Chapter 43 Muscle Pain Syndromes
Muscle pain is common in clinical practice. The most frequently described muscle pain syndromes are myofascial pain syndrome (MPS) and fibromyalgia syndrome (FMS). Many question whether these two syndromes exist,13,158 but in the past 2 decades many well-controlled studies have strongly supported their existence. In light of this controversy, it is best to consider myofascial pain and fibromyalgia as clinical syndromes rather than distinct disease entities.
Etiology and Classification of Muscle Pain
Pathophysiologic Considerations in Muscle Pain
Muscle pain can be elicited by any irritation of the pain pathway from the muscle to the cerebral cortex. This can include peripheral sensitization of nociceptors in the muscle or central sensitization in the central nervous system (CNS).4,37,149
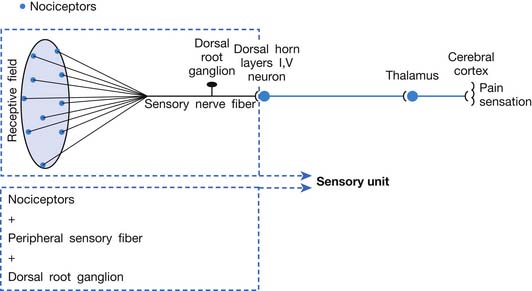
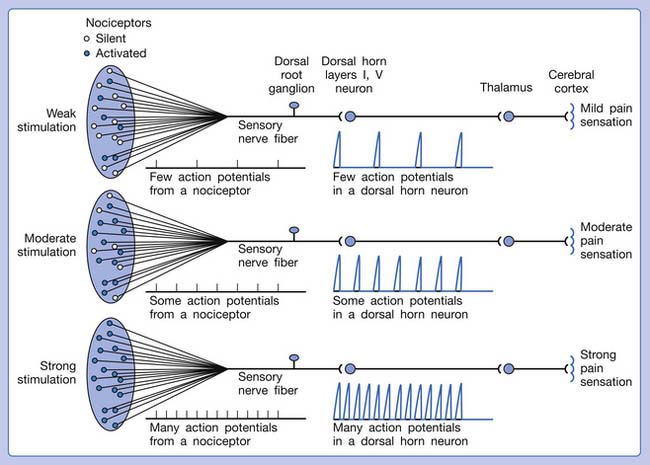
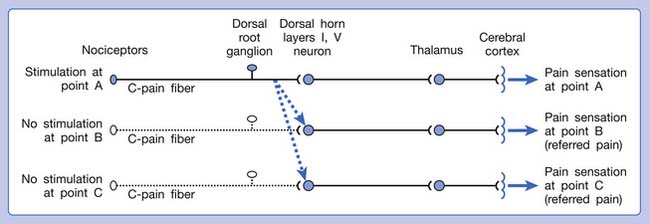
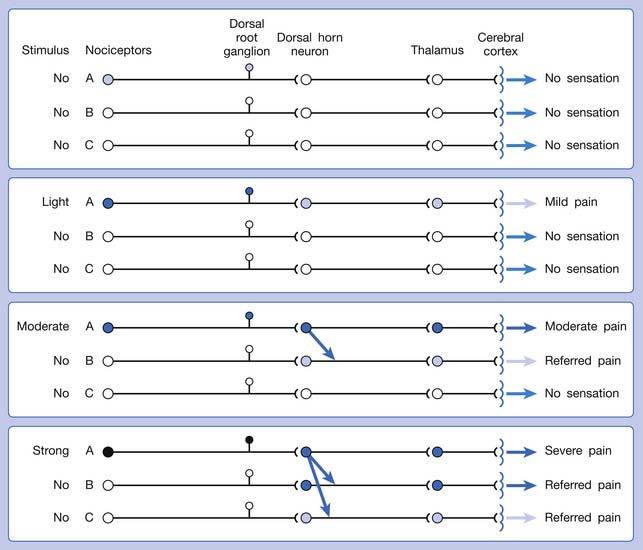
A basic peripheral “sensory unit” is defined as the dorsal root ganglion plus the peripheral nerve fibers (the peripheral axon and the centrally extended dendrite) and the supplied nociceptors (Figure 43-1). The location of pain is determined by the traditional physiologic concept of the law of projection. The cerebral cortex can perceive pain at a certain site via transmission of stimuli from the corresponding sensory unit to the dorsal horn of the spinal cord, and then via the spinothalamic tract to the thalamus and brain (Figure 43-2). The intensity of pain is determined by both the spatial and temporal summation of action potentials generated by the sensory unit (Figure 43-3). The discrimination of the character of pain is based on the simultaneous stimulation on two or more different sensory units with different entities (Figure 43-4).
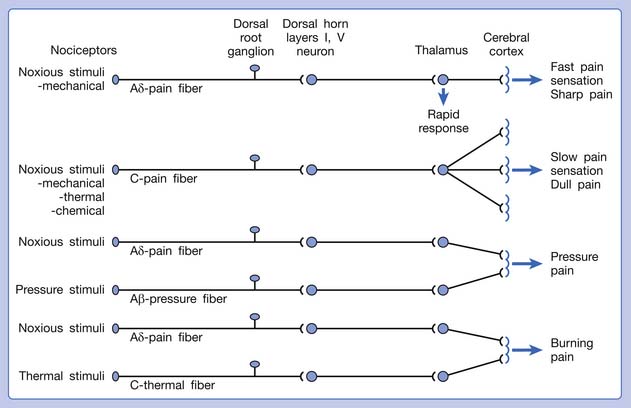
The pain pathway for muscle pain appears to be different from that of skin. In fact, there is no evidence for the existence of an ascending tract that exclusively mediates muscle pain.149 After the first synapse with the dorsal horn cell in the spinal cord, the nociceptive information from muscle is largely mixed with information from other tissues. The dorsal horn cells receiving information from muscles are convergent neurons (both wide dynamic range neurons and nociceptive-specific neurons). The spinothalamic tract is the main ascending pathway for muscle pain in the spinal cord, but it also mediates pain from other tissues. A specific cortical “center” for muscle pain does not seem to exist.149 The cortical perception of the site, intensity, or characteristic of muscle pain is a complex process probably involving areas such as the anterior cingulate cortex, in addition to the primary sensory cortex.149
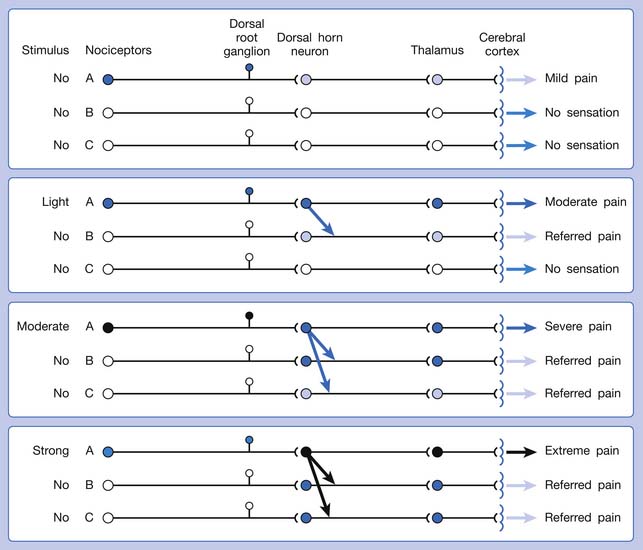
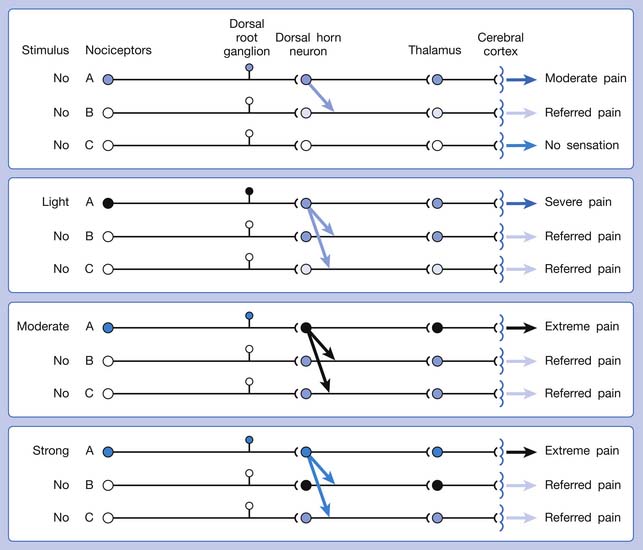
The ability of the CNS to react to a short-duration input of noxious stimulation with a long-lasting deviation from normal synaptic function (neuroplasticity) is particularly obvious in the case of muscle pain.4,149 Increased muscle sensitivity can be manifested as (1) increased pain intensity evoked by a noxious stimulus (hyperalgesia), (2) pain evoked by an innocuous stimulus (allodynia), or (3) increased size and number of referred pain areas with associated somatosensory changes.4
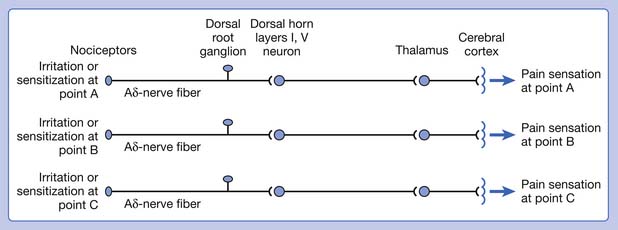
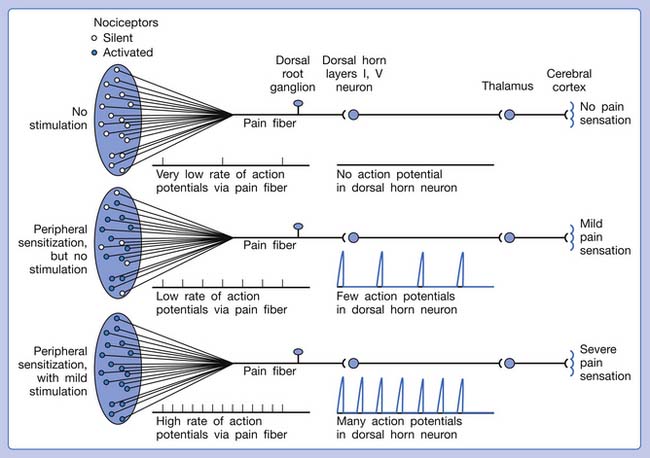
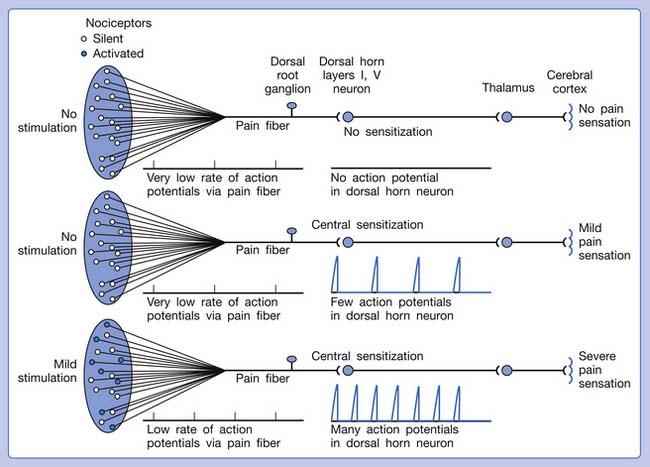
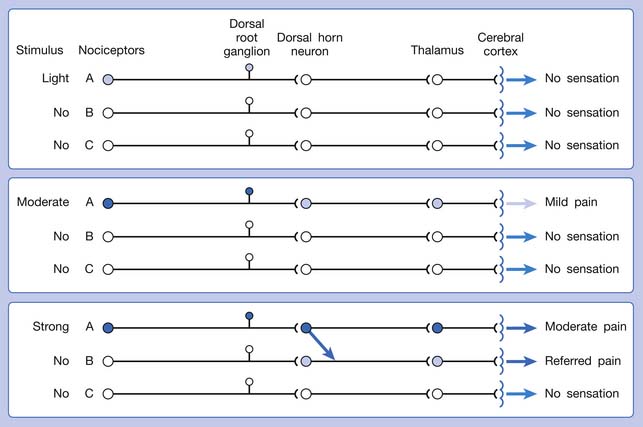
Referred pain can be elicited by a strong stimulation to a sensory unit with spread to the sensory pathways of different sensory units at the spinal cord (or higher) level of the CNS (Figure 43-5). Peripheral sensitization (nociceptor sensitization) occurs when there is an increase of excitability in the nociceptors of a sensory unit as a result of peripheral irritation (Figure 43-6). Central sensitization occurs when there is an increase of excitability of the spinal dorsal horn neuron (or higher center) for a long period.4,149 This is why a mild stimulus to a sensory unit can cause a cortical perception of severe pain in its receptive field (Figure 43-7).
Etiologic Classification of Muscle Pain
Based on its site along the pain pathway, muscle pain can be divided into five categories:
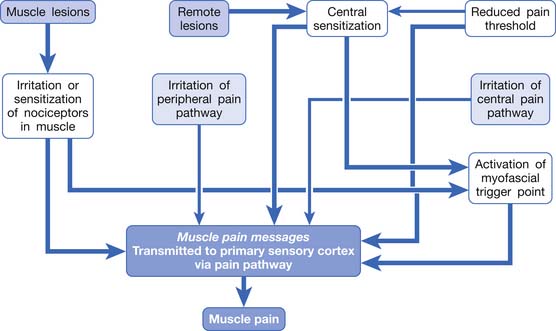
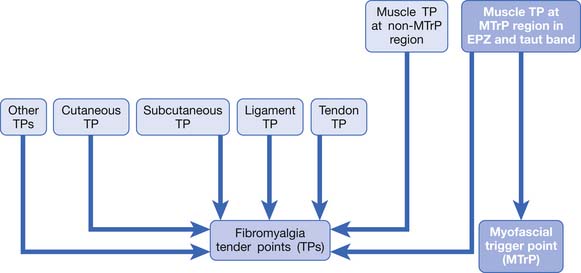
In summary, any irritation or sensitization of the muscle nociceptors or the muscle pain pathway can result in muscle pain (Figure 43-8). The causes of muscle pain are listed in Box 43-1. Russell has developed a system to classify soft tissue pain.162,163 In his system, focal muscle pain is localized pain, myofascial pain syndrome is regional pain, and fibromyalgia is generalized pain. Although this classification is convenient for clinical practice, it cannot provide the mechanism of muscle pain.
BOX 43-1 Etiologic Classification of Muscle Pain
Peripheral irritation or sensitization of the nociceptors in the muscle
Peripheral irritation (mechanical or chemical) to the peripheral nerve
Myofascial Pain Syndrome
Definition of Myofascial Pain Syndrome
Clinically MPS has been defined as a regional pain syndrome characterized by muscle pain caused by MTrPs.∗ In a broader sense, however, MPS includes a regional muscle pain syndrome of any soft tissue origin that is associated with muscle tenderness.53,149 In this chapter, MPS includes any pain phenomenon that is due to the activation of latent MTrPs. This can be secondary to pathologic conditions such as chronic repetitive minor muscle strain, poor posture, systemic diseases, and neuromusculoskeletal lesions (such as strain, sprain, enthesopathy, bursitis, arthritis, spinal disk lesion, etc.).87,98 Considerable evidence exists to suggest that myofascial pain syndrome is frequently caused by or related to a lesion in another soft tissue, as described in the next section.
