of patients.16 Neurovascular compromise, however, can occur with any displaced fracture. The median nerve is involved in half of the cases and is associated with posterolateral displacement. The radial nerve is involved in almost one third of patients and is associated with posteromedial displacement. Brachial artery injuries, including arterial entrapment, laceration, intimal tears, thrombosis, and compression from compartment syndrome, occur in approximately 40% of patients and are found with either type of displacement. Fortunately, the brachial artery has many branches around the elbow, and flow to the forearm and hand can be maintained even when the brachial artery is injured.
Despite the frequency of neurovascular deficits immediately after the injury, most nerve palsies are caused by stretching or contusion and resolve spontaneously. The typical course for nerve injuries is complete resolution. Although motor function usually returns within 12 weeks, sensory function may not return for 6 months or longer.17 If clinical or electromyographic evidence of nerve recovery is lacking after 5 months, exploration and neurolysis are indicated.18 Volkmann’s ischemic contracture (contracture deformity of the fingers, hand, and wrist) and permanent limb disability are the end result of untreated vascular injury. This complication is extremely rare and easily prevented by close observation and evaluation for the development of compartment syndrome. A few supracondylar fractures heal with a “gunstock” deformity; however, the combination of varus, hyperextension, and medial rotation of the limb is not a functional problem and, except in severe cases, requires no treatment. Severe cases can be corrected by humeral osteotomy.
Monteggia’s Fracture-Dislocation
Monteggia’s fracture-dislocations are characterized by a fracture of the proximal third of the ulna plus dislocation of the radial head. The radiographic evidence can be subtle, with only a minor greenstick fracture or bowing of the ulna. Isolated ulna fractures are rare in children; therefore, with all such fractures, AP and lateral radiographs of the elbow should be obtained to rule out dislocation of the radial head. The radial head should align with the capitellum on all radiographs of the elbow; if it does not, a Monteggia injury should be suspected (Fig. 176-17).
Nursemaid’s Elbow
In one study, radial head subluxation, or nursemaid’s elbow, was the most common upper extremity injury in children younger than 6 years presenting to a pediatric ED.19 It typically occurs when axial traction is placed on an extended and pronated arm, as when the child is pulled up or swung by the arms. It also may occur when the child falls onto the outstretched arm, sustains minor direct trauma to the elbow, or simply twists the arm. In infants, radial head subluxation can occur when an extended arm is caught beneath the infant’s body while being rolled over. In pathoanatomic terms, subluxation occurs when the annular ligament becomes loosened from the head of the radius and slips into the radiocapitellar joint, where it becomes entrapped (Fig. 176-18).
Nursemaid’s elbow occurs in children a few months to 5 years of age and has a peak incidence between 2 and 3 years of age.20 It has been reported in children younger than 6 months20 and has been seen in children as old as 9 years. This injury has a slight predilection for girls.
The diagnosis of nursemaid’s elbow is made clinically, and radiographs are not necessary. If, however, significant point tenderness, swelling, or ecchymosis is present or if the history suggests another injury, such as a supracondylar fracture, radiographs should be obtained. Although it is not commonly used as a diagnostic procedure, ultrasound imaging may demonstrate a widened space between the radial head and the capitellum.21
Radial head subluxation is an orthopedic injury that is easily reduced without sequelae. Classically, the affected elbow is gripped with the clinician’s thumb over the radial head, and with the other hand, the clinician flexes and supinates the patient’s arm. As the radial head relocates, the clinician feels it click or “clunk” under the thumb. Hyperpronation of the forearm also is effective in reducing radial head subluxation. As is done in the flexion-supination maneuver, the child’s affected elbow is held with the clinician’s thumb over the radial head, but then flexion-supination is replaced with hyperpronation of the forearm. Success rates range from 8020 to 92%22 with supination and from 9323 to 98%24 with pronation. Pronation also may be less painful to the patient24,25 and is the reduction method of choice.24 After successful reduction, the child typically uses the arm normally within 10 minutes. This may be delayed in younger children and when the injury occurred more than 4 to 6 hours before reduction. Neither splinting nor orthopedic referral is required after a successful reduction.
Parents should be cautioned to avoid traction on the forearm and elbow because recurrence rates of radial head subluxation range from 5 to 39%, depending on the referral population studied.20,22,24 With recurrent subluxations, immobilization in a posterior splint with the elbow maintained at 90 degrees and the forearm supinated may be warranted. The need for open reduction or repair of the annular ligament is exceedingly rare.
Toddler’s Fracture
AP and lateral radiographs may reveal a spiral or oblique fracture extending downward and medially through the distal third of the tibia (Fig. 176-19). An internal oblique radiograph is helpful if evidence of fracture is absent on the AP or lateral view. If findings on all views are normal, consideration should be given to fractures elsewhere in the limb. If no fracture is apparent, the child should be splinted for comfort and radiographs repeated in 10 days, at which time periosteal new bone or sclerosis of the fracture edges will make the fracture visible. If findings on these radiographs are normal and the child is still limping, further evaluation should be undertaken to rule out osteomyelitis and malignant neoplasm. Bone scans often are helpful in the assessment of a limping toddler and are more sensitive than plain radiographs for detection of fractures. Ultrasonography is also emerging as an imaging option in the detection of occult fractures in toddlers as well as in older children.26 Treatment of a toddler’s fracture consists of a below-knee walking cast for approximately 3 weeks.
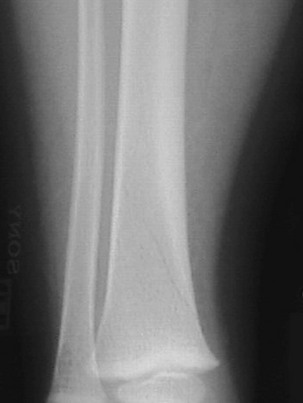
Figure 176-19 Toddler’s fracture.
Skeletal Aspects of Nonaccidental Trauma
Fractures are the second most common manifestation of child physical abuse, second only to soft tissue injury, and are present in up to 70% of physically abused children. Fractures associated with child abuse occur in the very young, 50% in children younger than 12 months27 and 94% in children younger than 3 years.28 With child abuse, a timely and accurate diagnosis is imperative because children who are returned to an abusive home face a 35% chance of repeated abuse and a 10% chance of death; more than 20% of children who were diagnosed with a fracture from nonaccidental trauma had at least one previous physician visit at which abuse was missed.29
No fracture is pathognomonic for abuse, but certain fracture patterns are more worrisome than others. Any fracture in a child younger than 1 year, fractures at different stages of healing, and bilateral or multiple fractures indicate a need for thorough assessment for nonaccidental trauma (see Chapter 66). Injuries that are especially concerning include complex skull fractures, rib fractures, metaphyseal fractures, and vertebral fractures or subluxations. Midshaft humeral and scapular fractures are nearly always associated with abuse, as are approximately 70% of femoral fractures in children younger than 1 year.
Specific Disorders and Injuries
Diaphyseal Fractures: Although multiple fractures at different stages of healing are strongly suggestive of child abuse, the most common manifestation is an isolated diaphyseal fracture, which occurs four times as often as classic metaphyseal fractures. The humerus, femur, and tibia are the most frequently fractured long bones; the radius and ulna are the most infrequently fractured. With the exception of supracondylar fractures, all fractures of the midshaft of the humerus in children younger than 3 years are strongly suggestive of abuse.
Metaphyseal Fractures: Although less common than diaphyseal fractures, metaphyseal fractures are more specific for child abuse. Metaphyseal fractures most commonly affect the tibia, femur, and proximal end of the humerus. Corner fractures and bucket-handle fractures, which are probably the same fracture viewed in two different projections, result from violent shaking or forceful pulling or twisting of an infant’s limb. The diagnosis is made by careful evaluation of high-quality plain radiographs. The tight adherence of the periosteum at the metaphysis precludes an active periosteal response, making these fractures difficult to diagnose even in the healing stages. In addition, after healing, these fractures may not be visible because of rapid bone remodeling. Bone scans, although sometimes helpful, are difficult to interpret because of the normally increased radionuclide uptake in the metaphyseal area.
