11
Muscle and Tendon Injuries
1. Recognize the macrostructure and microstructure of muscle and tendon.
2. Describe the two main types of muscle fibers.
3. Describe the three mechanisms for a muscle injury.
4. Describe the injury mechanism’s associated tendon pathology.
5. Name the functional unit of a tendon and its structural significance.
6. Describe the difference between a supraphysiologic and a subfailure load and how each type may contribute to an injury.
7. Define how a muscle strain differs from a ligament sprain.
8. Describe the differences between a tendonitis and a tendinopathy.
9. Describe the effects of aging on tendons.
10. Name and describe the three phases of connective tissue healing.
11. Describe the effects of immobilization on connective tissue.
12. Discuss clinical applications of therapeutic interventions based on the stages of connective tissue healing.
MUSCLE AND TENDON FUNCTIONAL ANATOMY
Macrostructure of Muscle and Tendons
Microstructure of Muscle
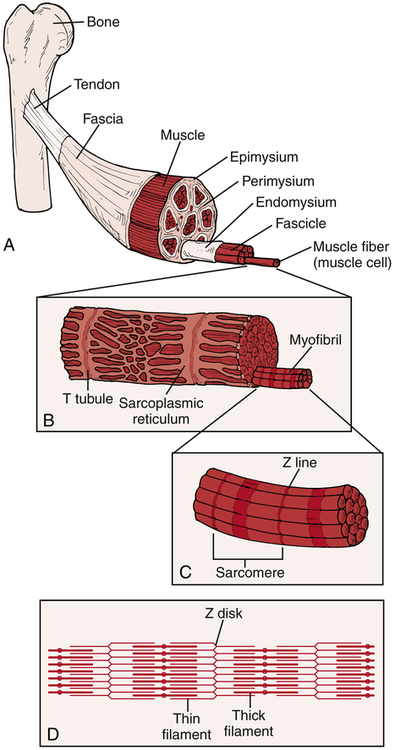
The muscle consists of muscle fibers and connective tissue (Fig. 11-1). There are three layers of connective tissue: the epimysium, the perimysium, and the endomysium. The epimysium surrounds the muscle and (along with the perimysium and the endomysium) is continuous with the muscle’s tendons. Within the muscle, groups of muscle fibers (known as a fasciculus) are surrounded by the perimysium. Within each fasciculus, each individual muscle fiber is surrounded by the endomysium.
A muscle fiber (muscle cell) is further divided into myofibrils and myofilaments (see Fig. 11-1). Each muscle fiber is surrounded by a sarcolemma, the muscle fiber’s cellular membrane. Within the muscle fiber, the myofibrils are contained within the sarcoplasm. The myofibrils are bundles of the contractile proteins myosin and actin grouped together as a sarcomere (the basic functional unit of the myofibril). The interaction between the myosin and actin during the muscle’s contraction contributes to functional movement. The structural relationship between the contractile and noncontractile components allows skeletal muscles to perform both concentric (shortening) and eccentric (lengthening) muscle contractions as well as the ability to return to a resting position.18
Muscle Fiber Types
Whereas the type I (ST) muscle fibers possess a high endurance capacity, the type II (FT) muscle fibers differ in that they are specialized for more anaerobic activities. There are several types of FT fibers that are differentiated by their resistance to fatigue, their functional use, and the type of myosin heavy chain (MHC) protein complex present (Table 11-1).27
Table 11-1
| Parameter | Type I | Type IIA | Type IIB |
| Cell body size | Small | Medium | Large |
| Conduction velocity | Slow | Fast | Fast |
| Number of muscle fibers | Few | More | Most |
| Rate of force development | Slow | Moderate | Fast |
| Absolute force generation | Low | Moderate | High |
| Resistance to fatigue | High | Moderate | Low |
| Mitochondrial density | High | High | Low |
