CHAPTER 12 Multisystem Metastases and Assessment of Treatment Efficacy
A major focus in the management of cancer patients is the monitoring of response to treatment, whether chemotherapy, hormone therapy, or biologics. Two important issues are the accurate assessment of therapeutic efficacy and the provision of prognostic information. Clinical signs and symptoms can be subjective and difficult to evaluate because of the side effects of treatment. Serum tumor markers may lack sensitivity or specificity. Imaging offers an objective, and often quantifiable, method of measuring response. CT, MRI, positron emission tomography (PET), and bone scintigraphy have all demonstrated both advantages and disadvantages for patient monitoring and should be viewed as complementary modalities. Chemotherapeutic efficacy can be assessed in different contexts, such as neoadjuvant (i.e., presurgical) applications and in the setting of metastatic disease (measurable distant disease).
NEOADJUVANT
Neoadjuvant chemotherapy was initially applied to locally advanced breast cancer but has more recently been used in patients presenting with smaller, resectable breast cancers.1 Studies identifying the amount of breast and axillary tumor after neoadjuvant chemotherapy have demonstrated prognostic value in predicting disease-free and overall survival.2–5 In a study using fluorodeoxyglucose (FDG) PET, Bellon and coworkers found that internal mammary nodal involvement in patients with locally advanced breast cancer was predictive of regional or systemic failure.6 Modalities with exquisite anatomic and localization capabilities, such as CT and MRI, rely on a change in lesion size to assess efficacy. It is well recognized that a change in size may lag for weeks or months. CT scans are not routinely used to monitor the efficacy of chemotherapy in the neoadjuvant setting, although they may be very helpful to investigate a new finding during this time period. MRI has shown promise, but early results require larger, confirmatory studies. Dynamic contrast-enhanced MRI allows analysis of both tumor size and contrast enhancement pattern. Although these parameters may ultimately prove to be of great value in separating responders from nonresponders, underestimation of residual tumor size, comparing baseline and follow-up studies, could produce false-negative results, especially in larger tumors.7,8 In the neoadjuvant setting, imaging is used primarily to assess local response.
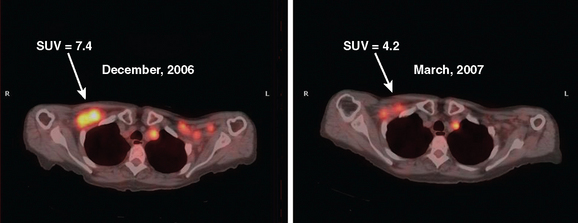
Metabolic imaging, specifically FDG PET, has shown encouraging results in the neoadjuvant setting.9–12 PET can be assessed visually or semiquantitatively. The most widely used quantitative method is measurement of the standardized uptake value (SUV) (Figure 1). This measures the amount of FDG uptake in a region of interest (i.e., tumor), divided by the injected dose corrected for patient weight. Many factors can affect this measurement, including lesion size, time interval between injection of FDG and time of imaging, and serum glucose level. Despite these and other variables, the SUV has proved useful for sequential assessment. Changes in SUV may be detected as early as after one cycle of chemotherapy. A significant fall in SUV separates responders from nonresponders and is also used prognostically. These data have been validated against histopathology.11,13
METASTATIC
Monitoring response to chemotherapy in the metastatic setting is also an evolving challenge for imaging. Supporting data from large, wellcontrolled, prospective studies are lacking for both soft tissue and bone metastases. Interest in this particular aspect of breast cancer treatment has been sparked by the continued development of new chemotherapeutic agents, as well as the significant potential toxicity of these drugs. As in the neoadjuvant setting, CT and MRI reliably provide data on lesion size changes. However, changes in size may not occur for months in patients who are responding to treatment. Additionally, various cytostatic agents may never result in a decrease in size, even if efficacious in prolonging survival. Preliminary data on the use of FDG PET are encouraging, with some authors relying on a change in SUV to separate responders from nonresponders.13–15 Significant differences have been shown between responders and nonresponders, with responders showing declining FDG uptake, as compared with minimal to no change in nonresponders. These changes have been demonstrated as early as after one cycle of chemotherapy. In the area of bone metastases, FDG PET appears to offer an advantage over conventional imaging (CI), including bone scintigraphy. Both CI and scintigraphy have difficulty assessing change in size of bone metastases; however, demonstration of significantly decreased or resolution of hypermetabolic activity on FDG PET provides valuable information.16
A possible caveat in the use of FDG PET to monitor response to therapy may be in patients treated with hormonal therapy. Initial studies have shown a transient increase in glucose use in responding patients, resulting in a “metabolic flare.” This may be due to an initial agonist effect rather than an antagonist effect of therapy. In two reports, responders showed this early increase in FDG uptake, whereas nonresponders did not.17,18
Skeletal scintigraphy is covered in Chapter 8. Although whole-body bone scanning is a sensitive test for the identification of osteoblastic metastases, inherent problems with the physiologic basis for a positive study limit its utility for assessing efficacy of chemotherapy. As discussed previously, bone-seeking radiopharmaceuticals concentrate in large part owing to localized osteoblastic activity. Even relatively small increases in osteoblastic activity result in focal hot spots, often weeks to months before these lesions become visible on plain radiography or CT. However, the healing response alone, even in the absence of viable tumor, typically elicits increased radiopharmaceutical uptake, indistinguishable from active metastases. The added issue of suboptimal specificity (i.e., inability to separate benign from malignant disease) limits the role of bone scintigraphy as a modality to assess chemotherapy response. Two exceptions would be the disappearance of previously identified bony metastases on scintiscan and the development of new sites while receiving treatment. Under these circumstances, more definitive statements can be made.