Rib Fractures: Rib fractures are present in 5 to 27% of cases of child abuse; 90% of such fractures occur in children younger than 2 years. The young pediatric rib cage is compliant, and considerable force is required to break a rib. Accordingly, rib fractures are seldom seen in unintentional injury and are almost never seen after cardiopulmonary resuscitation.30 When present, postresuscitation rib fractures are anterior and also may be multiple.30 In a child younger than 3 years, the positive predictive value of a rib fracture as an indicator of intentional trauma approaches 100%.31
Skull Fractures: Skull fractures are the second most frequent injury in abuse and occur more commonly in abuse cases than from unintentional trauma.26 Eighty percent occur in infants younger than 1 year, and although complex skull fractures are more suspicious for abuse, linear skull fractures are the most common type. Standard skull radiographs may not be adequate for diagnosis and are reported to miss more than 25% of head injuries. Children who meet high-risk criteria (the presence of rib fractures, multiple fractures or facial injury, or age younger than 6 months) should undergo CT or MRI for assessment of occult head injury.32
Periosteal New Bone Formation: Periosteal new bone formation, which is one of the most common findings in cases of abuse, reflects separation of the periosteum from the bone and may be the only manifestation of orthopedic trauma related to child abuse. It may be present with or without a fracture. It results from shaking, from acceleration-deceleration forces applied to an unsupported limb, or from forceful gripping.
Diagnostic Strategies: Radiology.: Conventional skeletal radiography is the screening examination of choice in cases of suspected physical abuse. Unsuspected fractures are found in 22% of physically abused children and are more common in the very young. A complete skeletal survey is recommended for all physically abused children younger than 3 years and for all infants younger than 1 year with evidence of abuse or neglect. Complete skeletal surveys are rarely indicated in children older than 5 years; in children between 3 and 5 years of age, the need for a skeletal survey is determined on a case-by-case basis. Although standard practice has been to obtain skeletal surveys in infants with skull fractures without intracranial injury or suspicious clinical findings, their routine use has come into question as they have been found to find additional fractures in only 1.4% of patients.33
Differential Considerations.: Although most cases of intentional injury are easily diagnosed with a thorough history and physical examination, certain conditions can be confused with child abuse. Metaphyseal cupping and spurring and periosteal new bone formation are two normal variants that radiographically are almost identical to the findings in cases of child abuse. These features are seen in more than 40% of normal infants. They appear between 2 and 3 months of age and may persist until 8 months of age. These findings can be differentiated from child abuse by subtle radiographic clues: normal-variant infantile metaphyseal spurs are in continuity with normal bone and cortex, and physiologic periostitis is bilateral, confined to the diaphysis, and smooth and lamellar in appearance. Physiologic periostitis also tends to be more obvious on the medial aspect of the bone and most commonly involves the femur, although it also is seen in the humerus and tibia. Radiographic differentiation between normal variants and trauma-related injury is extremely difficult, and the clinical findings must be taken into account.
Osteogenesis imperfecta (OI), a heritable disorder of connective tissue, is an alternative cause of multiple fractures with minimal trauma. In the United States, the estimated incidence is 1 in 20,000 persons. This estimate includes cases in children in whom the condition is diagnosed within 1 year of birth but does not include milder forms of the disease that are not diagnosed until later in life. Accordingly, the true incidence of OI may be significantly higher. Most types of OI have been linked to mutations in type I collagen genes that interfere with either the synthesis or the construction of collagen subunits. Clinical features include bone fragility, ligament laxity, defective dentinogenesis, short stature, scoliosis, middle ear deafness, blue sclera and tympanic membranes, and misshapen skull. On radiographs, the bones are diffusely osteopenic, with thin cortices and attenuated trabecular patterns. The long bones have narrow diaphyses, and bowing and fractures are common (Fig. 176-20).
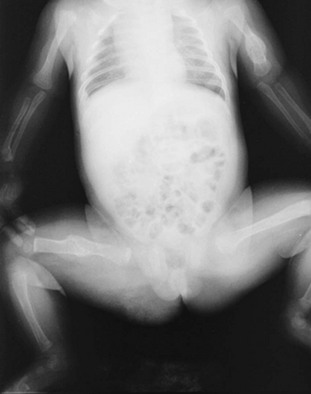
Figure 176-20 Radiograph obtained in a child with osteogenesis imperfecta. Note the narrow diaphyses, osteopenia, and multiple fractures.
In addition to musculoskeletal complaints, children with OI are predisposed to abdominal pain and neurologic abnormalities. The abdominal pain is thought to be related to the trefoil-shaped pelvis and acetabular protrusion associated with OI. These structural abnormalities narrow the pelvic outlet, resulting in partial rectosigmoid obstruction and subsequent constipation or obstipation. Neurologic abnormalities associated with OI result from basilar impression. Basilar impression, or elevation of the floor of the posterior cranial fossa, is reported to occur in 25% of patients with OI.34 The signs of basilar impression (nystagmus, facial spasm, nerve paresis, pyramidal signs, and papilledema) may predate the symptoms (headache, neuralgia, imbalance, weakness, and incontinence). The most serious outcomes with basilar impression include brainstem compression, respiratory arrest, and sudden death. Any neurologic changes in a patient with OI mandate neurosurgical evaluation.
Developmental Dysplasia of the Hip
Developmental dysplasia of the hip (DDH), formerly known as congenital dislocation of the hip, is abnormal formation of the hip joint that occurs between organogenesis and fetal maturity.35 It encompasses a spectrum of disease ranging from “subluxatable” (“loose”) hips to frank dislocation of the hips.
In white neonates, the incidence of dysplasia is 1%, and the incidence of dislocated hips is 0.1%. DDH is more common in Native American populations and less common in African American, Korean, and Chinese populations. An apparent familial predilection for the development of DDH has been reported; one British study noted that more than 20% of children who required treatment for DDH had a positive family history.35 DDH is more common in girls and most frequently is unilateral (80%). With unilateral involvement, a predilection for the left side is characteristic. Associated birth factors include oligohydramnios, breech presentation, torticollis, talipes equinovarus, metatarsus adductus, and first-born status.36 Postnatally, swaddling of infants with their hips and knees in extension predisposes to dislocation.
Clinical Features
DDH may be diagnosed at birth, or despite frequent and appropriate physical examinations, it may not be discovered until later in life. In one study, 6% of children with documented DDH had normal findings on physical examination at birth.35 Conversely, in more than 50% of infants found to have unstable hips at birth, spontaneous stabilization occurs within 3.5 days.37
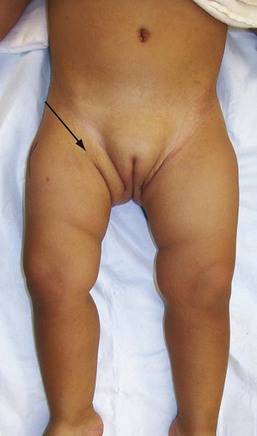
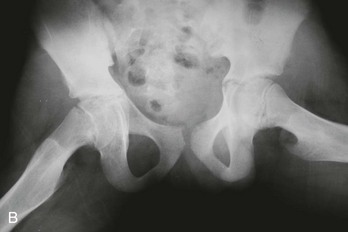
The clinical manifestations of and physical examination findings with DDH are as diverse as the disease itself. This variability is due to differences in the severity of the dysplasia and the progressive changes that occur over time. Up to 4 to 6 months of age, the diagnosis of DDH is based on physical examination findings of leg length, skinfold, and range-of-motion asymmetry and abnormal findings on the Barlow provocative test and the Ortolani reduction maneuver. Skinfold asymmetry (Fig. 176-21) can be noted in the groin, below the buttock, and along the thighs. Although skinfold asymmetry is not specific for DDH, being present in approximately 30% of infants with normal hips, it is sensitive, and the diagnosis of DDH is unlikely in infants with normal skinfold symmetry. Range of hip motion also is helpful in diagnosis of DDH; any asymmetry in hip flexion, abduction, and external rotation should prompt further investigation. Although the general physical examination can be helpful in assessment of hip stability, the physical diagnostic cornerstones for diagnosis of DDH in young infants are the Ortolani reduction maneuver and the Barlow provocative test. The Ortolani reduction maneuver is performed in an attempt to reduce a dislocated hip into normal position, and the Barlow provocative test detects a subluxatable or dislocatable hip (Box 176-1). Abnormal findings are the presence of a clunk with the Ortolani test and any abnormal movement between the femoral head and the acetabulum with the Barlow maneuver.
