7 MUSCLE TISSUE
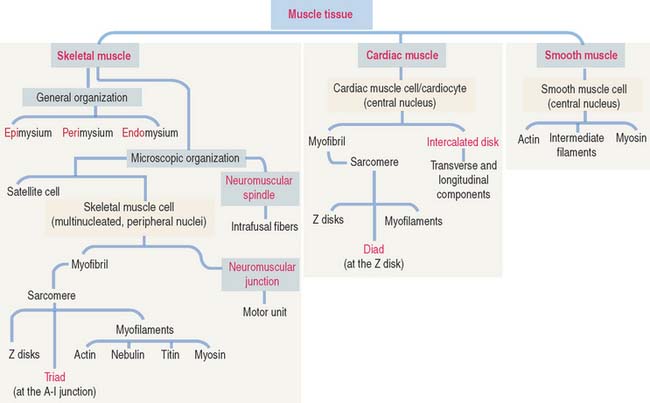
Muscle is one of the four basic tissues. There are three types of muscle: skeletal, cardiac, and smooth. All three types are composed of elongated cells, called muscle cells, myofibers, or muscle fibers, specialized for contraction. In all three types of muscle, energy from the hydrolysis of adenosine triphosphate (ATP) is transformed into mechanical energy.
SKELETAL MUSCLE
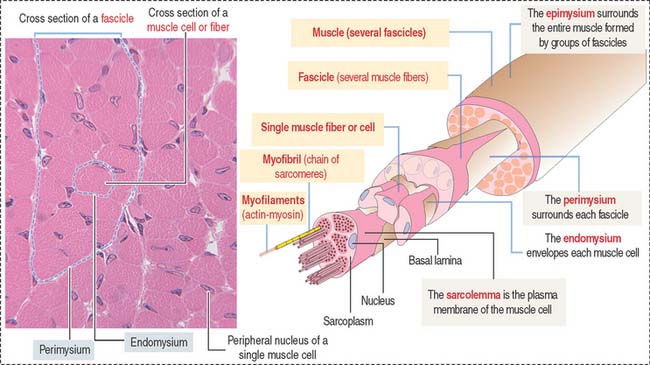
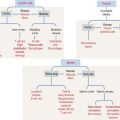
Muscle cells or fibers form a long multinucleated syncytium grouped in bundles surrounded by connective tissue sheaths and extending from the site of origin to their insertion (Figure 7-1). The epimysium is a dense connective tissue layer ensheathing the entire muscle. The perimysium derives from the epimysium and surrounds bundles or fascicles of muscle cells. The endomysium is a delicate layer of reticular fibers and extracellular matrix surrounding each muscle cell. Blood vessels and nerves use these connective tissue sheaths to reach the interior of the muscle. An extensive capillary network, flexible to adjust to contraction-relaxation changes, invests individual skeletal muscle cell.
Characteristics of the skeletal muscle cell or fiber
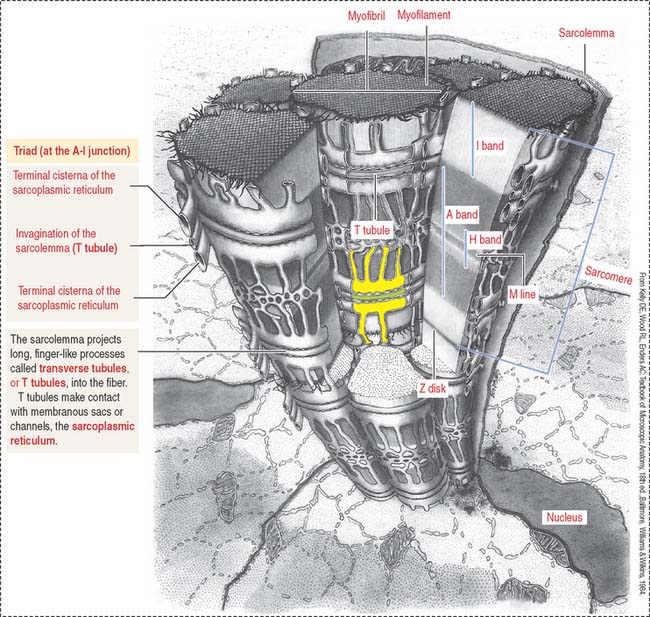
The plasma membrane (called the sarcolemma) of the muscle cell is surrounded by a basal lamina and satellite cells (Figure 7-2). We discuss the significance of satellite cells in muscle regeneration. The sarcolemma projects long, finger-like processes—called transverse tubules or T tubules—into the cytoplasm of the cell—the sarcoplasm. T tubules make contact with membranous sacs or channels, the sarcoplasmic reticulum. The sarcoplasmic reticulum contains high concentrations of Ca2+. The site of contact of the T tubule with the sarcoplasmic reticulum cisternae is called a triad because it consists of two lateral sacs of the sarcoplasmic reticulum and a central T tubule.