1 Bonadonna G, Valagussa P, Zucali R, et al. Primary chemotherapy in surgically resectable breast cancer. CA Cancer J Clin. 1995;45:227-243.
2 Mankoff DA, Dunnwald LK. Changes in glucose metabolism and blood flow following chemotherapy for breast cancer. Avril N, editor. PET Clinics. Philadelphia: WB Saunders; 2006;1:71-81. No. 1
3 Machiavelli MR, Romero AD, Perez JE, et al. Prognostic significance of pathological response of primary tumor and metastatic axillary lymph nodes after neoadjuvant chemotherapy for locally advanced breast carcinoma. Cancer J Sci Am. 1998;4(2):125-131.
4 Wolmark N, Wang J, Mamounas E, et al. Preoperative chemotherapy in patients with operable breast cancer: nine year results from national surgical adjuvant breast and bowel project B-18. J Natl Cancer Inst Monog. 2001;30:96-102.
5 McCready DR, Hortobagyi GN, Kau SW, et al. The prognostic significance of lymph node metastases after preoperative chemotherapy for locally advanced breast cancer. Arch Surg. 1989;124:21-25.
6 Bellon JR, Livingston RB, Eubank WB, et al. Evaluation of the internal mammary lymph nodes by FDG-PET in locally advanced breast cancer (LABC). Am J Clin Oncol. 2004;27(4):407-410.
7 Rieber A, Brambs HJ, Gabelmann A, et al. Breast MRI for monitoring response of primary breast cancer to neoadjuvant chemotherapy. Eur Radiol. 2002;12(7):1711-1719.
8 Martincich L, Montemurro F, De Rosa G, et al. Monitoring response to primary chemotherapy in breast cancer using dynamic contrast-enhanced magnetic resonance imaging. Breast Cancer Res Treat. 2004;83(1):67-76.
9 Wahl RL, Zasadny K, Helvie MA, et al. Metastatic monitoring of breast cancer chemohormonotherapy using positron emission tomography: initial evaluation. J Clin Oncol. 1993;11:2101-2111.
10 Bassa P, Kim EE, Inove T, et al. Evaluation of preoperative chemotherapy using PET with {fluorine-18} fluorodeoxyglucose in breast cancer. J Nucl Med. 1996;37:931-938.
11 Schelling M, Avril N, Nahrig J, et al. Positron emission tomography using [18F]–fluorodeoxyglucose for monitoring primary chemotherapy in breast cancer. J Clin Oncol. 2000;18:1689-1695.
12 Biersack HJ, Palmedo H. Locally advanced breast cancer: is PET useful for monitoring primary chemotherapy? J Nucl Med. 2003;44(11):1815-1817.
13 Jansson T, Westlin J, Ahlstrom H, et al. Positron emission tomography studies in patients with locally advanced and/or metastatic breast cancer: a method for early therapy evaluation? J Clin Oncol. 1995;13:1470-1477.