Association of Myofascial Pain and Other Lesions (The Underlying Pathologic Lesions)
It can be observed clinically that although myofascial pain can be suppressed by an effective myofascial pain therapy, such as an MTrP injection, the pain often recurs a few days or weeks later if the related pathologic lesion is not eliminated.84,87,88 When the underlying etiologic lesion is completely eliminated, the MTrPs can be inactivated “permanently” unless reinjured. The underlying pathologic lesions are usually found in other regions remote to the activated MTrP. This remote activation of MTrP is due to central sensitization. Overuse or inappropriate use of a muscle can activate the MTrP in that muscle as a result of peripheral sensitization. Repetitive use of a muscle can reduce the pain threshold of the MTrP in that muscle.25 A recent study demonstrated that an MTrP (a sensitive spot in a palpable taut band with reduced pressure–pain threshold) of the extensor digitorum muscle can be induced by repeated eccentric exercise of that muscle.120 The MTrP can be successfully inactivated, however, by the avoidance of overuse or inappropriate use. Persistent or recurrent MTrPs are usually related to remote lesions, rather than to lesions in the affected muscle.
It has been reported that the number and pain intensity of MTrPs were significantly reduced after physical therapy or surgery for lumbar disk herniation.202 The association of active MTrPs with cervical disk lesions,111 cervical facet lesions,12 cervical radiculopathy,19 lumbar disk lesions,170 osteoarthritis of knee,6 teres minor tendinitis,199 lateral epicondylitis,47 floating kidney,109 septic arthritis,196 and herpes zoster26 has been demonstrated. Spinal adjustment107 and local injection132 of a cervical facet can effectively relieve the pain caused by MTrPs. It has been suggested that facet nociceptors and MTrP nociceptors might be connected in the spinal cord and might use the same nociceptive pathway to the higher center.12 Suppressing the facet pain suppresses the MTrP pain, and vice versa. However, during physical examination, compression of the related level facet joint can elicit pain in the MTrP regions, but needle stimulation to the associated MTrP can rarely induce pain in the correlated facet joint. Therefore facet dysfunction could be one of the important causes of the activation of MTrPs. It appears that, at least in some cases, MTrP pain is caused by the facet lesion or another musculoskeletal cause. It less frequently occurs as a consequence of primary muscle lesion.89,90,98 Activation of MTrPs can cause pain to avoid any movement that could interfere with the healing process of the primary lesion. Muscle pain can be an important defense mechanism to avoid further injury before complete healing of the etiologic lesion.89,98
Myofascial Pain Syndrome Controversies
The most important controversy in MPS is the problem of the lack of a set of specific diagnostic criteria. This set of agreed-on specific diagnostic criteria is required if different clinicians are to be able to agree on when it is present (with acceptable interrater reliability).152,158 Acceptable interrater reliability of many of the indicators of MTrPs appears to be obtainable only after the examiners have undergone special training.59
Although identification of an MTrP is clinically difficult, recent advances in MTrP research provide strong evidence that MTrPs exist. The electrophysiologic and morphologic findings of MTrPs cannot realistically be part of the criteria for the diagnosis of MTrP184 because they are expensive and time consuming. The practical diagnostic procedure still has to depend on a palpatory examination, which requires special clinical skills. Nonexpensive and simple devices for the measurement of MTrP characteristics are essential, and it is hoped that they can be developed in the near future.
Myofascial Trigger Point
Definition
Travell and Simons defined MTrP as the most tender (hyperirritable) circumscribed spot in a palpable taut band of skeletal muscle fibers.184,193 Pressure stimulation of a typical MTrP can elicit pain, referred pain, and LTR (brisk contraction of the muscle fibers in its taut band). The pain elicited by compression of this spot is familiar to the patient as the usual pain complaint (pain recognition).174
Latent MTrPs are tender but not spontaneously painful.184,193 They can be identified in most normal adult skeletal muscles but not in newborns or babies less than 1 year old.119 Active MTrPs are painful spontaneously or in response to movement of the involved muscle and are often so painful on palpation that the patient jumps (the “jump sign”). A latent MTrP can be observed clinically to evolve into an active MTrP. When an active MTrP is suppressed with treatment, it is still tender but not spontaneously painful because it has become a latent MTrP. Compression of the MTrP can reproduce or aggravate the patient’s usual complaint (pain recognition174), and elimination of the MTrP (or more appropriately, inactivation of the MTrP) can relieve the pain and other symptoms.
A myofascial pain patient can have many active MTrPs. Typically the syndrome begins with the patient having only one active MTrP in the affected muscle as a result of a soft tissue lesion. If it is not appropriately treated or the associated underlying pathologic lesion is not eliminated, the pain region can expand to other regions and develop additional active MTrPs. The original MTrP is called the primary MTrP or key MTrP, and the ones that occur later are called secondary MTrPs or satellite MTrPs.184 Inactivation of a key MTrP can subsequently eliminate the satellite MTrPs.84,184
Characteristics of Myofascial Trigger Points
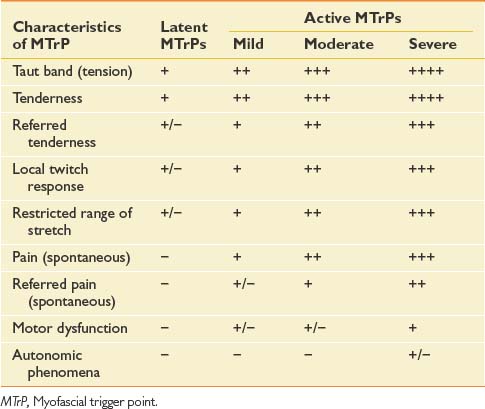
The chief characteristics of latent and active MTrPs are listed in Table 43-1. In clinical practice, active MTrPs can be further classified into categories based on the severity of pain and other characteristics. A clear-cut distinction exists between a latent MTrP and an active one, that being the existence of spontaneous pain. No clear distinctions, however, exist to categorize the severity of active MTrPs.
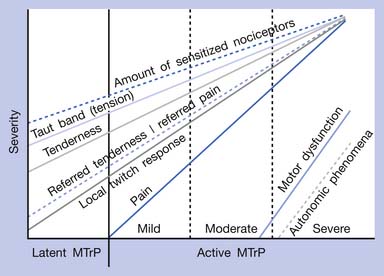
The separation of active MTrPs into three categories (mildly, moderately, and severely active MTrPs, listed in Table 43-1) is artificial. The common characteristics of any MTrP include taut band, spot tenderness, referred tenderness, LTR, and restricted range of stretch. Referred tenderness, LTR, and restricted range of stretch, however, are not always elicited in latent MTrPs. In a mildly active MTrP, it is painful, but the referred pain is often not obvious. In a moderately active MTrP, referred pain usually develops. Patients with severely active MTrPs typically show motor dysfunction and autonomic phenomena. As seen in Figure 43-9, for MTrPs the change in symptom severity is continuously variable. The degree of sensitized nociceptors in an MTrP region is the most important factor in determining the degree of MTrP severity.90 Any MTrP phenomenon (characteristic) shown in Figure 43-9 can be continuously variable and not necessarily follow the straight lines seen in the figure.
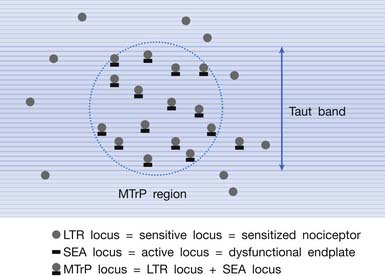
MTrPs are usually located within the end-plate zone because end-plate noise (EPN) can usually be recorded electromyographically in an MTrP region much more frequently than in other areas of normal muscle tissue.174,180,181,184 Simons defined this typical MTrP occurring in the end-plate zone as a central MTrP, and the trigger points in other locations in the muscle or tendon attachment region as attachment trigger points (A-TrP).184 The A-TrPs do not have all the characteristics of central MTrPs. A-TrPs are always located at the end of a taut band. Pain, referred pain, and LTR can be elicited, but EPN cannot be recorded from an A-TrP region of muscle.
Clinical Characteristics of Myofascial Trigger Points
Painful or Tender Spot
The most important characteristic of an MTrP is a circumscribed spot in the muscle with pain or tenderness. For most muscles, this spot can be identified in approximately the same region in different persons.193,194 The exact location of the MTrP in almost every skeletal muscle has been demonstrated in the “Trigger Point Manual.”184,193,194
Taut Band
Simons et al.174,184 have considered that an MTrP is always found in a taut band of skeletal muscle fibers, and a taut band is the precursor of an MTrP. A taut band is an essential component of the definition of an MTrP.174,184,193,194 Restriction of stretch with reduced range of motion can occur in a muscle with one or more tense taut bands that produced pain during stretch. This is different from muscle shortening caused by contraction because no muscle action potentials can be recorded from the muscle fibers in a taut band.149
Referred Tenderness and Referred Pain
Referred tenderness occurs when a distant muscle has pain in response to compression of an MTrP. Referred pain occurs when spontaneous pain is referred to remote sites from an MTrP.184 The occurrence of referred tenderness depends on two factors: the irritability of the MTrP and the pressure of compression.82,95,97 “Spontaneous” referred pain usually occurs clinically in relatively severe cases of MPS (see Figure 43-9).82 Each muscle tends to have the same area of referred pain in different persons.184,193,194 Figure 43-10 shows one example of the distribution of referred pain from an MTrP in the upper trapezius muscle. Regarding the location and referred pain pattern for all the exact muscles, please refer to the Travell and Simons texts.184,193,194
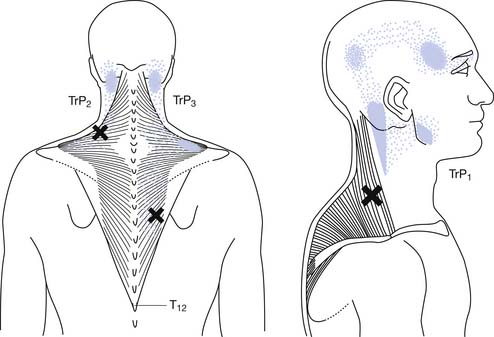
FIGURE 43-10 Referred pain pattern of myofascial trigger points in the upper trapezius muscle.
(Modified from Simons DG, Travell JG, Simons LS: trapezius muscle.In Travell & Simons’ myofascial pain and dysfunction: the trigger point manual, vol 1, ed 2, Baltimore, 1999, Williams & Wilkins, with permission.)
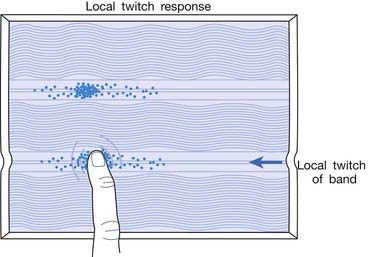
Local Twitch Responses
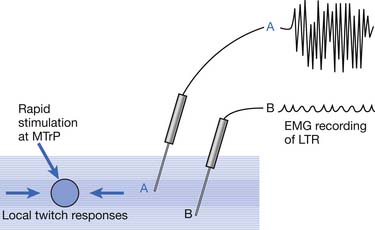
LTR is a sudden brisk contraction of a group of muscle fibers (usually in a taut band) in response to snapping palpation (quick compression across the muscle fibers perpendicularly of the MTrP) (Figure 43-11).184,193 The occurrence of the LTR also depends on the irritability of the MTrP and the pressure applied for eliciting LTR. High pressure is required to elicit an LTR in an MTrP with low irritability, and vice versa. A needle tip can provide high pressure stimulation to the MTrP and can elicit LTR much easier than using finger palpation.97,179
Motor Dysfunction
The clinically observed reduced muscle strength (weakness) caused by an MTrP is neither a true neurogenic nor a myogenic weakness. It is a pain-induced weakness and usually occurs only in severe cases of myofascial pain. Disuse muscle atrophy occurs rarely, mainly in cases of MPS with persistent severe pain.184 Other motor dysfunctions related to MTrP include increased responsiveness (muscle hyperactivity, referred muscle hyperactivity, referred inhibition), delayed relaxation, and increased fatigability (accelerated fatigability, delayed recovery).4,149 Mense and Simons149 called this hyperresponsiveness “muscle spasm” and “referred muscle spasm” and defined muscle spasm as involuntary contraction (with electromyographic [EMG] activity) of a muscle that is not dependent on posture.
Autonomic Phenomena
In extremely severe cases of MPS, autonomic phenomena (including abnormal sweating, abnormal tearing, abnormal salivation, increased vasomotor response, and increased pilomotor response) can be observed. For example, an MTrP in the sternocleidomastoideus muscle with high irritability can result in a discharge of tears and redness of the conjunctiva.193
The Basic Science of Myofascial Trigger Points
Myofascial Trigger Points Have Multiple Sensitive Loci
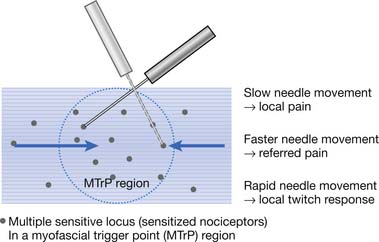
The concept of multiple sensitive loci in an MTrP region was first described by Hong88 and was based on clinical observations during MTrP injections. Using the traditional technique for MTrP injection with slow movement of the needle, pain and sometimes referred pain can be elicited when the needle tip encounters the tiny sensitive locus, but LTR can rarely be elicited by low-pressure stimulation (Figure 43-12). Hong84,86 has suggested a new injection technique by moving the needle in and out in a straight track and rapidly to avoid damage to the muscle fibers caused by side movement of the sharp-edged needle or resulting from the grabbing of the needle by an elicited LTR.184 An LTR can be easily elicited during rapid needle insertion (high-pressure stimulation because pressure = force/area, and force = mass × acceleration) when the needle tip encounters a sensitive locus (see Figure 43-12). Many LTRs can be elicited by needle insertions into different sensitive loci. More LTRs can be elicited in a painful MTrP region than in a region having low-grade pain. This is because the more sensitive loci (sensitized nociceptors) are located in the MTrP with high irritability rather than low irritability (see Figure 43-9). The sensitive locus has also been defined as an LTR locus because an LTR can be elicited by a strong pressure stimulation on this tiny locus.98 Histologic study revealed a nerve ending (nociceptor) at the LTR locus (Figure 43-13).93 Injection of certain dyes into the area of the nociceptors can cause the dye to spread up to the sensory neurons.128
Animal Models for Myofascial Trigger Point Studies
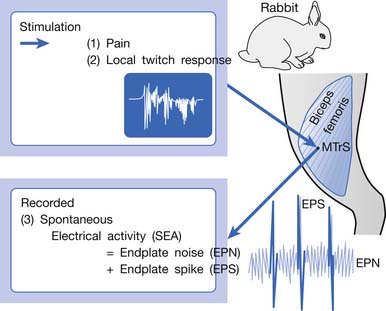
An animal model for myofascial pain study was initially reported in 1994.99 A sensitive spot could be found in the biceps femoris muscle of a rabbit. When this sensitive spot was compressed before anesthesia, the rabbit kicked and exhibited signs of pain. This spot was marked, and under anesthesia, a brisk muscle twitch that was similar to LTRs elicited in human skeletal muscle could be elicited by needle stimuli in the marked spot but rarely in other unmarked spots.99 Similar to human studies, spontaneous electrical activity (SEA), including EPN and end-plate spike (EPS), could be frequently recorded in this marked sensitive spot, but rarely from other sites in the end-plate zone and never in the non–end-plate zone.180,181 To distinguish it from human MTrP, this sensitive spot in the rabbit was defined as a myofascial trigger spot (MTrS).99 This animal model provided at least three important characteristics similar to human MTrP: pain, LTR, and SEA (Figure 43-14). Other similarities exist between human MTrP and rabbit MTrS as described below.98
Studies on Taut Band
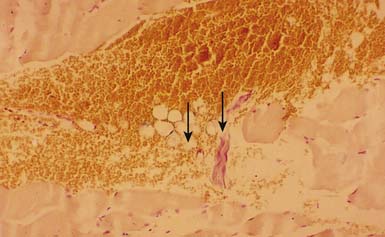
Electron microscopy has shown morphologic evidence of taut bands and contraction knots (or locally shortened sarcomeres) in the MTrP region (end-plate zone) of human muscles.157 In a light microscopic study with trichrome stain on canine muscles, Simons and Stolov182 demonstrated the contraction knot (Figure 43-15). A light microscopic study on rat muscles,150 an ultrasonic study on human muscles,58 and an magnetic resonance elastography study on human muscles24 have all shown the existence of taut band.