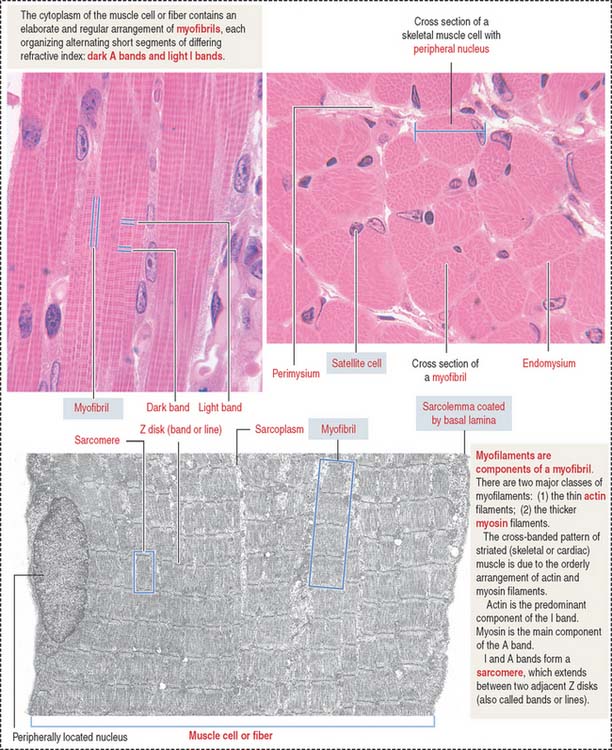
About 80% of the sarcoplasm is occupied by myofibrils surrounded by mitochondria (called sarcosomes). Myofibrils are composed of two major filaments formed by contractile proteins: thin filaments contain actin, and thick filaments are composed of myosin (see Figure 7-2).
The myofibril: A repeat of sarcomere units
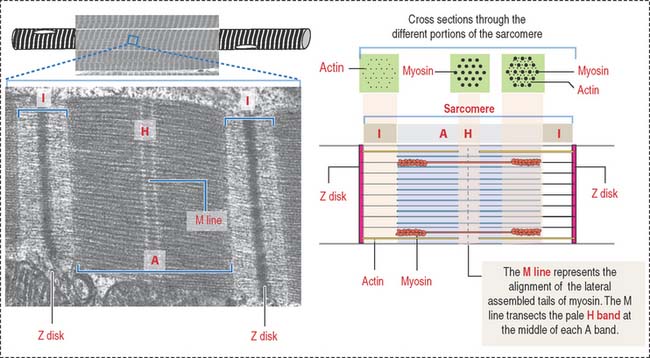
The sarcomere is the basic contractile unit of striated muscle (Figure 7-3). Sarcomere repeats are represented by myofibrils in the sarcoplasm of skeletal and cardiac muscle cells.
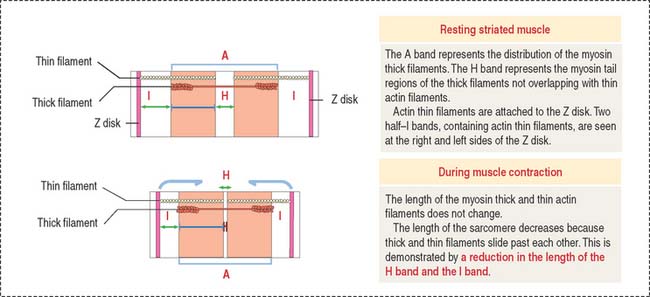
The arrangement of thick (myosin) and thin (actin) myofilaments of the sarcomere is largely responsible for the banding pattern observed under light and electron microscopy (see Figures 7-2 and 7-3). Actin and myosin interact and generate the contraction force. The Z disk forms a transverse sarcomeric scaffold to ensure the efficient transmission of the generated force.
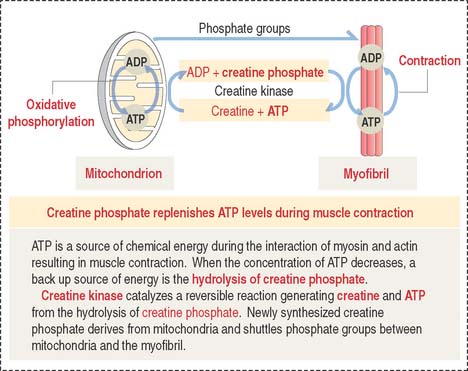
The A band is bisected by a light region called the H band (see Figures 7-3; 7-4). The major component of the H band is the enzyme creatine kinase, which catalyzes the formation of ATP from creatine phosphate and adenosine diphosphate (ADP). We discuss later how creatine phosphate maintains steady levels of ATP during prolonged muscle contraction.
Components of the thin and thick filaments of the sarcomere
F-actin, the thin filament of the sarcomere, is double-stranded and twisted. F-actin is composed of globular monomers (G-actin; see Cytoskeleton in Chapter 1, Epithelium). G-actin monomers bind to each other in a head-to-tail fashion, giving the filament polarity, with barbed (plus) and pointed (minus) ends. The barbed end of actin filaments inserts into the Z disk.