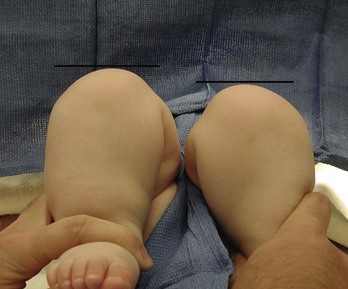
After approximately 4 to 6 months of age, soft tissue contractures develop, the Ortolani and Barlow tests yield less information in detection of unstable hips, and range-of-motion abnormalities become more apparent. Parents may notice limited or asymmetrical leg movements or difficulty with diapering. Findings on examination include limited abduction (Fig. 176-22), relative shortening of the ipsilateral femur (Galeazzi’s sign; Fig. 176-23), and skinfold asymmetry. In children with bilateral DDH, the diagnosis is even more difficult beyond the first few months of life because of the absence of asymmetry. After the development of contractures, physical findings in bilateral DDH include widening of the perineum, abduction of less than 60 degrees, and the appearance of abnormally short thigh segments.
Diagnostic Strategies
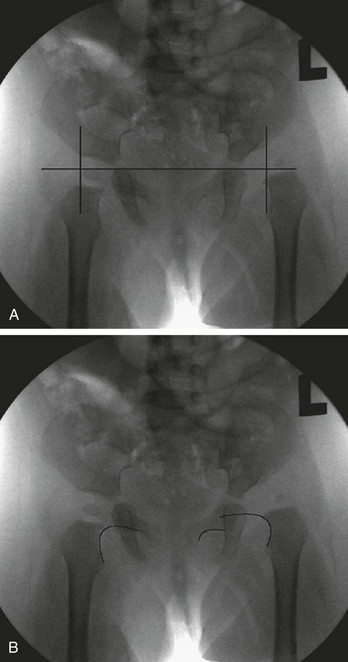
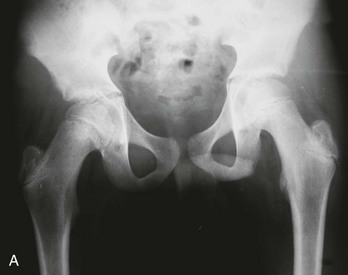
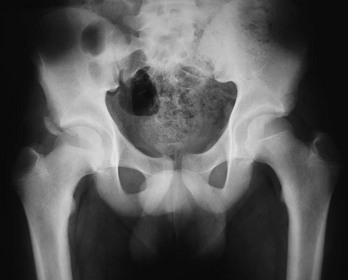
Radiographs of infant hips are extremely difficult to interpret and may provide a false sense of security if the findings seem normal. Before the child is 3 to 6 months old, at which time the femoral head ossifies, an abnormal relationship between the upper end of the femur and the acetabulum may not be apparent. In addition, in infants with unstable but nondislocated hips, radiographs will show the hip in position, so its instability goes undetected. Before femoral head ossification, a better diagnostic test is ultrasonography.38 Because a large percentage of infants will have abnormal ultrasound findings in the first week of life, with many of these abnormalities resolving within a few weeks,35 it is best to delay ultrasound imaging in children with nondislocated but possibly unstable hips until 4 to 6 weeks of age. Children with dislocated hips require immediate ultrasound examination. Plain radiographs are useful after the age of approximately 6 months. A standard AP radiographic view of the pelvis with both legs extended in neutral abduction is then sufficient for diagnosis. Radiographic findings may include displacement of Shenton’s line (a curvilinear line defined by the medial border of the femoral neck and the superior border of the obturator foramen) and a widened acetabular angle; angles greater than 30 degrees are abnormal, and those greater than 40 degrees indicate dislocation (Fig. 176-24).
Management
In the first 6 months of life, use of the Pavlik harness is the mainstay of treatment. It is a dynamic splint that allows movement while preventing hip extension or adduction. Other treatment options include application of the Craig and von Rosen splints.39 If these modalities are unsuccessful, application of a hip spica cast usually is the next choice. Beyond 6 months of age, use of a hip spica cast or fixed orthosis is required. Surgical release of contracted muscles may be necessary in older infants and children, and open surgical reduction is required if complete closed reduction is not achieved. Femoral or pelvic osteotomy (or both) may be necessary to reduce and to stabilize dislocated hips in children older than 18 months.40 Beyond the age of 4 years in bilateral cases and 8 years in unilateral cases, reduction should not be attempted. The risk of aseptic necrosis and the potential for a poor result are too high.36
Hip Pain in Children
Hip pain in children is an extensive topic with myriad causes (Box 176-2). The extensive differential diagnosis precludes an in-depth review of the topic in this chapter, but an overview of some of the more common causes of hip pain in children is presented next.
Specific Disorders and Injuries
Transient Synovitis: Transient synovitis is the most common cause of hip pain in childhood.41 It is a self-limited condition caused by a nonpyogenic inflammatory response of the synovium. Although it has been reported in children as young as 3 months and occasionally occurs in adults,42 its peak incidence is between 3 and 6 years of age. Transient synovitis of the hip affects boys more commonly than girls and has a slight predilection for the right side. Less than 5% of cases are bilateral. Approximately half of the patients are seen acutely, usually within the first 3 days of the onset of symptoms, whereas the other half have a more insidious course, with symptoms occurring for weeks to months before medical evaluation.43 Although transient synovitis most commonly affects the hip, it also can affect the knee.
The etiology of transient synovitis is unknown. Current theories imply an association with active or recent infection, trauma, or allergic hypersensitivity. At least half of the children with transient synovitis have or recently have had an upper respiratory illness.44 One study demonstrated a fourfold rise in viral titers in 45% of patients and elevated serum interferon levels, consistent with a concurrent viral infection, in 43% of patients with transient synovitis.45 Trauma commonly is associated with transient synovitis, but no clear relationship has been demonstrated. Similarly, a causal association with an allergic reaction to an infectious agent has been suggested but not proved.
The diagnosis of transient synovitis is one of exclusion and relies on the history and physical examination in combination with limited laboratory testing and AP and frog-leg lateral radiographs of the pelvis. Laboratory tests are used to help distinguish children with transient synovitis from those with septic arthritis. In transient synovitis, laboratory values may be normal or may reveal mild elevations in the white blood cell count and erythrocyte sedimentation rate (ESR), both consistent with a nonspecific inflammatory process. Multiple studies have attempted to define criteria to help differentiate transient synovitis from septic arthritis. Clinical decision rules have included the presence or absence of fever, the ability to bear weight, white blood cell counts, the inflammatory markers ESR and C-reactive protein (CRP), side-to-side differences in the width of the joint space on radiographs, and previous visits to a health care provider for related symptoms. Kocher and colleagues found four independent multivariant predictors of septic arthritis: fever, inability to bear weight, ESR of 40 mm/hr or higher, and serum white blood cell count higher than 12,000 cells/µL. Patients with three of the four predictors had a 93% chance of having septic arthritis, and those with all four had a 99% likelihood.46 A subsequent study to validate these findings showed that patients with none of the predictors had a 2% probability of septic arthritis; with one of four predictors, a 9.5% probability; with two of four predictors, a 35% probability; with three of four predictors, a 73% probability; and with all four predictors, a 93% probability of septic arthritis.47 When this algorithm was tested at another institution, however, the presence of all four Kocher criteria predicted septic arthritis only 59% of the time.48 In general, in children with transient synovitis and septic arthritis, the overlap of laboratory values and historical features is too great to provide a foolproof diagnostic algorithm.
Although radiographs of the hip and pelvis tend to be normal in appearance with transient synovitis, they are helpful to exclude other diseases. Radiographic findings consistent with transient synovitis include medial joint space widening, accentuated pericapsular shadow, and Waldenström’s sign, which is lateral displacement of the femoral epiphysis with surface flattening secondary to effusion. However, these findings also are apparent in Legg-Calvé-Perthes disease and, if present, mandate close follow-up or further investigation with MRI. If joint aspiration is necessary to clarify the diagnosis, ultrasonography can be used to guide hip joint aspiration. Effusions are present in 60 to 70% of cases of transient synovitis; however, they also are present with septic arthritis, osteomyelitis, acute slipped capital femoral epiphysis (SCFE), Legg-Calvé-Perthes disease, rheumatoid and infectious arthritis, malignant disease, and osteoid osteoma. Accordingly, the presence of an effusion on ultrasonography cannot be used to distinguish transient synovitis from other causes of hip pain.49 Nuclear scintigraphy in patients with transient synovitis is helpful in differentiation of transient synovitis from osteomyelitis (with or without septic arthritis), Legg-Calvé-Perthes disease, and SCFE. Scintigraphy may demonstrate differential uptake in the capital femoral epiphysis, with decreased uptake early in the course of the disease and increased uptake later on. The early changes also are seen with very early Legg-Calvé-Perthes disease and suggest that some children with transient synovitis experience ischemia of the capital femoral epiphyses. This ischemia may be caused by an intracapsular effusion that tamponades vessels along the femoral neck. These ischemic findings have not yet been shown to have clinical significance.