14 Gennari A, Donati S, Salvador B, et al. Role of 2-[18F]–fluorodeoxyglucose (FDG) positron emission tomography (PET) in the early assessment of response to chemotherapy in metastatic breast cancer patients. Clin Breast Cancer. 2002;1:156-161. discussion 162–163
15 Dose Schwartz J, Bader M, Jenicke L, et al. Early prediction of response to chemotherapy in metastatic breast cancer using sequential 18F-FDG-PET imaging. J Nucl Med. 2005;46(7):1144-1150.
16 Hoh CK, Schiepers C. 18-FDG imaging in breast cancer. Semin Nucl Med. 1999;29:49-56.
17 Dehdashti F, Flanagan FL, Mortimer JE, et al. Positron emission tomographic assessment of “metabolic flare” to predict response of metastatic breast cancer to antiestrogen therapy. Eur J Nucl Med. 1999;26:51-56.
18 Mortimer JE, Dehdashti F, Siegel BA, et al. Metabolic flare: indicator of hormone responsiveness in advanced breast cancer. J Clin Oncol. 2001;19:2797-2803.
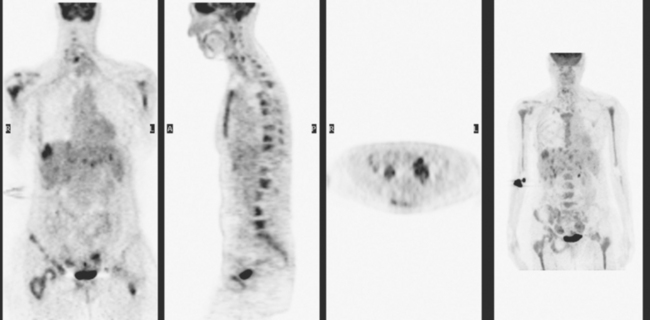
CASE 1 Recurrent PET-positive activity in LABC and bone metastases with increased tumor markers
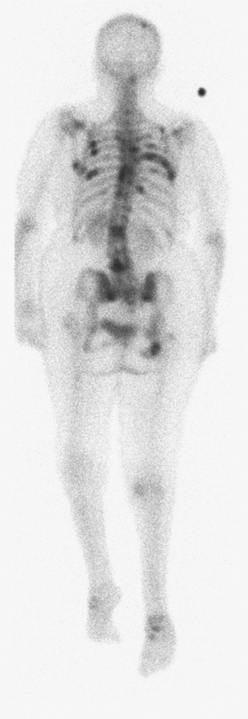
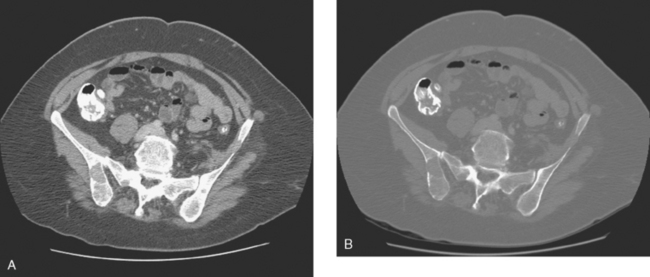
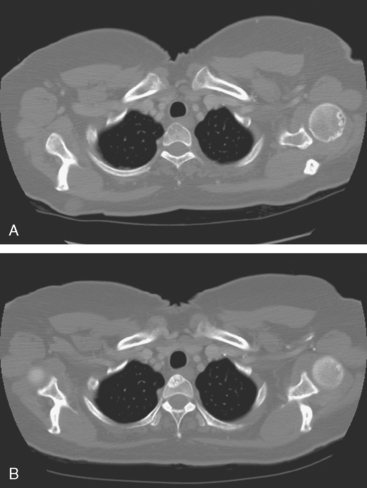
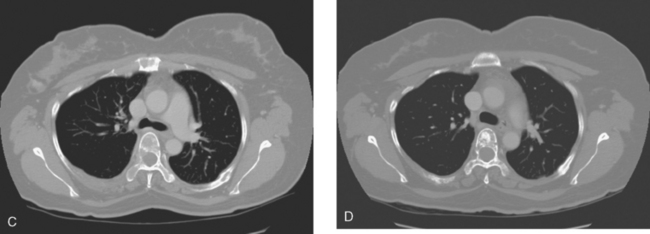
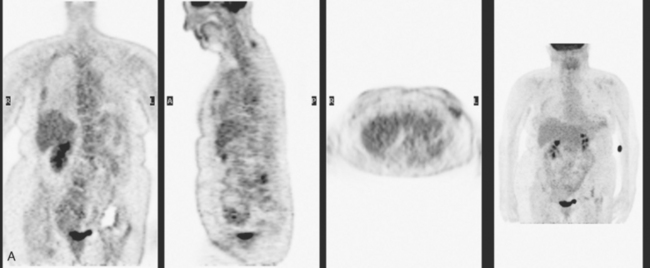
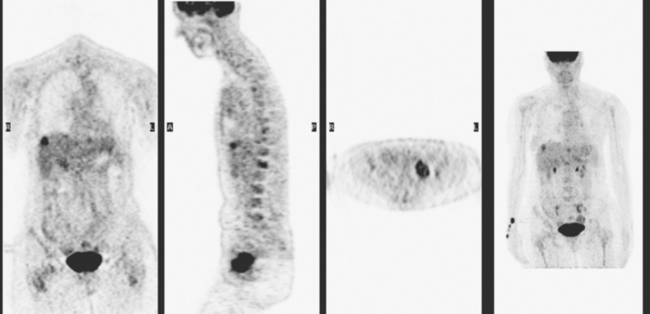
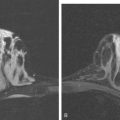
A 51-year-old woman presented with a greater than 1 year’s history of a central, firm, and retracted left breast mass, involving the nipple, with fungation and spontaneous drainage. The patient had not sought medical attention, having a generalized aversion because of a history of multiple surgeries as a child for congenital orthopedic anomalies. When she developed hip pain, she saw her primary care physician, at which time the breast mass was noted. A palpation-guided biopsy confirmed poorly differentiated infiltrating ductal carcinoma. Surgical, oncologic, and imaging evaluations of her locally advanced breast cancer (LABC) showed a palpable, dominant lobulated and spiculated mass, with nipple retraction, skin thickening, malignant calcifications, and pathologic axillary adenopathy. Because of the patient’s hip pain, a metastatic workup was performed, including bone scan; CT scans of the chest, abdomen, and pelvis; and positron emission tomography (PET)/CT imaging. Bone scan showed multiple abnormal