Studies on Myofascial Trigger Point Pain
To assess the effectiveness of a certain therapeutic modality or technique for myofascial pain relief, subjective pain intensity can be assessed with a numerical rating scale or a visual analogue scale (VAS). The scale usually ranges from 0 to 10, with 0 representing no pain and 10 the worst pain that could be experienced. The numerical rating scale is verbally reported by the patient based on the feeling of pain intensity. For VAS assessment, the patient is requested to mark the subjective feeling of pain at a 10-cm line on a card. The two ends of the line are marked with 0 and 10 to represent “no pain” and “worst pain,” respectively. The pain intensity is assessed by reading the distance between the 0 point and the marked point using the scale on the reverse side of the card.
Pressure algometer, developed by Andrew Fischer, is a useful tool to document MTrP irritability.49,51,82 The pressure applied on the MTrP can be read from a scale on this device as soon as the patient reports pain. Three consecutive measurements separated by intervals of at least a few minutes are required to confirm the consistency of repeated measures. This device can provide a semiobjective measurement because the patient cannot see the scale on the algometer. It has been reported that the pressure algometer is a reliable and valid device to help assess myofascial pain.154 The pressure algometer is also useful in assessing the effectiveness of MTrP therapy.∗
Spontaneous Electrical Activity Assessment
Recent studies have suggested that the irritability of an MTrP is proportional to the prevalence129 and the amplitude29 of EPN recorded from that MTrP region. It is likely that the measurement of SEA (including EPN) in an MTrP region can be used for the assessment of the effectiveness of a certain therapeutic method.†
Studies on Referred Pain
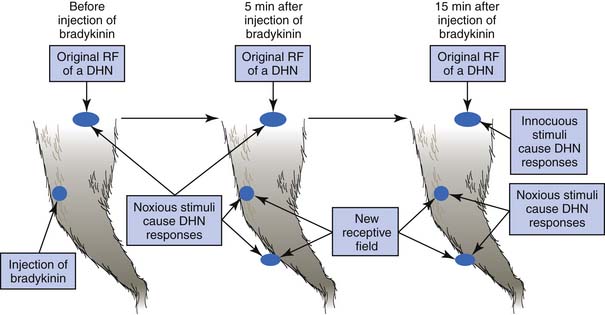
Referred pain from a muscle to another distant muscle has been demonstrated in animal studies.80,81,144–150 Pain signals from peripheral stimuli can be electrophysiologically recorded from sensory neurons in the dorsal horn of the spinal cord in a rat. The receptive field of a dorsal horn neuron can be identified by mapping the peripheral sites receiving stimuli. In a study by Hoheisel et al.81 (Figure 43-16) the original receptive field could be expanded to other sites 5 minutes after a noxious chemical substance was injected into another distant muscle. It appears that the brain can perceive pain at other sites in addition to the originally stimulated site. Fifteen minutes after a noxious injection, an innocuous stimulation could also induce a response in the original receptive field (allodynia). This phenomenon cannot be simply explained by the traditional “convergence–projection theory” (synaptic connections of a dorsal horn neuron with two separate innervation areas) because the size, number, and nature (high threshold or low threshold) of receptive fields for a dorsal horn neuron can be changed rapidly in the presence of noxious stimuli to muscle.80,112,149 Mense144,146,147 considered this mechanism to be secondary to the unmasking of formerly ineffective synaptic connections among neurons corresponding to different receptive fields. Many synaptic connections described in the convergence–projection theory are not functionally effective in a normal situation. However, some of these connections can become effective under the influence of certain conditions, such as a longstanding painful stimulus or a particularly strong painful stimulus.149 A strong noxious stimulus can send the impulse to the corresponding dorsal horn neuron and induce it to release substance P and calcitonin gene-related peptide (CGRP), which diffuse to other dorsal horn neurons and promote silent synaptic connections.148 This is a type of central sensitization.
In human studies, referred tenderness could also be elicited from a latent MTrP region or even normal muscle tissues if a strong pressure was applied.95,197 The pressure required to elicit referred pain from a compressed site is proportional to the degree of irritability (amount of sensitized nociceptors) at that site (Figures 43-17 to 43-21). The concept demonstrated in Figure 43-9 has been developed based on the above important studies.95,197 However, it is unclear why referred pain can also be elicited from a non-MTrP region, especially for that near an active MTrP. The non-MTrP region might also contain few nociceptors (see Figure 43-18). It is also likely that the high-pressure compression can indirectly stretch the muscle fibers in the taut band containing the MTrP.
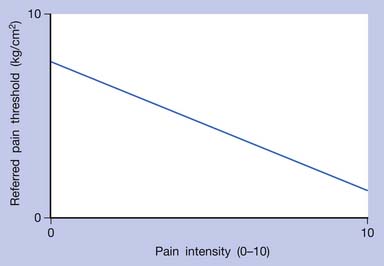
FIGURE 43-17 Referred pain threshold is proportionate to pain intensity of a myofascial trigger point.
Studies on the Local Twitch Response
EMG activity of an LTR elicited by stimulation of an MTrP can be recorded in the taut band containing that MTrP.54,179 LTR is most easily elicited when the MTrP, but not any other site, is mechanically stimulated (Figure 43-22) and is best recorded electromyographically in the taut band (Figure 43-23).99 In one case study, the LTR could not be electromyographically recorded from the extensor digitorum communis muscle in a patient with brachial plexus injury involving the posterior cord.91 The injured nerve partially recovered 6 months later, and then the LTR could be partially recorded.
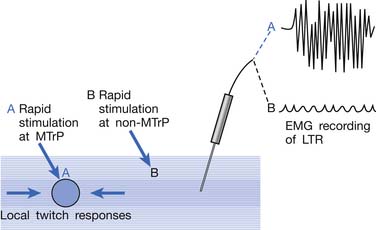
FIGURE 43-22 Local twitch responses (LTR) can be recorded only when the myofascial trigger point (MTrP) is stimulated.
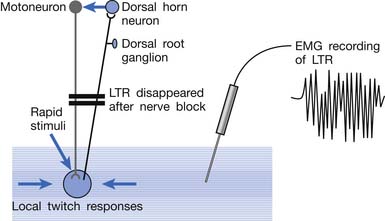
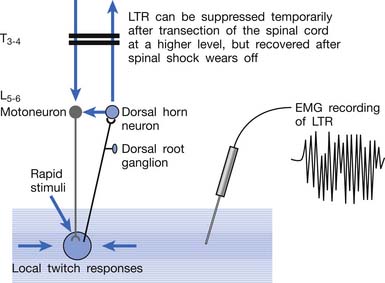
Animal studies have also demonstrated that LTRs can be elicited and electromyographically recorded from a muscle only if the innervated nerve is intact with a complete connection with the spinal cord (Figure 43-24).99 LTRs recorded from a muscle could be transiently suppressed after a complete transection of the spinal cord at a level higher than that providing innervation to that muscle but nearly completely recovered after the spinal shock period (Figure 43-25).100 It has been concluded that LTR is mediated via a spinal cord reflex.85,98,100 LTR can also be monitored by sonography.58
Surface Electromyographic Studies on Myofascial Trigger Point–Related Motor Dysfunction
As demonstrated in surface EMG, MTrP, as well as other soft tissue lesions such as ligamentous sprain or articular dysfunction, can induce muscle hyperactivity (so-called “muscle spasm”) in associated muscles.41,61,76,78,117 Because of muscle hyperactivity, the surface EMG amplitude recorded over a muscle with MTrP was 20% greater than the asymptomatic muscle. Headley76 further demonstrated that when pressure was applied on the MTrP of the supraspinatus muscle, a referred muscle hyperactivity could identified from surface EMG recordings in a distant muscle. Carlson et al.20 also found a significant reduction in surface EMG activity of the masseter muscle after MTrP injection of the trapezius muscle. On the other hand, referred inhibition of gluteal muscle hyperactivity by an active MTrP in the quadratus lumborum was demonstrated in surface EMG recordings.78 In studies on experimental acute muscle pain by Arendt-Nielsen’s group, reduced activation of the painful muscle was frequently observed.4,45,46,66,190 It is unclear why this finding is opposite to that seen in patients with chronic pain conditions.
Delayed relaxation of a muscle with active MTrP can occur during a repetitive exercise (alternative contraction and relaxation), and loss of relaxation at each end of contraction can be found.77,149
Acceleration fatigability with delayed recovery has been demonstrated in surface EMG studies.73,77,149 Evidence of initial fatigue (increased amplitude and reduced median power frequency of surface EMG activity) was found in a work tolerance study on the upper trapezius muscle with painful MTrPs compared with the contralateral pain-free muscle.73
Electrophysiologic Findings in a Myofascial Trigger Point Region: Spontaneous Electrical Activity (End-Plate Noise + End-Plate Spikes)
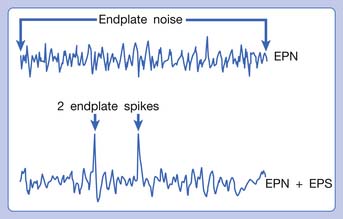
Hubbard and Berkoff114 were the first to record SEA from an MTrP region of the upper trapezius muscle in 1993. They suggested that SEA were potentials recorded from a muscle spindle.113 SEA can be recorded only in the end-plate zone, more frequently from an MTrP region than from other regions, including normal muscle tissues.∗ Further studies by Simons et al.180,181 indicated that there are two components in the SEA recorded from the MTrP region, including the low-grade continuous electrical activity and few sharp spikes with much higher amplitude (Figure 43-26). The low-grade continuous activity is the EPN, which is an accumulation of nonpropagated miniature end-plate potentials (MEPPs), and the spike is an EPS, which is a propagated action potential generated from the end-plate.174,176 EPS is probably elicited by a strong irritation of the recording needle on the “hyperirritable” end-plate because it occurs much frequently in hyperirritable active MTrPs than latent ones.129,174,176,180 When the SEA is recorded by an EMG needle, the patient always complains of a sharp pain sensation.180 This painful locus from where SEA is recorded was originally defined as an active locus,181 and later as an SEA locus98 or EPN locus.127 Simons174 has suggested that the occurrence of EPN indicates excessive leakage of acetylcholine (ACh) in the end-plate region based on extensive reviews of old literature. Previous studies indicated that only MEPPs were observed in a normal end-plate, and EPN were recorded only after a mechanical or chemical stimulation to the end-plate that induced excessive ACh leakage.116,138 These ACh molecules can cause calcium release from the t-tubules of the sarcomeres in the end-plate zone but not in other portions of muscle fibers. This is because EPN potentials are not propagated action potentials (no action potential can be recorded from a taut band). Muscle tension from a taut band is completely different from that caused by muscle hyperactivity (“spasm”) because propagated action potentials can be recorded from a muscle with hyperactivity secondary to a hyperirritable active MTrP.
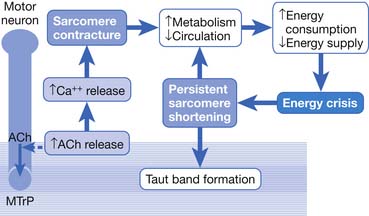
This focal contracture of sarcomeres can produce a contraction knot as shown in the electron microscopic findings (see Figure 43-15).182 Based on this finding, Simons183 brought an “energy crisis” hypothesis to explain the formation of a taut band (Figure 43-27). Sarcomere contracture can cause an increase in metabolism and a decrease in local circulation, so that the sarcomere cannot relax because of an inadequate energy supply. Sarcomere shortening occurs only in the end-plate region, but the sarcomeres outside the end-plate zone in either direction become somewhat elongated (because the muscle fiber length is unchanged) (see Figure 43-15). In this way, the muscle tension in the end-plate region is increased.
In an animal study, SEA persisted after transection of peripheral nerve and a high-level spinal cord.101 This results in the leakage of ACh in the end-plate region not being under the immediate control of the nervous system. In a single-fiber EMG study in rabbits, Kuan et al.130 found no increase in neuromuscular jitter in the MTrS region and suggested that the neuromuscular transmission itself was not impaired. This would indicate that the excessive ACh leakage is a secondary phenomenon and not caused by an abnormality in neuromuscular transmission. These findings support the theory that energy crisis in the MTrP region is a focal reaction and not related to neural controls. Once the nociceptors are involved in the integrated mechanism (see below), however, the spinal cord is involved in the mechanism of central phenomena such as pain, referred pain, and LTR. In a recent single-fiber EMG study, Chang et al.21 found evidence of degeneration in motor nerve endings in the MTrP region. Further study is required to clarify whether any motor nerve lesion is involved in the pathogenesis of MTrP.
Biochemicals Associated With Pain and Inflammation in a Myofascial Trigger Point Region
Using a microanalytic technique, Shah et al.171,172 measured biochemicals (associated with pain and inflammation) at GB-21 (acupuncture point in the MTrP region) in the upper trapezius muscle in subjects identified as active (having neck pain and MTrP), latent (no neck pain but with MTrP), or normal (no neck pain and no MTrP). They found that concentrations of all analyzed biochemicals were significantly higher in active than latent or normal subjects. It appears that substances related to either pain or inflammation are elevated in the vicinity of active MTrPs. These findings strongly support Simons’ integrated hypothesis of “energy crisis.”171,172 They also found a marked elevation of biochemicals during the LTR followed by a slow, variable return to baseline. Substance P and CGRP were the only two analytes for which concentrations during the recovery period after the LTR were significantly below the baseline concentrations.172 This interesting finding could possibly explain the immediate relief of pain (reduced substance P and CGRP) after eliciting LTRs during MTrP injection, but the overall mechanism is still unclear.