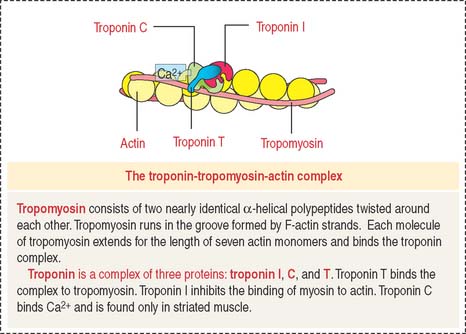
Tropomyosin consists of two nearly identical α-helical polypeptides twisted around each other. Tropomyosin runs in the groove formed by F-actin strands. Each molecule of tropomyosin extends for the length of seven actin monomers and binds the troponin complex (Figure 7-5).
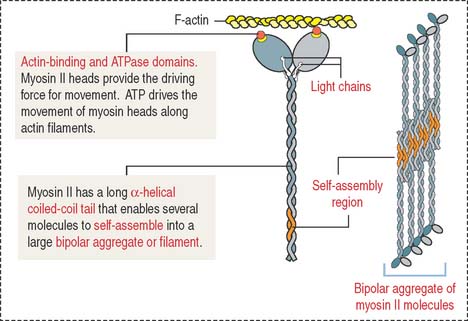
Myosin II consists of two identical heavy chains and two pairs of light chains (Figure 7-6; see Cytoskeleton in Chapter 1, Epithelium). At one end, each heavy chain forms a globular head. Two different light chains are bound to each head: the essential light chain and the regulatory light chain. The globular head has three distinct regions: (1) an actin-binding region; (2) an ATP-binding region; and (3) a light chain–binding region. Myosin II, like the other molecular motors kinesins and dyneins, use the chemical energy of ATP to drive conformational changes that generate motile force. As you recall, kinesins and dyneins move along microtubules. Myosins move along actin filaments to drive muscle contraction.
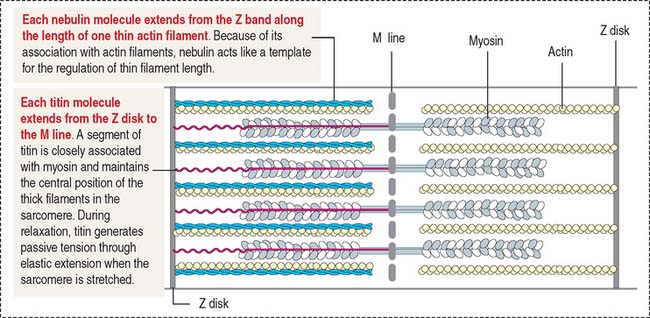
Nebulin (Figure 7-7) is associated with thin (actin) filaments; it inserts into the Z disk and acts as a template for determining the length of actin filaments.
Titin (see Figure 7-7) is a very large protein with a molecular mass in the range of millions. Each molecule associates with thick (myosin) myofilaments and inserts into the Z disk, extending to the bare zone of the myosin filaments, close to the M line. Titin controls the assembly of the myosin myofilament by acting as a template. Titin has a role in sarcomere elasticity by forming a spring-like connection between the end of the thick myofilament and the Z disk.
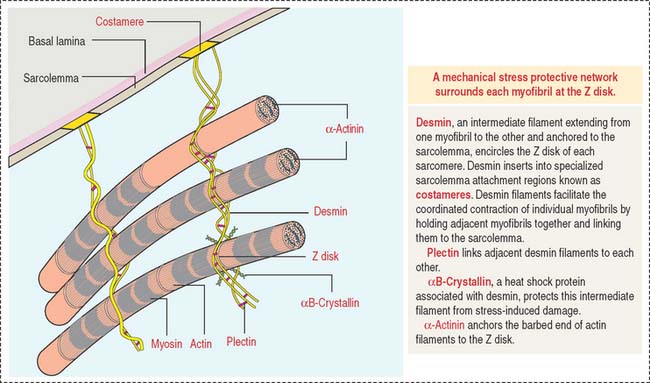
Desmin is a 55-kd protein that forms intermediate (10-nm) filaments. Desmin filaments encircle the Z disks of myofibrils and are linked to the Z disk and to each other by plectin filaments (Figure 7-8). Desmin filaments extend from the Z disk of one myofibril to the adjacent myofibril, forming a supportive latticework. Desmin filaments also extend from the sarcolemma to the nuclear envelope.
Mechanism of muscle contraction
During muscle contraction, the muscle shortens about one third of its original length. The relevant aspects of muscle shortening are summarized in Figure 7-9 as follows:
Creatine phosphate: A back up energy source
Figure 7-10 provides a summary of the mechanism of regeneration of creatine phosphate, which takes place in mitochondria and diffuses to the myofibrils, where it replenishes ATP during muscle contraction.