sites of activity (Figure 1), consistent with metastatic disease, which corresponded to abnormal sites of hypermetabolism on PET/CT. CT images showed corresponding lytic lesions (Figure 2). Breast MRI showed multiple foci of abnormal signal intensity in bone (Figure 3). MRI showed the left breast to be extensively occupied by a massive neoplasm, with central necrosis, nipple extension and retraction, and skin thickening and enhancement. Abnormal enhancement and extension into the pectoral muscle was also noted.
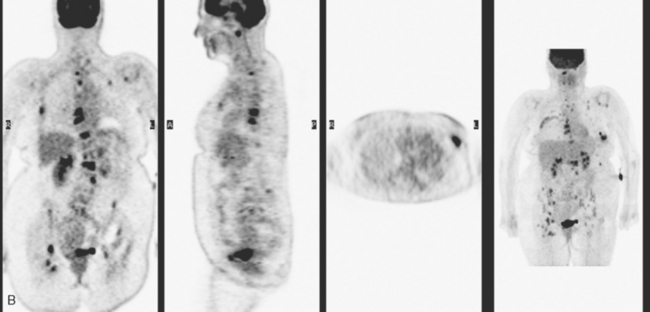
The patient was started on chemotherapy for locally advanced and metastatic breast cancer. Clinically, the patient had an initial excellent response to chemotherapy (six cycles of trastuzumab [Herceptin], docetaxel [Taxotere], carboplatin, and zoledronic acid [Zometa]), with improved bone pain and partial regression of the dominant breast mass. PET scan hypermetabolism at both the breast and bony levels improved dramatically. The corresponding CT scans showed previously lytic bone lesions to convert to a sclerotic appearance, presumably a treatment response (Figure 4). She was maintained on a regimen of Herceptin, Zometa, and letrozole (Femara) every 3 weeks for nearly a year with continued clinical response, before her tumor markers were noted to rise, at which time she was reimaged with CT and PET. Hypermetabolism in the index breast mass and in bone metastases on PET, which had markedly regressed on PET, now showed increased metabolic activity (Figure 5). The patient’s hormonal therapy was stopped, and vinorelbine (Navelbine) chemotherapy was started.
TEACHING POINTS
This patient presented with a neglected breast cancer and extensive bone metastases. Bone scan, PET, CT, and MRI findings were all in agreement. With chemotherapy, the patient’s bone pain, tumor markers, and PET scan findings markedly improved. The lesions on CT changed, showing sclerosis where previously there was lytic change. This indicated a healing response to the therapy. Of course, these patients are not cured per se; rather, their disease is held in check by chemotherapy or hormonal therapy. PET/CT is the most efficient whole-body means of evaluating such patients for relapse, which may be suspected based on increased tumor markers, as in this case, or by the development of new signs or symptoms.
