Chapter 10. Multisystem failure
Shock: The Basic Mechanisms
In the early stages, the extremities are cool as blood is diverted to more important areas, the pulse increases and the blood pressure starts to fall.
Once established, shock causes a cold clammy skin, tachycardia and increased respiratory rate. Oxygen saturations decrease. The urine output is reduced due to impaired blood flow to the kidneys. The release of lactic acid from ischaemic, poorly perfused tissues leads to an acidosis. Acidosis in turn impairs cardiac function and leads to further falls in the blood pressure.
In severe shock, reduced perfusion to the brain leads to agitation, restlessness and eventual coma. By this stage, the reduced blood supply to the kidneys means there may be little or no urine output.
Box 10.1
• A systolic blood pressure of less than 90mmHg
• A tachycardia
• An increased respiratory rate (above 20 breaths/min) and reduced oxygen saturations
• Mottled, cool and clammy skin
• Evidence of hypovolaemia: dry mouth, dry axillary skin, postural hypotension, thirst
• Low urine output (less than 0.5ml/kg per h)
• Confusion or agitation progressing to coma
 |
| Fig. 10.1 |
There are three forms of shock: cardiogenic shock, hypovolaemic shock and redistributive (low-resistance) shock.
Cardiogenic shock
In cardiogenic shock, the heart as a pump fails to propel blood around the circulation. The common causes of cardiogenic shock are:
1. Heart muscle damage – a myocardial infarction severe enough to damage a large part of the left ventricle will reduce the cardiac output sufficiently to produce shock.
2. Severe arrhythmia – an acute rapid arrhythmia, most commonly atrial fibrillation, can reduce the blood pressure enough to cause shock. This commonly occurs in the setting of a pre-existing acute illness such as DKA or pneumonia.
3. Valve disease – acute valve failure is an important reversible cause of cardiogenic shock. Sudden mitral regurgitation can complicate myocardial infarction and acute aortic incompetence can complicate bacterial endocarditis.
4. Output obstruction – this is seen in massive pulmonary embolus: the output from the right side of the heart is suddenly cut off by a clot lodging in the main pulmonary arteries, leading to sudden profound shock.
Hypovolaemic shock
Hypovolaemic shock is probably the most important type to recognise; it is relatively easy to treat and is rapidly fatal if allowed to progress. In hypovolaemic shock, there is a reduction in the circulating blood volume due to either external fluid loss or ‘internal loss’ by fluid being sequestrated within the body’s internal compartments.
Common medical causes of acute external fluid loss are:
• upper gastrointestinal bleeding (Table 10.1)
| Clinical observations | |||||||
|---|---|---|---|---|---|---|---|
| % of total blood volume | BP | Pulse | Respiratory rate | Mental state | IV therapy | ||
| BLOOD LOSS | 750ml | 15% | N | 90 | N | THIRSTY | SALINE |
| 1500ml | 30% | LOW | 120 | >20 | ANXIOUS CONFUSED | BLOOD | |
• severe diarrhoea and vomiting
• diabetic ketoacidosis
Internal fluid loss is seen in:
• acute pancreatitis
• severe paralytic ileus
Redistributive (low-resistance) shock
Redistributive shock is due to the effect of circulating toxins disturbing the normal distribution of blood flow within the body. Some vessels, notably those in the skin, open up and provide little or no resistance to blood flow. Other vessels shut down in a pattern in which organs such as the kidney are starved of blood. In contrast to the other forms of shock, in redistributive shock the overall resistance to blood flow is reduced: the cardiac output is therefore high, the pulse is bounding and the peripheries are warm. The patient appears flushed and warm rather than grey and clammy. Without treatment, however, the progression is the same as that in other forms of shock – to kidney failure and to increasingly severe acidosis.
The main causes of redistributive shock are:
• sepsis, especially from gut and renal tract infections
• anaphylaxis
Acute Severe Hypotensive Collapse
Clinically, patients with shock present to the Acute Medical Unit as a problem of acute severe hypotensive collapse. Given this clinical situation, there are five groups of conditions to consider:
Case Studies 10.1
CASE 1
A 70-year-old woman is admitted with collapse.
Initial observations
She is coughing frothy sputum and cannot lie flat. She has pulse 160 beats/min, blood pressure 90/60mmHg, saturations 80%, respiratory rate 35 breaths/min, and temperature 37.4°C.
• This is pulmonary oedema due to acute heart failure – cardiorespiratory collapse
• She needs to be sat up and given immediate oxygen
• Her low blood pressure and high pulse are due to the heart problem
CASE 2
An 80-year-old man with a urinary catheter is admitted with confusion and rigors.
Initial observations
He is hot and delirious, with rigors. He has pulse 115 beats/min, blood pressure 70/40mmHg, saturations 82%, respiratory rate 30 breaths/min, and temperature 38.5°C.
• This is septicaemic shock
• He needs urgent oxygen, i.v. fluids and immediate antibiotics
• Progress can be judged from the blood pressure and urine output
CASE 3
A 50-year-old man has collapsed after vomiting a large amount of fresh blood.
Initial observations
He is cold, clammy and very restless. He has pulse 130 beats/min, blood pressure 100/50mmHg lying and unrecordable on sitting, saturations 88%, respiratory rate 40 breaths/min, and temperature 36.5°C.
• This is hypovolaemic shock
• He needs urgent oxygen, a large cannula, rapid i.v. fluids then blood
• Progress can be judged from the falling pulse rate and increasing blood pressure
2. massive pulmonary embolus
3. septicaemia (→ Case 2 in Case Studies 10.1)
4. hypovolaemia (→ Case 3 in Case Studies 10.1)
5. anaphylaxis
Importance of Immediate Resuscitation
These deficiencies in the first critical hours of admission have an impact on the patient’s chance of recovery, especially, as in Case Study 10.2, where there is sepsis. Once the condition deteriorates to septic shock, the mortality rate approaches 50%. In this case, the priority should have been to correct the oxygen saturation and try and restore a blood pressure within the first half hour of admission. The admitting nurse and doctor must share their information from the initial assessments in order to draw up an effective management plan. There are critical time intervals between the admission and the first:
• dose of antibiotics
• treatment with oxygen
• initiation of i.v. fluids
• correction of the blood pressure
Case Study 10.2
A patient came in at 13.30h and was clerked in at 16.30h. Although the nursing notes record a blood pressure of 90/40mmHg, this is not recorded in the doctor’s clerking. Blood gases were taken on admission, but the results were not recorded in the notes.The chest X-ray showed a right upper lobe pneumonia.
At 19.30h the registrar was asked to see the patient because of persistent hypotension. A blood pressure of 60/40mmHg was noted, as were the abnormal blood gases and a high creatinine (indicating a renal problem). Intensive i.v. fluids were started.
The patient had a central line put in at 01.30h, 12h after admission, and was transferred to the ITU. Noradrenaline (norepinephrine) was needed to restore the blood pressure. By the second day, the whole of the right upper lobe had consolidated due to the rapid progression of pneumococcal pneumonia.The pneumococcus bacterium was grown in two sets of blood cultures taken on admission.
Resuscitation of the patient with hypotensive collapse
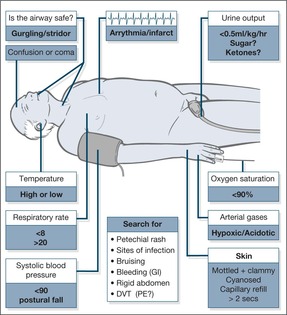
It is important to identify the patient who needs a high priority for resuscitation. This is done by the triage system, ABCDE. In these critically ill patients, resuscitation will commence before the full diagnosis is apparent.
Rapid assessment in the critically ill: the importance of ABCDE
Look. A respiratory rate of < 8 breaths/min (depressed respiration) or > 20 breaths/min suggests a serious problem.
• Remember that an increased respiratory rate is seen in any cause of shock, even if the lungs are normal.
• Measure the oxygen saturation: a level below 90% indicates severe hypoxia.
• Does breathing look like hard work or is the patient tired and losing interest?
• Does this look like acute asthma?
In LVF, the patient will be sitting upright and coughing frothy sputum.
Listen. Gurgling indicates aspiration and needs urgent aspiration of the mouth and oropharynx (attempting to aspirate the larynx or trachea may trigger laryngospasm) with a rigid Yankauer suction catheter.
• Acrowing sound or a harsh inspiratory stridor suggests acute upper airway obstruction, which is seen in acute epiglottitis or severe tonsillitis
• Snoring suggests an unsafe airway and is seen in coma or stroke
• Wheezing occurs in COPD, asthma, LVF and massive pulmonary embolus
Feel. A finger sweep may be needed to clear an airway or obstruction. Well-fitting dentures can be left, as they will make it easier to apply a mask and maintain an airway.
C: The circulation. An impalpable pulse suggests a systolic blood pressure of less than 80mmHg. Hypotension is the major finding in shock and, if severe, will be associated with an increased respiratory rate, anxiety and confusion due to reduced blood flow to the brain. Other features of a low blood pressure will be cool and clammy skin, reduced capillary refill and a low hourly urine output (< 0.5ml/kg per hour). Capillary refill time provides an important assessment of the state of the circulation: after digital pressure has been applied to a finger tip for 5s, the capillaries should refill in less than 2s – a delay indicates an impaired circulation and is a useful sign, especially in hypovolaemic shock.
Important: An increased pulse rate may only appear as a late sign of shock, especially in otherwise fit young patients, and occasionally the response to a major internal bleed may be a slowing of the pulse. An increase in respiratory rate is frequently the first sign in shock.