| Aerobic capacity | High | Moderate | Low |
| Myoglobin content | High | Low | Low |
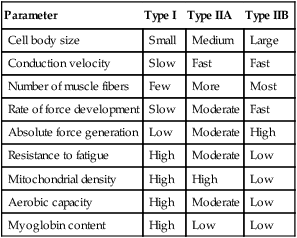
Within the literature, there are at least eight types of named muscle fibers: I, IC, IIA, IIB, IIC, IID, IIX (IIB), and IIAX.7,9,12,15,27,30,31,35 However, this does not mean that there are eight distinct fiber types. Rather, there appears to be an inconsistency in the literature as to how the fibers are named.7,9,12,15,27,30,31,35 While the PTA can appreciate that there is variability among sources about the actual number of different fiber types, it is more important to appreciate the two broad categories of muscle fibers: type I and type II. Type I are the slow, oxidative fibers and type II are the fast, glycolytic fibers. There are significant differences between the two main types that have functional implications. The exercise prescription strategy used during different phases of the healing process should take into account the primary fiber composition of the injured skeletal muscle. The characteristics of the three most common fiber types in human skeletal muscle characteristics—types I, IIA, and IIB—will be compared in this chapter (see Table 11-1).7,31,35
Microstructure of Tendon
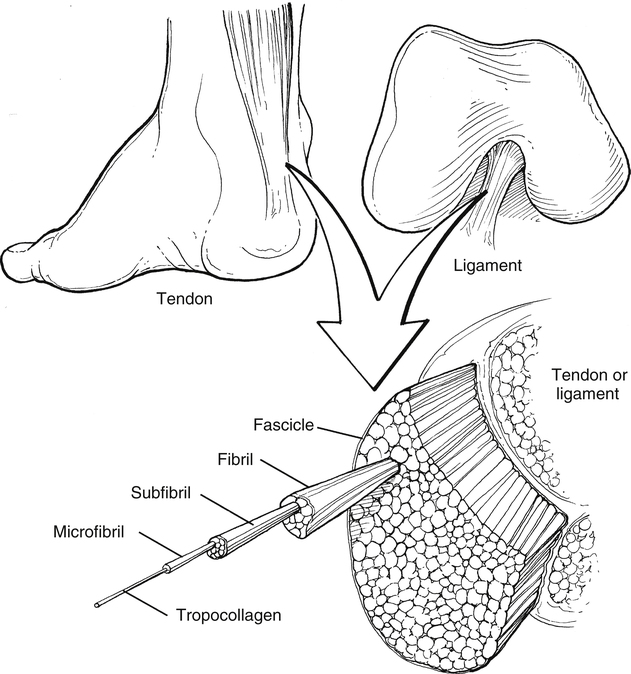
Tendons are dense connective tissue consisting of fibroblasts (cells) and an extracellular matrix composed of collagen fibers, elastin, and ground substance (Fig. 11-2). The fibroblasts are responsible for synthesizing components of the extracellular matrix.28 The ground substance provides support to collagen fibers.28 The collagen fibers, which are oriented in a parallel arrangement, provide strength to resist tensile forces.
The structural hierarchy of tendons is similar to skeletal muscle. Collagen fibrils are grouped together into the tendon’s basic functional unit, the collagen fiber. Groups of collagen fibers (primary fiber bundle) form a subfascicle.16 Several subfascicles grouped together form a fascicle (secondary fiber bundle).16 Several fascicles grouped together form a tertiary bundle.16 These three groups (bundles) are surrounded by a connective tissue called the endotenon.16 The epitenon surrounds the entire tendon as a fine, loose connective tissue sheath.16,28 Blood vessels and nerves are also contained within the epitenon.28 The paratenon is a loose areolar connective tissue attaching to the outer portion of the epitenon.16,28 The function of the paratenon is to allow gliding over adjacent structures.16
The transition from tendon into its osseous attachment site includes several connective tissue changes.16 The first change is from the primary tendon structure into fibrocartilage. Next, the fibrocartilage converts into a mineralized fibrocartilage. The mineralized fibrocartilage secures the attachment of the tendon to the bone. In addition to this arrangement, there are collagen fibers from the tendon that pass through these transition zones and attach to the bone. These fibers are known as Sharpey’s fibers.