CASE 2 Gastrointestinal metastases from breast cancer
An abdominal and pelvic CT scan was subsequently obtained and showed the colon to be diffusely abnormal as well as the stomach. There were CT findings of a diffuse colitis with colonic wall thickening from cecum to rectum. The stomach also showed diffuse mural thickening. Colonoscopy showed markedly thickened transverse colonic mucosa. The colon was noted to be extremely rigid, and the endoscope could not be further advanced. Biopsies from the distal transverse colon showed a noncolorectal infiltrating carcinoma, compatible with metastatic breast primary (Figure 1).
CASE 3 Peritoneal breast carcinomatosis
A 52-year-old woman with a history of treated breast cancer with a subsequent axillary recurrence presented with rising tumor markers and abdominal distention. Her original diagnosis of breast cancer was 14 years before, a left breast infiltrating ductal carcinoma with extensive ductal carcinoma in situ, which was treated with mastectomy, chemotherapy (six cycles of Cytoxan, methotrexate, 5-fluorouracil (CMF)) and radiation. Margins were close, and 0 of 12 lymph nodes were involved. Five years later, the patient developed a biopsy-proved axillary lymph node recurrence, which was surgically excised. She took tamoxifen for 8 years and was changed to letrozole (Femara) for rising tumor markers, without much response.
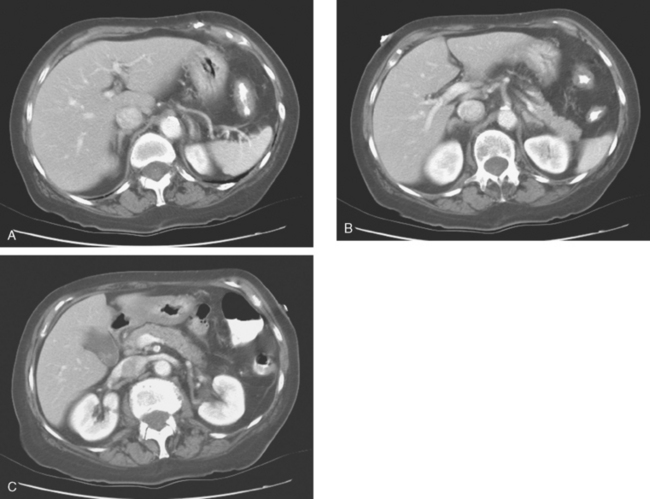
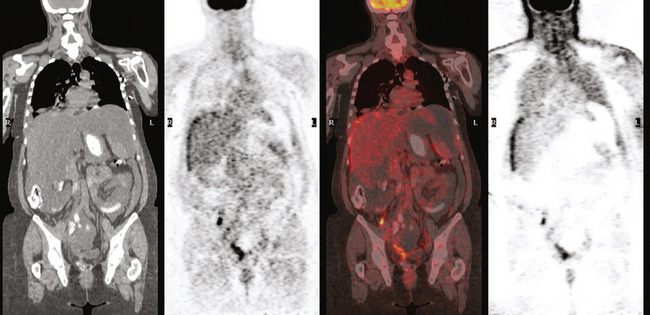
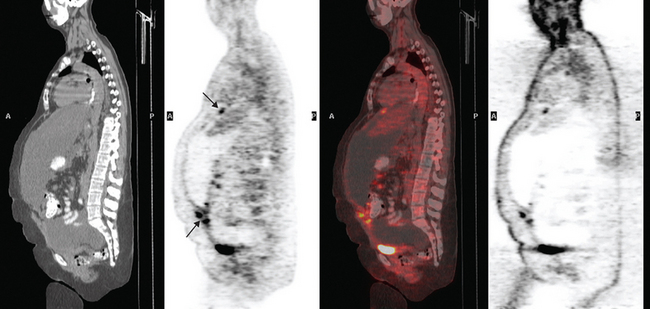
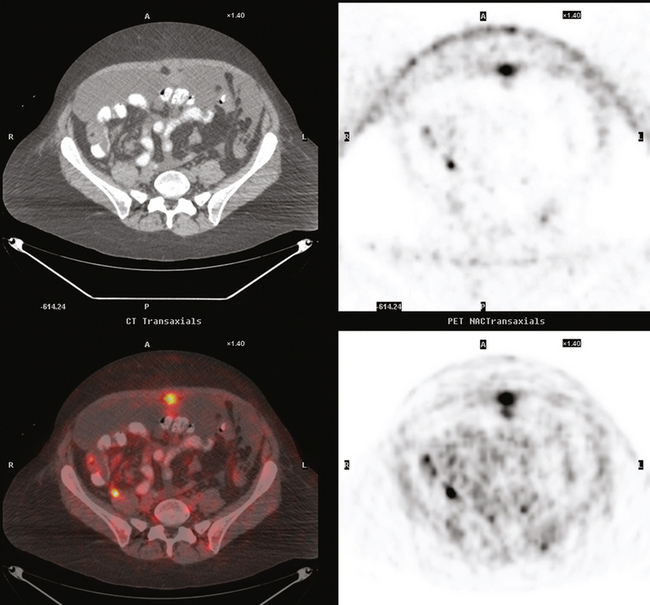
She was restaged with positron emission tomography (PET) and CT for rising tumor markers and abdominal distention and was found to have pleural effusions, ascites, and peritoneal tumor implants (Figures 1, 2, 3, 4, 5, 6, and 7). Analysis of pleural and peritoneal fluid confirmed adenocarcinoma, consistent with breast primary, estrogen receptor