D: Disability. Check the consciousness level quickly using the AVPU score (alert, responding to voice/pain, unresponsive; →Chapter 4: Acute neurological problems; p. 104). Use the GCS for a later more detailed assessment if the conscious level is impaired.
Shocked patients are restless and often uncooperative due to impaired circulation to the brain.
Common examples of the critically ill
Cardiac arrhythmia. A man comes in with very rapid atrial fibrillation and hypotension after suffering a painless myocardial infarction at home.
Massive pulmonary embolus. An obese woman in her 20s collapses with breathlessness, syncope and hypotension a week after a caesarean section.
Anaphylaxis. A young man with nut allergy collapses with wheeze and hypotension after inadvertently consuming peanuts during a Chinese meal.
Gastrointestinal bleed. A man in his 40s vomits a pint of fresh blood and passes a large melaena stool. He comes in with hypotensive collapse.
Acute abdomen. A woman aged 35 years, known to have gallstones, comes in with a 12-h history of increasing abdominal pain, hypotension and signs of acute pancreatitis.
Septicaemia. An elderly lady has a week’s history of vomiting and diarrhoea and is admitted to hospital with hypotension and renal failure. Her blood cultures yield Salmonella.
Pneumonia. A 35-year-old man becomes increasingly breathless and confused during an influenza epidemic. He is hypotensive and hypoxic. The chest film shows pneumonia.
Soft tissue infection. A 60-year-old woman with multiple sclerosis is admitted with hypotension and drowsiness. She has necrotic infected sacral pressure sores and positive blood cultures.
Recognition of a deteriorating patient
Chapter 1 outlined the basis of using simple nursing observations in scoring systems which can be used to recognise the patient who is deteriorating and is at risk. The scores are used to document clinical progress and to trigger corrective measures, particularly through the involvement of Critical Care Outreach Teams.
The assessment aims to recognise what is initially reversible that, unless corrected, will progress to:
1. persistent hypotension
2. increasing acidosis
3. kidney failure
4. cardiorespiratory failure
5. cardiorespiratory arrest
Box 10.2 summarises the clinical findings that need urgent attention on the Acute Medical Unit.
Box 10.2
N.B. Patients who have a cardiac arrest often go through a warning period of unrecognised deterioration in their vital signs.
Threats to the airway
• Impending obstruction: anaphylaxis with swollen tongue, epiglottitis
• Unsafe airway: impaired consciousness level or stroke
Respiratory failure
• Respiratory arrest
• Respiratory rate low (< 8 breaths/min) or very high (> 30 breaths/min)
• Poor arterial gases: pO2 less than 8.0 kPa on at least 60% oxygen pCO2 more than 6.5 kPa pH less than 7.2
Circulatory failure
• Pulse less than 40 or greater than 140 beats/min
• Systolic blood pressure less than 90mmHg
• Urine output (i.e. circulation to the kidneys) of less than 30ml/h
Falling level of consciousness
• GCS of less than 12 or a fall of 2 or more
Critical nursing tasks in assessing hypotensive collapse
Maintain the safety of the patient
Secure the airway and maintain oxygenation.
Measure the respiratory rate and the oxygen saturations
Choose the most appropriate oxygen delivery system to bring the saturations above 90%. When there is doubt, administer continuous high-flow oxygen at 15 L/min through a tight-fitting mask with a reservoir bag. The doctors will do urgent blood gas measurements to:
• assess the adequacy of the oxygen therapy
• look at the degree of acidosis (low pH and low bicarbonate)
• use the CO2 levels as a measure of adequate ventilation
• measure the blood lactate levels (high) as evidence of poor overall tissue perfusion
These critical results must be communicated to the medical staff.
Measure the radial and apical pulse rates
If the pulse is weak, use the monitor or an ECG trace. Rapid atrial fibrillation will give a deficit between the apical and radial pulses.
Measure the lying blood pressure and look for any postural decrease
Hypotension will, in general, require i.v. fluid replacement or drugs to support the heart. A patient with a systolic blood pressure of less than 90mmHg will show signs of reduced blood flow to the brain – restlessness, confusion and a reduced conscious level. Reduced blood flow to the kidneys results in reduced hourly urine output.
Ensure large-bore venous access
Venous access must be obtained immediately, as the priority in shock is often the need for urgent fluid replacement.
Obtain an immediate 12-lead ECG
This will exclude myocardial infarction and acute arrhythmias as the cause of the hypotension.
▪ Maintain the safety of the patient
▪ Measure the respiratory rate and oxygen saturations
▪ Measure the radial and apical pulse rates
▪ Measure the lying blood pressure and look for any postural decrease
▪ Ensure large-bore venous access
▪ Obtain an immediate 12-lead ECG
▪ Keep the relatives on the ward
Keep the relatives on the ward
You may require urgent information concerning:
• the recent history (in the elderly, any sudden deterioration can be due to sepsis)
• previous health and levels of dependence, history of substance or alcohol abuse
• details of medications and allergies
• information to inform future discussions regarding resuscitation status
Important nursing tasks in assessing hypotensive collapse
Monitor the heart
For the best trace, use adhesive electrodes placed over bony prominences. The skin should be shaved and cleaned with alcohol. The red electrode should be placed below the right clavicle, the yellow one in the same position on the left, and the green electrode below the left rib margin.
Assess the site and severity of any pain
Examples of pain in sudden hypotensive collapse are:
• severe chest pain: myocardial infarction or pulmonary embolus
• severe abdominal pain: perforation/acute pancreatitis/leaking abdominal aneurysm
Reassure the patient about the management plan and initiate symptom relief
• Relieve breathlessness: give oxygen and sit the patient up if he wishes (unless hypovolaemic)
• Ensure the mask is comfortable and tight-fitting and that the patient understands that he is to keep it on
• Prepare the patient for possible further procedures:
— blood gas sampling (wrist arterial puncture)
— central venous line placement (via internal jugular or subclavian veins)
— urinary catheterisation
— further tests (endoscopy, CT, ultrasound). It is critical that resuscitative measures are not compromised while the patient is off the ward
Explain
Explain what has gone wrong and what will be the signs of improvement, e.g.:
| Acute LVF: | less dyspnoeic, falling pulse, good diuresis |
| Upper gastrointestinal bleeding: | less thirsty, less light-headed, no further vomiting, clearing of melaena |
Speak to the relatives
• Explain what they will see: drips, monitors, urinary catheters, masks
• Explain the management plan
• Explain the signs of improvement or deterioration
• Forewarn the relatives that the patient may need to be transferred to an HDU/ITU
Access further information
Obtain the previous hospital records and any previous laboratory results urgently.
An example of the immediate management of hypotensive collapse in a man with abdominal pain is given in Case Study 10.3.
Case Study 10.3
A 30-year-old man was admitted with a 3-day history of upper abdominal pain. He was reluctant to give many details and denied any significant past history, but he was clearly in considerable discomfort and was very agitated. His wife was with him and added that he had been drinking a litre of strong cider a day for several years and that, 6 months previously, he had been investigated for alcohol-related liver damage and told that he should stop drinking for good.
Initial assessment was of a wasted man lying restlessly in bed with severe upper abdominal pain. His GCS was 15 but he scored only 7/10 on an abbreviated mental test score. His respiratory rate was 30 breaths/min and oxygen saturations 88%. His pulse rate was regular at 120 and his blood pressure was 90/70mmHg lying and 70/50mmHg sitting up. On general exposure there was scattered bruising and he appeared moderately jaundiced. His abdomen was distended, tense and silent but only moderately tender.The BM stix was satisfactory at 7.0mmol/L.
1. He was given high-flow oxygen at 60% with immediate improvements in his saturation to 98%
2. Venous access was obtained with difficulty and blood was taken for:
— FBC – to look for bleeding (low Hb) and infection (high white cell count)
— platelets – he is bruised, and alcoholics have low platelet counts
— biochemistry:
• to check for dehydration (U&E)
• to exclude acute pancreatitis
• to check the liver function tests
— Clotting – bruising can indicate a serious coagulation disturbance
3. Arterial blood gases were taken to look at the oxygen level and to identify acidosis (a common problem resulting from hypotension in the setting of an acute serious illness)
4. While awaiting initial results a small dose of i.v. opiates was administered to provide immediate pain relief and he was reassured that further analgesia would be available once the test results had been seen.
The investigations showed a very high amylase (1500 IU), abnormal liver function and early kidney failure. Combined with the clinical assessment the overall picture was of acute pancreatitis complicated by redistributive shock:
• hypotension
• confusion
• hypoxia
• poor kidney function due to hypotension
Basic management plan
• Administer continuous oxygen immediately
• Provide effective analgesia
• Bring up the blood pressure urgently
— assess the i.v. fluid requirements with a central venous pressure line
— administer sufficient i.v. fluid to bring the CVP up to 15 cmH2O of water
— if this does not bring up the blood pressure consider inotropic support
• Use the urine output to assess the response to the improved blood pressure (catheter)
• Rest the gastrointestinal tract with nasogastric intubation on free drainage
Nursing observations to monitor the response
• Hourly urine output to aim at > 50ml/h
• Hourly pulse
• Hourly blood pressure
• Hourly pain score
• 2-hourly oxygen saturations
• 2-hourly temperature (shock, central lines and the underlying disease can all lead to sepsis)
• Careful charting of input and output
• Continuous cardiac monitoring
In this man’s case the initial urine output was 10ml in the first 3h, in spite of vigorous fluid replacement, and his blood pressure remained low. He was therefore started on i.v. dopamine at 5μg/kg per min. His blood pressure responded, followed after 2h by an increase in his urine output.
When do we use dopamine, dobutamine and noradrenaline (norepinephrine) in patients with shock?