Studies on Myofascial Trigger Points in Early Life and Formation of Myofascial Trigger Points
Kao172 was the first to report that no latent MTrPs could be identified in children less than 1 year of age. It appears that latent MTrPs develop as the child is growing up. It is still unclear, however, when and how the nociceptors in the MTrP region become sensitized in later life. Gunn68,69 suggested that the formation of MTrP is due to minor lesions in the peripheral nerve, especially in the nerve root. This hypothesis cannot be generalized for all kinds of MTrPs because MTrPs can be activated as a result of a lesion other than nerve.83,98 It is not unlikely, however, that the formation of a latent MTrP in early life is related to minor peripheral nerve injury during the growing-up period.83 In clinical observation, the author has found that many adult patients with diffuse MTrPs in the upper back and shoulders related to cervical facet lesions have related neck injuries in early life.
Summary of Myofascial Pain Theory
Multiple Myofascial Trigger Point Loci in the Myofascial Trigger Point Region
It has been hypothesized that there are multiple MTrP loci in an MTrP region (Figure 43-28).98 An MTrP locus contains a sensory component (the sensitive locus or LTR locus) and a motor component (the active locus or SEA locus). An LTR locus is a sensitized nociceptor (free nerve ending),93 and an SEA locus is a dysfunctional end-plate.174,176 An SEA locus is in the close vicinity of an LTR locus. They interact mutually for the formation of a taut band. Stimulation of an LTR locus can elicit pain, referred pain (as a result of central sensitization), and LTR (via spinal cord reflex).
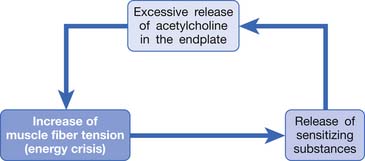
Simons’ Integrated Hypothesis of Myofascial Trigger Point
Based on the studies mentioned above, Simons et al.149,184 has postulated three essential features of MTrPs: excessive ACh release, sarcomere shortening, and release of sensitizing substances. These three essential features relate to one another in a positive feedback cycle (Figure 43-29) that is self-perpetuating once it is started (but which can be interrupted at several points in the cycle in a number of ways).149,177,178,184 An increased ACh release in the neuromuscular junctions (motor end-plates) can cause an increase of the muscle fiber tension. This results in a taut band that contains an MTrP and subsequently can cause “energy crisis” with increased metabolism and local ischemia with hypoxia, which can then induce secretion of sensitizing substances to cause pain. The sensitizing substances can then cause abnormal ACh release so that a vicious cycle is activated. It is unclear, however, whether the abnormal ACh release initially occurs to trigger the sensitization of nociceptors (peripheral sensitization) or the inflammatory reaction initially causes the release of inflammatory and pain substance171,172 and then induces abnormal ACh release. This is a “chicken–egg” question. In fact, Shah’s findings171,172 can support either one because the inflammation reaction can be secondary to the ischemic reaction in the contracture knot.
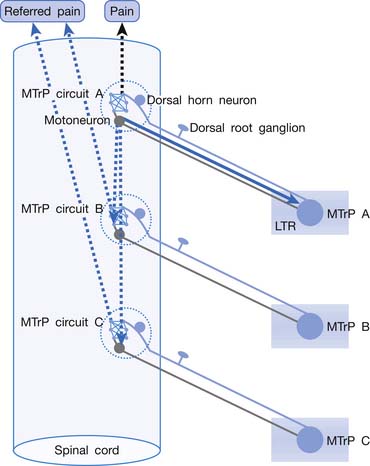
“MTrP Circuit”—Spinal Cord Mechanism of Pain, Referred Pain, and Local Twitch Response
Irritation or sensitization of nociceptors can cause spontaneous pain. Central sensitization can also cause spontaneous pain without stimulation of the nociceptors. Central sensitization is the main cause of MTrP activation in a remote lesion. Nociceptors in an MTrP region connect to a group of dorsal horn cells (sensory neurons) in the spinal cord. These MTrP-related sensory neurons are responsible for central sensitization and for transmission of pain information to the brain. The neural network with connections among these MTrP-related sensory neurons is defined as an MTrP circuit (Figure 43-30).87,92 These MTrP-related sensory neurons also send nerve branches to connect with the other groups of dorsal horn neurons corresponding to other MTrPs. These connections are silent (ineffective synaptic connections) in normal situations. When a strong stimulation is applied on a nociceptor of this MTrP, however, strong impulses are transmitted to these MTrP-related sensory neurons and also spread to MTrP-related sensory neurons corresponding to other MTrPs to induce referred pain, and even spread to corresponding motor neurons in the anterior horn to elicit an LTR via the spinal cord reflex. The MTrP circuit for an MTrP is responsible for all MTrP phenomena, including pain, referred pain, LTR, motor dysfunction, and autonomic phenomena.
Evidence for the Existence of Myofascial Trigger Points
Based on the above scientific findings, the existence of MTrP is obvious. The evidence for the existence of MTrP is summarized in Box 43-2.
BOX 43-2 Summary of the Evidence for the Existence of Myofascial Trigger Points
Clinical observations
Diagnosis of Myofascial Pain
Diagnostic Criteria for the Myofascial Trigger Point
In clinical practice, the identification of MTrPs depends on manual palpation and clinical judgment. Because manual palpation has been considered to be an unreliable technique,152 special training is usually required to obtain interrater reliability and common agreement regarding the palpation criteria.59
It has been suggested that spot tenderness, taut band, and pain recognition are the three most important criteria for the diagnosis of MTrP (Box 43-3).59 In other words, an exquisitely tender spot in a taut band with pain in response to digital compression that can induce or aggravate the patient’s usual clinical complaint is an MTrP responsible for the clinical symptoms. Referred pain and LTRs can be confirmatory signs of MTrP diagnosis.59 For an MTrP with low irritability, high-pressure stimulation is required to elicit a referred pain or an LTR. Applying high-pressure compression during palpation is not recommended because some patients cannot tolerate the high pressure.
The tender spot can always be pointed out by the patient using one finger. When it is associated with a referred pain, however, it can be difficult to point out this spot clearly because the pain has spread out to the surrounding area. When performing palpatory examination on the MTrP, it is important to confirm that the pain elicited from this tender spot is similar to the patient’s usual clinical complaint (pain recognition) so that the correct MTrP can be treated.174,184 In some cases, two or more MTrPs in one or more muscles are responsible for the clinical pain, and the patient might not be able to point out those spots exactly and thus reports that the whole area is painful.
To identify a primary (key) MTrP among many MTrPs, provocative testing can be applied. Compression of a primary MTrP can also cause pain in secondary (satellite) MTrPs. Compression of any secondary MTrP, however, cannot elicit pain in the primary MTrP. In many cases, secondary MTrPs are found in the referred zone (area of referred pain) of the primary MTrP.84
Laboratory Findings of Myofascial Trigger Points
Routine blood chemistries and radiographic studies are not helpful for the diagnosis of myofascial pain. Thermography has been used to assess MTrPs, but its use is still controversial.125,184,191 The use of innovative techniques for MTrP assessment includes the measurement of biochemicals associated with pain or inflammation in the MTrP region,171,172 the sonographic study of MTrP,58 and the magnetic resonance elastography for taut band images.24 Although these techniques might become important in the objective assessment of MTrP in the future, they are relatively expensive at this time.
Identification of the Underlying Pathologic Lesion of Myofascial Pain
As soon as a patient has confirmed MTrPs, the treating physician should attempt to identify the underlying etiologic lesion. This requires a careful medical history and physical examination as described below, and includes the items listed in Box 43-4.
BOX 43-4 Identification of Underlying Etiologic Lesion-Induced Myofascial Trigger Points
Identification of Etiologic Lesions Based on Clinical Presentation of Active Myofascial Trigger Point
Mapping the distribution of MTrPs can help discover the basic pathologic lesion causing activation of MTrPs. MTrPs found in the muscles corresponding to a certain myotome can be caused by radiculopathy.170 MTrPs around a joint can be caused by a lesion of that joint.6 MTrPs in a group of agonists can be caused by a lesion in the common tendon of those muscles. For example, MTrPs in the wrist and hand extensors are often due to lateral or medial epicondylitis of the elbow. MTrPs in the upper trapezius and rhomboid muscles are most frequently related to cervical facet joint lesions.12,92,132 MTrPs in the gluteal muscles are frequently secondary to lumbar facet joint lesions.92
An MTrP in a specific muscle can be associated with a specific level of a facet joint lesion. This can be confirmed by provocative tests that irritate the facet joint to reproduce or aggravate pain in that MTrP. Irritation of a facet joint can be performed by a direct finger compression (for a cervical facet joint) or by immobilizing the lower vertebra of that facet joint and then rotating the neck to the involved side, followed by an extension movement of the neck (facet sign).28,92,132 An MTrP in the upper trapezius or levator scapula muscle is frequently related to a C4–C5 facet joint lesion, and an MTrP in the rhomboid minor muscle is often related to a C5–C6 facet joint lesion.12 In the lower limb, an MTrP in the gluteus minimus muscle is often related to a lesion in the L4–L5 facet joint. An MTrP in the piriformis muscle is frequently related to a lesion in the L5–S1 facet joint.92
Management of Myofascial Pain Syndrome
Basic Principles in the Management of Myofascial Pain
Treating the underlying etiologic lesions that cause the activation of MTrPs is the most important strategy in MPS therapy.∗ If the underlying pathology is not appropriately and completely treated, the MTrP can only be inactivated temporarily, and never completely. Treatment (inactivation) of active MTrPs, however, is necessary in certain situations (Box 43-5), including the presence of severe intolerable pain, pain or discomfort that interferes with functional activities, persistent pain and tightness (can interfere with the healing process of the injured tissue) in the MTrP region after the underlying etiologic lesion is appropriately treated, difficulty in identifying the underlying pathology, and failure in treating the underlying pathology. Release of muscle tightness after inactivation of MTrPs often improves the local circulation to facilitate the healing process of the underlying etiologic lesion. Whether treating active MTrPs or their underlying pathology, conservative treatment (such as manual therapy or physical therapy modalities) should be performed before more aggressive therapy (such as needling), especially for acute or mild lesions.14,87,92,123 It is also important to eliminate any perpetuating factor that might cause persistent existence or recurrence of active MTrPs, and to provide adequate education and home programs to patients, so that recurrent or chronic pain can be avoided. 57,184
Treatment of the Underlying Pathologic Lesions Producing Myofascial Pain
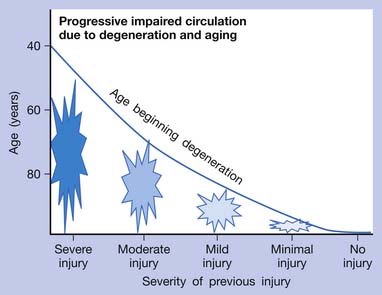
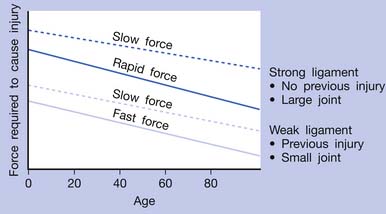
If an acute lesion is not well controlled (usually as a result of severe tissue damage, repeat injury, or inadequate treatment), it can become a chronic lesion with progressive scar tissue formation. This scar tissue is the major cause of degenerative lesions, which can be a source of MTrP activation in later life.18 The pathogenesis of soft tissue lesions is demonstrated in Figure 43-31. Degeneration can develop at middle age or even younger if previous injury was severe or not treated appropriately (Figure 43-32). Degenerative tissue resulting from previous injury is vulnerable to reinjury, especially in elderly persons (Figure 43-33). Consequently, appropriate treatment of an injured soft tissue lesion is important to avoid chronic myofascial pain.
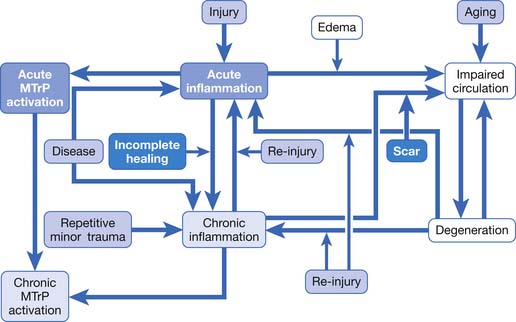
FIGURE 43-31 Pathogenesis of soft tissue lesions as related to activation of myofascial trigger points.
The suggested physical therapy for an injured soft tissue (without active bleeding or acute inflammation) includes thermotherapy and manual therapy.18,92 Local steroid injection is often helpful if the injured lesion responds poorly to conservative treatment. Spinal facet joint injection can inactivate many MTrPs simultaneously if those active MTrPs can be triggered by spinal facet irritation based on provocative tests.87,92,132
Perpetuating Factors
Two major categories of factors that can perpetuate pain are the mechanical and the systemic.57,184,193 Mechanical factors include abnormal posture, leg length discrepancy, scoliosis, etc.57,193 These factors can cause repetitive minor trauma to muscles, tendons, and ligaments that can perpetuate the activation of MTrPs. It has been suggested that systemic factors include nutritional deficiency, vitamin deficiency, anemia, and endocrine disorders (e.g., hypothyroidism, estrogen deficiency).57,193 For chronic or recurrent myofascial pain, it is important to identify any perpetuating factors and eliminate them if possible to avoid persistence or recurrence of active MTrPs.
Inactivation of Active Myofascial Trigger Points
Commonly Applied Techniques to Inactivate (Immediately Release) Myofascial Trigger Points
The management of myofascial pain caused by MTrPs has been extensively reviewed by many authorities.∗ The commonly used methods for inactivating MTrPs are listed in Box 43-6. The conservative programs for myofascial pain recommended by Simons et al.184 and Travell and Simons193 include intermittent cold and stretch (spray and stretch), deep pressure soft tissue massage, trigger point pressure release, and postisometric relaxation. Simons et al.184 also recommend the use of thermotherapy, electrotherapy, laser therapy, muscle energy technique (contract–relax), reciprocal inhibition, or spinal manipulation as an adjunct for myofascial pain therapy. Needling of MTrP (MTrP injection, dry needling, or acupuncture) can be effective techniques for MTrP pain control if applied appropriately and accurately.† A combination of various methods is typically used to treat myofascial pain in clinical practice because there is no single treatment that is successful in every case.
Physical Therapy Modalities for Inactivating Myofascial Trigger Points
Effective modalities for treating myofascial pain have traditionally included ultrasound and electrotherapy.‡ It has been demonstrated that ultrasound can provide immediate pain relief94,131 or immediate increase of pressure pain threshold.188 In addition to the thermal effect, ultrasound might also provide mechanical stimulation from the sound waves.