and progesterone receptor positive, HER-2/neu negative. The patient was begun on docetaxel (Taxotere) and gemcitabine (Gemzar), with improvement on repeat PET/CT 2 months later. Subsequent evaluations over the next year showed development of PET-positive retroperitoneal nodal disease and, later, a brain metastasis.
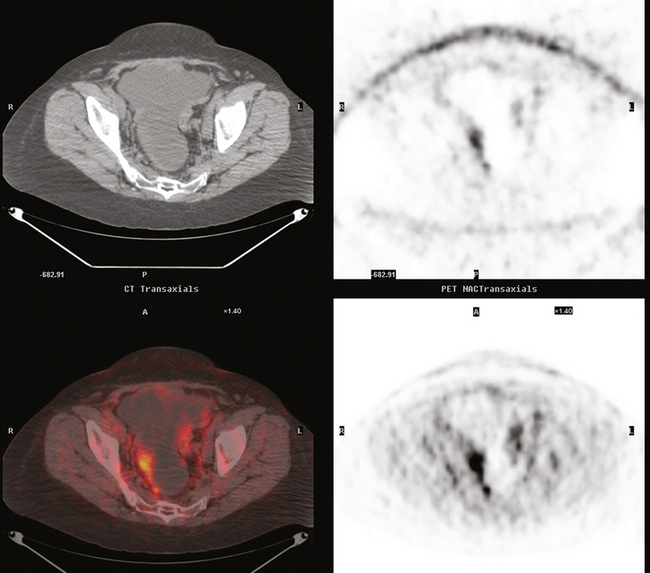
FIGURE 7 Additional findings of peritoneal carcinomatosis are seen in the pelvis, especially notable on the right posterolaterally (corresponding to Figure 3).
TEACHING POINTS
The lymphatic system is thought to be the dominant route of breast cancer spread to the thorax, rather than hematogenous. A seminal detailed necropsy study published in 1979 by Thomas and colleagues of 26 patients who died of disseminated breast cancer looked at the frequency of tumor involvement of thoracic lymph node groups, lungs, pleura, and pericardium. Based on the findings of this study, spread from involved internal mammary nodes through lymphatic communications to intercommunicating nodal groups in the mediastinum and secondary lymphatic involvement of lung, pleura, and pericardium was implicated. The direction of spread to the lung would be centrifugal, from tracheobronchial lymph nodes peripherally. Lymphatics penetrating the diaphragm would be implicated in a case like this, with both pleural and peritoneal involvement. Potential routes include communications described between breast and liver through pericardial lymph nodes and superior liver surface drainage to inferior internal mammary lymph nodes. This can be characterized as an Internet-like system of collaterals extending from scalp to inguinal nodes without valves, with distant lymphatic spread more likely to occur with bulky axillary tumor and with prior axillary surgery or radiation and with inflammatory carcinomas, namely conditions resulting in lymphatic obstruction.