Which one to use?
Dopamine. Dopamine is used in the range 2–20mg/kg per min. At the lower dose it acts to improve kidney perfusion (although the clinical value of this is questionable), in the mid range it also begins to stimulate the heart, and at the higher doses it begins to constrict peripheral arteries to produce additional beneficial effects on the blood pressure. It is the most widely used ‘general-purpose’ inotrope. It is usual to start with the lower dose and increase accordingly.
Dobutamine. Dobutamine only stimulates the heart and tends to be reserved for cardiogenic shock.
How to monitor their effectiveness
• The blood pressure
• The mental state (brain blood flow)
• The hourly urine output (kidney blood flow), which should be kept above 0.5ml/kg per h
• The blood lactate levels (lactate levels fall as tissue perfusion improves)
There are two adverse effects that must be identified:
1. if the vasoconstrictor effect on the peripheral vessels is too strong, the patient can develop ischaemic tips to their fingers and toes, i.e. fixed blue/black discolouration
2. the cardiac stimulation can lead to marked tachycardia and then arrhythmias: these patients need close cardiac monitoring
Ensuring Adequate Oxygen Delivery to Vital Organs: Maintaining the Oxygen Saturation and the Blood Pressure
Oxygen therapy
The main indications for emergency oxygen therapy are:
• oxygen saturations of less than 90%
• systolic blood pressure of less than 100mmHg
• respiratory distress (respiratory rate of more than 20 breaths/min)
• cardiac or respiratory arrest
• acidosis on blood gas results
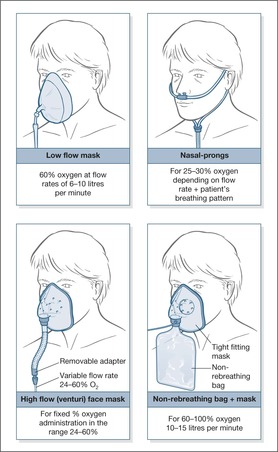
There is understandable anxiety about the correct dose of oxygen in patients with COPD, but in general the problem in acutely ill medical patients is not giving too much oxygen, but in giving too little. The aim, whatever the circumstances, should be to give sufficient oxygen to raise the oxygen saturation above 90%. Oxygen is a drug and, unless in an emergency, it should be prescribed – the prescription should specify the mask, the flow rate and, in the case of the Venturi mask, the oxygen concentration. A target oxygen saturation should also be specified. The aim should be for a normal saturation (94% to 98%) in most acutely ill medical patients and for saturations of 88% to 92% in those (usually patients with severe COPD) who are at risk from Type II (hypercapnic) respiratory failure. The different techniques of oxygen administration are illustrated in Fig. 10.2.
 |
| Fig. 10.2 |
Oxygen masks
• Simple face masks (Hudson masks, MC masks) – these provide up to 60% oxygen at flow rates of 6–10 L/min, but underperform at rates of less than 5 L/min. These masks are suitable for most situations in which emergency oxygen is needed.
• Nasal prongs – the percentage of oxygen delivered by nasal prongs at 2 L/min is between 25% and 30%. At 4 L/min around 40% will be delivered. Nasal prongs are less intrusive than masks and tend to be better tolerated in uncooperative patients. Although oxygen delivery with nasal prongs may be inadequate for a critically ill patient, they are suitable for most situations in which emergency oxygen is needed.
• Non-rebreathing mask with reservoir bag – Depending on their fit, these masks can administer from 60% to 100% oxygen with flow rates set at 15 L/min. Proper use requires some expertise because the mask must fit tightly, the reservoir bag must be filled before the patient starts to breathe and the flow rate requires adjustment to ensure the bag remains at least half full throughout the respiratory cycle. Non-rebreathing masks with reservoir bags are used in the critically ill to correct severe hypoxia.
• Oxygen therapy during nebuliser treatment – In patients with acute asthma the nebuliser can be driven by oxygen at 6 L per minute or by compressed air combined with nasal prongs to maintain target saturations. In COPD oxygen should not be used to drive the nebuliser but nasal prongs at 2 L min during air-driven nebulisation should keep the saturations between 88 and 92%.
The blood pressure
There are two main reasons why the blood pressure can suddenly fall in the critically ill. First, the heart may start to fail as a pump – examples would be an acute myocardial infarction or the toxic effects of septicaemia. Secondly, there may not be enough fluid returning to the heart to fill it sufficiently; examples would be rapid blood or fluid loss or the sudden loss of vascular tone seen in overwhelming sepsis. Improving the circulation of the blood by restoring the blood pressure is as important as correcting the amount of oxygen contained in it. It is critical to ensure there is sufficient circulating volume to carry any additional oxygen to the tissues. If there is hypotension and evidence of inadequate tissue perfusion – notably oliguria, poor capillary refill and impaired conscious level – an urgent challenge with 500–1000ml of i.v. saline should be considered.
When is a central line used?
• To assess the state of the circulation from the central venous pressure
• To gain access to major veins:
— when there is no peripheral access
— for administration of irritant infusions or drugs, e.g. dopamine and amiodarone
• To insert a temporary pacemaker
Inserting a central venous line: subclavian and internal jugular venous cannulation
These are the two preferred sites for inserting central venous lines. Placement involves the Seldinger technique, in which the vein is first cannulated with a needle, through which is pushed a guide wire. The needle is removed, leaving the wire in place, and then the cannula is fed into the vein over the guide wire, which itself is then withdrawn. Success rates have improved and complications are less common with the use of portable ultrasound machines to locate the central veins before cannulation.
The procedure
1. Are the results of clotting studies available to the operator?
2. This is a sterile procedure: masks, gowns, drapes, gloves and full skin preparation are required.
3. 2% aqueous chlorhexidine is more effective as a skin disinfectant than povidone-iodine
4. The patient must be 20° head-down to prevent air entering the cannula (air embolus) during insertion.
5. The operator must check the trolley and packs before starting the procedure.
6. The infusion should be run through and ready.
7. If a multi-lumen catheter is inserted, the staff must be pre-briefed about its correct use.
8. Decide beforehand what dressings are to be used to secure the site.
9. Explain and reassure the patient (and the relatives) about the procedure:
— the rationale for needing a central line
— the fact that it is a routine method of monitoring/administering certain treatments
— local anaesthetic will be used
— the doctor will provide an appropriate level of information on the benefits and risks
— it would be normal practice to obtain written consent if the patient is well enough.
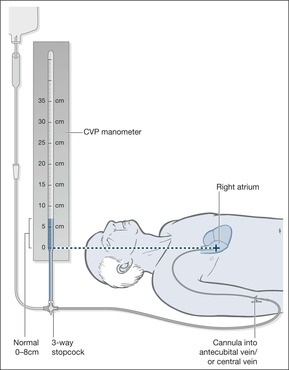
The central venous pressure
The CVP is a simple measurement that gives important information about the overall state of the circulation, and specifically about the degree of hydration. The measurement is taken from the height of a vertical column of fluid that is in free communication with a central venous catheter (→Fig. 10.3 and Fig. 10.4). If the patient is lying flat, the reference point from which the measurement is made is in the mid-axillary line at a point vertically below the sternal angle. This coincides with the position of the right atrium.
 |
| Fig. 10.3 |
 |
| Fig. 10.4 |
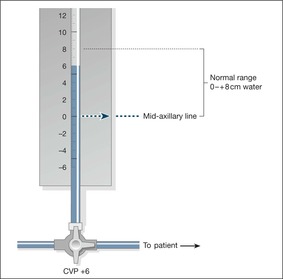
The normal central venous pressure is from 0 to + 8 cmH2O
The central venous pressure is abnormally high in congestive cardiac failure and in overtransfusion; it is too low in hypovolaemic and septic shock, in which values of −3 cmH2O or less will be found.
In a sick patient with a low blood pressure, the CVP provides invaluable information on the fluid requirements, which may not be obvious from simple observation. In particular, patients who are septic tend to be dehydrated:
• they have a poor intake
• they have been sweating
• the respiratory rate is increased, so there is increased respiratory fluid loss
• patients with sepsis often have diarrhoea
In these patients, a fluid challenge can be given with confidence if there is a combination of hypotension and a low CVP. Conversely, if the blood pressure is low but the CVP is high, the cause may be cardiac and a fluid challenge would be inappropriate.
Fluid challenge
Hypovolaemia is the most likely cause of hypotension (systolic blood pressure < 90mmHg) in the critically ill patient and in these circumstances, once the airway is secured and oxygen saturations are corrected, the circulating volume is expanded using relatively large amounts of fluid over a short period of time. The term ‘fluid challenge’ applies to the combination of aggressive volume expansion and a careful assessment of its effect on the patient. Either a crystalloid (500–1000ml of isotonic saline or 5% dextrose) or a colloid (300–500ml of polygeline [Haemaccel]) is given over 30 min and if necessary repeated. The response is evaluated using the vital signs, capillary refill, and urine output. The patient is observed for signs of fluid overload: overfilling of the neck veins (increased JVP), and new-onset breathlessness with wheezing and crackles (pulmonary oedema) at the lung bases.
Which Critically Ill Patients May Need Transfer to Intensive Care/HDU?
• When there has been a respiratory arrest – these patients remain at marked risk until the underlying problem (pneumonia, asthma, etc.) has been treated.
• Arespiratory rate of less than 8 or more than 40breaths/min – extremes of respiratory rate indicate severe illness with impending cardiorespiratory arrest.
• Oxygen saturations that remain less than 90% in spite of at least 50% oxygen by mask – these patients will need extra respiratory support, possibly even intubation and ventilation of the lungs.