Electrical stimulation to the nerve, such as transcutaneous nerve stimulation, can assist in pain control via the gate-control mechanism if low-intensity currents are given or via counterirritation (hyperstimulation analgesia) if high-intensity currents are applied. Electrical stimulation to the muscle, such as interferential current therapy, can provide a massage effect from intermittent muscle contractions. It has been suggested that electrical muscle stimulation can be used for release of tight muscles with taut bands.110 Combined therapy with electrotherapy and ultrasound can provide better effectiveness together than when used alone.131 It is also suggested that thermotherapy be applied before and after any manual therapy.87,184 Application of heat can improve focal circulation through reflex vasodilatation.121
Low-power laser therapy has also been applied in treating MTrPs with good results in some studies,30,71,74,185,186 but no significant effect in other studies.3,42 This discrepancy could be due to the different dosages applied in different studies. The mechanism of laser therapy is still unclear. In a recent animal study, Chen et al.23 showed that the prevalence of EPN in the trigger point region was significantly reduced after application of low-power laser on the trigger spot (animal equivalent to the human MTrP). Although the studies on the use of low power laser on an MTrP are still unclear, it is frequently helpful in practice in the author’s experience.
Shockwave therapy is another newly developed device used to help inactive MTrPs.9 Its efficacy and the mechanism involved in the treatment of MTrPs are also still unclear.
Manual Therapy for the Inactivation of Myofascial Trigger Points
Manual therapy is commonly used for myofascial pain therapy and has been described by various authorities.∗ Frequently applied techniques include stretch with intermittent cold spray,184 deep pressure massage,94 ischemic compression,103,123 trigger point pressure release (modified myotherapy),134 manipulation,106,107,134 and postisometric relaxation.135,137
The traditional method of stretch with intermittent cold spray is the “spray and stretch” technique recommended by Travell and Simons.193 The taut band is often released immediately after stretch with cold spray. The cold spray can inhibit cutaneous nociceptors that can cause reflex muscle hyperactivity during stretch.193 Stretch with intermittent ice massage is an alternative method of releasing muscle tightness.
Deep pressure massage on the MTrP region can provide a focal stretch effect in addition to improving local circulation. One controlled study has shown that deep pressure massage was the most effective treatment for an immediate relief of pain of the MTrP compared with the use of hot packs, ultrasound, and stretch.94 Ischemic compression might not be favorable for some patients because it can cause severe pain with local ecchymosis.184 Many of the commonly used myofascial release techniques are modified massage therapies.
Spinal manipulation can provide immediate relief of certain types of pain caused by MTrPs.106,107,134 The strong mechanical stimulation to the facet joint during a quick manipulative thrust might provide an effect similar to needling (hyperstimulation analgesia).143
Alternating contraction–relaxation techniques include muscle energy techniques, postisometric relaxation, reciprocal inhibition, and some myofascial release techniques.184 It has been suggested that the postisometric relaxation technique developed by Lewit et al.135,137 is effective for stretch of a taut band and for pain relief. It is a combination of contraction and relaxation alternately. The tight muscle fibers can typically be stretched easily immediately after an isometric contraction.
Needling for Inactivation of Myofascial Trigger Points
The traditional MTrP injection technique was described by Travell and Simons.193 The needle technique was “move in-and-out to multiple directions” to encounter the sensitive loci in an MTrP region. MTrP pain can typically be eliminated nearly completely immediately after injection of local anesthetic. Hong88 has modified the traditional MTrP injection method with quick needle movement as mentioned above. This new injection technique has been recommended by Simons184 and will be described in detail in the next section.
Gunn69 used an acupuncture needle and Chu31 used an EMG needle to perform dry needling to avoid the tissue damage that can be done by the sharp edge of the injecting needle. This technique has been further modified by adding electrical stimulation during treatment (electrical twitch-obtaining intramuscular stimulation).34,35 This modified technique is similar to electrical acupuncture. The technique of superficial dry needling (inserting the needle into the subcutaneous but not the muscle tissues) has also been suggested for treating myofascial pain.7,8,63
Several authors reported the therapeutic effectiveness of pain control by MTrP injection with botulinum toxin A.1,27,62,200,203 Botulinum toxin A can presynaptically block the ACh release and relieve the taut band in the MTrP region. In a controlled animal study, EPNs recorded in the MTrP region were suppressed after the injection of botulinum toxin A.127 The effectiveness of botulinum toxin A on the myofascial pain relief, however, is probably only due to the needling effect because some studies indicated no benefit from botulinum toxin injection compared with dry needling118,133 or bupivacaine injection.64
Chou et al.28 have recently developed a new technique of acupuncture therapy. This technique is similar to MTrP dry needling by insertion of the acupuncture needle into multiple sites of the MTrP region with a fast insertion speed (high pressure) to elicit LTRs. Simultaneous rotation of the needle (fast screwed-in and screwed-out technique) is also performed to facilitate the needle movement and to avoid bending of the small-diameter acupuncture needle. Use of a small-diameter acupuncture needle can reduce focal tissue damage and decrease postinjection pain or discomfort. Because the temporary postinjection discomfort was much stronger in patients with FMS than in others, and lasted longer in patients with FMS compared with patients with simple MPS,96 it was suggested that this new treatment might be particularly beneficial to patients with FMS.28
Many authors have indicated the importance of eliciting LTRs during needling to obtain immediate and complete pain relief.∗ When an LTR is elicited, the patient usually feels a sharp pain with referred pain and muscle twitching. Such feeling is similar to that when a “De-Qui” effect is obtained during acupuncture therapy. Eliciting an LTR indicates that a sensitive locus (nociceptor) is encountered by the needle tip. In an animal study, both LTR and SEA were suppressed after repeated needling on the same locus in the rabbit MTrS region.22,180 It appears that after a sensitive locus is encountered and an LTR is elicited, the irritability of this sensitive locus (nociceptor) can be suppressed. Needling of a key MTrP could also inhibit the irritability of satellite MTrPs as a result of the central desensitization phenomenon.108
The most likely mechanism of pain relief by needle stimulation is hyperstimulation analgesia.143 The strong pressure stimulation to the MTrP loci (nociceptors) can provide strong neural impulses to the dorsal horn cells in the spinal cord, which might then break the vicious cycle of the “MTrP circuit.”87,92
Recommended Myofascial Trigger Point Injection Techniques
Basic Principles of Myofascial Trigger Point Injection
The important principles of MTrP injection are listed in Box 43-7. Before injecting an MTrP, it is important to confirm that the underlying pathologic lesion has been eliminated and conservative therapies for MTrP inactivation have been tried. Then the key MTrP in the muscle should be identified, and pain recognition on this MTrP should be confirmed. During MTrP injection, the exact location of the MTrP should be identified. Then the needle tip is directed toward the MTrP region and moved in and out quickly to ensure that side-to-side movement of the needle is minimized. The quick needle movement can minimize tissue damage and can also provide a high-pressure stimulation to facilitate the occurrence of LTRs. After completion of MTrP injection, both the skin-penetrating site and needle insertion sites should be compressed firmly to reduce bleeding (excessive bleeding can cause postinjection pain or soreness).
Preparation for Myofascial Trigger Point Injection
The recommended solution for MTrP injection is 0.5% lidocaine without epinephrine.84,184 Injection of local anesthetic might reduce the postinjection sore pain.86 Usually 0.5 to 1 mL of solution is enough to inject one MTrP region. A 25-gauge needle that is 1.5 inches long can be used for deep muscles, or a 27-gauge needle that is 1.25 inches long can be used for superficial muscles. A 5- or 10-mL syringe can be held most comfortably for the MTrP injection.
The patient should be recumbent or in a comfortable sitting position for injection.84 A recumbent position is recommended for a patient with no previous experience in MTrP injection to avoid vasovagal syncope. The physician who performs MTrP injection should be in a comfortable sitting position with elbows (or forearms) and wrists (or hands) well supported. A pillow or blanket can be placed on the patient’s body part to aid this support if the physician has to put the wrist or hand on a certain body part of the patient.
Myofascial Trigger Point Injection Procedure
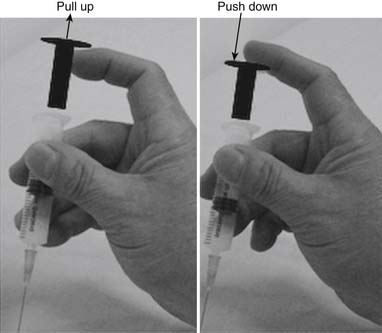
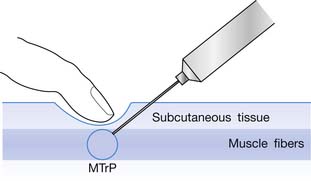
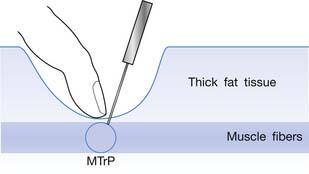
The injection procedure is outlined in Box 43-8. The exact site of the MTrP should be carefully located for needle penetration by the index or long finger of the nondominant hand. The fingertip compresses the MTrP region firmly to direct the needle tip placement. The syringe is held tightly by the dominant hand with its thumb and long fingers, allowing the index finger to control the moving part of syringe for either a pushing or pulling movement (Figure 43-34). After skin sterilization, the needle is pushed through the skin at a site about 0.5 to 1.0 cm (depending on the depth of the MTrP) from the center of the MTrP with the needle approximately 45 degrees oblique to the skin surface. The oblique angle can be smaller if a deep muscle is injected. The needle is initially placed in the subcutaneous tissue layer in a direction toward the MTrP region under the site compressed by the directing the fingertip of the nondominant hand (Figures 43-35 and 43-36). Then the needle is moved quickly (about 20 to 30 mm/s) into the MTrP region. The solution is then injected into the sensitive locus when an LTR is elicited. A drop of solution (about 0.02 to 0.05 mL) is enough to inject into a sensitive locus (and the needle track). Injection of the solution into a site with no LTR elicited (nonsensitive locus) is not necessary but is also not harmful. As soon as a drop of solution is injected, the needle is quickly pulled back to the subcutaneous layer. Then the needle is turned into a different direction for another in-and-out movement to search for another sensitive locus. This in-and-out needle movement is repeated many times to elicit as many LTRs as possible. Excessive insertions are unnecessary, however, and can cause tissue damage with subsequent excessive bleeding. If no LTRs can be elicited in several subsequent insertions in different sites (loci), the needle can be pulled out, and the injection procedure is completed. An experienced physician can make an accurate judgment about the adequacy of needle insertions by palpation of the MTrP to feel the consistency of the taut band. Immediately after injection, both epidermal and intramuscular sites of needle insertion should be compressed firmly for at least 3 minutes. A cold pack can be applied if there is any swelling at the injection site or excessive bleeding.
BOX 43-8 Procedure of Myofascial Trigger Point Injection
Preventing Adverse Effects of Myofascial Trigger Point Injection
The possible adverse effects of MTrP injection are listed in Box 43-9. The most likely complication of MTrP injection is the damage to muscle fibers with subsequently scar tissue formation (fibrosis). The muscle fiber is much smaller than the needle, and a direct cut of the muscle fibers is unlikely. A large-bore needle or a side movement of the needle, however, can cause a stretching injury that can break the muscle fibers. This can be prevented by straight, quick needle movements and by using a needle with a small diameter.
Another possible complication is an injury to the nerve fibers. Nerve fibers are even smaller than the muscle fibers. Nerve injury is prevented by the same methods used above to prevent muscle injury. When a nerve is penetrated or encountered by the injection needle, the patient will complain of a tingling sensation similar to electrical shock. The feeling only lasts for a few seconds and then completely subsides.
Potential Causes of an Unsuccessful Myofascial Trigger Point Injection
Cause of Failure in Immediate Pain Relief
Most patients can have nearly complete pain relief immediately after an injection using the above technique (Box 43-10). If the palpation technique is unsatisfactory, however, the precise location of MTrP cannot be located, and either no or an inadequate number of sensitive loci will be encountered to elicit LTRs. Excessive needle insertions can cause muscle fiber damage and bleeding. Other factors are related to the inaccurate diagnosis of the key MTrP responsible for all clinical complaints (pain recognition) as a result of inappropriate provocative tests or inaccurate information from the patient. Patients with FMS, neurogenic pain, or central pain typically cannot have complete pain relief immediately after an MTrP injection.
Causes of Early Recurrence
If the underlying etiologic lesion cannot be eliminated, the duration of effectiveness of one MTrP injection is usually about 2 weeks.84,86 For the reasons listed in Box 43-11, however, MTrP pain recurrence after injection can occur earlier. The most likely factors causing an early return of the pain are incomplete inactivation of sensitive loci, excessive needle insertions that can cause bleeding, and inadequate hemostasis after injection. The self-care and home care are also essential in avoiding early recurrence of pain. Some conditions such as long-standing myofascial pain, FMS, neuropathic pain, or central pain, as well as existence of perpetuating factors, can also cause early recurrence after injection.
Summary of Myofascial Pain Therapy
The first step to treat myofascial pain is identifying and treating the underlying pathologic lesions that cause activation of MTrPs. To inactivate an MTrP, conservative treatments including manual therapy combined with physical therapy modalities should be tried before aggressive procedures such as needling. Thermotherapy should be given before and after any manual therapy. Needling can provide immediate and complete relief of myofascial pain if it is performed appropriately and accurately. Selection of treatment procedures should be individualized.
Fibromyalgia Syndrome
Definition of Fibromyalgia Syndrome
FMS is a generalized chronic pain syndrome characterized by widespread pain and tenderness to palpation at multiple anatomically defined soft tissue body sites.163 Its comorbidities include depression, anxiety, insomnia, cognitive dysfunction, chronic fatigue, endocrinopathies, irritable bowel syndrome, and dysfunction of the autonomic system. Primary FMS is defined as “pure” FMS having no association with any other medical condition. In some patients, FMS can accompany and complicate a variety of medical conditions, such as rheumatoid arthritis, systemic lupus erythematosus, and hypothyroidism. FMS associated with another medical condition is secondary FMS. The diagnosis of FMS is based on the American College of Rheumatology (ACR) 1990 criteria for the classification of FMS (Box 43-12).201 The major problem with these criteria is the lack of a gold standard diagnostic test for FMS. However, the development of ACR criteria has facilitated research regarding this common painful condition, and these criteria have been applied to community clinical care.11
BOX 43-12 The 1990 American College of Rheumatology Criteria for the Classification of Fibromyalgia Syndrome
Modified from Wolfe F, Smythe HA, Yunus MB, et al: The American College of Rheumatology 1990 criteria for the classification of fibromyalgia, Arthritis Rheum 33:160-172, 1990.