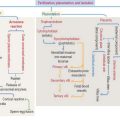
A depolarization signal travels inside the muscle by T tubules
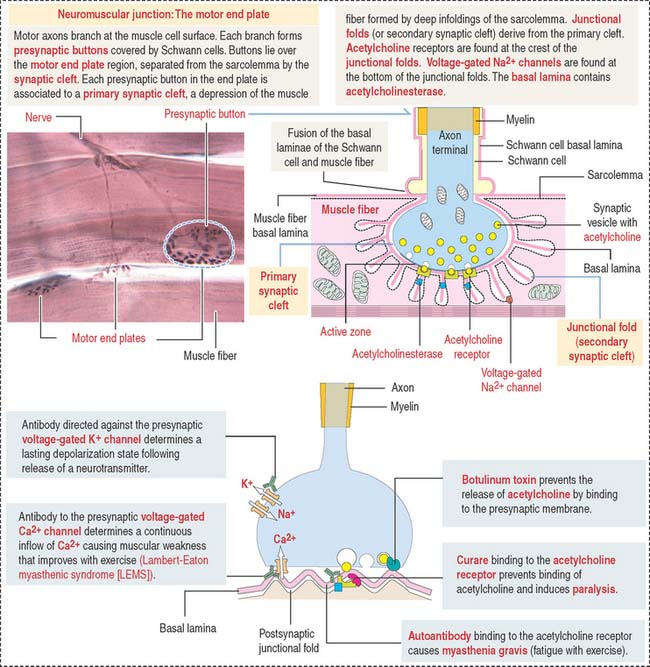
An excitation-contraction signal is generated by acetylcholine, a chemical transmitter released from a nerve terminal in response to an action potential. Acetylcholine diffuses into a narrow gap, called the neuromuscular junction, between the muscle and a nerve terminal (Figure 7-11). The action potential spreads from the sarcolemma to the T tubules, which transport the excitation signal to the interior of the muscle cell. Remember that T tubules form rings around every sarcomere of every myofibril at the A-I junction.
Box 7-A Functional types of muscle fibers
NEUROMUSCULAR JUNCTION: MOTOR PLATE
Synaptic buttons occupy a depression of the muscle fiber, called the primary synaptic cleft. In this region, the sarcolemma is thrown into deep junctional folds (secondary synaptic clefts). Acetylcholine receptors are located at the crests of the folds and voltage-gated Na+ channels are down into the folds (see Figure 7-11).
Clinical significance: Disorders of neuromuscular transmission
Synaptic transmission at the neuromuscular junction can be affected by curare and botulinum toxin (see Figure 7-11).
Curare binds to the acetylcholine receptor and prevents binding of acetylcholine. Curare derivatives are used in surgical procedures in which muscle paralysis is necessary.
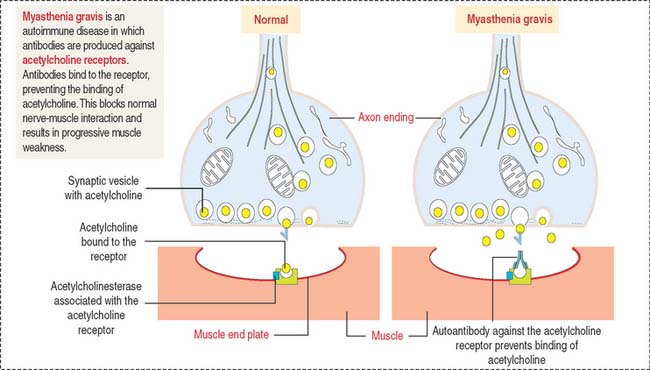
Myasthenia gravis is an autoimmune disease in which antibodies are produced against acetylcholine receptors (Figure 7-12). Autoantibodies bind to the receptor, preventing the binding of acetylcholine. This blocks normal nerve-muscle interaction and results in progressive muscle weakness.
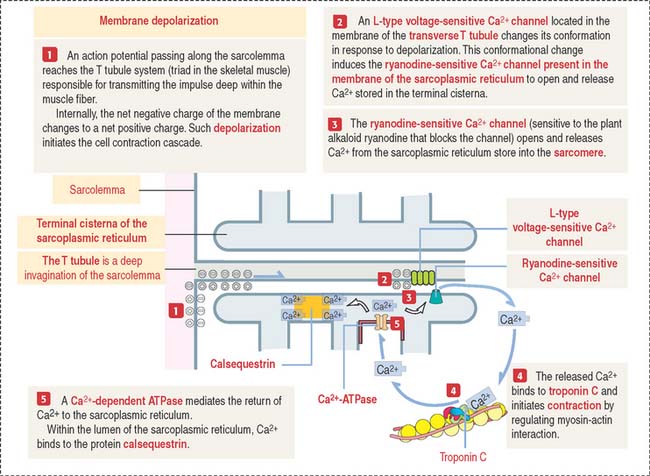
Calcium controls muscle contraction
When a depolarization signal arrives, Ca2+ exits the terminal cisternae of the sarcoplasmic reticulum with the help of the ryanodine-sensitive Ca2+ channel (Figure 7-13). In the sarcomere, Ca2+ binds to troponin C and causes a change in configuration of the troponin-tropomyosin complex. As a result, the myosin-binding site on the actin filament is exposed. Myosin heads bind to the actin filament, and hydrolysis of ATP occurs. We have seen that steady levels of ATP rely on the mitochondrial supply of creatine phosphate and the availability of creatine kinase (see Figure 7-10).