• Apulse rate of less than 40 or greater than 140beats/min in spite of treatment – this can be evidence of circulatory failure, although clearly straightforward uncontrolled atrial fibrillation would not come into this category.
• Asystolic blood pressure that stays below 90mmHg in spite of fluid replacement – this suggests established shock and may need support with inotropic drugs.
• An unexpected and sudden fall in the conscious level – (a fall in GCS of more than 2 points). This may be a manifestation of falling cerebral blood flow due to persistent shock.
• Increasing arterial carbon dioxide tension with acidosis – deteriorating blood gases in spite of treatment is a warning of impending respiratory arrest
• Any patient giving cause for concern in whom there is a reasonable expectation of recovery
Surviving Sepsis
There is increasing concern about the rising incidence of severe sepsis as a cause of critical illness and the very high associated mortality – approaching 40% in the UK – some of which can be reversed with better early resuscitation, particularly in the first 6h of admission. The key is to recognise:
• significant sepsis-induced tissue under-perfusion
• increased respiratory rate
• hypotension
• increased blood lactate levels (> 2–4mmol/L)
Once sepsis is recognised, initial therapy with aggressive fluid challenge (20ml/kg of saline), oxygen, early antibiotics and if necessary dobutamine/noradrenaline (norepinephrine) will significantly improve the outlook.
Anaphylactic Shock
Anaphylaxis is the term used for a severe generalised allergic reaction. Allergic diseases in general are on the increase, and a growing number of acute anaphylactic reactions are now being seen – often in patients with a history of asthma.
The most common causes are:
• foods, especially nuts
• insect stings
• drugs, especially antibiotics, aspirin and ACE inhibitors
• latex allergy, especially in health-care workers
From 5 min to an hour after exposure, the patient develops a generalised reaction with swelling, redness and itch. In cases of oral ingestion in food allergy, the swelling usually starts in the mouth and tongue and progresses to upper airway obstruction. The time course depends on the route of exposure: it may take 30 min for latex to be absorbed sufficiently by the skin to trigger a reaction. Occasionally, the delay from exposure to reaction is as long as 6h, or there may be an early reaction that clears, to be followed by a delayed reaction 1–2 days later.
The two potentially fatal complications are:
• airway obstruction (laryngeal obstruction and an asthmatic reaction; →Case Study 10.4)
Case Study 10.4
Ten minutes after finishing a bowl of cereal, a 30-year-old woman developed sudden itching and discomfort in her throat.Within a further few minutes there was a generalised itchy red blotchy rash and she started to wheeze. She felt faint and collapsed on the kitchen floor. Her husband dialled 999.
In casualty she was still wheezy and distressed, with a widespread urticarial rash. She was given i.v. piriton, hydrocortisone and nebulised salbutamol, and admitted to hospital.
On admission to the Acute Medical Unit, the following initial assessment was made:
ABCDE: the safety of the patient
• The patient was drowsy, but responding to the spoken voice
• The face, lips and tongue were swollen and the voice was hoarse
• The patient was having difficulty in swallowing her saliva
• There was a loud harsh wheeze
• The oxygen saturation was 85% on air and respiratory rate was 35 breaths/min
• Pulse was 105 beats/min, with a lying blood pressure of 75/40mmHg
• There was a widespread raised urticarial rash
The assessment was of acute anaphylaxis with impending upper airway obstruction (unable to swallow, swollen tongue, voice change).There was bronchospasm, hypoxaemia and circulatory collapse.
Management
The patient already had venous access. High-flow 60% oxygen was given. Intramuscular adrenaline (epinephrine), 0.5ml of 1:1000 (0.5mg), was administered immediately.This was followed by i.v. chlorpheniramine and hydrocortisone.A litre of isotonic saline was given over 30min, followed by 1L over 1h.
The recovery was uneventful, but the patient was referred on to the allergy service as a case of probable nut allergy, was fully assessed and advised appropriately before discharge. She now wears a Medicalert bracelet and carries an EpiPen to self-administer adrenaline (epinephrine) should it be necessary.
• circulatory collapse (severe hypotension with syncope)
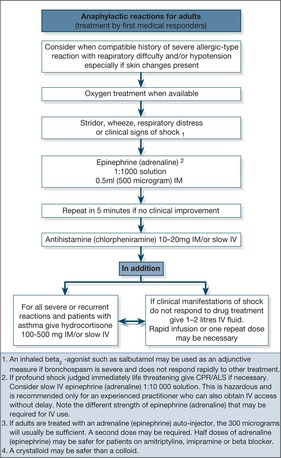
Management of anaphylactic shock (→Box 10.3, Fig. 10.5)
Intramuscular adrenaline (epinephrine). Adrenaline (epinephrine) is the most important part of the management and must be given early. It is important to be familiar with the different preparations of adrenaline (epinephrine) and to understand that in anaphylaxis the preferred route is intramuscularly and NOT subcutaneous or intravenous – and that the dose is NOT ‘one ampoule’ but 0.5ml of 1:1000 strength adrenaline (epinephrine).
Box 10.3
• Intramuscular adrenaline (epinephrine) 0.5ml of 1:1000 (0.5mg or 500μg)
• Intravenous chlorpheniramine 10mg
• Intravenous hydrocortisone 200mg
• Intravenous fluids
• Oxygen
Intravenous adrenaline (epinephrine)? Severe bronchospasm, increasing upper airway obstruction or continuing hypotension are indications for the careful use of i.v. adrenaline (epinephrine). The adrenaline (epinephrine) is diluted to 1:100 000 and infused at 10–20mg/min.
Oxygen. The patients are hypoxic and shocked.
Fluids. Intravenous fluids and adrenaline (epinephrine) should restore the blood pressure.
Bronchodilators. Patients can die from the asthmatic component of the anaphylactic reaction: treatment is with nebulised salbutamol or, in the critically ill, i.v. aminophylline.
Antihistamines. This will clear the rash and the itch.
Steroids. Steroids will not have an immediate effect, but will help any asthmatic component and prevent a prolonged or delayed response.
Prevention. There must be effective education in allergen avoidance and good control of any underlying asthma by optimising the use of prophylactic anti-inflammatory treatment with inhaled steroids. Many patients with food allergy are supplied with adrenaline (epinephrine) auto-injectors (EpiPen) and trained in their use.
Answering Relatives’ Questions in Shock
What do you mean by shock? This is not the same as emotional shock. By shock, we mean a drastic change in the overall function of the body due to a fall in the patient’s blood pressure. The kidneys are not producing enough urine to flush out waste products, and the brain is at risk of being starved of oxygen.
What else can go wrong? The damage to some of the organs could become more serious. The kidneys and lungs are at particular risk and we are paying particular attention to these.
How are you treating the main causes? We are using drugs and intravenous fluids to try and keep the blood pressure up. The extra oxygen is to make up for the problems with the circulation. We are giving antibiotics to combat infection and we are replacing any lost blood or body fluids.
How will we know that he is going to recover? Within 24–48h of starting treatment we should see a general improvement: less drowsiness, a better urine output and an increase in the blood pressure. Any fever should start to fall and you may see that we are easing off on the drugs and the intensity of the nursing observations.
What can we do to help? It is reassuring to have somebody familiar at hand particularly if, as commonly happens in shock, there is an element of confusion. Try and keep him calm. Keep the oxygen mask in place and explain what it is for. Keep visitors down to a few very close relatives, because for the first 48h there will be a lot of clinical activity going on in the form of observations, changing drip bags, administering drugs and so forth.
Emergency Blood Transfusion in Shock
There are two main indications for transfusion on the Acute Medical Unit: acute blood loss and to improve oxygen transport in patients with anaemia.
• Acute blood loss – this is the most common indication and may require massive transfusion, with its associated risks
• Improving oxygen transport in patients with anaemia – this is of uncertain benefit in the acute situation unless the haemoglobin levels are particularly low – probably down to less than half normal
Transfusion is often regarded with suspicion by patients and their relatives. This has been exacerbated by the recent discovery that two patients have developed new variant CJD transmitted in blood from infected donors. Fortunately the risks associated with transfusion are surprisingly low. The chance of catching AIDS through transfusion is around 1 in a million. In fact they are 100 times more likely to be given the wrong blood (as a result of human error), but even this risk is extremely low. The most common reactions are minor febrile reactions and rashes that require symptomatic treatment and reassurance. The risks involved in blood transfusion are listed in Table 10.2.
| Risk/reaction | Risks per unit |
|---|---|
| Urticarial rash | 1 in 50 |
| Febrile reaction | 1 in 100 |
| Pulmonary oedema | 1 in 5000 |
| Wrong blood | 1 in 30 000 |
| Bacterial contamination | 1 in 50 000 |
| Hepatitis B | 1 in 100 000 |
| Hepatitis C | 1 in 200 000 |
| Major reaction | 1 in 600 000 |
| HIV | 1 in 3 000 000 |
It is important to reassure the patient and provide adequate information (if he is well enough to discuss it):
• indications for and purpose of the transfusion
• risks to the patient
• benefits to the patient
• the patient’s right to refuse
• alternative treatments to transfusion
• a National Transfusion Service information leaflet should be given to the patient or relatives
Transfusion Reactions
Clinical features and basic management
It is important to monitor transfusion using a standard protocol. The patients must be told to report:
• shivering
• rashes
• flushing
• breathlessness
• loin and limb pains
The temperature, pulse and blood pressure are recorded before the start of the transfusion, after 15–20 min and at the end of delivery of each unit of blood. This information is recorded and dated separately from the routine observations. Most transfusion reactions start within 15 min of starting the blood, so the early observations are critical.