Pathogenesis of Fibromyalgia Syndrome
The cause of FMS is still unclear. In the past, FMS was considered to be a somatic manifestation of an affective disorder.115,155 More evidence is accumulating, however, that FMS patients have reactive depression in response to this devastatingly painful illness.72,79,163,164
Some evidence has shown anatomic abnormality139 and energy deficit10,140 in the skeletal muscles of people with FMS, but others have considered these abnormal findings the result of the chronic deconditioning that typically occurs in FMS.173,204 It has been suggested that patients with FMS have low chemical energy levels because lower-than-normal adenosine triphosphate levels in red blood cells have been detected.43,169
Neuroendocrine dysfunctions, such as functional abnormalities in the hypothalamic–pituitary–adrenal axis, sympathoadrenal gland, and in the hypothalamic–pituitary–growth hormone axis, have been reported.2,39,48,67 Some FMS patients complain of dizziness and lightheadedness resulting from orthostatic hypotension.15,163 Measures of heart rate variability (ratio of low frequency band divided by high frequency band = sympathetic activity/ parasympathetic activity) have shown persistently high sympathetic activity at night.141 This finding could be related to insomnia, morning stiffness, and daytime fatigue in FMS patients.163
The above phenomena could be directly related to or secondary to the basic cause of fibromyalgia. The most important characteristic finding relating to the pathogenesis of FMS is neurosensory dysfunction.163 The neurochemicals probably involved in the pathogenesis of FMS are serotonin (5-HT) and substance P. Serotonin is a neurotransmitter for the descending antinociceptive system from brainstem raphe nuclei to the spinal cord. Activated Aδ and C fiber afferent neurons release substance P into the spinal cord dorsal horn. Substance P can contact with the neurokinin-1 receptors and facilitate nociception. In the spinal cord, 5.HT can inhibit the release of substance P. A dysfunction of the descending antinociceptive system is the most likely mechanism for the pain of fibromyalgia.5,36,163
In 1978, Moldofsky and Warsh151 first suggested that 5-HT might be involved in the pathogenesis of FMS. This was based on the finding of a clinical correlation between FMS pain and the plasma concentration of tryptophan that can be metabolized by oxidative decarboxylation to 5-HT.151 The serum and CSF concentrations of tryptophan were lower in FMS patients than in normal subjects.166,168 Low serum concentration of 5HT was also found in FMS patients.105,165
It was found that the CSF concentration of substance P was higher in FMS patients than in normal subjects.167 It has been suggested that CSF substance P is integrally related to changes in the severity of the symptomatic pain of FMS.163
A new development was the finding of elevated CSF level of nerve growth factor in some primary FMS patients but not in secondary FMS patients.60 Nerve growth factor can facilitate the growth of substance P–containing neurons and is involved in the process of neuroplasticity. It is likely that nerve growth factor can be important for the initiation or perpetuation of the painful symptoms of FMS.163
Genetic predisposition in FMS patients has been found in a few studies.16,17,156,160,205 Evidence produced by family studies has suggested that heritable, etiologic factors are shared by FMS and psychiatric disorders that tend to co-occur within individuals and their family members.16 Recent studies of the genetic linkage of FMS clinical patterns has provided strong support for the concept that there are subgroups of FMS, which present with unique but overlapping clinical manifestations.16,36,162
A much higher prevalence of FMS among females than among males can be explained by the fact that females have lower antinociceptive activity than males.163 This is supported by a study showing that a higher conversion rate of 5-HT to methylated 5-hydroxyindole acetic acid was significantly lower in women than in men.153
Fibromyalgia Versus Myofascial Pain
The difference between myofascial pain syndrome and FMS is the pathogenesis: MPS is soft tissue injury induced by activation of MTrPs, whereas FMS is caused by a lower pain threshold related to dysfunction of the descending antinociceptive system. Because FMS has lower pain threshold, all nociceptors in the body are hyperirritable.163,198 In addition to the sensitized nociceptors in the MTrP region, a patient with FMS also has sensitized nociceptors (usually centrally sensitized) in other tissues (Figure 43-37). Theoretically the pain intensity should be symmetrical. Many FMS patients have asymmetrical pain, however, because of the FMS pain being superimposed on previous trauma. Before the onset of fibromyalgia, those affected might have no symptomatic pain (“cured”) from previous injury, but they still have lower threshold at the tender spots in the injured region. When they suffer FMS with generalized decrease of pain threshold, this previously injured region should be much more painful than the noninjured spots in the other side.
Tender Point Versus Trigger Point
In a broad sense, any point with tenderness is a tender point. Rheumatologic definition of a tender point, however, is “one of the eighteen designated soft tissue body sites that, when tender to palpation, help to identify fibromyalgia.”149 In fact, some of these defined tender points are MTrPs (points 5, 6, 7, 8, 11, 12, 13, and 14 in Box 43-12). A tender point should contain nociceptors and can have some characteristics of MTrP such as pain, referred tenderness, and referred pain.82 In many FMS patients, however, the pain distribution is so intense and widespread that referred pain cannot be perceived.
Clinical Manifestation of Fibromyalgia Syndrome
A typical FMS patient complains of chronic widespread severe pain involving bilateral body sites including the posterior neck, anterior chest, upper and lower back, shoulders, buttock, and extremities.163 Most patients have pain for longer than 3 months. The pain description includes aching, throbbing, stabbing, lancing, or burning.163 The other common symptoms associated with FMS are listed in Box 43-13. These symptoms can “mix or match” at different times in a given patient, but none of them is required for classifying a patient as having FMS.163
Management of Fibromyalgia Syndrome
The suggested approach to an FMS patient is summarized in Box 43-14.163 Both the treating physician and the patient should accept the concept and the fact of FMS before management can be successful. The next step is to establish a diagnosis of FMS based on medical history, as well as careful examination according to the criteria suggested by ACR (see Box 43-12). Laboratory and imaging studies should be used as needed to exclude other possible diseases. After that, the patient and family should be educated regarding the nature of FMS, options of treatment, prognosis, self-care programs, family support, interaction with the society, etc. Many education materials are now available.163,189 It is important to educate the patient to take responsibility for the overall management of FMS.
BOX 43-14 Suggested Management of Fibromyalgia Syndrome
Modified from Russell IJ: Fibromyalgia syndrome. In Mense S, Simons DG, editors: Muscle pain: understanding its nature, diagnosis, and treatment, Philadelphia, 2001, Lippincott Williams & Wilkins.
A recently developed cognitive behavior therapy has been used in the control of pain for FMS patients.44 Patients treated with this cognitive behavior therapy program needed significantly fewer analgesics, and they achieved better responses in respect to depression and mental health than the control subjects.44
Many FMS patients have MTrPs in the painful regions and can be treated with physical therapy as mentioned above. A general exercise program has been recommended for FMS patients.142 It has been demonstrated that conditioning exercise can activate the endogenous opioid system.104 Unaccustomed physical exertion, however, can induce severe body pain for several days.163
The most commonly advocated medication programs involve the use of low-dose tricyclic, sedative, hypnotic medication and analgesic-level dosage of a nonsteroidal antiinflammatory drug (NSAID).163 Amitriptyline, cyclobenzaprine, alprazolam, trazodone, and fluoxetine can increase serotonin availability. Hypnotic medication is used for sleep disorder, and low-dose NSAID is used for pain. Recent studies suggest that different subgroups of FMS patients might respond to different drugs, and some patients can benefit from the concurrent use of education, cognitive behavior, and exercise programs that address the comorbid problems frequently seen in FMS patients.36 Close and regular follow-up evaluation is important in the management of these patients.
1. Acquadro M.A., Borodic G.E. Treatment of myofascial pain with botulinum A toxin [letter], Anesthesiology. 1994;80:705-706.
2. Adler J.E., Kinsley B.T., Hurwetz S., et al. reduced hypothalamic-pituitary and sympathoadrenal responses to hypoglycemia in women with fibromyalgia syndrome. Arthritis Rheum. 1996;39(suppl 9):S276.
3. Altan L., Bingol U., Aykac M., et al. Investigation of the effect of GaAs laser therapy on cervical myofascial pain syndrome. Rheumatol Int. 2005;25(1):23-27.
4. Arendt-Nielsen L., Graven-Nielsen T. Muscle pain: sensory implications and interaction with motor control. Clin J Pain. 2008;24:291-298.
5. Arendt-Nielsen L., Sluka K.A., Nie H.L. Experimental muscle pain impairs descending inhibition. Pain. 2008;140:465-471.
6. Bajaj P., Bajaj P., Graven-Nielsen T., et al. Trigger points in patients with lower limb osteoarthritis. J Musculoskeletal Pain. 2001;9(3):17-33.
7. Baldry P.E. Superficial dry needling at myofascial trigger point sites. J Musculoskeletal Pain. 3(3), 1995. 117–26
8. Baldry P.E. Superficial dry needling. In Chaitow editor. In: Fibromyal syndrome: a practitioner’s guide to treatment. Edinburgh: Churchill Livingston; 2000.
9. Bauermeister W. The diagnosis and treatment of myofascial trigger points using shockwaves in patients with “idiopathic” low back pain. J Musculoskeletal Pain. 2004;12(suppl 9):13.
10. Bengtsson A., Henriksson K.G., Larsson J., et al. Reduced high energy phosphate levels in the painful muscles of patients with primary fibromyalgia. Arthritis Rheum. 1986;29:817-821.
11. Bennett R.M. Diagnostic criteria and differential diagnosis of the fibromyalgia syndrome. J Musculoskeletal Pain. 2004;12(3/4):59-64.
12. Bogduk N., Simons D.G. Neck pain: joint pain or trigger points? In: Vaeroy H., Mersky H., editors. Progress in fibromyalgia and myofascial pain, Vol. 6 Pain research and clinical management. Amsterdam: Elsevier, 1993.
13. Bohr T.W. Fibromyalgia syndrome and myofascial pain syndrome. Do they exist? Neurol Clin. 1995;13(2):365-384.
14. Borg-Stein J., Simons D.G. Myofascial pain. Arch Phys Med Rehabil. 2002;83(suppl 1):S40-47.
15. Bou-Holaigah I., Calkins H., Flynn J.A., et al. Provocation of hypotension and pain during up-right tilt table testing in adults with fibromyalgia. Clin Exp Rheumatol. 1997;15:239-246.
16. Bradley L.A. Family and genetic influences on fibromyalgia syndrome. J Musculoskeletal Pain. 2008;16(1/2):49-57.
17. Buskila D., Neumann L., Hazanov I., et al. Familial aggregation in the fibromyalgia syndrome. Semin Arthritis Rheum. 1996;26:605-611.
18. Cailliet R. Soft tissue pain and disability. Philadelphia: FA Davis; 1977.
19. Cannon D.E., Dillingham T.R., Miao H., et al. Musculoskeletal disorders in referral for suspected cervical radiculopathy. Arch Phys Med Rehabil. 2007;88:1256-1259.
20. Carlson C.R., Okeson J.P., Falace D.A., et al. Reduction of pain and EMG activity in the masseter region by trapezius trigger point injection. Pain. 1993;55:397-400.
21. Chang C.-W., Chen Y.-R., Chang K- F. Evidence of neuroaxonal degeneration in myofascial pain syndrome: a study of neuromuscular jitter by axonal microstimulation. Eur J Pain. 2008;12:1026-1030.
22. Chen J.-T., Chung K.-C., Hou C.-R., et al. Inhibitory effect of dry needling on the spontaneous electrical activity recorded from myofascial trigger spots of rabbit skeletal muscle. Am J Phys Med Rehabil. 2001;80:729-735.
23. Chen K.-H., Hong C.-Z., Kuo F.-C., et al. Electrophysiologic effects of a therapeutic laser on myofascial trigger spots of rabbit skeletal muscle. Am J Phys Med Rehabil. 2008;87:1006-1014.
24. Chen Q., Bensamoun S., Basford J.R., et al. Identification and quantification of myofascial taut bands with magnetic resonance elastography. Arch Phys Med Rehabil. 2007;88:1658-1661.
25. Chen S.-M., Chen J.-T., Kuan T.-S., et al. Decrease in pressure pain thresholds of latent myofascial trigger points in the middle finger extensors immediately after continuous piano practice. J Musculoskeletal Pain. 2000;8(3):83-92.
26. Chen S.-M., Chen J.-T., Wu Y.-C., et al. Myofascial trigger point in intercostal muscles secondary to herpes zoster infection to the intercostal nerve. Arch Phys Med Rehabil. 1998;79:336-338.
27. Cheshire W.P., Abashian S.W., Mann J.D. Botulinum toxin in the treatment of myofascial pain syndrome. Pain. 1994;59:65-69.
28. Chou L.-W., Hong J.Y., Hong C- Z. A new technique of acupuncture therapy and its effectiveness in treating fibromyalgia: a case report. J Musculoskeletal Pain. 2008;16(3):193-198.
29. Chou L.-W., Hsieh Y.-L., Kao M.-J., et al. Remote influences of acupuncture on the pain intensity and the amplitude changes of endplate noise in the myofascial trigger point of the upper trapezius muscle. Arch Phys Med Rehabil. 2009;90:905-912.
30. Chow R.T., Heller G.Z., Barnsley L. The effect of 300 mW, 830 nm laser on chronic neck pain: a double-blind, randomized, placebo-controlled study. Pain. 2006;124(1-2):201-210.
31. Chu J. Does EMG (dry needling) reduce myofascial pain symptoms due to cervical nerve root irritation? Electromyogr Clin Neurophysiol. 1997;37:259-272.
32. Chu J. Dry needling (intramuscular stimulation) in myofascial pain related to lumbar radiculopathy. Eur J Phys Med Rehabil. 1995;5:106-121.
33. Chu J. Twitch-obtaining intramuscular stimulation: observation in the management of radiculopathic chronic low back pain. J Musculoskeletal Pain. 7(4), 1999. 131–46
34. Chu J., Neuhauser D., Schwartz I., et al. The efficacy of automated/electrical twitch-obtaining intramuscular stimulation (ATOIMS/ETOIMS) for chronic pain control: Evaluation with statistical process control methods. Electromyogr Clin Neurophysiol. 2002;42:393-401.
35. Chu J., Yuen K., Wang B., et al. Electrical twitch-obtaining intramuscular stimulation in lower back pain. Am J Phys Med Rehabil. 2004;83:104-111.
36. Clauw D.J. Mechanistic studies and their implication for the management of fibromyalgia syndrome. J Musculoskeletal Pain. 2008;16(1/2):59-66.
37. Coderre T.J., Katz J., Vaccarino A.I., et al. Contribution of central neuroplasticity to pathological pain: review of clinical and experimental evidence. Pain. 1993;52:259-285.
38. Couppe C., Midttun A., Hilden J., et al. Spontaneous needle electromyographic activity in myofascial trigger points in the infraspinatus muscle. J Musculoskeletal Pain. 2001;9:7-17.
39. Crofford L.I., Pillemer S.R., Kalogeras K.T., et al. Hypothalamic-pituitary-adrenal axis perturbations in patients with fibromyalgia. Arthritis Rheum. 1994;37:1583-1592.
40. Cummings T.M., White A.R. Needling therapies in the management of myofascial trigger point pain: a systemic review. Arch Phys Med Rehabil. 2001;82:986-992.
41. Donaldson C.C.S., Skubick D.L., Clasby R.G., et al. The evaluation of trigger point activity using dynamic EMG techniques. Am J Pain Manage. 1994;4:118-122.
42. Dundar U., Evcik D., Samli F., et al. The effect of gallium arsenide aluminum laser therapy in the management of cervical myofascial pain syndrome: a double blind, placebo-controlled study. Clin Rheumatol. 2007;26(6):930-934.
43. Eisinger J., Plantamura A., Ayavou T. Glycolysis abnormalities in fibromyalgia. J Am Coll Nutr. 1994;13:144-148.
44. Falcao D.M., Sales L., Leite J.R., et al. Cognitive behavior therapy for the treatment of fibromyalgia syndrome: a randomized controlled trial. J Musculoskeletal Pain. 2008;16(3):133-140.
45. Falla D., Farina D., Kanstrup Dahl M., et al. Muscle pain induces task dependent changes in cervical agonist/antagonist activity. J Appl Physiol. 2007;102:601-609.