The energy of hydrolysis of ATP produces a change in the position of the myosin head, and the thin filaments are pulled past the thick filaments. Contraction results in the complete overlap of the A and I bands (see Figure 7-9). The contraction continues until Ca2+ is removed.
In summary, the sarcoplasmic reticulum, a network of smooth endoplasmic reticulum surrounding each myofibril (see Figure 7-4), stores Ca2+. In response to depolarization signals, the sarcoplasmic reticulum releases Ca2+. When membrane depolarization ends, Ca2+ is pumped back into the sarcoplasmic reticulum with the help of Ca2+-dependent ATPase, and binds to the protein calsequestrin (see Figure 7-13). Contraction can no longer take place.
Clinical significance: Muscular dystrophies
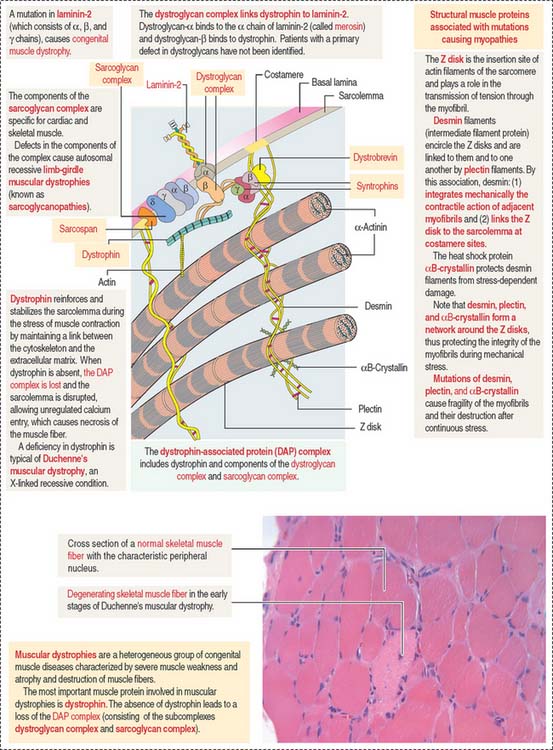
Muscular dystrophies are a group of congenital muscular diseases characterized by muscle weakness, atrophy, elevation of serum levels of muscle enzymes, and destructive changes of muscle tissue (Figure 7-14).
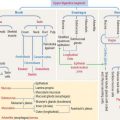
Muscular dystrophies are caused by a deficiency in the dystrophin-associated protein (DAP) complex. The DAP complex consists of dystrophin and two subcomplexes: the dystroglycan complex (α and β subunits), and the sarcoglycan complex (α, β, γ, δ,  , and ζ subunits; for simplicity, only four subunits are shown in Figure 7-14). Additional proteins include syntrophins (α β1, β2, γ1, and γ2 subunits), dystrobrevin, and sarcospan. Dystrophin, syntrophins, and dystrobrevin are located in the sarcoplasm; dystroglycans, sarcoglycans, and sarcospan are transmembrane glycoproteins. Patients with a primary defect in dystroglycans and syntrophins have not been identified.
, and ζ subunits; for simplicity, only four subunits are shown in Figure 7-14). Additional proteins include syntrophins (α β1, β2, γ1, and γ2 subunits), dystrobrevin, and sarcospan. Dystrophin, syntrophins, and dystrobrevin are located in the sarcoplasm; dystroglycans, sarcoglycans, and sarcospan are transmembrane glycoproteins. Patients with a primary defect in dystroglycans and syntrophins have not been identified.
The most important muscle protein involved in muscular dystrophies is dystrophin, a 427-kd cytoskeletal protein associated to F-actin, dystroglycans, and syntrophins (see Figure 7-14). The absence of dystrophin determines the loss of components of the DAP complex. The function of dystrophin is to reinforce and stabilize the sarcolemma during the stress of muscle contraction by maintaining a mechanical link between the cytoskeleton and the extracellular matrix. Deficiencies of dystrophin are characteristic of Duchenne’s muscular dystrophy (DMD). Most patients die young (in their late teens or early twenties) due to an involvement of the diaphragm and other respiratory muscles.