Urticarial rashes
Urticarial rashes may occur during transfusion. If the patient develops an acute allergic response to any protein in the donated blood product, urticaria, an intense itchy red eruption, will develop. It is more common with platelet transfusions or FFP than with whole blood. Urticaria is frightening for the patient, but not serious. The best treatment is i.v. chlorpheniramine 10mg, an antihistamine. Provided that the reaction is not associated with major symptoms or changes in the pulse or blood pressure, the transfusion does not have to be stopped. The observations should be increased to hourly.
Febrile reactions
These may occur towards the end of a transfusion or within hours of its completion. The patient reacts against the white cells in the donated blood. It can be simply treated with paracetamol to bring the temperature down. If patients have had reactions before, they can be premedicated with antihistamines. Rarely, a patient can develop signs of septic shock during transfusion – hypotension, increased respiratory rate and a fever – which is due to bacterial contamination of the blood; platelet transfusions are the most likely to be contaminated.
Pulmonary oedema
This is not always due to overtransfusion – there is a form of acute transfusion-related lung injury that is a direct toxic effect of stored blood. The symptoms are the same as cardiac pulmonary oedema: increasing breathlessness, increased respiratory rate, tachycardia and hypoxia. Platelets and FFP are the most dangerous in this regard.
Wrong blood
Within the first few millilitres of the transfusion, a reaction may occur that is due to the patient rejecting the blood. Usually this is a case of AB blood being incorrectly given to an O group patient. It is vital to recognise this type of transfusion reaction.
Nursing assessment to identify a severe transfusion reaction
When to suspect a reaction
• Patient complains of symptoms
• The basic observations change (pulse, temperature and blood pressure)
What action to take
Any of the following may lead you to suspect a severe reaction:
• a reaction starts within minutes of the start of blood
• an error is discovered
• the patient develops acute loin or limb pain
• there is fever, hypotension and tachycardia
Avoiding human error
The most important risk in blood transfusion is that of simple human error leading to the administration of the wrong blood. The mistakes are predictable:
• when blood samples are taken for cross-matching they are mislabelled at the bedside
• the wrong blood is collected from the blood bank
• the blood is given to the wrong patient (identification bracelets must be fitted on admission)
It is important to work to defined protocols for taking blood for cross-matching, collecting the correct blood from the bank, receiving the correct blood on the ward and administering it to the correct patient.
An example of good practice. Take the transfusion request form to the patient.
1. Ask the patient to give their name and date of birth.
2. Confirm this with the identification bracelet.
3. Check these details and the hospital number with the form.
4. Take blood and then label the tube at the bedside, but not with an addressograph label.
Transfusion should start as soon as the blood is delivered to the ward. If blood has been out of the refrigerator for more than 30 min and is not about to be used, the hospital blood bank should be consulted and the blood returned.
The bedside check before the start of the transfusion is vital. There must be exact matches:
patient → blood transfusion compatibility report form → compatibility label on blood pack → prescription or fluid balance chart → medical notes
Keep the blood transfusion compatibility report form readily available during the transfusion, in case there is a reaction.
Massive Blood Transfusion
Acute massive blood loss on the Acute Medical Unit is usually due to upper gastrointestinal bleeding. The term is usually used when a patient loses around half his blood volume (2–3 L) over 2–3h. Surprisingly, there is a tendency to underestimate the total volume loss. Massive blood loss requires large-volume transfusions with cross-matched blood. In extreme life-or-death situations, blood from ‘universal donors’ – group O rhesus negative – can be used while awaiting the emergency cross-match (saline or polygeline [Haemaccel] may be used while awaiting blood).
The aim of emergency transfusion is:
• to normalise the blood pressure
• to maintain a urine output of at least 30ml/h
Stored blood carries very little clotting factor, and so massive transfusion can lead to a clotting deficiency.
• Full clotting studies (platelet count, prothrombin time, KCCT and fibrinogen levels) must be checked before the start of the transfusion
• These are repeated after every 3 units of blood
• The prothrombin time and KCCT must be kept at no more than 1.5 times the normal value using FFP – probably 1 unit of FFP every 30 min for 4 units. (Note: FFP takes 30 min to thaw out)
• Platelets will need to be replaced after more than 20 units of blood
• Low fibrinogen levels will need correction with cryoprecipitate
Occasionally, rather than a clotting deficiency, massive transfusion triggers off spontaneous clotting within the small arteries and veins – this is DIC. There are five reversible factors that increase the risk of DIC and which are, to some extent, amenable to standard resuscitation measures:
• massive blood loss
• hypoxia
• hypovolaemia
• administration of excessively cold fluids
• failure to correct the clotting factors and platelets
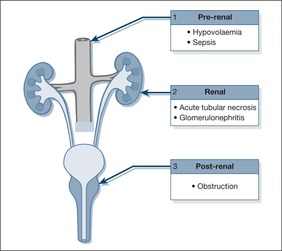
Acute Kidney Injury
The term Acute Kidney Injury (AKI) applies to situations where there is a risk of acute renal failure and also to situations where renal failure is fully established. The term AKI has replaced Acute Renal Failure to emphasise the progressive loss of renal function that occurs if urgent preventative action is not taken. There are three stages in the deterioration from “at risk of” to “established” renal failure. They are defined by the urinary output (Table 10.3):
| Stages of Acute Kidney Injury | Urine output |
|---|---|
| 1. Risk | < 0.5ml/kg/hr for > 6 hours |
| 2. Injury | < 0.5ml/kg/hr for > 12 hours |
| 3. Failure | < 0.3ml/kg/hr for > 24 hours or anuric for 12 hours |
To function, the kidneys require:
1. an adequate blood pressure
2. sufficient functioning glomeruli to filter the blood
3. intact renal tubules to reabsorb water and exchange electrolytes
4. an unobstructed urinary tract leading from the kidneys to the outside of the body
Fig. 10.6 summarises the basic mechanisms of acute kidney injury.
 |
| Fig. 10.6 |
In the Acute Medical Unit, problems with kidney function are commonly the result of an inadequate blood pressure – pre-renal failure – which occurs in the setting of acute fluid depletion. A useful concept is “volume-responsive” acute kidney injury as it will usually respond to fluid replacement.
Uncorrected pre-renal failure may progress to acute tubular necrosis. Acute tubular necrosis can also be the result of direct renal toxicity from drugs such as NSAIDs, from the products of acute muscle damage (rhabdomyolysis), and from sepsis.
Urinary tract obstruction is clearly important, because it is easy to treat and is a fully reversible cause of kidney failure.
Diseases of the glomeruli – acute glomerulonephritis – are rare causes of acute renal failure on the Acute Medical Unit, although they are important to recognise because the patients usually need specialist assessment and care.
While acute kidney injury is an important clinical area, it is often handled with limited confidence on the Acute Medical Unit. Once a set of very abnormal kidney function tests comes back from the biochemistry laboratory, the tendency is to phone the renal unit and hope that they will take the problem over. This is unfortunate, because many of the cases are straightforward and will recover – with fluid replacement, with catheterisation, or simply with time – provided that immediate problems of electrolyte disturbance and fluid balance are addressed.
Which patients are at particular risk from acute kidney injury?
• Patients with fluid depletion – severe diarrhoea, major upper gastrointestinal bleed
• Patients with septic shock
• Patients with a vulnerable blood supply to the kidneys – acute and chronic heart failure, vascular disease, hypertension
• Patients taking diuretics, NSAIDs, ACE inhibitors and those undergoing chemotherapy.
• Patients who already have chronic renal failure
• The elderly frail and those who have several medical conditions, particularly if those include diabetes and hypertension
Illustrative case studies
The following case studies (Case Study 10.5, Case Study 10.6 and Case Study 10.7) illustrate the typical presentation and immediate management of acute renal failure. The main features of emergency management of acute renal failure are summarised in Fig. 10.7.
Case Study 10.5
A 34-year-old woman was admitted with a bilateral pneumonia. On admission, the temperature was 35.5°C, blood pressure 90/60mmHg, pulse 100 beats/min and respiratory rate 15 breaths/min; the saturations were 90% on high-flow oxygen. On admission the WCC was 1.2 × 109/L, urea 10.7mmol/L and creatinine 102μmol/L. She was treated with fluids and antibiotics, but was markedly oliguric, passing less than 10ml/h.Within 24h her WCC had increased to 28.2 × 109/L, her urea had increased to 24.3mmol/L and her creatinine was 259μmol/L. Her respiratory rate increased to 25 breaths/min and she was transferred to the ITU with a view to ventilation.
Case Study 10.6
A 70-year-old woman was awoken in the early hours of the morning with severe constant pain in the right loin.This was described as being as severe as labour pain. Her pain persisted for 72h, during which time she developed rigors and passed absolutely no urine. In the past she was known to have a chronically damaged left kidney from childhood infections.
She was admitted to hospital where she was found to be pyrexial and dehydrated, with a pulse rate of 110 beats/min and a blood pressure of 70/60mmHg when lying. On palpating her abdomen, there was an enlarged tender right kidney. Investigations showed a high WCC (15 × 109/L) and a low platelet count.There was mild renal insufficiency. An urgent ultrasound confirmed hydronephrosis (an obstructed kidney) due to a large stone blocking off the ureter.
She was treated with fluid replacement and i.v. antibiotics. Her subsequent management included an emergency nephrostomy to allow drainage of urine from the obstructed right kidney, followed some time later by surgical removal of the stone.
Case Study 10.7
A 50-year-old man with a 2-week history of malaise was admitted because of swelling of his eyes and ankles.Three weeks previously he had had an acute sore throat.
On admission there was generalised oedema. His blood pressure was 202/114mmHg. His ward urine testing was strongly positive for blood and protein. A subsequent MSU showed red cell casts.