MUSCLE AND TENDON INJURIES
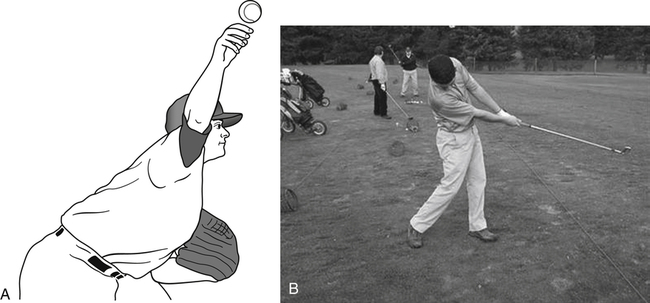
Examples from the world of sports will help illustrate the significant loads and stresses that may be experienced by some athletes. During the deceleration phase of pitching a baseball, the pitcher’s shoulder rotates up to 7000° per second with 400 newtons (N) of posterior shear forces, 300 N of inferior shear forces, and 1000 N of compressive forces experienced at the glenohumeral joint (Fig. 11-3, A).20 Over time, these shear and compressive forces may increase the baseball pitcher’s risk of sustaining a shoulder injury.20,21,34 During the golf swing, a golfer may experience compressive loads in the lumbar spine that are eight times his or her body weight (Fig. 11-3, B).11 To provide some perspective, a distance runner will experience compressive loads that are equal to three times his or her body weight during the run. To reduce the risk of injury, muscles and tendons must possess the ability to stretch or deform in response to the applied load. When an applied load supersedes the muscle or tendon’s ability to resist tensile forces, the muscle or tendon is at a higher risk of injury.
Muscle and Tendon Injury Mechanisms
A direct muscle injury will occur when an external force applied to the body results in a trauma. Examples of direct muscle injuries include a contusion caused by a blunt trauma or a deep laceration caused by a sharp implement. In each of these examples, the applied supraphysiologic load was greater than the muscle’s maximum tissue tolerance. An indirect muscle injury occurs independent of an applied external force. Indirect muscle injuries are the result of either a supraphysiologic load (e.g., a violent or excessive muscular stretch or excessive muscular stretch combined with an eccentric muscular contraction) or repeated insults (e.g., overuse injury) to the tissue without proper time for recovery.8,18 Indirect muscle injuries primarily occur at the musculotendinous junction; however, they may also occur in the belly itself.18
A muscle strain, frequently referred to as a muscle tear, is an indirect injury (Box 11-1). A pulled hamstring (or sometimes referred to as a hammy) is an example of a common muscle strain experienced in sports.5,6,26 Muscle strains can cause significant pain and greatly reduce function.5,6,26 Hamstring injuries can plague an athlete with a lengthy rehabilitation period and a slow return to sport.25 Muscles that cross two joints, like the hamstrings, are frequently strained.8,25 Sports medicine specialists believe that these muscles may become injured in response to an intense eccentric load.8 Many of the most frequently strained muscles are composed of type II muscle fibers, the fibers that are least resistant to fatigue.8
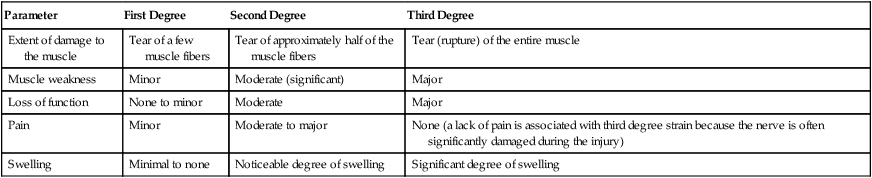
Muscle strains are frequently described by the degree of damage to the muscle. Table 11-2 presents the characteristics associated with a first degree, second degree, or third degree muscle strain.
Table 11-2
Characteristics of First Degree, Second Degree, and Third Degree Muscle Strains
| Parameter | First Degree | Second Degree | Third Degree |
| Extent of damage to the muscle | Tear of a few muscle fibers | Tear of approximately half of the muscle fibers | Tear (rupture) of the entire muscle |
| Muscle weakness | Minor | Moderate (significant) | Major |
| Loss of function | None to minor | Moderate | Major |
| Pain | Minor | Moderate to major | None (a lack of pain is associated with third degree strain because the nerve is often significantly damaged during the injury) |
| Swelling | Minimal to none | Noticeable degree of swelling | Significant degree of swelling |
Tendon injuries range from acute tendinitis to chronic tendinopathy (Table 11-3). Tendinitis is an acute injury of the tendon associated with an inflammatory response. A patient with a “true” tendonitis may present to physical therapy with pain, functional loss, and signs associated with inflammation (pain, warmth, swelling, redness). A patient with tendinitis often describes performing an unfamiliar, repetitive activity from 1 day to a week before seeking medical attention. If a patient presents with a tendon injury that lacks inflammatory signs, it is likely that the patient has a tendinopathy. It is not uncommon to have a patient describe that he or she has experienced pain for many months to years before seeking medical attention. The Achilles tendon, the quadriceps tendon, and the supraspinatus tendon are common locations that one might develop a tendinopathy. The manner in which each condition is treated differs greatly. A tendon may also rupture, requiring surgical repair.