The prognosis for children with transient synovitis is excellent. Up to 75% of patients have complete resolution of pain within 2 weeks and 88% within 4 weeks. The remainder may have less intense but persistent pain for up to 8 weeks. Relapse is possible, although infrequent, and usually occurs within 6 months. In general, long-term sequelae of transient synovitis are infrequent; these may include asymptomatic coxa magna (enlargement and deformity of the femoral head and neck caused by hypertrophy of cartilage secondary to inflammation) and mild degenerative cystic changes of the femoral neck. These changes persist for years and may be accompanied by radiographic degenerative change, but they do not tend to cause long-term functional disability. In addition, a small number (2%) of cases of transient synovitis follow a clinical and radiographic course consistent with Legg-Calvé-Perthes disease. Whether this is due to ischemia of the capital femoral epiphyses during the early stages of synovitis or reflects an initial misdiagnosis is unclear. It is recommended that children with persistent symptoms undergo ultrasonography to evaluate for the presence of an effusion. Persistent joint effusion beyond 4 to 6 weeks may be associated with the subsequent development of Legg-Calvé-Perthes disease.50 Some experts recommend routine 6-month follow-up radiographs for all children with transient synovitis,43 whereas others reserve radiographs for children who remain symptomatic.51
Acute Septic Arthritis: Septic arthritis refers to microbial invasion and infection of the joint space. Bacterial pathogens are common in patients with acute septic arthritis, whereas fungal and mycobacterial pathogens tend to be associated with a more indolent septic arthritis. Acute septic arthritis occurs in all age groups but is more common in children; 70% of cases occur in children younger than 4 years, and the peak incidence is between 6 and 24 months. Boys are affected twice as frequently as girls. Predisposing factors include preceding viral infection, trauma, immunodeficiency, hemoglobinopathy, hemophilia with recurrent hemarthroses, diabetes, injection drug abuse, rheumatoid arthritis, and intra-articular injections or operations. In 75% of cases of septic arthritis, the pathologic process involves the joints of the lower extremity, with the knee being most commonly and the hip second most commonly involved. Other affected joints, in order of involvement, include the ankle, elbow, shoulder, and wrist. More than 90% of the cases are monarticular.
The most common bacterial causes of septic arthritis are listed in Table 176-5. Overall, Staphylococcus aureus is the most common cause of septic arthritis and infection in children; community-acquired methicillin-resistant S. aureus (MRSA) accounts for up to 65% of S. aureus infections.52 In neonates, group B streptococci and gram-negative organisms are common causes. In addition to the organisms listed in Table 176-5, additional causative organisms include Neisseria gonorrhoeae in neonates and sexually active adolescents, Pseudomonas aeruginosa and Candida species in injection drug abusers, Salmonella species in children with sickle cell disease, and gram-negative bacteria in immunosuppressed children. Kingella kingae, a fastidious gram-negative coccobacillus that colonizes the respiratory and oropharyngeal tracts in children, has been implicated as a common cause of osteoarticular infections in young children. K. kingae was found to be the causative agent in up to 50% of osteoarticular infections.53,54 In previously well children, more than 95% of K. kingae infections are diagnosed between 6 months and 4 years of age; it may also cause infections in older children with underlying medical conditions.55 Fortunately, K. kingae is susceptible to a wide array of antibiotics that are given empirically to young children for septic arthritis.
Table 176-5
Septic Arthritis Pathogens and Treatment
| AGE | ORGANISM | TREATMENT |
| Birth to 2 months | Group B streptococci Staphylococcus aureus Gram-negative rods Neisseria gonorrhoeae |
Nafcillin, 50 mg/kg, and cefotaxime, 50-75 mg/kg |
| 2 months to 5 years | Staphylococcus aureus Streptococcus pneumoniae Streptococcus pyogenes Kingella kingae Haemophilus influenzae |
Nafcillin, 50 mg/kg, and ceftriaxone, 50 mg/kg (consider vancomycin, 10 mg/kg) |
| 5 years to 12 years | Staphylococcus aureus Streptococcus pyogenes |
Nafcillin, 50 mg/kg, and ceftriaxone, 50 mg/kg (consider vancomycin, 10 mg/kg) |
| >12 years | Staphylococcus aureus Neisseria gonorrhoeae |
Nafcillin, 50 mg/kg, and ceftriaxone, 50 mg/kg (consider vancomycin, 10 mg/kg) |
Laboratory studies helpful in diagnosis of septic arthritis include a white blood cell count, ESR, CRP assay, blood cultures, and evaluation of joint fluid. Use of the white blood cell count and ESR is discussed in the section on transient synovitis. The ESR rises 24 hours or more after the onset of signs and symptoms of infection, so it may not be helpful during the first day of illness. The CRP level may be a better monitor than the ESR for septic arthritis. The CRP assay is a simpler test that requires only a finger-stick sample of blood. The CRP level rises more quickly than the ESR, typically is elevated at the time of the initial evaluation, and with appropriate therapy will normalize within a week; by contrast, the ESR will not normalize for more than a month.56 In patients with septic arthritis, the peripheral white blood cell count, ESR, and CRP level generally are elevated, although white blood cell counts and acute-phase reactants may be normal, especially with K. kingae infection.55,57
Blood cultures should be included in the evaluation of a child with suspected septic arthritis. Culture results are positive in 20 to 50% of cases.56 When culture results are positive, they not only help direct antibiotic treatment but also provide an organism for serum bactericidal testing as the child progresses to oral antibiotics.
Evaluation of synovial fluid is the standard modality for diagnosis of septic arthritis. If septic arthritis is being considered, joint aspiration should be performed without delay and the sample sent for Gram’s stain, aerobic and anaerobic cultures, cell count with differential count, and glucose determination. The synovial fluid in patients with septic arthritis tends to be turbid or grossly purulent, with a white blood cell count higher than 40,000 cells/µL and a predominance of polymorphonuclear cells (Table 176-6). Synovial glucose concentration may be low (synovial fluid glucose/blood glucose ratio of less than 0.5) and protein and lactate levels elevated. Because of the intrinsic immunoglobulins in the synovial fluid, results on culture of the fluid will be positive in only half of the children in whom the clinical picture is consistent with septic arthritis. Joint fluid should be inoculated directly into blood culture bottles to enhance identification of fastidious organisms such as K. kingae.52,57 Culture specimens may need to be incubated for a week or longer.54
Table 176-6
Synovial Fluid Findings in Different Types of Arthritis
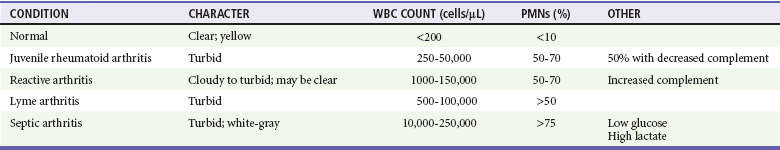
PMNs, polymorphonuclear leukocytes (neutrophils); WBC, white blood cell.
In septic arthritis of the hip, plain radiographs may be normal in appearance or, in the presence of a large joint effusion, may show periarticular soft tissue swelling, widening of the joint space, obliteration or displacement of the gluteal lines, and asymmetrical fullness of the iliopsoas and obturator soft tissue planes. Late in the course of infection, subchondral bone erosions and narrowing of the joint space are seen. Ultrasonography is much more sensitive than plain radiography for detection of hip effusion and provides direct visualization of the fluid and needle during joint aspiration. Scintigraphy also may be useful in diagnosis of septic arthritis; during the “blood pool” or delayed images of the joint, symmetrical uptake in periarticular tissue on both sides of the joint is seen. Scintigraphy is diagnostic of septic arthritis earlier than other imaging techniques are and is a useful adjunct in identification of associated osteomyelitis or avascular necrosis of the femoral head. CT can confirm the presence of an effusion but does not differentiate septic from nonseptic arthritis. Changes in signal intensity and femoral epiphysis perfusion seen on MRI may provide diagnostic clues in attempting to differentiate septic arthritis from transient synovitis.58,59
Septic arthritis requires immediate hospital admission, antibiotics, and surgical intervention. Surgical options range from needle aspiration to open surgical drainage, but no randomized, controlled trials have compared these two treatment approaches. Some experts recommend surgical drainage in all infants and young children with septic arthritis because needle aspiration has been shown to be inferior in this population.60 Indications for surgical drainage in children with septic arthritis include involvement of the hip joint, presence of large amounts of pus or debris in the joint, loculated fluid, recurrence of joint fluid after four or five aspirations, and lack of clinical improvement within 3 days of the initiation of appropriate therapy.61–64 In joints other than the hip, the need for surgical drainage is determined on a case-by-case basis.