46. Farina D., Arendt-Nielsen L., Merletti R., et al. Effect of experimental muscle pain on motor unit firing rate and conduction velocity. J Neurophysiol. 2004;91:1250-1259.
47. Fernandez-Carnero J., Fernandez-de-las-Penas C., de la Llave-Rincon A.I., et al. Prevalence of and referred pain from myofascial trigger points in the forearm muscles in patients with lateral epicondylalgia. Clin J Pain. 2007;24(3):353-360.
48. Ferraccioli G., Gavalieri F., Salaffi F., et al. Neuroendocrinologic findings in primary fibromyalgia and in other chronic rheumatic conditions. J Rheumatol. 1990;17:869-873.
49. Fischer A.A. Documentation of myofascial trigger points. Arch Phys Med Rehabil. 1988;69:286-291.
50. Fischer A.A. New approach in treatment of myofascial pain. Phys Med Rehabil Clin N Am. 1997;8(1):153-169.
51. Fischer A.A. Pressure threshold meter: Its use for quantification of tender spots. Arch Phys Med Rehabil. 1986;67:836-838.
52. Fischer A.A. Treatment of myofascial pain. J Musculoskeletal Pain. 7(1/2), 1999. 131–42
53. Fricton J.R. Myofascial pain syndrome. Neurol Clin N Am. 1989;7(2):413-427.
54. Fricton J.R., Auvinen M.D., Dykstra D., et al. Myofascial pain syndrome: electromyographic changes associated with local twitch response. Arch Phys Med Rehabil. 1985;66:314-317.
55. Gerwin R. Differential diagnosis of trigger points. J Musculoskeletal Pain. 2004;12(3/4):23-28.
56. Gerwin R.D. Myofascial aspects of low back pain. Neurosurg Clin N Am. 1991;2(4):761-784.
57. Gerwin R.D. The management of myofascial pain syndromes. J Musculoskeletal Pain. 1993;1(3/4):83-94.
58. Gerwin R.D., Duranleau D. Ultrasound identification of the myofascial trigger point [letter]. Muscle Nerve. 1997;20:767-768.
59. Gerwin R.D., Shannon S., Hong C.-Z., et al. Identification of myofascial trigger points: interrater agreement and effect of training. Pain. 1997;69:65-73.
60. Giovengo S.S.L., Russell I.J., Larson A.A. Increased concentration of nerve growth factor in cerebrospinal fluid of patients with fibromyalgia. J Rheumatol. 1999;26:1564-1569.
61. Glaros A.G., Glass E.G., Brockman D. Electromyographic data from TMD patients with myofascial pain and from matched control subjects: evidence for statistical, not clinical, significance. J Orofac Pain. 1997;11:125-129.
62. Gobel H., Heinze A., Reichel G., et al. Efficacy and safety of a single botulinum type A toxin complex treatment (Dysport) for the relief of upper back myofascial pain syndrome: Results from a randomized double-blinded placebo-controlled multicentre study. Pain. 2006;125(1-2):82-88.
63. Goddard G., Karibe H., McNeill C., et al. Acupuncture and sham acupuncture reduce muscle pain in the myofascial pain patients. Orofac Pain. 2002;16(1):71-76.
64. Graboski C.L., Gray D.S., Burnham R.S. Botulinum toxin A versus bupivacaine trigger point injections for the treatment of myofascial pain syndrome: a randomized double blind crossover study. Pain. 2005;118:170-175.
65. Graff-Radford S.B., Reeves J.L., Baker R.L., et al. Effects of transcutaneous electrical nerve stimulation on myofascial pain and trigger point sensitivity. Pain. 1989;37(1):1-5.
66. Graven-Nielsen T., Svensson P., Arendt-Nielsen L. Effects of experimental muscle pain on muscle activity and co-ordination during static and dynamic motor function. Electroenc Clin Neurogr. 1997;105:156-164.
67. Griep E.N., Boersma J.W., deKloet E.R. Evidence for neuroendocrine disturbance following physical exercise in primary fibromyalgia syndrome. J Musculoskeletal Pain. 1993;1(3/4):217-222.
68. Gunn C.C. Radiculopathic pain: Diagnosis and treatment of segmental irritation or sensitization. J Musculoskeletal Pain. 1997;5(4):119-134.
69. Gunn C.C. Treatment of chronic pain. Intramuscular stimulation for myofascial pain of radiculopathic origin. London,: Churchill Livingstone; 1996.
70. Gunn C.C., Milbrandt W.E., Little A.S., et al. Dry needling of motor points for chronic low-back pain: a randomized clinical trial with long-term follow-up. Spine. 1980;5:279-291.
71. Gur A., Sarac A.J., Cevik R., et al. Efficacy of 904 nm gallium arsenide low level laser therapy in the management of chronic myofascial pain in the neck: a double-blind and randomize-controlled trial. Lasers Surg Med. 2004;35(3):229-235.
72. Hader N., Rimon D., Kinarty A., et al. Altered interlejkin-2 secretion in patients with primary fibromyalgia syndrome. Arthritis Rheum. 1991;34:866-872.
73. Hagberg H., Kvarnstrom S. Muscular endurance and electromyographic fatigue in myofascial shoulder pain. Arch Phys Med Rehabil. 1984;65:522-525.
74. Hakguder A., Birtane M., Gurcan S., et al. Efficacy of low level laser therapy in myofascial pain syndrome: an algometric and thermographic evaluation. Lasers Surg Med. 2003;33(5):339-343.
75. Harden R.N. Muscle pain syndromes. Am J Phys Med Rehabil. 2007;86(Suppl):S47-S58.
76. Headley B.J. Evaluation and treatment of myofascial pain syndrome utilizing biofeedback. In: Cram J.G., editor. Clinical EMG for surface recording, vol, 2. Nevada City, CA: Clinical Resources, 1990.
77. Headley B.J. Physiologic risk factors. In: Sanders M., editor. Management of cumulative trauma disorders. London: Butterworth-Heineman; 1997:107-127.
78. Headley B.J. The use of biofeedback in pain management. Phys Ther Pract. 1993;2(2):29-40.
79. Hernanz W., Valenzuela A., Quijada, et al. Lymphocyte subpopulations in patients with primary fibromyalgia. J Rheumatol. 1994;21:2122-2124.
80. Hoheisel U. Mense S. Long-term changes in discharge behavior of cat dorsal horn neurons following noxious stimulation of deep tissues. Pain. 1989;36:239-247.
81. Hoheisel U., Mense S., Simons D.G., et al. Appearance of new receptive fields in rat dorsal horn neurons following noxious stimulation of skeletal muscle: a model for referral of muscle pain? Neurosci Lett. 1993;153:9-12.
82. Hong C.-Z. Algometry in evaluation of trigger points and referred pain. J Musculoskeletal Pain. 1998;6(1):47-59.
83. Hong C.-Z. Comment on Gunn’s “radiculopathy model of myofascial trigger points,”. J Musculoskeletal Pain. 2000;8(3):133-135.
84. Hong C.-Z. Consideration and recommendation of myofascial trigger point injection. J Musculoskeletal Pain. 1994;2(1):29-59.
85. Hong C.-Z. Current research on myofascial trigger points: pathophysiological studies. J Musculoskeletal Pain. 1999;7(1/2):121-129.
86. Hong C.-Z. Lidocaine injection versus dry needling to myofascial trigger point: the importance of the local twitch response. Am J Phys Med Rehabil. 1994;73:256-263.
87. Hong C.-Z. Myofascial pain therapy. J Musculoskeletal Pain. 2004;12(3/4):37-43.
88. Hong C.-Z. Myofascial trigger point injection. Crit Rev Phys Rehabil Med. 1993;5(2):203-217.
89. Hong C.-Z. Myofascial trigger points: pathophysiology and correlation with acupuncture points. Acup Med. 2000;18:41-47.
90. Hong C.-Z. Pathophysiology of myofascial trigger point. J Formos Med Assoc. 1996;95:93-104.
91. Hong C.-Z. Persistence of local twitch response with loss of conduction to and from the spinal cord. Arch Phys Med Rehabil. 1994;75:12-16.
92. Hong C.-Z. Treatment of myofascial pain syndrome. Curr Pain Headache Rep. 2006;10:345-349.
93. Hong C.-Z., Chen J.-T., Chen S.-M., et al. Histological findings of responsive loci in a myofascial trigger spot of rabbit skeletal muscle from where localized twitch responses could be elicited [abstract]. Arch Phys Med Rehabil. 1996;77:962.
94. Hong C.-Z., Chen Y.C., Pon C.H., et al. Immediate effects of various physical medicine modalities on pain threshold of the active myofascial trigger points. J Musculoskeletal Pain. 1993;1(2):37-52.
95. Hong C.-Z., Chen Y.-N., Twehous D., et al. Pressure threshold for referred pain by compression on the trigger point and adjacent areas. J Musculoskeletal Pain. 1996;4(3):61-79.
96. Hong C.-Z., Hsueh T.-C. Difference in pain relief after trigger point injections in myofascial pain patients with and without fibromyalgia. Arch Phys Med Rehabil. 1996;77:1161-1166.
97. Hong C.-Z., Kuan T.-S., Chen J.-T., et al. Referred pain elicited by palpation and by needling of myofascial trigger points: a comparison. Arch Phys Med Rehabil. 1997;78:957-960.
98. Hong C.-Z., Simons D.G. Pathophysiologic and electrophysiologic mechanism of myofascial trigger points. Arch Phys Med Rehabil. 1998;79:863-872.
99. Hong C.-Z., Torigoe Y. Electrophysiologic characteristics of localized twitch responses in responsive bands of rabbit skeletal muscle fibers. J Musculoskeletal Pain. 1994;2(2):17-43.
100. Hong C.-Z., Torigoe Y., Yu J. The localized twitch responses in responsive bands of rabbit skeletal muscle fibers are related to the reflexes at spinal cord level. J Musculoskeletal Pain. 1995;3(1):15-33.
101. Hong C.-Z., Yu J. Spontaneous electrical activity of rabbit trigger spot after transection of spinal cord and peripheral nerve. J Musculoskeletal Pain. 1998;6(4):45-58.
102. Hou C.-R., Chung K.-C., Chen J.-T., et al. Effects of calcium channel blocker on electrical activity in myofascial trigger spots of rabbit. Am J Phys Med Rehabil. 2002;81:342-349.
103. Hou C.-R., Tsai L.-C., Cheng K.-F., et al. Immediate effects of various physical therapeutic modalities on cervical myofascial pain and trigger point sensitivity. Arch Phys Med Rehabil. 2002;83:1406-1414.
104. Howlett T.A. Hormonal responses to exercise and training: a short review. Clin Endocrinol. 1987;26(6):723-742.
105. Hrycaj P., Stratz T., Muller W. Platelet 3H-imipramine uptake receptors density and serum serotonin in patients with fibromyalgia syndrome [Letter]. J Rheumatol. 1993;20:1986-1987.
106. Hsieh C.-Y., Adams A.H., Tobis J.S., et al. Effectiveness of four treatments for subacute low back pain: a randomized clinical trial. Spine. 2002;27(11):1142-1148.
107. Hsieh C.-Y., Hong C- Z. Effect of chiropractic manipulation on the pain threshold of myofascial trigger point. In Proceedings of the 1990 International Conference of Spinal Manipulation. Los Angeles: Los Angeles College of Chiropractic; 1990.
108. Hsieh Y.L., Kao M.-J., Kuan T.-S., et al. Dry needling to a key myofascial trigger point may reduce the irritability of satellite MTrPs. Am J Phys Med Rehabil. 2007;86:397-403.
109. Hsu H.-C., Hong C- Z. Floating kidney with chronic myofascial pain syndrome in the abdominal muscles as the major clinical manifestation: a case report. J Musculoskeletal Pain. 2008;16(3):199-204.
110. Hsueh T.-C., Cheng P.-T., Kuan T.-S., et al. The immediate effectiveness of electrical nerve stimulation and electrical muscle stimulation on myofascial trigger points. Am J Phys Med Rehabil. 1997;76(6):471-476.
111. Hsueh T.-C., Yu S., Kuan T.-S., et al. Association of active myofascial trigger points and cervical disc lesion. J Form Med Assoc. 1998;97:174-180.
112. Hu J.W., Sessle B.J., Raboisson P., et al. Stimulation of craniofacial muscle afferents induces prolonged facilitatory effects in trigeminal nociceptive brainstem neurons. Pain. 1992;48:53-60.
113. Hubbard D.R. Chronic and recurrent muscle pain: pathophysiology and treatment, and review of pharmacologic studies. J Musculoskeletal Pain. 1996;4(1/2):123-143.
114. Hubbard D.R., Berkoff G.M. Myofascial trigger points show spontaneous needle EMG activity. Spine. 1993;18:1803-1807.
115. Hudson J.I., Hudson M.S., Plineer L.F., et al. Fibromyalgia and major affective disorder: a controlled phenomenology and family history study. Am J Psychiatry. 1985;142:441-446.
116. Ito Y., Miledi R., Vincent A. Transmitter release induced by a ‘factor’ in rabbit serum. Proc R Soc Lond B. 1974;187:235-241.
117. Jensen R., Bendtsen L., Olesen J. Muscular factors are of importance in tension-type headache. Headache. 1998;38:10-17.
118. Kamanli A., Kaya A., Ardicoglu O., et al. Comparison of lidocaine injection, botulinum toxin injection, and dry needling to trigger points in myofascial pain syndrome. Rheumatol Int. 2005;25(8):604-611.
119. Kao M.-J., Han T.-I., Kuan T.-S., et al. Myofascial trigger points in early life. Arch Phys Med Rehabil. 2007;88:251-254.
120. Kawakita K., Itoh K., Okada K. Experimental model of trigger points using eccentric exercise. J Musculoskeletal Pain. 2008;16(1/2):29-35.
121. Kellogg D.L.Jr. In vivo mechanisms of cutaneous vasodilatation and vasoconstriction in humans during thermoregulatory challenges. J Appl Physiol. 2006;100(5):1709-1718.
122. Kostopoulos D., Nelson A.J., Ingber R.S., et al. Reduction of spontaneous electrical activity and pain perception of trigger points in the upper trapezius muscle through trigger point compression and passive stretching. J Musculoskeletal Pain. 2008;16(4):266-278.
123. Kostopoulos D., Rizopoulos K. The manual of trigger point and myofascial therapy. Thorofare, NJ: SLACK Incorporated; 2001.
124. Kraft GH [consulting editor], Stanton DF, Mein EA [Guest Editors]: Manual Medicine. Phys Med Rehabil Clin N Am 7(4):679–932, 1996.
125. Kruse R.A., Christiansen J.A. Thermographic imaging of myofascial trigger points: a follow-up study. Arch Phys Med Rehabil. 73, 1992. 819–23
126. Kuan T.-S., Chang Y.-C., Hong C- Z. Distribution of active loci in rat skeletal muscle. J Musculoskeletal Pain. 1999;7(4):45-54.
127. Kuan T.-S., Chen J.-T., Chen S.-M., et al. Effect of botulinum toxin on endplate noise in myofascial trigger spots of rabbit skeletal muscle. Am J Phys Med Rehabil. 2002;81:512-520.