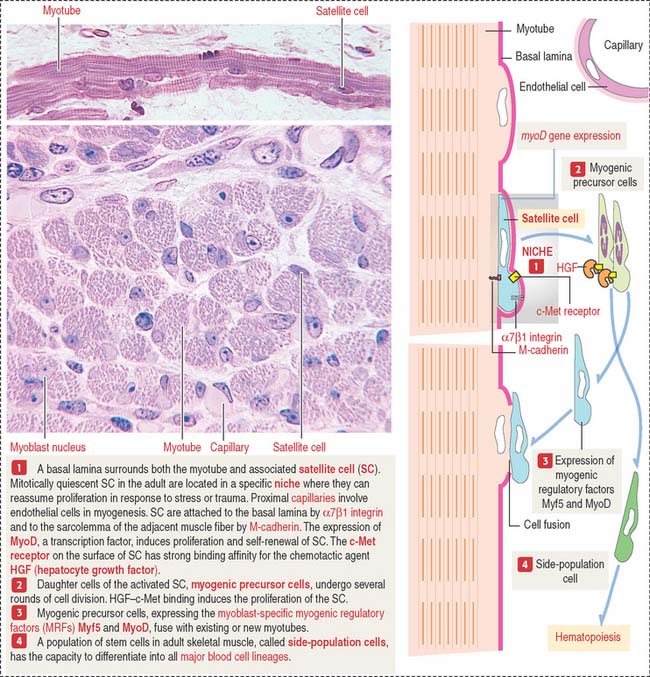
Clinical significance: Satellite cells and muscle regeneration
Satellite cells are a stem cell population distinct from the myoblasts. They attach to the surface of the myotubes before a basal lamina surrounds the satellite cell and myotube (Figure 7-15). Satellite cells are of considerable significance in muscle maintenance, repair, and regeneration in the adult. The function of satellite cells is regulated by their specific niche. A niche is a specific site where stem cells reside for an indefinite period of time and produce a cell progeny while self-renewing. The basis for the regulation of the satellite cell population is the attachment within the niche. Satellite cells express α7β1 integrin, linking F-actin to the basal lamina, and M-cadherin, a calcium-dependent adhesion molecule attaching the satellite cell to the sarcolemma of the adjacent muscle fiber. Capillaries are located close to the satellite cells.
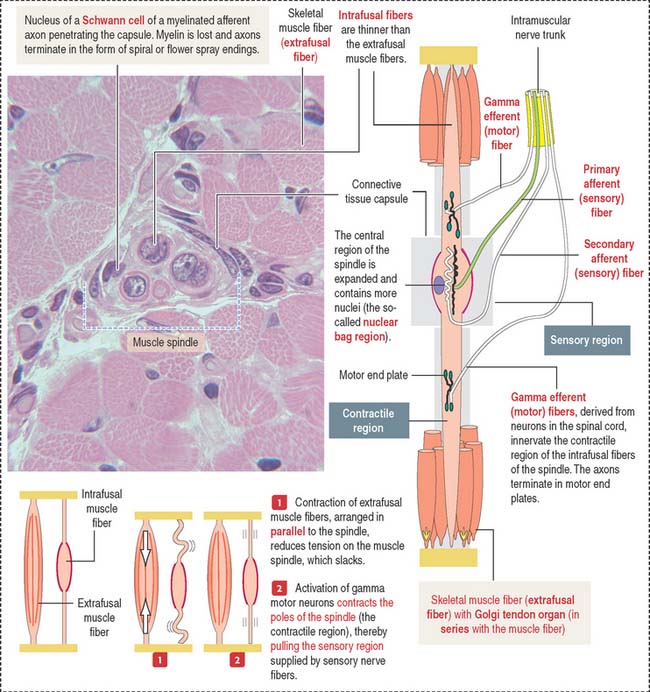
NEUROMUSCULAR SPINDLE
The central nervous system continuously monitors the position of the limbs and the state of contraction of the various muscles. Muscles have a specialized encapsulated sensor called the neuromuscular spindle that contains sensory and motor components (Figure 7-16).
There are two kinds of intrafusal fibers designated by their histologic appearance: (1) nuclear bag fiber, consisting of a central sensory (noncontractile) baglike region, and (2) the nuclear chain fiber, so-called because its central portion contains a chain-like array of nuclei. The distal portion of both nuclear bag and nuclear chain fibers consists of striated muscle with contractile properties.
Sensory nerve endings are arranged around the central nuclear region and sense the degree of tension of the intrafusal fibers.
CARDIAC MUSCLE
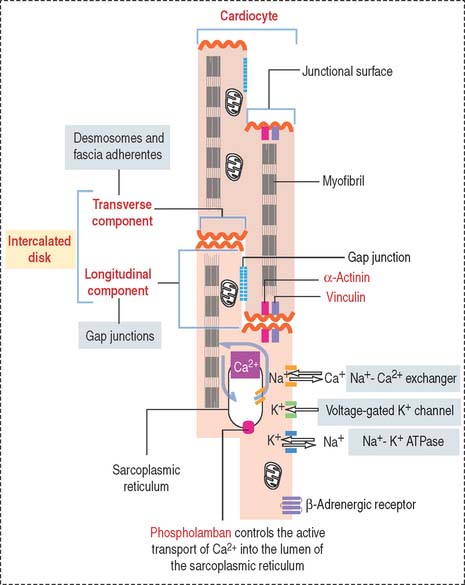
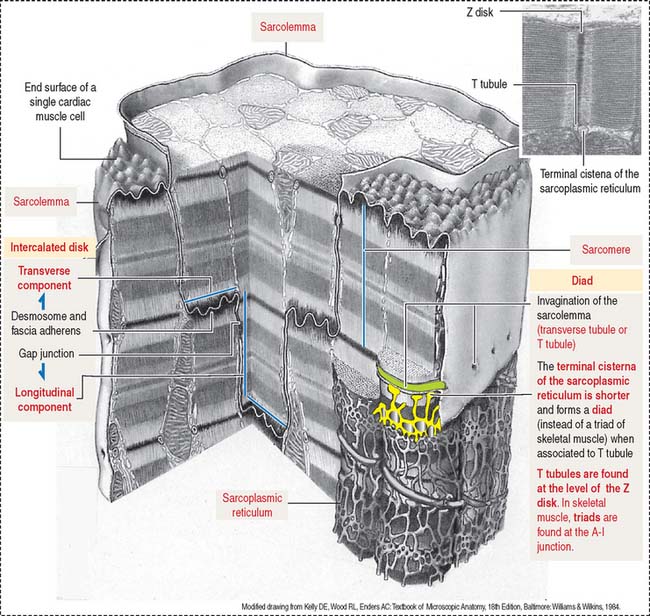
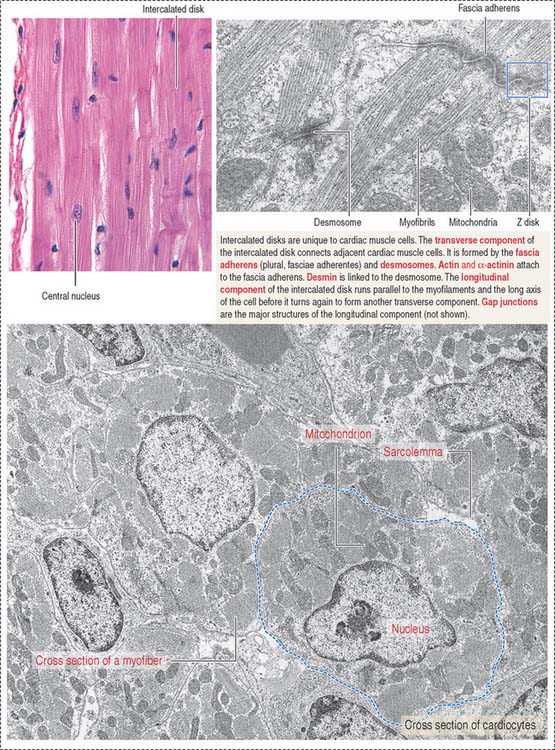
Cardiac cells (or cardiocytes) are branched cylinders, 85 to 100 μm long, approximately 15 μm in diameter (Figures 7-17 and 7-18), with a single centrally located nucleus (Figure 7-19).