Empirical antibiotic therapy for septic arthritis is directed against the most likely organisms as dictated by the patient’s age and comorbid conditions (see Table 176-5). Treatment may then be changed after culture and sensitivity results are known. If a pathogen is not isolated but the patient is improving, initial antibiotic therapy is continued. If a pathogen is not identified and the patient is not improving, reaspiration of the joint or the possibility of a noninfectious process should be considered. To maximize culture results, antibiotics should not be given until a specimen of joint fluid is obtained. Initial treatment should be parenteral to ensure adequate serum antibiotic concentrations. In neonates, the entire course of antibiotics should be administered parenterally. Patients older than 1 month can be transitioned to oral antibiotics after they have been afebrile for 48 to 72 hours and have improved signs and symptoms of infection, a white blood cell count that has normalized, and decreasing inflammatory markers (CRP and ESR). In general, doses two to three times those used for mild infections are sufficient. Response to therapy is measured by clinical improvement and assays for acute-phase reactants (CRP and ESR).
Legg-Calvé-Perthes Disease: Idiopathic avascular necrosis of the proximal femoral epiphysis, also known as Legg-Calvé-Perthes disease, is named after the men who independently described it in the early 1900s. It usually occurs between the ages of 3 and 12 years, with the peak incidence between the ages of 5 and 7 years. Legg-Calvé-Perthes disease has been reported in teenagers as well as in children as young as 2 years. Boys are affected three to five times more frequently than girls, and the disease is familial approximately 10% of the time. The disorder is bilateral in up to 20% of cases. Trauma often is related to the onset of symptoms, but a direct relationship between the two has not been clearly established. Thrombophilia also may be associated with Legg-Calvé-Perthes disease; however, studies are conflicting.
Despite extensive research, the etiology of Legg-Calvé-Perthes disease remains unclear. Ponseti and colleagues65 suggested that the disease is a local manifestation of a transient generalized disorder of epiphyseal cartilage. Their histologic studies showed thickening and disorganization of the growth plate, which could block penetration of the blood vessels and render the femoral epiphysis avascular. Other etiologic theories involve abnormalities in the vascular anastomotic network around the femoral epiphysis, increased blood viscosity leading to infarction, and abnormalities in growth hormone.
Legg-Calvé-Perthes disease is diagnosed and staged by plain radiographs obtained in the AP and frog-leg lateral views. This radiographic survey shows the extent of epiphyseal involvement and the stage of the disease and also provides prognostic information. Radiographic “head at risk” signs that are associated with poor results include a radiolucent V-shaped defect in the lateral epiphysis and adjacent metaphysis (Gage’s sign), speckled calcification lateral to the capital epiphysis, diffuse metaphyseal reaction (metaphyseal cysts), lateral capital femoral epiphysis subluxation, and horizontal physis. These prognostic signs help guide treatment. In early Legg-Calvé-Perthes disease, radionuclide bone scanning may be diagnostic before the development of abnormalities on plain film. Bone scintigraphy also has been shown to provide accurate information concerning the extent of the necrotic process as well as the degree of vascularization and hence the stage of the disease. Scintigraphy, however, was not able to predict disease outcome.66 Compared with plain radiography and bone scanning, MRI gives earlier and more reliable information about the extent of necrosis of the femoral head. MRI also is better than scintigraphy at showing revascularization. Despite the usefulness of MRI in early Legg-Calvé-Perthes disease, its role in healed Legg-Calvé-Perthes disease is limited because it provides no additional information about the configuration and structure of the femoral head beyond that from plain radiography.66 Arthrography is useful in delineation of flattening of the femoral head, demonstration of the hinge abduction phenomenon with abduction of the leg, and, in conjunction with plain films or CT, diagnosis of osteochondritis dissecans after Legg-Calvé-Perthes disease.
There are four radiographic classification stages of Legg-Calvé-Perthes disease: initial, fragmentation, reossification, and healed. Radiographic findings in the initial stage include a femoral head that appears smaller than the opposite unaffected femoral head, widening of the medial joint space, subchondral lucent zone (subchondral collapse, the so-called crescent sign; Fig. 176-25), irregular physeal plate, and blurry and radiolucent metaphysis. In the fragmentation phase, the repair aspects of the disease become more prominent. The epiphysis begins to fragment, and areas of increased radiolucency appear as new bone forms as well as areas of increased radiodensity. During the reossification stage, the repair process continues as normal bone density returns, radiodensities replace radiolucencies, and alterations in the shape of the femoral head and neck become apparent. The healed stage is the final radiographic stage of Legg-Calvé-Perthes disease, and radiographs of the proximal third of the femur obtained during this stage of the illness will demonstrate any residual deformities.
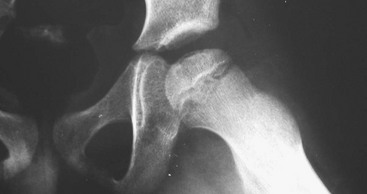
Figure 176-25 Crescent sign (subchondral lucent zone) in early Legg-Calvé-Perthes disease. (Courtesy Marianne Gausche-Hill, MD.)
Residual deformities resulting from Legg-Calvé-Perthes disease follow four patterns: coxa magna (enlargement and deformation of the head of the femur), premature physeal arrest, irregular head formation, and osteochondritis dissecans. Despite these deformities, most patients with childhood hip disease are capable of bone remodeling with subsequent improvement in femoral head deformities. During their early years, regardless of radiographic appearance, patients with Legg-Calvé-Perthes disease tend to do well.67 A majority (70-90%) of patients are active and pain free and have good range of motion 20 to 40 years after the onset of symptoms. Only patients with flattened irregular femoral heads at the time of primary healing or premature physeal closure experienced clinical deterioration and increasing pain.68 Later in life, function becomes markedly reduced, and degenerative joint disease develops in a majority of patients by their 50s and 60s.67 With noncontainment treatment, however, the outcome is not as good. Yrjonen found that at an average of 35 years of follow-up, 48% of patients had evidence of degenerative joint disease and 17% either had undergone total hip arthroplasty or had clinical symptoms that warranted total hip arthroplasty.69
Slipped Capital Femoral Epiphysis: SCFE refers to posterior and inferior slippage of the proximal femoral epiphysis on the metaphysis. The femoral head sits securely in the acetabulum, whereas the epiphysis separates from the femoral neck through the growth plate. The average annual incidence of SCFE ranges from 0.2 (in Japan)70 to 10.8 (in the United States) per 100,000 children; boys are affected almost twice as frequently as girls are.71 The peak incidence is during the adolescent growth spurt: boys between 12 and 16 years of age (mean age, 13.5 years) and girls between 10 and 14 years of age (mean age, 11.5 years). The age at diagnosis decreases with increasing obesity. A majority of children with SCFE have delayed skeletal maturation, with bone age being as much as 20 months behind chronologic age. African American and Hispanic children are affected more frequently than white children are. The literature reports SCFE to be bilateral in up to 80% of cases, although 30 to 40% of these cases are asymptomatic and discovered only on screening radiographs. In unilateral cases, the left hip is affected twice as often as the right.
The traditional classification of SCFE was based on the duration of symptoms, with acute slippage defined as symptom duration of less than 3 weeks, chronic slippage defined as symptoms for 3 weeks or longer, and acute-on-chronic slippage defined as symptom duration of more than 3 weeks with a recent sudden exacerbation. This classification has been replaced by one based on stability: in stable SCFE, ambulation is possible (with or without crutches), whereas in unstable SCFE, ambulation is not possible (with or without crutches).72 This classification system is preferred to the traditional classification because it does not rely on patient or parent recall for duration of symptoms and provides information about prognosis.