Table 11-3
| Injury | Classification |
| Tendinitis | Acute injury to the tendon with associated inflammatory response |
| Tendinosis | Degeneration to the tendon, not associated with inflammatory process, due to one or more factors (e.g., microtrauma, age-related changes) |
| Peritenonitis | Inflammation of the peritenon only |
| Tenosynovitis | Inflammation of the tendon’s synovial membrane |
| Tenovaginitis | An inflamed, thickened tendon sheath |
Age Effects on Tendons
Over time, the aging tendon has an increased risk of sustaining an injury.33 PTAs will frequently provide treatment for patients who are 40 years and older who have experienced a tendon injury. In some cases, patients will receive conservative physical therapy treatment, whereas in other cases patients may require invasive procedures such as injections or surgical repair.
Pathomechanics Associated with the Aging Tendon
As one ages, the stresses that would have been tolerated in youth now can contribute to an overuse injury or a tendon rupture. The aging tendon is usually smaller in size (compared with its size during youth or when a young adult) with a decrease in total number of collagen fibers. In addition, the ability of the aging tendon to turn over collagen fibers decreases.28,33 The inability to add new collagen fibers at the same rate, or at all, limits the body’s effectiveness in repairing microtrauma. Over time, repeated subfailure loads to a tendon will result in a tendon rupture. In addition, because of the smaller overall size, the tendon will have a lower capacity to resist certain tensile loads.
INJURY AND HEALING
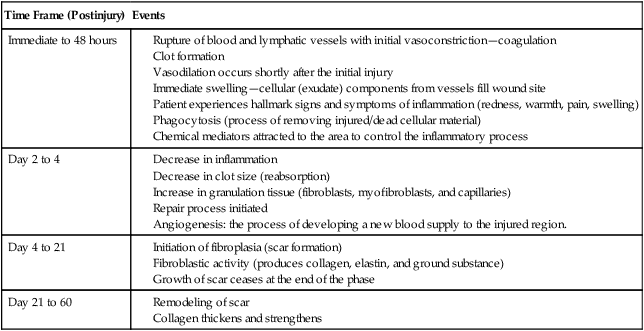
As an immediate response to a muscle or tendon injury, the human body will initiate the healing process. For a patient to optimally recover from a muscle and or tendon injury, his or her body must successfully complete the three stages of healing: acute, subacute, and chronic.1,23,28 The events that occur during each of these three stages are fairly consistent among connective tissues. Table 11-4 provides a timeline overview of events that occur during the healing process. A chronic pain syndrome may develop if the body fails to appropriately progress through these three stages.
Table 11-4
Timeline of Cellular Events Occurring During the Healing Process
Effects of Immobilization
Muscle atrophy also results in a decrease in muscle weight. Muscle that has atrophied and lost weight also loses its ability to generate force and tension. The greatest amount of atrophy occurs within the first week of immobilization, and muscle fiber size decreases by approximately 17% within 3 days of immobilization. Box 11-2 depicts the various physiologic changes that occur in skeletal muscle when immobilized in a shortened position.
Muscle Repair and Heterotopic Bone Formation
Occasionally, injury to a muscle (e.g., severe blunt injury, deep contusion, surgical exposures, and certain fractures) may result in heterotopic bone formation (ectopic bone formation). Heterotopic bone formation after blunt trauma to muscle is known as myositis ossificans.22 Generally, if the contusion is severe (deep versus superficial), there may be a periosteal reaction that stimulates undifferentiated mesenchymal cells to proliferate during the first 3 to 4 days after the trauma. During the next 5 to 8 days, cartilage develops with gradual calcification and neovascularization with subsequent bone deposition.