128. Kuan T.-S., Hong C.-Z., Chen J.-T., et al. The spinal cord connections of myofascial trigger spots. Eur J Pain. 2007;11:624-634.
129. Kuan T.-S., Hsieh Y.-L., Chen S-M., et al. The myofascial trigger point region: correlation between the degree of irritability and the prevalence of endplate noise. Am J Phys Med Rehabil. 2007;86:183-189.
130. Kuan T.-S., Lin T.-S., Chen J.-T., et al. No increased neuromuscular jitter at rabbit skeletal muscle trigger spot spontaneous electrical activity sites. J Musculoskeletal Pain. 2000;8(3):69-82.
131. Lee J.C., Lin D.T., Hong C- Z. The effectiveness of simultaneous thermotherapy with ultrasound and electrotherapy with combined AC and DC current on the immediate pain relief of myofascial trigger point. J Musculoskeletal Pain. 1997;5(1):81-90.
132. Lee P.-S., Lin P., Hsieh L.-F., et al. Facet injection to control the recurrent myofascial trigger points: a case report. J Rehabil Med Assoc ROC. 1998;26(1):41-45.
133. Lew H.L., Lee E.H., Castaneda A., et al. Therapeutic use of botulinum toxin type A in treating neck and upper-back pain of myofascial origin: a pilot study. Arch Phys Med Rehabil. 2008;89:75-80.
134. Lewit K. Manipulative therapy in rehabilitation of the locomotor system, Ed 2. Oxford, uk: Butterworth Heinemann; 1991.
135. Lewit K. Postisometric relaxation in combination with other methods of muscular facilitation and inhibition. Manual Med. 1986;2:101-104.
136. Lewit K. The needle effect in relief of myofascial pain. Pain. 1979;6:83-90.
137. Lewit K., Simons D.G. Myofascial pain: relief by post-isometric relaxation. Arch Phys Med Rehabil. 1985;65:452-456.
138. Liley A.W. An investigation of spontaneous activity at the neuromuscular junction of the rat. J Physiol. 1956;132:650-666.
139. Lindman R., Hagberg M., Bengtsson A., et al. Capillary structure and mitochondrial volume density in the trapezius muscle of chronic trapezius myalgia, fibromyalgia and healthy subjects. J Musculoskeletal Pain. 1995;3(3):5-22.
140. Lund N., Bengtsson A., Thorborg P. Muscle tissue oxygen pressure in primary fibromyalgia. Scand J Rheumatol. 1986;15:165-173.
141. Martinez-Lavin M., Hermosillo A.G., Rosas M., et al. Circadian studies of autonomic nervous balance in patients with fibromyalgia: a heart rate variability analysis. Arthritis Rheum. 1998;41:1966-1971.
142. McCain G.A., Bell D.A., Mai F.M., et al. A controlled study of the effects of a supervised cardiovascular fitness training program on the manifestations of primary fibromyalgia. Arthritis Rheum. 1988;31(9):1135-1141.
143. Melzack R. Myofascial trigger points: relation to acupuncture and mechanism of pain. Arch Phys Med Rehabil. 1981;62:114-117.
144. Mense S. Considerations concerning the neurobiological basis of muscle pain. Can J Physiol Pharmacol. 1991;69(5):610-616.
145. Mense S. Nervous outflow from skeletal muscle following chemical noxious stimulation. J Physiol. 1977;267:75-88.
146. Mense S. Nociception from skeletal muscle in relation to clinical muscle pain. Pain. 1993;54:241-289.
147. Mense S. Peripheral mechanisms of muscle nociception and local muscle pain. J Musculoskeletal Pain. 1993;1(1):133-170.
148. Mense S. Referral of muscle pain: new aspects. Am Pain Soc J. 1994;3:1-9.
149. Mense S., Simons D.G. Muscle pain. Understanding its nature, diagnosis, and treatment. Philadelphia: Lippincott Williams & Wilkins; 2001.
150. Mense S., Simons D.G., Hoheisel U., et al. Lesions of rat skeletal muscle following local block of acetylcholinesterase and neuromuscular stimulation. J Appl Physiol. 2003;94:2494-2501.
151. Moldofsky H., Warsh J.J. Plasma tryptophan and musculoskeletal pain in nonarticular rheumatism (fibrositis syndrome). Pain. 1978;5:65-71.
152. Myburgh C., Larsen A.H., Hartvigsen J. A systemic, critical review of manual palpation for identifying myofascial trigger points: evidence and clinical significance. Arch Phys Med Rehabil. 2008;89:1169-1176.
153. Nishizawa S., Benkelfat C., Young S.N., et al. Differences between males and females in rates of serotonin synthesis in human brain. Proc Natl Acad Sci U S A. 1997;94:5308-5313.
154. Ohrbach R., Gale E.N. Pressure pain thresholds, clinical assessment, and differential diagnosis: reliability and validity in patients with myofascial pain. Pain. 39, 1989. 157–69
155. Payne T.C., Leavitt F., Garron D.C., et al. Fibrositis and psychologic disturbance. Arthritis Rheum. 1982;25:213-217.
156. Pellegrino MJ, Waylonis GW, Sommer A: Familial occurrence of primary fibromyalgia—Department of Physical Medicine, Ohio State University, Columbus. Arch Phys Med Rehabil 70:61-63, 1989.
157. Pongratz D., Vorgerd M., Schoser B.G.H. Scientific aspects and clinical signs of muscle pain. J Musculoskeletal Pain. 2004;12(Suppl 9):9.
158. Robinson J.P., Arendt-Nielsen L. Muscle pain syndrome. In: Braddom R.L., editor. Physical medicine and rehabilitation. ed 3. St Louis: Elsevier; 2007:989-1020. Chapter 44
159. Rosen N.B. The myofascial pain syndrome. Phys Med Rehabil Clin N Am. 1993;4(Feb):41-63.
160. Rosenblatt R.M., Reich J., Dehring D. Tricyclic antidepressants in treatment of depression and chronic pain. Anesth Analg. 1984;63(11):1025-1032.
161. Rubin D. Myofascial trigger point syndromes: an approach to management. Arch Phys Med Rehabil. 1981;62(3):107-110.
162. Russell I.J. Developments in the fibromyalgia syndrome. J Musculoskeletal Pain. 2004;12(3/4):47-57.
163. Russell I.J. Fibromyalgia syndrome. In: Mense S., Simons D.G., editors. Muscle pain. Understanding its nature, diagnosis, and treatment,. Philadelphia: Lippincott Williams & Wilkins, 2001.
164. Russell I.J., Flecher E.M., Michalek J.E., et al. Treatment of primary fibrositis/fibromyalgia syndrome with ibuprofen and alprazolam: A double-blind, placebo-controlled study. Arthritis Rheum. 1991;34:552-560.
165. Russell I.J., Michalek J.E., Vipraio G.A., et al. Platelet 3H-imipramine uptake receptors density and serum serotonin in patients with fibromyalgia syndrome. J Rheumatol. 1992;19:104-109.
166. Russell I.J., Michalek J.E., Vipraio G.A., et al. Serum amino acids in fibrositis/fibromyalgia syndrome. J Rheumatol. 1989;19(suppl):158-163.
167. Russell I.J., Orr M.D., Littman B., et al. Elevated cerebrospinal fluid levels of substance P in patients with fibromyalgia syndrome. Arthritis Rheum. 1994;37:1593-1601.
168. Russell I.J., Vipraio G.A., Acworth I. Abnormalities in the central nervous system metabolism of tryptophan to 3-hydroxy kynurenine in fibromyalgia syndrome. Arthritis Rheum. 1993;36(9):S222.
169. Russell I.J., Vipraio G.A. Red cell nucleotide abnormalities in fibromyalgia syndrome. Arthritis Rheum. 1993;36(9):S223.
170. Samuel A.S., Peter A.A., Ramanathan K. The association of active trigger points with lumbar disc lesions. J Musculoskeletal Pain. 2007;15(2):11-18.
171. Shah J.P. Uncovering the biochemical milieu of myofascial trigger points. Using in vivo microdialysis. J Musculoskeletal Pain. 2008;16(1-2):17-20.
172. Shah J.P., Danoff J.V., Desai M.J., et al. Biochemicals associated with pain and inflammation are elevated in sites near to and remote from active myofascial trigger points. Arch Phys Med Rehabil. 2008;89:16-23.
173. Simms R.W. Is there muscle pathology in fibromyalgia syndrome? Rheum Dis Clin North Am. 1996;22:245-266.
174. Simons D.G. Clinical and etiological update of myofascial pain from trigger points. J Musculoskeletal Pain. 1996;4(1/2):93-121.
175. Simons D.G. Diagnostic criteria of myofascial pain caused by trigger points. J Musculoskeletal Pain. 1999;7(1/2):111-120.
176. Simons D.G. Do endplate noise and spikes arise from normal motor endplates? Am J Phys Med Rehabil. 2001;80:134-140.
177. Simons D.G. New aspects of myofascial trigger points: etiological and clinical. J Musculoskeletal Pain. 2004;12(3/4):15-21.
178. Simons D.G. New views of myofascial trigger points: etiology and diagnosis (commentary). Arch Phys Med Rehabil. 2008;89:157-159.
179. Simons D.G., Dexter J.R. Comparison of local twitch responses elicited by palpation and needling of myofascial trigger points. J Musculoskeletal Pain. 1995;3(1):49-61.
180. Simons D.G., Hong C.-Z., Simons L.S. Endplate potentials are common to midfiber myofascial trigger points. Am J Phys Med Rehabil. 2002;81:212-222.
181. Simons D.G., Hong C.-Z., Simons L.S. Prevalence of spontaneous electrical activity at trigger spots and at control sites in rabbit skeletal muscle. J Musculoskeletal Pain. 1995;3(1):35-48.
182. Simons D.G., Stolov W.C. Microscopic features and transient contraction of palpable bands in canine muscle. Am J Phys Med. 1976;55:65-88.
183. Simons D.G., Travell J.G. Myofascial trigger points, a possible explanation. Pain. 1981;10:106-109.
184. Simons D.G., Travell J.G., Simons L.S. Travell & Simons’ myofascial pain and dysfunction: the trigger point manual, vol. 1, ed 2. Baltimore: Williams & Wilkins; 1999.
185. Snyder-Mackler L., Barry A.J., Perkins A.I., et al. Effects of helium-neon laser irradiation on skin resistance and pain in patients with trigger points in the neck or back. Phys Ther. 1989;69(5):336-341.
186. Snyder-Mackler L., Bork C., Bourbon B., et al. Effect of helium-neon laser on musculoskeletal trigger points. Phys Ther. 1986;66(7):1087-1090.
187. Sola A.E., Bonica J.J., et al. Myofascial pain syndromes. In Bonica J.J., Loeser J.D., Chapman C.R., editors: The management of pain, ed 2, Philadelphia: Lea & Febiger, 1990.
188. Srbely J.Z., Dickey J.P. Randomized controlled study of the antinociceptive effect of ultrasound on trigger point sensitivity: novel applications in myofascial therapy? Clin Rehabil. 2007;21:411-417.
189. Starlanyl D., Copeland M.E. Fibromyalgia & chronic myofascial pain: a survival manual, ed 2. Oakland, CA: New Harbinger Publications, Inc; 2001.
190. Svensson P., Arendt-Nielsen L., Houe L. Muscle pain modulates mastication: an experimental study in humans. J Orofac Pain. 1998;12:7-16.
191. Swerdlow B., Dieter J.N. An evaluation of the sensitivity and specificity of medical thermography for the documentation of myofascial trigger points. Pain. 48, 1992. 205–213
192. Tanrikut A., Özaras N., Kaptan H.A., et al. High voltage galvanic stimulation in myofascial pain syndrome. J Musculoskeletal Pain. 2003;11(2):11-15.
193. Travell J.G., Simons D.G. Myofascial pain and dysfunction: the trigger point manual, vol. 1. Baltimore: Williams & Wilkins, 1983.
194. Travell J.G., Simons D.G. Myofascial pain and dysfunction: the trigger point manual, vol. 2,. Baltimore: Williams & Wilkins, 1992.
195. Tsai C.-T., Hsieh L.-F., Kuan T.-S., et al. Remote effects of dry needling on the irritability of the myofascial trigger point in the upper trapezius muscle. Am J Phys Med Rehabil. 2010;89(2):133-140.
196. Tsai W.-C., Wang T.-G., Hong C- Z. Myofascial trigger points in the ipsilateral gluteal muscles associated with pyogenic sacroiliitis: a case report. J Musculoskeletal Pain. 1999;7(3):73-82.
197. Vecchiet L., Galleti R., Giamberardino M.A., et al. Modifications of cutaneous, subcutaneous, and muscular sensory and pain thresholds after the induction of an experimental algogenic focus in the skeletal muscle. Clin J Pain. 1988;4:55-59.
198. Vecchiet L., Giamberardino M.A., de Bigontina P., et al. Comparative sensory evaluation of parietal tissues in painful and nonpainful areas in fibromyalgia and myofascial pain syndrome. In: Gebhart G.F., Hammond D.L., Jensen T.S., editors. Proceedings of the 7th World Congress on Pain: Progress in Pain Research and Management,, Vol. 2,. Seattle: IASP Press, 1994:177-249.
199. Wang J.-F., Chen M., Lin M.-T., et al. Teres minor tendinitis manifested with chronic myofascial pain syndrome in the scapular muscles: a case report. J Musculoskeletal Pain. 2006;14(1):39-43.
200. Wheeler A.H., Goolkasian P., Gretz S.S. A randomized, double-blind, prospective pilot study of Botulinum toxin injection for refractory, unilateral, cervicothoracic, paraspinal, myofascial pain syndrome. Spine. 1998;23:1662-1667.
201. Wolfe F., Smythe H.A., Yunus M.B., et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Arthritis Rheum. 1990;33:160-172.
202. Wu C.-M., Chen H.-H., Hong C- Z. Myofascial trigger points in patients with lumbar radiculopathy due to disc herniation before and after surgery. J Surg Assoc ROC. 1997;30:175-184.
203. Yue S.K. Initial experience in the use of botulinum toxin A for the treatment of myofascial related muscle dysfunctions. J Musculoskeletal Pain. 1995;3(suppl 1):22.
204. Yunus M.B., Kalyan-Ramam U.P., Masi A.T., et al. Electron microscopic studies of muscle biopsy in primary fibromyalgia syndrome: a controlled and blinded study. J Rheumatol. 1989;16:97-101.
205. Yunus M.B., Rawlings K.K., Khan M.A., et al. Genetic studies of multicase families with fibromyalgia syndrome with HLA typing. Arthritis Rheum. 1995;38(suppl):S247.
∗ References 53, 56, 75, 84, 98, 149, 159, 184, 187, 193.
∗ References 86, 103, 106, 108, 110, 131.
† References 22, 23, 102, 122, 127, 195.
∗ References 38, 126, 175, 176, 180, 181, 184.
∗ References 55, 84, 87, 92, 98, 184.
∗ References 14, 50, 52, 53, 57, 75, 87, 123, 149, 159, 161, 184, 187, 193.
† References 7, 8, 31–33, 40, 50, 52, 68–70, 84, 86–88, 92, 136, 193.
‡ References 14, 65, 87, 94, 110, 131, 184, 192.