His chest film showed mild pulmonary oedema. His creatinine was 128μmol/L, urea 11.6mmol/L.The total 24-h urinary excretion of 1.14g. His ASO titre was greater than 800 units/ml (indicative of a recent streptococcal infection).
He was treated with frusemide and fluid restriction. At follow-up 3 months later, his creatinine had fallen to 105μmol/L, his urea to 6.9mmol/L, and his blood pressure was normal. His urine testing showed microscopic haematuria.
Management of Acute Kidney Injury
Ensuring the safety of the patient
The safety of the patient is established with attention to the airway, oxygenation and the circulation. There are two life-threatening complications that require urgent action: hyperkalaemia and pulmonary oedema.
Hyperkalaemia
Hyperkalaemia, or an increase in the serum potassium level, is the most serious complication of acute renal failure because it has a dramatic effect on the heart. Levels above 6.0mmol/L lead to cardiac arrest. It is critical to measure and document serial changes in the potassium in any patient with established or threatened kidney failure. Hyperkalaemia produces characteristic changes on the ECG (peaking of the T waves and slurring of the QRS complexes), if these are present with a potassium level of 6.0mmol/L (or above 6.5mmol/L even if there are no ECG changes), urgent action must be taken to protect the heart and to bring the potassium concentration down.
Emergency (acute renal failure) management of hyperkalaemia
1. Give 10ml of 10% calcium gluconate over 5 minutes and repeat until the ECG starts to improve. Calcium gluconate protects the heart, but does not reduce the potassium. It can be repeated after 5 minutes.
2. Give 10 units of neutral insulin solution (Actrapid) in 50ml of 50% glucose over 5 min. This will reduce the potassium level by 2.0mmol/L over 1h.
3. Give 15g of calcium resonium orally every 6h. This takes 4h to work.
Pulmonary oedema
This occurs because of the inability of the failing kidney to get rid of fluid. Pulmonary oedema is often triggered by over-enthusiastic i.v. fluid replacement in the critically ill, particularly if saline has been used and the CVP has not been monitored. The evidence for pulmonary oedema will be obtained from the initial assessment of a patient whose blood tests have shown kidney failure:
• low oxygen saturation
• increased respiratory rate
• unable to lie flat because of breathlessness
• in advanced pulmonary oedema, the patient is restless and confused
Completing the clinical assessment
Assessing fluid balance:
• What fluid has been lost?
— a history of vomiting, diarrhoea, gastrointestinal bleeding?
— is there internal fluid loss, as in acute pancreatitis?
• Is there any postural hypotension on sitting the patient up?
• What is the CVP?
• What is the urine output?
Is there evidence of obstruction?
• There may be a history of prostatism:
— hesitancy in starting
— poor stream
— terminal dribbling
— frequent small amounts.
• Adistended bladder may be palpable.
• Sudden complete cessation of urine output or overflow with constant dribbling.
• Pain over the kidneys due to distension and back pressure.
• If the patient has a catheter – is it blocked? (gentle flush and aspiration with sterile water).
What was the previous kidney function?
• Examine old notes and laboratory reports.
• The GP’s surgery may have results of prior renal function.
• A flow chart of results and their dates is invaluable.
Is there a comprehensive drug list?
In particular:
• diuretics
• NSAIDs
• ACE inhibitors
What does ward urine testing show?
Urine testing is usually negative in pre-renal failure. In acute tubular necrosis there is usually a combination of light to moderate proteinuria and microscopic haematuria. In acute glomerulonephritis, the characteristic findings are haematuria and heavy proteinuria. Should a fresh MSU also show copious red cells which have been moulded into the shape of the renal tubules (red cell “casts”), the diagnosis is acute glomerulonephritis, and a specialist opinion will be required. Send a spot urine sample for osmolality (‘concentration’) and urinary sodium. In hypovolaemia, the kidneys are trying to retain fluid and sodium to keep up the blood pressure – osmolality will be high (in excess of 450 mosmol/L) and sodium low (less than 10mmol/L). The reverse is the case once acute tubular necrosis becomes established (osmolality less than 300 mosmol/L and sodium more than 20mmol/L).
Establishing a management plan
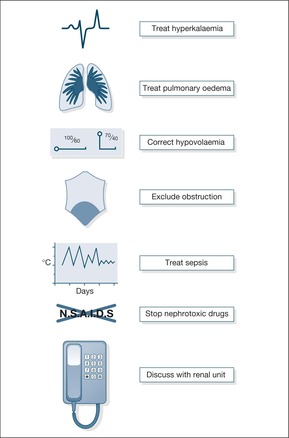
Assuming there has been emergency treatment of acute hyperkalaemia and pulmonary oedema, the priorities are:
• To correct hypovolaemia – if hypovolaemia is present, prompt fluid replacement should immediately increase the blood pressure and increase the urine output to more than 0.5ml/kg per h. Depending on the frailty of the patient and their cardiac status, fluid replacement may require between 250 and 1000ml of isotonic saline, aiming for a systolic blood pressure of 100mmHg. If the urine output remains poor in spite of adequate hydration, a fluid challenge may be indicated.
• To remove any obstructive component – this will range from bladder catheterisation to percutaneous drainage of an obstructed kidney. There should be 24 hour access to renal ultra-sound as obstruction must be recognised and treated immediately.
• To stop any nephrotoxic drugs – this relies on a full drug history, with details from both hospital and GP sources. The ward pharmacist will advise on nephrotoxicity and the need for dose modifications in the presence of renal failure.
• To identify and treat sepsis – sepsis is an important cause of renal failure and is easy to miss, especially in the elderly. Symptoms may simply consist of an abrupt change in health. Sites for ‘hidden’ sepsis include the perineum, the joints, the spine and the soft tissues, including infected bed sores. It is important to take at least two sets of blood cultures if sepsis is suspected: each set consists of 5ml into each of two culture bottles – one aerobic and one anaerobic.
The management of pre-renal failure and acute tubular necrosis is outlined in Box 10.4.
Box 10.4
• Correct hypoxaemia
• Conduct emergency management of hyperkalaemia or pulmonary oedema
• Ensure the patient is well hydrated
• Administer a fluid challenge, usually 5% dextrose:
1. 250ml over an hour
2. Watch urine output for next hour
— If output is less than 0.5ml/kg per h:
3. 5% dextrose 250ml/h for 2h
— If the output remains less than 0.5ml per kg per hour, then consider:
4. Dopamine 2.5μg/kg per min and frusemide 50mg/h
Critical nursing tasks in acute renal failure (see box)
▪ Prepare to start treatment for hyperkalaemia
▪ Carry out basic management of pulmonary oedema
▪ Identify sepsis: strong clues are fever, malaise, rigors and perhaps dysuria
▪ Identify hypovolaemia:
— symptoms of thirst and dizziness
— signs of postural fall in blood pressure
— low CVP
Important nursing tasks in acute kidney injury
Confirm that the problem is not a simple outflow obstruction
• Enlarged tender bladder
• Blocked catheter
Take a full history
• Any history to indicate hypovolaemia?
— severe diarrhoea
— vomiting
— bleeding
• Any history to suggest sepsis?
— dysuria
— rigors
— fever
• Any history to suggest obstruction?
— absolute anuria
• Past history
— chronic kidney disease
— diabetes
— hypertension
• Drug history
— NSAIDs
— ACE inhibitors
— diuretics
Exclude rhabdomyolysis in prolonged immobility/coma
• Be aware of the possibility in coma, alcoholics and overdoses.
• Look for tense swollen legs and areas of bruising suggesting soft tissue damage. In rhabdomyolysis, the blood creatinine kinase level will be very high.
▪ Confirm that the problem is not a simple outflow obstruction
▪ Take a full history
▪ Exclude rhabdomyolysis in prolonged immobility/coma
▪ Check the urine
▪ Ensure that there is a system for reviewing the abnormal urea and electrolyte results on the ward
Check the urine
• Appearance (e.g. a muddy appearance is typical of myoglobinuria – rhabdomyolysis)
• Dip for blood and protein (in rhabdomyolysis the urine is positive for blood on dip-stick but no red blood cells are seen on microscopy)
• Send for microscopy to look for casts (hyaline casts are from normal urinary constituents and occur in pre-renal failure because the urine is concentrated. Red cell casts indicate glomerulonephritis; granular casts indicate acute tubular necrosis)
Ensure that there is a system for reviewing the abnormal urea and electrolyte results on the ward
There should be flow charts that follow sequential tests in a critically ill patient. The most common cause for a delayed diagnosis of renal failure on the Acute Medical Unit is a failure to see and act on the abnormal results when they come back to the ward.
Answering Relatives’ Questions in Acute Kidney Injury
What does acute kidney failure mean? The kidneys filter impurities from the blood, so they need a constant stream of blood and an effective filtering mechanism. If anything too drastic happens to either mechanism, the kidneys become inefficient and eventually, as a result, impurities build up and poison the body – this is known as kidney failure.
Will the kidneys recover? The kidneys have good powers of recovery, particularly if they were working normally before this happened. We are checking previous hospital records and blood tests to see if the kidneys were completely normal up until the present illness. In many cases of acute kidney failure, the kidneys start to show signs of recovery within a few days of starting treatment.
Why are you not sending him to a kidney unit? The doctors use the unit for advice during the initial period of stabilisation. If the kidneys do not show the expected rate of recovery or if complications occur that could be more effectively dealt with in a kidney unit, we can transfer the patient at that stage.