CASE 4 Bone, liver, pericardial, and ovarian metastases
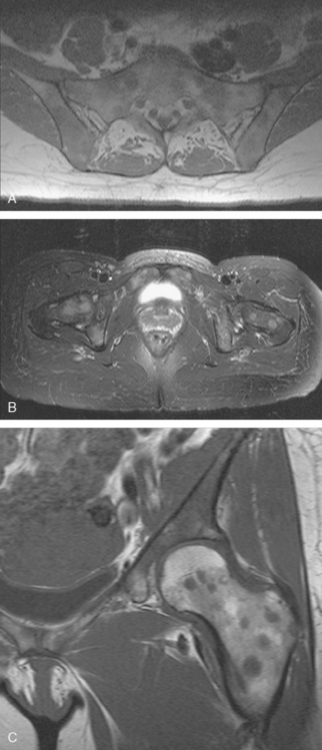
A 48-year-old woman, who had been treated for breast cancer 6 years earlier with mastectomy, chemotherapy and chest wall radiation, presented with low back and progressive groin pain. The original tumor was detected on mammographic screening, and was a left breast, T1N1 (3 of 21 lymph nodes positive, with extracapsular extension), estrogen receptor–negative, progesterone receptor weakly positive, HER-2/neu-positive, 1.1-cm infiltrating ductal carcinoma (IDC) with an extensive intraductal component. Hormone therapy after chemotherapy had been refused.
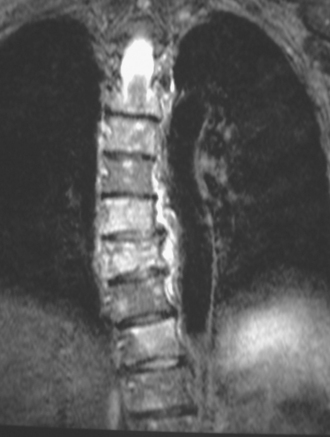
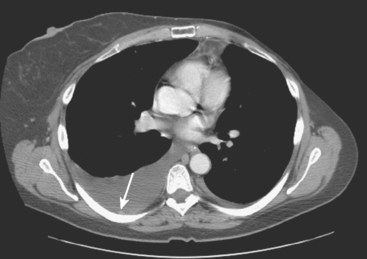
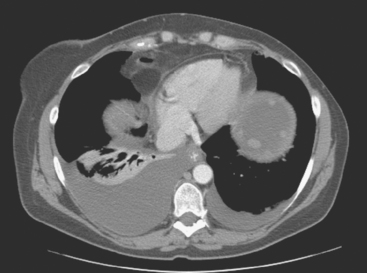
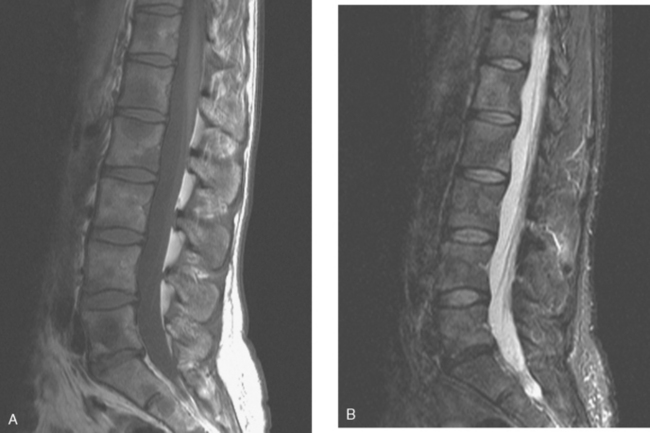
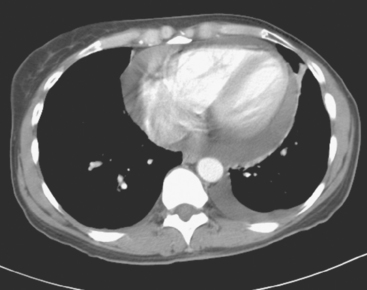
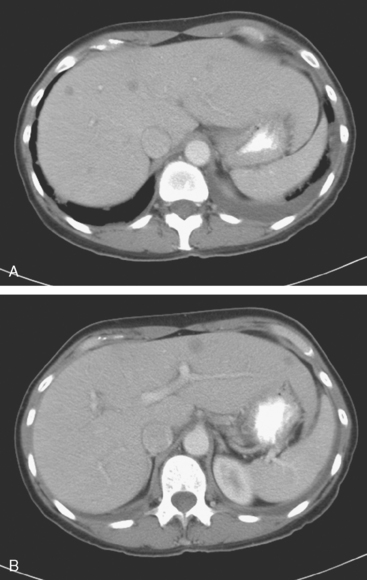
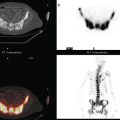
Lumbar spine and pelvis MRI, obtained to evaluate complaints of back and pelvic pain, showed extensive bone metastases (Figures 1 and 2). The patient was restaged with CT and bone scans, which showed pleural and pericardial effusions (Figure 3), liver metastases (Figure 4), and the extensive bone metastases. The pericardial effusion was sizable, and echocardiographic evaluation suggested early tamponade. A pericardial window was created surgically, and 800 mL of serous fluid was drained. The pericardium appeared normal, and the histology was benign. The effusion was positive for metastatic adenocarcinoma, with cytologic features consistent with the patient’s IDC. The patient was treated with chemotherapy, and her response was monitored by CT and positron emission tomography (PET)/CT scans (Figures 5, 6, 7, and 8).