The cardiocytes are joined end-to-end by specialized junctional complexes called intercalated disks (see Figure 7-17). Intercalated disks have a steplike arrangement, with transverse components that run perpendicular to the long axis of the cell and longitudinal components running in parallel to the cardiocyte for a distance that corresponds to one or two sarcomeres before it turns again to form another transverse component.
The transverse component consists of (1) desmosomes, which mechanically link cardiac cells, and (2) fasciae adherentes, which contain α-actinin and vinculin and provide an insertion site for the actin-containing thin filaments of the last sarcomere of each cardiocyte.
The terminal fibers of the conducting system of the heart are specialized, glycogen-rich Purkinje fibers. Compared with the contractile fibers, Purkinje fibers are larger, paler-stained, and contain fewer myofibrils (see Chapter 12, Cardiovascular System, for additional details).
Clinical significance: Transport proteins on the sarcolemma of cardiocytes
The sarcolemma of the cardiocyte contains specific transport proteins (see Figure 7-17) controlling the release and reuptake of ions critical for systolic contractile function and diastolic relaxation.
Active transport of Ca2+ into the lumen of the sarcoplasmic reticulum by Ca2+-dependent ATPase is controlled by phospholamban. The activity of phospholamban is regulated by phosphorylation. Changes in the amount and activity of phospholamban—regulated by thyroid hormone—may alter diastolic function during heart failure and thyroid disease. An increase in heart rate and cardiac output is observed in hyperthyroidism. We discuss the role of phospholamban in Graves’ disease (hyperthyroidism) in Chapter 19, Endocrine System.
Additional transporters, including the Na+-Ca2+ exchanger and voltage-gated K+ channels, regulate the intracellular levels of K+ and Na+. β-Adrenergic receptor is also present in the sarcolemma.
Clinical significance: Myocardial infarction
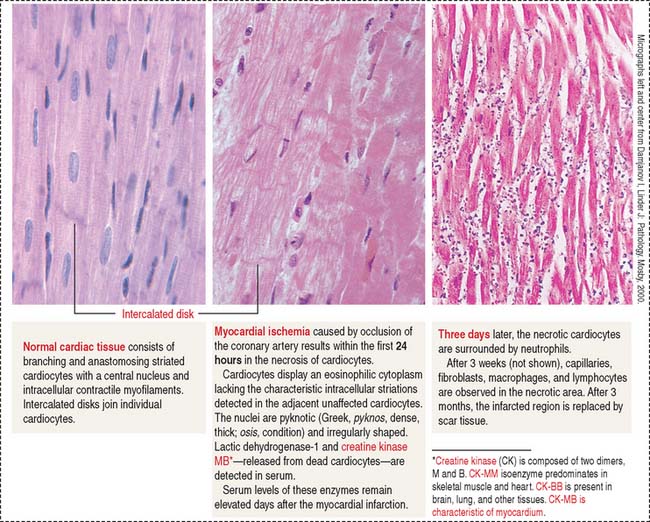
Irreversible damage of cardiocytes occurs when the loss of blood supply lasts more than 20 minutes. If blood flow is restored in less than 20 minutes—an event known as reperfusion—cardiocyte cell viability is maintained. Timing is critical for implementing early therapy to reestablish blood flow by using thrombolytic agents. The histologie changes of myocardial infarction are summarized in Figure 7-20.