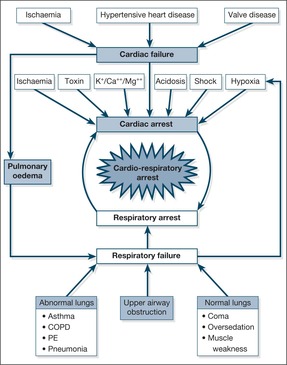
Sudden Collapse and Cardiac Arrest
It is important to understand the events that can precede and progress to a cardiac arrest.
• Arespiratory arrest may lead to a cardiac arrest, and vice versa. In most arrest situations the term cardiorespiratory arrest is appropriate
• Most cardiac arrests occur as an early complication of myocardial infarction. These have the most successful outcomes
• However, cardiorespiratory arrest should also be viewed as an end-point in acute illnesses other than myocardial infarction. Examples include acute severe asthma, septicaemic shock and uncorrected acidosis. By recognising and treating them at an early stage, the risk of progressing to cardiorespiratory arrest can be reduced. Several studies have now shown that ‘sudden death’ on Acute Medical Units is often preceded by a period of potentially reversible deterioration – most notably unrecognised hypoxaemia and hypotension. Competent assessment and early intervention in the critically ill (Chapter One) should prevent this sequence.
Common causes of the sudden collapse (→Fig. 10.8)
Cardiac. In 80% of cases, sudden pulseless collapse is due to ventricular fibrillation or ventricular tachycardia. In the remainder, the problem is a severe bradycardia or asystole. Pulmonary embolism is a classical cause of sudden severe collapse.
Respiratory
• Tension pneumothorax
• Aspiration/upper airway obstruction; this can cause a major deterioration, especially in coma
• Massive haemoptysis, usually as a terminal event in lung cancer
Gastrointestinal. A catastrophic bleed, usually from oesophageal varices. A ruptured abdominal aortic aneurysm.
Anaphylaxis. A characteristic setting with a recognised trigger, wheeze, oropharyngeal swelling and severe hypotension.
Assessment of collapse
What is the setting in which it occurred?
1. New patient just arrived on ward: this could be anything.
2. A patient admitted earlier in the day with unexplained breathlessness: pulmonary embolus.
3. A patient with known heart disease/chest pain: a major arrhythmia.
4. A patient admitted with deliberate self-harm: an arrhythmia or a respiratory arrest.
Diagnosing cardiac arrest
There are four common scenarios:
1. pulseless and collapsed with ventricular fibrillation or ventricular tachycardia – cardiac arrest
2. pulseless and collapsed with a flat trace – cardiac arrest
3. pulseless and collapsed but with an electrical rhythm (pulseless electrical activity or PEA) – cardiac arrest
4. flat trace but patient looks well – has an electrode come off?
If this is a pulseless cardiac arrest, the cause is almost certainly ventricular fibrillation or ventricular tachycardia. With immediate defibrillation, these patients have a 23% chance of survival. If ventricular fibrillation deteriorates into asystole or the initial rhythm is asystole (a flat trace) or PEA, the chances of survival decrease to 2%.
The Chain of Survival
This is the term used to describe the sequence of events from prevention, in the form of the recognition and treatment of the critically ill patient, through early resuscitation integrating CPR and urgent defibrillation to the final link of effective post resuscitation care. The key to survival is the immediate recognition of a cardiac arrest and institution of BLS. The aim is to keep the patient alive and neurologically undamaged (to ‘buy time’) until defibrillation can be carried out to restart the heart.
Basic life support
Basic life support describes the process of:
• the initial assessment of the collapsed patient
• the techniques to keep the airway open
• the use of expired air ventilation and chest compression – CPR
The equipment used in BLS comprises a simple airway and a face mask to facilitate mouth to mouth breathing.
If the cause of the collapse is a respiratory arrest, then BLS alone may succeed. In most cases, however, the patient has had a cardiac arrest, the rhythm is ventricular fibrillation (VF arrest) or pulseless ventricular tachycardia (VT) and BLS is a holding operation until the defibrillator arrives and the process moves into that of ALS. Advanced life support involves following established management algorithms for treating resistant arrhythmias and for maintaining ventilation with endotracheal intubation or other airway adjuncts.
Providing immediate and effective BLS markedly increases the chances of success in a cardiac arrest:
• 2 or 3 minutes’ delay will halve the chances of survival
• Interruptions to chest compression must be kept to an absolute minimum. For example, using a manual defibrillator, the pause between stopping and re-starting chest compression while the shock is being administered should be no more than five seconds.
BLS must be started at the time of the arrest and, except during defibrillation, it must be continued throughout the resuscitation procedure until the return of a spontaneous circulation.
• Start BLS
• The primary helpers continue active resuscitation
• Secondary helpers may need to:
— fetch the crash trolley
— make the referral letter and the medical and nursing notes readily available
— ensure laboratory results are fed back to the arrest team
— put any recent X-rays up on the viewing box (is this a pneumothorax?)
— see to other patients in the ward and move any patient if appropriate
— make the paramedics’ assessment documents available
— send for any old hospital notes
— handle the relatives: phone calls and direct contact
Sequence of actions in BLS (→Fig. 10.9)
1. Ensure safety of rescuer and patient.
2. Shout for help. Check the patient and see if he responds: gently shake his shoulders and ask loudly, ‘Are you all right?’
3. If he responds by answering or moving:
— place him in the recovery position
— check his condition (ABCDE), give oxygen, secure IV access and attach monitors
— reassess him regularly, recording vital signs.
— hand over to the Emergency Medical Team using a standardised communication framework (e.g. RSVP: Reason, Story, Vital signs, Plan of management)
If he does not respond:
— turn the patient on to his back
— open his airway by tilting his head and lifting his chin:
• place your hand on his forehead and gently tilt his head back, keeping your thumb and index finger free to close his nose if rescue breathing is required
• at the same time, with your fingertip(s) under the point of the patient’s chin, lift the chin to open the airway
• remove any visible obstruction from the patient’s mouth, including dislodged dentures, but leave well-fitting dentures in place.
4. Keeping the airway open, look, listen and feel for normal breathing (occasional gasps, slow and laboured or noisy breathing are abnormal):
— look for chest movements
— listen at the patient’s mouth for breath sounds
— feel for air on your cheek
— look, listen and feel for no more than 10 s before deciding that breathing is absent.
5. If he is breathing normally or if you are in any doubt (occasional, irregular (‘agonal’) gasps commonly occur after a cardiac arrest and must not be confused with normal breathing):
— turn him into the recovery position
— check for continued breathing
If he is not breathing normally:
6. Assess the patient for signs of a circulation and for signs of life:
— look for any movement, including swallowing and coughing
— take no more than 10s to do this
7. If you are confident that you can detect signs of a circulation within 10 s (ie this is a respiratory arrest):
— start rescue breathing (Box 10.5), if necessary until the patient starts breathing on his own
Box 10.5
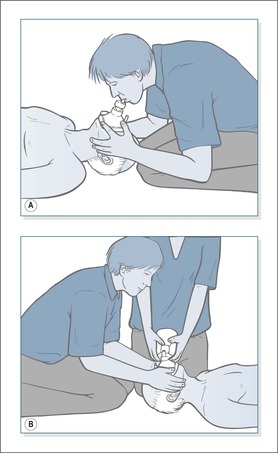
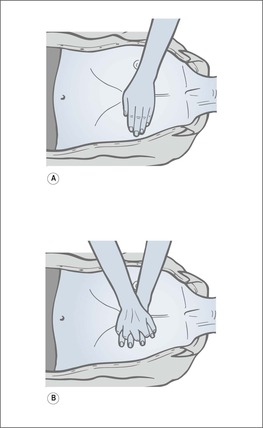
The Laerdal Pocket mask (→Fig. 10.10A)
Rescue breathing is usually started with a Laerdal Pocket mask, which is an acceptable and safe way to administer mouth to mouth ventilation until the self-inflating bag-mask and reservoir is available.
• The patient is approached from behind the head
• The thumbs and index fingers press and seal the mask onto the face
• The remaining fingers perform the jaw thrust to keep the airway open
• Blow through the inspiratory valve for 1 s and watch the chest rise
• Apply oxygen to the input valve at 10 L/min
Guedel or oropharyngeal airway
The Guedel airway is used with head tilt and jaw thrust to ensure the airway remains patent. It will not be tolerated by a conscious patient. In the correct position it stops the tongue falling back and blocking the upper airway.
1. Select the size (from small 2, medium 3, or large 4) by matching an airway to the vertical distance between the incisors and the angle of the jaw.
2. Remove any loose material from the patient’s mouth.
3. Insert the airway upside down and, when it is in position with the flat part between the patient’s teeth, turn it the right way up.
The self-inflating bag-mask and reservoir (→Fig. 10.10B)
During resuscitation expired air alone supplies the patient with only 16% oxygen.This can be significantly enriched using a Laerdal mask, but only with a bag-mask and reservoir can the levels approach 100%.The technique, however, requires two people: one to secure a tight seal and maintain a clear airway using the Laerdal mask technique, and the other person, utilising both hands, to squeeze the bag. Ventilation at 10 breaths per minute can be continued without interrupting chest compression.
— every 10 breaths check for a circulation, take no more than 10 s each time
— if the patient starts to breathe on his own but remains unconscious, turn him into the recovery position. Check his condition and be ready to turn him onto his back and restart rescue breathing if he stops breathing
If there are no signs of a circulation or signs of life, or you are at all unsure:
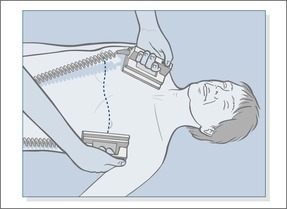
— start chest compression rather than initial ventilation (→Fig. 10.11):
• Place the heel of one hand in the centre of the victims chest (in the middle of the lower half of the sternum)
• Place the heel of the other hand on top of the first hand.