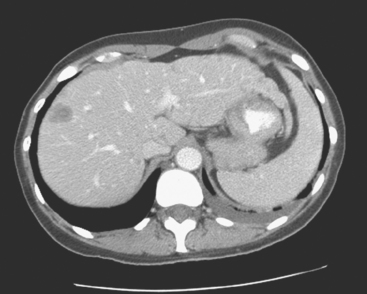
FIGURE 5 Repeat enhanced, PV phase abdominal CT scan shows a larger hypodense liver metastasis, with perilesional enhancement, in the right lobe. Note new nodularity of the liver contour, best seen in the left lobe (contrast with Figure 4, 1 year earlier). The localized capsular contraction coincides with sites of subjacent liver metastases.
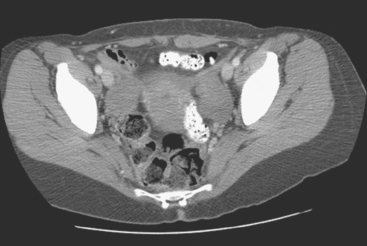
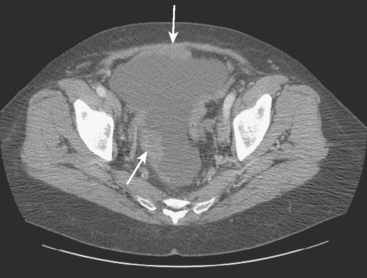
FIGURE 6 Image from the pelvis from the same enhanced CT scan in Figure 5 shows bilateral ovarian enlargement, left greater than right.
TEACHING POINTS
Once the diagnosis of metastatic breast cancer was established, the patient was begun on chemotherapy, and her response was monitored with periodic CT and PET/CT scans. The initial liver CT illustrated here showed liver metastases, and a comparable study a year later demonstrated evolving findings. Lesions seen on the first study were successfully treated, leaving behind areas of capsular contraction, imparting a nodular contour to the liver. This post-treatment appearance has been termed pseudocirrhosis. Unfortunately, these changes do not signify eradication of liver metastases, but rather local response and a degree of local control. More dramatic post-treatment changes (see Case 3 in Chapter 9) can be difficult to assess based solely on anatomic imaging modalities. Correlation with PET imaging can be very helpful in assessing the active tumor burden.
In a case like this, with multisystem metastatic disease, the whole-body capability of PET is most valuable. The adnexal activity seen here in the pelvis localizes to enlarged ovaries. The constellation of imaging findings in this context is most compatible with ovarian metastases. Although benign ovarian hypermetabolism can be seen in premenopausal patients because of corpus luteum cysts, the bilaterality and intensity of the activity seen here, as well as the abnormal size and appearance of the ovaries on the CT, strongly suggest metastatic involvement. See Case 17 in Chapter 3 for an example of benign pelvic PET activity due to a corpus luteum cyst.
Gupta AA, Kim DC, Krinsky GA, et al. CT and MRI of cirrhosis and its mimics. Am J Roentgenol. 2004;183:1595-1601.
Nascimento AB, Mitchell DG, Rubin R, et al. Diffuse desmoplastic breast carcinoma metastases to the liver simulating cirrhosis at MR imaging: report of two cases. Radiology. Oct 2001;221:117-121.
Young ST, Paulson EK, Washington K, et al. CT of the liver in patients with metastatic breast carcinoma treated by chemotherapy: findings simulating cirrhosis. AJR Am J Roentgenol. 1994;163:1385-1388.