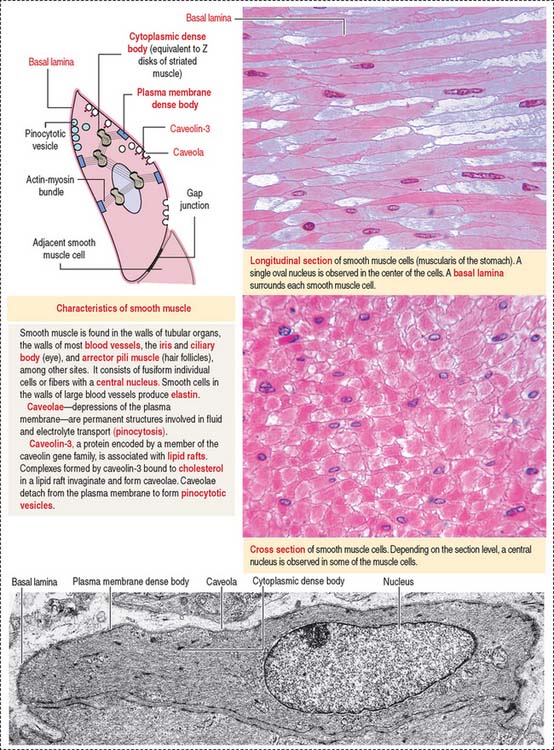
SMOOTH MUSCLE
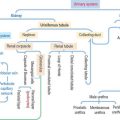
Smooth muscle differs from skeletal and cardiac muscle: smooth muscle cells are spindle-shaped, tapering cells with a central nucleus (Figure 7-21). The perinuclear cytoplasm contains mitochondria, ribosomes, rough endoplasmic reticulum, a Golgi apparatus, a latticework of thick myosin filaments, thin actin filaments, and intermediate filaments composed of desmin and vimentin. Actin and intermediate filaments insert into cytoplasmic and plasma membrane-associated structures rich in α-actinin, called dense bodies.
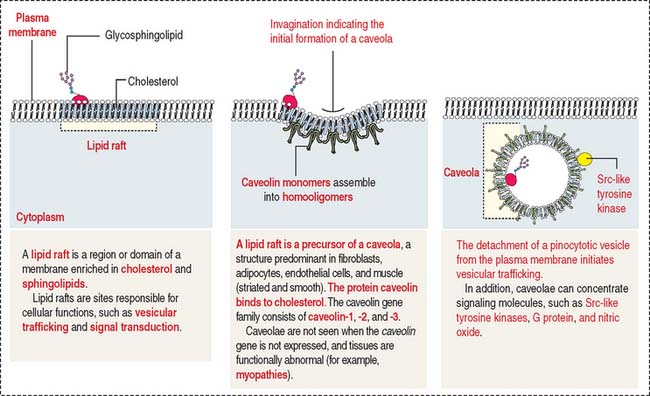
Invaginations of the plasma membrane, called caveolae, act as a primitive T tubule system, transmitting depolarization signals to the underdeveloped sarcoplasmic reticulum. The development of caveolae from lipid rafts and their diverse roles in several tissues are shown in Figure 7-22. Smooth muscle cells are linked to each other by gap junctions. Gap junctions permit synchronous contraction of the smooth muscle.
A basal lamina surrounds each muscle cell and serves to transmit forces produced by each cell.
Mechanism of smooth muscle contraction
We have seen that the sliding of the myosin-actin complex in striated muscle is the basis for contraction (see Figure 7-9). In smooth muscle, actin filaments and associated myosin attach to cytoplasmic and plasma membrane dense bodies, representing the equivalent of the Z disk of striated muscle. Dense bodies are attached to the plasma membrane through desmin and vimentin intermediate filaments. When the actin-myosin complex contracts, their attachment to the dense bodies determines cell shortening.
Calcium-dependent phosphorylation of myosin regulatory light chains is responsible for the contraction of smooth muscle. We have already discussed this mechanism in Chapter 1, Epithelium, when we analyzed the role of different myosins in the cell (review Figure 1-32).
Essential concepts
An excitation-contraction signal is produced by the release of acetylcholine from a presynaptic button into a primary synaptic cleft, an invagination on the surface of a muscle cell coated with basal lamina containing acetylcholinesterase. The primary synaptic cleft forms secondary synaptic clefts, also covered by basal lamina. Crests of the secondary synaptic clefts contain acetylcholine receptors.
 , and ζ). Sarcoglycanopathies (for example, limb-girdle muscular dystrophies) are caused by defects in components of the sarcoglycan complex.
, and ζ). Sarcoglycanopathies (for example, limb-girdle muscular dystrophies) are caused by defects in components of the sarcoglycan complex.