The signs and symptoms of SCFE vary with its stability. Children with stable SCFE have symptoms of intermittent limp and pain of several weeks to months in duration. Stable SCFE is found in approximately 95% of all cases.72 The pain of SCFE may be localized to the hip but more commonly is poorly localized to the thigh, groin, or knee. Atypical manifestations of SCFE include weakness and easy fatigability of the affected limb and limping on exertion. With continued slippage, internal rotation, flexion, and abduction are lost, and parents and children may note progressive external rotation and shortening of the involved lower extremity with subsequent difficulty in daily activities, such as tying shoes. On examination, children initially have a slight loss of internal rotation and experience pain only at the extremes of motion. Their gait is antalgic, and muscle atrophy is minimal. As the slip becomes more severe, the gait becomes more antalgic, internal rotation is lost, abduction and flexion of the hip increase, thigh and gluteal muscle atrophy is more pronounced, and leg-length discrepancy develops. A frequently seen sign associated with SCFE can be elicited during passive flexion of the affected hip: as flexion is increased from an extended position, the thigh abducts and externally rotates.
The diagnosis of SCFE is made with AP and lateral radiographs of both hips. With stable slippage, AP and frog-leg lateral pelvic radiographs should be obtained. When an unstable slip or a minimal slip is suspected, a cross-table radiograph replaces the frog-leg lateral view. Early in the course of SCFE, the initial slippage is posterior, so the AP view is normal in appearance or shows widening of the physis; the slip is better seen on the lateral projection (Fig. 176-26). Early findings on lateral radiographs include a minimal posterior step-off at the anterior physeal plate and widening of the growth plate. On AP radiographs, signs of slippage include Klein’s line and the blanch sign of Steele. Klein’s line is a line drawn along the superior margin of the femoral neck. With a normal hip, the line intersects with or falls within the epiphysis, whereas in a hip with a slipped epiphysis, the line does not come in contact with the epiphysis. The blanch sign of Steele is a crescent-shaped area of increased density in the proximal portion of the femoral neck that is created by superimposition of the posteriorly displaced epiphysis on the femoral neck. The femoral metaphysis of the affected hip also appears to be more laterally displaced from the medial wall of the acetabulum. As the slip continues, the epiphysis continues to be displaced inferiorly and posteriorly relative to the metaphysis. Over time, remodeling smoothes away the superior and anterior portions of the femoral neck, and in an attempt to buttress the slippage, callus is formed at the inferior and posterior portions of the femoral neck. Acute, unstable slips in children without previous symptoms will not be associated with evidence of bone healing or remodeling.
Slip severity is described in either of two ways. The simplest classification describes the amount of displacement of the femoral head on the femoral neck. In mild SCFE, the amount of displacement is less than one third; moderate SCFE has displacement between one third and one half; and severe SCFE is marked by displacement of the femoral head by more than half the width of the femoral neck. A more accurate description of the magnitude of slippage is obtained by measurement of the epiphyseal-shaft angle of Southwick. On a frog-leg lateral radiograph of the pelvis, a line is drawn between the anterior and posterior tips of the epiphysis at the physeal plate level. A second line is then drawn perpendicular to the epiphyseal line. Next, a line is drawn along the midshaft of the femur. The epiphyseal-shaft angle is formed by the intersection of the perpendicular and the femoral shaft lines. The magnitude of slip displacement is the angle of the involved hip minus the angle of the normal hip. If involvement is bilateral, 12 degrees is used as the control angle. Mild SCFE involves displacement of less than 30 degrees, a moderate slip is between 30 and 50 degrees, and severe displacement is more than 50 degrees.73
The two most worrisome short-term complications of SCFE are avascular necrosis and chondrolysis; the risk for either process increases with the severity of the initial slippage.74 Avascular necrosis has been reported to occur in 10 to 15% of children with SCFE. The risk is higher with unstable SCFE,75,76 greater degrees of slippage, and multiple attempts at reduction.76 In children with stable slips treated by single-screw internal fixation without reduction, reported rates of avascular necrosis are as low as 0 to 5%.77
Chondrolysis occurs in approximately 5% of patients with SCFE, with an increased incidence in children with greater degrees of slippage. Chondrolysis may develop before and as a result of treatment. It should be suspected when pain and loss of motion are disproportionate to the severity of the slip. On radiographs, loss of articular cartilage may be seen. When SCFE is treated by spica cast immobilization, the rate of chondrolysis is reported to range from 19 to 67%.78,79 Approximately 50% of cases of chondrolysis will resolve during the course of several years; however, in other cases, it may progress to such severe pain and contractures that hip arthrodesis is needed.
Apophyseal Injuries
Perspective and Principles of Disease
The true incidence of apophysitis is unknown. One study found an incidence of 18% in a population of 1000 patients seen at an urban general pediatric clinic during a 4.5-month period.80 Another study, which looked at patient visits to a sports medicine clinic, found an incidence of 31%.81 Children between 8 and 15 years of age most frequently are affected, with involvement of different apophyseal centers affected at different ages.
Specific Disorders and Injuries
Osgood-Schlatter Syndrome: In 1903, Osgood and Schlatter independently reported traumatically induced apophyseal injury to the tibial tubercle in adolescents.82 This entity, now known as Osgood-Schlatter syndrome, is the most common of the apophyseal disorders.83 It is found most commonly in boys between 10 and 15 years of age and in girls between 8 and 13 years of age and frequently is bilateral. Boys are affected more often than girls are, although this trend is changing as more girls are becoming increasingly involved in competitive sports. Bilateral involvement is found in 20 to 30% of patients.
Clinically, patients with Osgood-Schlatter syndrome have tenderness, pain, and swelling at the site of insertion of the patellar tendon on the tibial tubercle. The tibial tubercle may be prominent and the quadriceps tight. Pain is worsened with activities (such as running and jumping) that cause the quadriceps to contract, thereby stressing the tubercle. Extension of the knee against resistance causes pain, but resisted straight-leg raises are painless. Osgood-Schlatter syndrome is a clinical diagnosis, and radiographs generally are not indicated. If radiographs are obtained, however, lateral views of the tibial tubercle may appear normal or may show an enlarged, fragmented, and irregular tibial tuberosity with or without an overlying bony ossicle. These findings are nonspecific, however, and are not diagnostic on their own. Ultrasonography, which has been proposed as a diagnostic tool by some experts, may reveal pretibial swelling, fragmentation of the ossification center, insertional thickening of the patellar tendon, or excessive fluid collection in the infrapatellar bursa.84
Sever’s Disease: Sever’s disease is an apophysitis of the calcaneus due to traction by the gastrocnemius-soleus complex. It initially was described in 191285 and commonly is manifested as posterior heel pain in an 8- to 13-year-old athlete. It is bilateral in 60% of cases. As with other apophyseal injuries, pain is exacerbated by activity. Sever’s disease can be associated with growth or tight heel cords or other biomechanical abnormalities. Impact sports, especially those that involve running, and sports in which cleats are worn frequently are implicated in the development of Sever’s disease.
As in Osgood-Schlatter syndrome, treatment of Sever’s disease is conservative and symptomatic. Treatment consists of ice, massage, stretching of the plantar fascia and involved muscles (gastrocnemius-soleus complex and ankle invertors and evertors), nonsteroidal anti-inflammatory drugs, and addition of shock-absorbing shoe inserts. Heel cups also have been found to be helpful, but they should be accompanied by stretching to avoid exacerbation of the calf muscle contracture. Patients in whom conservative management fails have been found to have bone bruising and edema in the calcaneal metaphysis and apophysis, suggesting that calcaneal stress fractures may be the cause of persistent heel pain. Modification of activity and a trial of crutches for 3 to 4 weeks may be necessary.86 Plantar fasciitis can cause heel pain as well, but patients will experience tenderness along the plantar fascia, especially at the attachment of the fascia to the calcaneus. Treatment involves the use of anti-inflammatory agents, rest, and relaxation of the plantar fascia with heel support pads.
Little League Elbow: Little League elbow is a term used to describe a group of injuries of the elbow, including apophysitis, medial epicondylitis, and osteochondritis dissecans of the radial head and capitellum. The injury involves an overuse phenomenon resulting in inflammation at the site where the forearm flexor muscles originate. As its name suggests, it commonly affects baseball pitchers in children’s leagues and is associated with overhead arm motion. It also is seen in other skeletally immature overhand athletes, including tennis players. Little League elbow is caused by the excessive forces placed on the medial side of the elbow during the late cocking and acceleration phases of throwing.