EVIDENCE-BASED THERAPEUTIC INTERVENTIONS FOR INJURED MUSCLES AND TENDONS
Acute Stage: Treatment in the Physical Therapy Clinic
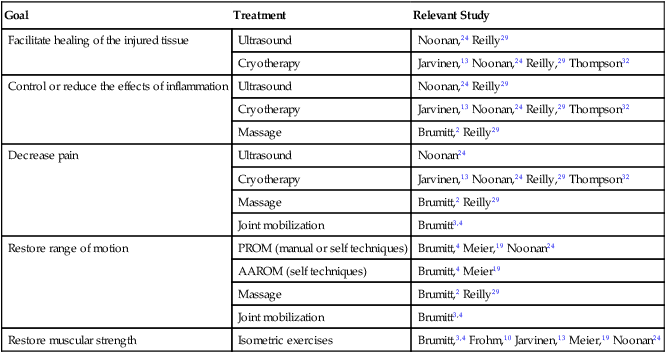
Physical agents are frequently used during the acute stage of healing to decrease pain, reduce inflammation, facilitate healing, and to restore muscular function. In addition, passive range of motion (PROM) and active assisted range of motion (AAROM) exercises are initiated to maintain or restore movement around the joint. Manual therapy techniques are performed to reduce pain and improve soft tissue and joint mobility. Isometric strengthening may also be performed during this phase to retard strength loss. Table 11-5 presents a list of evidence-supported treatments for patients in the acute stage of healing.
Table 11-5
Evidence-Supported Treatments for Patients in the Acute Stage of Healing
| Goal | Treatment | Relevant Study |
| Facilitate healing of the injured tissue | Ultrasound | Noonan,24 Reilly29 |
| Cryotherapy | Jarvinen,13 Noonan,24 Reilly,29 Thompson32 | |
| Control or reduce the effects of inflammation | Ultrasound | Noonan,24 Reilly29 |
| Cryotherapy | Jarvinen,13 Noonan,24 Reilly,29 Thompson32 | |
| Massage | Brumitt,2 Reilly29 | |
| Decrease pain | Ultrasound | Noonan24 |
| Cryotherapy | Jarvinen,13 Noonan,24 Reilly,29 Thompson32 | |
| Massage | Brumitt,2 Reilly29 | |
| Joint mobilization | Brumitt3,4 | |
| Restore range of motion | PROM (manual or self techniques) | Brumitt,4 Meier,19 Noonan24 |
| AAROM (self techniques) | Brumitt,4 Meier19 | |
| Massage | Brumitt,2 Reilly29 | |
| Joint mobilization | Brumitt3,4 | |
| Restore muscular strength | Isometric exercises | Brumitt,3,4 Frohm,10 Jarvinen,13 Meier,19 Noonan24 |
Subacute Stage: Treatment in the Physical Therapy Clinic
A primary goal of this phase is to progress and restore range of motion. During the acute phase, PROM and AAROM exercises are prescribed. As tolerated, the patient should be progressed to active range of motion (AROM) techniques and static stretching exercises. Typically, multiple sets and repetitions of AROM exercises are prescribed. Manual therapy techniques (joint mobilization, massage) may continue to be necessary to restore joint and soft tissue mobility. Static stretching exercises should initially be performed gently, holding each stretch for 30 seconds.13,24,37
The prescription of resisted exercises will assist the collagen orientation and improve tensile capabilities. Initially, exercises should be performed with high repetitions and low weights. Performing a muscular endurance program will allow the patient to gradually gain strength while reducing the risk of damaging the new collagen fibers. Consider patients performing 15 to 25 reps of 1 to 2 sets per exercise. The initial number of exercises prescribed by the PT will be dependent upon the patient’s presentation. The inclusion of neuromuscular electrical stimulation may further assist the restoration of muscular strength.19
Chronic Stage: Treatment in the Physical Therapy Clinic
Increasing the endurance capacity of the muscle may be continued as necessary, but now the therapy team should be able to progress strengthening as tolerated. Exercise prescription should be functional in nature and should account for muscle fiber structure within muscle groups. For example, muscles of the core (type I muscle fibers) should continue to be trained using endurance training strategies. Strength training variables may be applied to muscle groups containing higher percentages of type II muscle fibers. Once the patient is pain free, plyometrics or power training may be initiated if functionally necessary (e.g., athletes, industrial workers).4
Special Considerations: Tendon Repairs and Tendinopathy
Patients who have been referred with a tendinopathy diagnosis will benefit from performance of eccentric exercises. There is a growing body of evidence that suggests the inclusion of eccentric exercises will help to reduce pain and restore function in individuals with either a tendinosis at the Achilles tendon or patella tendon.10,14,17,36