To minimize the risk of medial epicondylitis in children’s league pitchers, the American Academy of Pediatrics recommends limiting the number of pitches to 200 per week or 90 pitches per outing87; however, the USA Baseball Medical and Safety Advisory Committee recommends lower pitch counts: 75 to 125 pitches per week or 50 to 75 pitches per outing, depending on age.88 Preseason conditioning, use of proper pitching mechanics, and gradual increases in the amount and intensity of throwing are other preventive measures.
Apophysitis of the Hip: Apophyseal injuries of the hip involve sites around the hip where major abdominal and hip muscles either originate or insert: the anterior superior iliac spine, the anterior inferior iliac spine, the iliac crests, and the ischial tuberosities. Dancers and distance runners most commonly are affected. Patients with apophysitis of the hip experience dull pain near the hip that is related to activity. Treatment includes strengthening and stretching of the abdominal and hip muscles and restriction of activity.
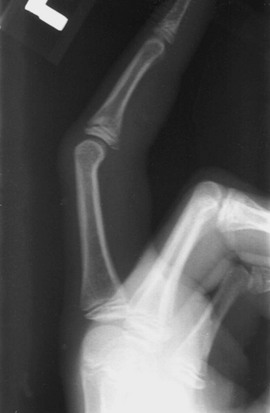
Avulsion Fractures: Apophysitis tends to have an insidious onset; therefore, any patient with sudden onset of apophyseal pain after an acute traumatic event should be evaluated for an avulsion fracture of the apophysis or the adjacent bone. Such fractures occur when the muscle attachments to the apophyses are pulled off during strong active contractions against resistance, such as with a single powerful throw. Examination will reveal localized tenderness and swelling. The diagnosis usually is readily apparent on plain films (Fig. 176-27); however, bone scan and ultrasonography may play a role when the diagnosis is in question. Ultrasonography is advantageous because it involves no radiation exposure, it can detect a fracture before the development of an ossification center, and it provides a dynamic examination. Findings consistent with an avulsion fracture include a hypoechogenic zone, increased distance to the apophysis, dislocation of the apophysis, and mobility of the apophysis on dynamic examination.89 Treatment of an avulsion fracture is based on the degree of separation. If the displacement is minimal (less than 2 cm with a hip avulsion and less than 5 mm with avulsion of the medial epicondyle90), immobilization for 4 to 6 weeks with subsequent slow resumption of activities usually is sufficient. With widely separated avulsion fractures, some experts recommend open reduction and fixation.90
References
1. Simon, TD, Bublitz, C, Hambidge, SJ. External causes of pediatric injury–related emergency department visits in the United States. Acad Emerg Med. 2004;11:1042.
2. Davidson, JS, et al. Simple treatment for torus fractures of the distal radius. J Bone Joint Surg Br. 2001;83:1173.
3. Solan, MC, Rees, R, Daly, K. Current management of torus fractures of the distal radius. Injury. 2002;33:503.
4. Firmin, F, Crouch, R. Splinting versus casting of “torus” fractures to the distal radius in the paediatric patient presenting at the emergency department (ED): A literature review. Int Emerg Nurs. 2009;17:173.
5. West, S, et al. Buckle fractures of the distal radius are safely treated in a soft bandage: A randomized prospective trial of bandage versus plaster cast. J Pediatr Orthop. 2005;25:322.
6. Khan, KS, et al. A randomized trial of “soft cast” for distal radius buckle fractures in children. Acta Orthop Belg. 2007;73:594.
7. Plint, AC, et al. A randomized, controlled trial of removable splinting versus casting for wrist buckle fractures in children. Pediatrics. 2006;117:691.
8. Boutis, K, et al. Cast versus splint in children with minimally angulated fractures of the distal radius: A randomized controlled trial. CMAJ. 2010;182:1507.
9. Randsborg, P, Sivertsen, EA. Distal radius fractures in children: Substantial difference in stability between buckle and greenstick fractures. Acta Orthop. 2009;80:585.
10. Hanby, CK, Pasque, CB, Sullivan, JA. Medial clavicle physis fracture with posterior displacement and vascular compromise: The value of three-dimensional computed tomography and duplex ultrasound. Orthopedics. 2003;26:81.
11. Gartland, JJ. Management of supracondylar fractures of the humerus in children. Surg Gynecol Obstet. 1959;109:145.
12. Williamson, D, et al. Normal characteristics of the Baumann angle: An aid in the assessment of supracondylar fractures. J Pediatr Orthop. 1992;12:633.
13. Worlock, P. Supracondylar fractures of the humerus: Assessment of cubitus varus by the Baumann angle. J Bone Joint Surg Br. 1986;68:755.
14. Davidson, RS, et al. Ultrasonographic evaluation of the elbow in infants and young children after suspected trauma. J Bone Joint Surg Am. 1994;76:1804.
15. Beltran, J, et al. Pediatric elbow fractures: MRI evaluation. Skeletal Radiol. 1994;23:277.
16. Campbell, CC, et al. Neurovascular complications resulting from supracondylar humerus fractures. J Pediatr Orthop. 1995;15:47.
17. Dormans, JP, Squillante, R, Sharf, H. Acute neurovascular complications with supracondylar humerus fractures in children. J Hand Surg Am. 1995;20:1.
18. Culp, RW, et al. Neural injuries associated with supracondylar fractures of the humerus in children. J Bone Joint Surg Am. 1990;72:1211.
19. Schutzman, SA, Teach, S. Upper extremity impairment in young children. Ann Emerg Med. 1995;26:474.
20. Schunk, JE. Radial head subluxation: Epidemiology and treatment of 87 episodes. Ann Emerg Med. 1990;19:1019.
21. Kosuwon, W, et al. Ultrasonography of pulled elbow. J Bone Joint Surg Br. 1993;75:421.
22. Quan, L, Marcuse, EK. The epidemiology and treatment of radial head subluxation. Am J Dis Child. 1985;139:1194.
23. McDonald, J, Whitelaw, C, Goldsmith, LJ. Radial head subluxation: Comparing two methods of reduction. Acad Emerg Med. 1999;6:715.
24. Macias, CG, Bothrer, J, Wiebe, R. A comparison of supination/flexion to hyperpronation in the reduction of radial head subluxation. Pediatrics. 1998;102:e10.
25. Green, DA, et al. Randomized comparison of pain perception during radial head subluxation reduction using supination-flexion or forced pronation. Pediatr Emerg Care. 2006;22:235.
26. Cho, K, Lee, S, Lee, Y, Suh, K. Ultrasound diagnosis of either an occult or missed fracture of an extremity in pediatric-aged children. Korean J Radiol. 2010;11:84.
27. Leonidas, J. Skeletal trauma in the child abuse syndrome. Pediatr Ann. 1983;12:875.
28. Herndon, WA. Child abuse in a military population. J Pediatr Orthop. 1983;3:73.
29. Ravichandiran, N, et al. Delayed identification of pediatric abuse-related fractures. Pediatrics. 2010;125:60.
30. Maguire, S, et al. Does cardiopulmonary resuscitation cause rib fractures in children? A systematic review. Child Abuse Negl. 2006;30:739.
31. Barsness, KA, et al. The positive predictive value of rib fractures as an indicator of nonaccidental trauma in children. J Trauma. 2003;54:1107.
32. Rubin, DM, et al. Occult head injury in high-risk abused children. Pediatrics. 2003;111:1382.
33. Wood, JN, Christian, CW, Adams, CM, Rubin, DM. Skeletal surveys in infants with isolated skull fractures. Pediatrics. 2009;123:e247.
34. Sillence, DO. Craniocervical abnormalities in osteogenesis imperfecta: Genetic and molecular correlation. Pediatr Radiol. 1994;24:427.
35. Marks, DS, Clegg, J, al-Chalabi, AN. Routine ultrasound screening for neonatal hip instability: Can it abolish late-presenting congenital dislocation of the hip? J Bone Joint Surg Br. 1994;76:534.
36. Novaceck, TF. Developmental dysplasia of the hip. Pediatr Clin North Am. 1996;43:829.
37. Barlow, TC. Early diagnosis and treatment of congenital dislocation of the hip. J Bone Joint Surg Br. 1962;44:292.
38. Boal, DK, Schwenkter, EP. The infant hip: Assessment with real-time ultrasound. Radiology. 1985;157:667.
39. Wilkinson, AG. The efficacy of the Pavlik harness, the Craig splint and the von Rosen splint in the management of neonatal dysplasia of the hip: A comparative study. J Bone Joint Surg Br. 2002;84:716.
40. Storer, SK, Skaggs, DL. Developmental dysplasia of the hip. Am Fam Physician. 2006;74:1310.
41. MacEwen, GD, Dehse, R. The limping child. Pediatr Rev. 1991;12:268.
42. Nyska, M, et al. Benign synovitis of the hip in adults. Br J Rheumatol. 1993;32:820.
43. Haueisen, DC, Weiner, DS, Weiner, SD. The characterization of “transient synovitis of the hip” in children. J Pediatr Orthop. 1986;6:11.
44. Koop, S, Quanbeck, D. Three common causes of childhood hip pain. Pediatr Clin North Am. 1996;43:1053.
45. Tolat, V, et al. Evidence for viral aetiology of transient synovitis of the hip. J Bone Joint Surg Br. 1993;75:973.
46. Kocher, M, Zurakowski, D, Kasser, J. Differentiating between septic arthritis and transient synovitis of the hip in children: An evidence-based clinical prediction algorithm. J Bone Joint Surg Am. 1999;81:1662.
47. Kocher, M, et al. Validation of a clinical prediction rule for differentiation between septic arthritis and transient synovitis of the hip in children. J Bone Joint Surg Am. 2004;86:1629.
48. Luhmann, S, et al. Differentiation between septic arthritis and transient synovitis of the hip in children with clinical prediction algorithms. J Bone Joint Surg Am. 2004;86:956.
49. Zamzam, MM. The role of ultrasound in differentiating septic arthritis from transient synovitis of the hip in children. J Pediatr Orthop B. 2006;15:418.
50. Eggl, H. Ultrasonography in the diagnosis of transient synovitis of the hip and Legg-Calvé-Perthes disease. J Pediatr Orthop B. 1999;8:177.
51. Hart, JJ. Transient synovitis of the hip in children. Am Fam Physician. 1996;54:1587.
52. Harik, NS, Smeltzer, MS. Management of acute hematogenous osteomyelitis in children. Expert Rev Anti Infect Ther. 2010;8:175.
53. Chometon, S, et al. Specific real-time polymerase chain reaction places Kingella kingae as the most common cause of osteoarticular infections in young children. Pediatr Infect Dis J. 2007;25:377.
54. Osteomyelitis/septic arthritis caused by Kingella kingae among day care attendees—Minnesota, 2003. MMWR Morb Mortal Wkly Rep. 2004;53:241.
55. Yagupsky, P, Porsch, E, St. Geme, JW. Kingella kingae: An emerging pathogen in young children. Pediatrics. 2011;127:557.
56. Kallio, MJT, et al. Serum C-reactive protein, erythrocyte sedimentation rate and white blood cell count in septic arthritis of children. Pediatr Infect Dis J. 1997;16:411.
57. Gutierrez, K. Bone and joint infections in children. Pediatr Clin North Am. 2005;52:779–794.
58. Kwack, KS, et al. Septic arthritis versus transient synovitis of the hip: Gadolinium-enhanced MRI finding of decreased perfusion at the femoral epiphysis. AJR Am J Roentgenol. 2007;189:437.
59. Yang, WJ, et al. MR imaging of transient synovitis: Differentiation from septic arthritis. Pediatr Radiol. 2006;36:1154.
60. Brower, AC. Septic arthritis. Radiol Clin North Am. 1996;34:193.
61. Syriopoulou, V, Smith, A. Osteomyelitis and septic arthritis. In Feigin R, Cherry J, eds.: Musculoskeletal Infections: Textbook of Pediatric Infectious Disease, 3rd ed, Philadelphia: WB Saunders, 1992.
62. Dunkle, LM. Toward optimum management of serious focal infections: The model of suppurative arthritis. Pediatr Infect Dis J. 1989;8:195.
63. Green, NE, Edward, K. Bone and joint infections in children. Orthop Clin North Am. 1987;18:555.
64. Nade, S. Acute septic arthritis in infancy and childhood. J Bone Joint Surg Am. 1983;65:234.
65. Ponseti, IV, et al. Legg-Calvé-Perthes disease: Histochemical and ultrastructural observations of the epiphyseal cartilage and physis. J Bone Joint Surg Am. 1983;65:797.
66. Kaniklides, C. Diagnostic radiology in Legg-Calvé-Perthes disease. Acta Radiol. 1996;406:1.
67. Weinstein, SL. Legg-Calvé-Perthes syndrome. In Morrisey RT, Weinstein SL, eds.: Lovell & Winter’s Pediatric Orthopedics, 4th ed, Philadelphia: Lippincott-Raven, 1996.
68. Weinstein, SL. Legg-Calvé-Perthes disease: Results of long-term follow up. In: Fitzgerald RH, Jr., eds. The Hip: Proceedings of the Thirteenth Open Scientific Meetings of the Hip Society. St. Louis: CV Mosby, 1985.
69. Yrjonen, T. Prognosis in Perthes’ disease after non-containment treatment: 106 hips followed for 28-47 years. Acta Orthop Scand. 1992;63:523.
70. Aronsson, DD, Loder, RT, Breur, GJ, Weinstein, SL. Slipped capital femoral epiphysis: Current concepts. J Am Acad Orthop Surg. 2006;14:666.
71. Lehmann, CL, Arons, RR, Loder, RT, Vitale, MG. The epidemiology of slipped capital femoral epiphysis: An update. J Pediatr Orthop. 2006;26:286.
72. Loder, RT. Controversies in slipped capital femoral epiphysis. Orthop Clin North Am. 2006;37:211.
73. Green, DW, et al. A modification of Klein’s Line to improve sensitivity of the anterior-posterior radiograph in slipped capital femoral epiphysis. J Pediatr Orthop. 2009;29:449–453.
74. Rattey, T, Piehl, F, Wright, JG. Acute slipped capital femoral epiphysis: Review of the outcomes and rate of avascular necrosis. J Bone Joint Surg Am. 1996;78:398.
75. Kennedy, JG, et al. Osteonecrosis of the femoral head associated with slipped capital femoral epiphysis. J Pediatr Orthop. 2001;21:189.
76. Tokmakova, KP, Stanton, RP, Mason, DE. Factors influencing the development of osteonecrosis in patients treated for slipped capital femoral epiphysis. J Bone Joint Surg Am. 2003;85:798–801.
77. Ward, WT, et al. Fixation with a single screw for slipped capital femoral epiphysis. J Bone Joint Surg Am. 1992;74:799.
78. Carney, BT, Weinstein, SL, Noble, J. Long term follow up of slipped capital femoral epiphysis. J Bone Joint Surg Am. 1991;73:667.
79. Meier, MC, Meyer, LC, Ferguson, RL. Treatment of slipped capital femoral epiphysis with a spica cast. J Bone Joint Surg Am. 1992;74:1522.
80. de Inocencio, J. Musculoskeletal pain in primary care pediatric care: Analysis of 1000 consecutive general pediatric clinic visits. Pediatrics. 1998;102:e63.
81. Micheli, LJ, Fehlandt, AF, Jr. Overuse injuries to tendons and apophyses in children and adolescents. Clin Sports Med. 1992;11:713.
82. Osgood, RB. Lesions of the tibial tubercle occurring during adolescence. Boston Med J. 1903;148:114.
83. Peck, DM. Apophyseal injuries in the young athlete. Am Fam Physician. 1995;51:1891.
84. Blankstein, A, et al. Ultrasonography as a diagnostic modality in Osgood-Schlatter disease: A clinical study and review of the literature. Arch Orthop Trauma Surg. 2001;121:536.
85. Sever, JW. Apophysitis of the os calcis. N Y Med J. 1912;95:1025.
86. Ogden, JA, Ganey, TM, Hill, JD, Jaakkola, JI. Sever’s injury: A stress fracture of the immature calcaneal metaphysis. J Pediatr Orthop. 2004;24:488.
87. Committee on Sports Medicine and Fitness, American Academy of Pediatrics. Risk of injury from baseball and softball in children. Pediatrics. 2001;107:782.
88. USA Baseball Medical and Safety Advisory Committee. Position Statement on Youth Baseball Pitching Injury: 2004. www.usabaseball.com/ot/usab-med-safety-list.html, 2008.
89. Lazovic, D, et al. Ultrasound for the diagnosis of apophyseal injuries. Knee Surg Sports Traumatol Arthrosc. 1996;3:234.
90. Bennet, JB, Tullos, HS. Ligamentous and articular injuries in the athlete. In: Morrey BF, ed. The Elbow and Its Disorders. Philadelphia: WB Saunders, 1985.