• position yourself vertically above the patient’s chest and, with your arms straight, press down on the sternum to depress it 5–6 cm
• release the pressure without breaking contact between the hands and the sternum, then repeat at a rate of about 100–120 times a minute (a little less than two compressions a second). Compression and release should take an equal amount of time
— combine rescue breathing and compression:
• after 30 compressions, tilt the head, lift the chin and give two effective breaths – these should be normal sized breaths (600 to 700ml) each given without force over 1 second with an equal time allowed for expiration, (this recommendation applies to any form of CPR ventilation including bag valve and mark). The two breaths should take no more than five seconds. Add supplemental oxygen as soon as possible
• return your hands immediately to the correct position on the sternum and give 30 further compressions, continuing compressions and breaths in a ratio of 30:2
— when using two-person BLS, the following points should be noted:
• when changing from single-person to two-person BLS, the second rescuer should take over chest compressions after the first rescuer has given two rescue breaths. During these rescue breaths, the incoming rescuer should determine the correct position on the sternum and should be ready to start compressions immediately after the second rescue breath has been given. It is preferable that the rescuers work from opposite sides of the patient.
• a ratio of thirty compressions to two rescue breaths should be used. By the end of each series of thirty compressions, the rescuer responsible for rescue breathing should be positioned ready to give a breath with the least possible delay. It is helpful if the rescuer giving compressions counts out aloud: ‘1-2-3-4-5…’
• chin lift and head tilt should be maintained at all times. Each rescue breath should take the usual 1 s, during which chest compressions should cease; they should be resumed immediately after inflation of the chest, waiting only for the rescuer to remove his or her lips from the patient’s face.
8. Continue resuscitation until the cardiac arrest team arrives to assist. Do not stop CPR to check the patient unless he starts to regain consciousness and shows clear signs of breathing normally again.
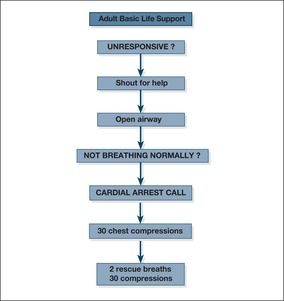 |
| Fig. 10.9 |
Notes on techniques of BLSBox 10.6 summarises the key to initial resuscitation.
• Only a small amount of resistance to breathing should be felt during rescue breathing, and each inflation should take about 1 s
• The tidal volume to be achieved is about 600–700ml in an adult, which is the amount normally required to produce visible lifting of the chest
• The rescuer should wait for the chest to fall fully during expiration before giving another breath. This should normally take about 1 s
• Once the airway is secured with an endotracheal tube or similar the lungs are ventilated at 10 breaths per minute without any pause in chest compression.
Box 10.6
• Airway control: head tilt, chin lift and jaw thrust
• Effective use of a Laerdal mask or a bag and reservoir
• Effective bagging needs two people
• If gurgling: suction must be used (Yankauer) but only to oropharynx
Chest compression
• Any interruptions in chest compression lessens the chance of recovery and must be kept to an absolute minimum.
• In an adult, the aim should be to press down approximately 5–6 cm and apply only enough pressure to achieve this.
• At all times the pressure should be firm, controlled and applied vertically. Erratic or violent action is dangerous.
• The recommended rate of compression is a rate, and not the number of compressions that are to be given in a minute; this will depend upon interruptions for rescue breathing.
• About the same time should be spent in the compression phase as in the released phase.
• As the chances are remote that effective spontaneous cardiac action will be restored by BLS without other techniques of ALS (including defibrillation), time should not be wasted by further checks for the presence of a pulse. Even short interruptions to chest compression have very adverse effects on the outcome so compression should not be interrupted.
• The presence of dilated pupils has, in the past, been variously used as a sign of cardiac arrest, failure of the circulation during resuscitation and the presence of established brain damage. This sign is unreliable and should not be used to influence management decisions before, during or after cardiopulmonary resuscitation.
Recovery position
When circulation and breathing have been restored, it is important to maintain a good airway and ensure that the tongue does not cause obstruction. It is also important to minimize the risk of inhalation of gastric contents.
For this reason, the patient should be placed in the recovery position (→Fig. 10.12). This allows the tongue to fall forward, keeping the airway clear.
• Remove the patient’s spectacles
• Kneel beside the patient and make sure that both his legs are straight
• Open the airway by tilting the head and lifting the chin
• Place the arm nearest you out at right angles to his body, elbow bent, with the palm of the hand uppermost
• With your other hand, grasp the far leg just above the knee and pull it up, keeping the foot on the ground
• Keeping his hand pressed against his cheek, pull on the leg to roll the patient towards you onto his side
• Adjust the upper leg so that both the hip and knee are bent at right angles
• Tilt the head back to make sure the airway remains open
• Adjust the hand under the cheek, if necessary, to keep the head tilted and facing downwards to allow liquid to drain from the mouth
• Check breathing regularly and ensure that oxygen is being administered
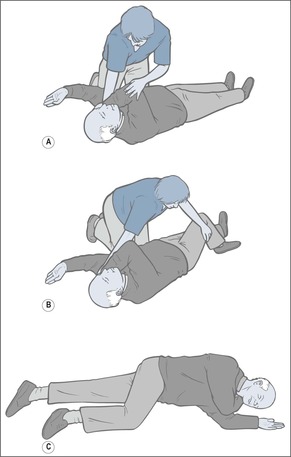 |
| Fig. 10.12 |
What the team leader of an arrest team should want to know from the ward nursing staff
• How, when and where the arrest occurred (use a common structured communication tool).
• Was there a witnessed account?
• What was the time from collapse to BLS?
• What was the initial rhythm?
• What is the time from arrest to defibrillation?
• Were there any special circumstances, e.g. tricyclic overdose/hypothermia?
• What is the DNAR status?
• Is there any equipment malfunction?
• Are the notes available for review
Problems to avoid and look for during cardiopulmonary resuscitation
• Ensure suction and wide-bore rigid suckers (Yankauer) are available
• Correct ampoules and drug doses
• Good i.v. lines that will not ‘tissue’, especially with sodium bicarbonate (all i.v. drug boluses should be followed by a 20ml N saline flush)
• Avoid letting fluids ‘run through’
• Avoid injury to the patient’s limbs and eyes from malposition and spillage
• Avoid staff electrocution
• Avoid burns to the patient during defibrillation – move oxygen masks or nasal cannulae at least a metre away from the patient’s chest
• Ensure effective chest compression by changing the operator every two minutes
One member of staff should be recording the events:
• timing of events
• number of shocks and strength
• administered drugs – dose and timing
• equipment problems
After basic life support
Although BLS is a holding measure that keeps the patient alive, it must be continued throughout the resuscitation attempt, except when a patient is undergoing defibrillation. Once the arrest team arrives the situation can be reviewed, with the first priority being to analyse the heart rhythm from the ECG monitor or, more rapidly, via the self adhesive defibrillation pads.
In a cardiac arrest, the heart rhythm falls into one of two categories:
1. Shockable rhythms: ventricular fibrillation and pulseless ventricular tachycardia (VF/VT). Patients in this group need immediate defibrillation.
2. Non-shockable rhythms: asystole (a straight line) and electrical complexes, but with no palpable pulse (Pulseless Electrical Activity – PEA, previously known as electromechanical dissociation – EMD). This group needs emergency drugs and continuing CPR.
Defibrillation strategy
Once VF/VT is recognised immediately give one shock (150–200 J bi-phasic defibrillator or 360 J monophasic defibrillator). Minimise the disruption to CPR by the delivery of the shock – with a manual de-fibrillator the time between stopping and re-starting CPR should be less than 5 seconds. Do not wait to check the rhythm or feel for a pulse but immediately re-start chest compression.
Continue CPR for two minutes in the ratio 30:2 and then check the monitor If there is still VF/VT give a second shock (150–360 biphasic) and restart CPR immediately
After two minutes recheck the monitor and if there is still VF/VT give a third shock and restart CPR immediately – while CPR is proceeding give 1mg of adrenaline (epinephrine) and 300mg of amiodarone intravenously. There must be no unnecessary interruption to chest compression (plan the next steps and re-charge the defibrillator during CPR).
Regardless of the rhythm adrenaline (epinephrine) is given every 3–5 minutes (i.e. immediately after alternate shocks) until there is return of a spontaneous circulation. If, during CPR, the patient shows signs of life and the monitor shows an organised rhythm – check the pulse and if it is present start post resuscitation care, if not continue CPR. Do not interrupt a two minute spell of CPR if there is an organised rhythm but no signs of life – continue CPR and only check the pulse at the end of the two minutes.
Technical points in defibrillation. For maximum success, the shocks must be applied without delay. If left, ventricular fibrillation deteriorates into asystole or PEA, which have a much worse prognosis. Effectively, defibrillation ‘stuns’ the heart, stops all electrical activity and, if successful, the heart re-starts with the natural pacemaker taking over to produce a normal rhythm. During CPR the time between stopping chest compression and administering the shock must be cut to the absolute minimum to maximise the chance of success. Self adhesive pads are much preferred to de-fibrillator paddles.
The correct defibrillation electrode (or paddle) positioning is critical – one is placed to the right of the upper sternum below the clavicle while the other is placed in the mid-axillary line approximately level with V6 ECG lead – avoiding breast tissue in a woman (→Fig. 10.13).
Early defibrillation after the recognition of cardiac arrest and the immediate institution of basic life support is the key to improved survival. This has been facilitated by the introduction of the Automated External Defibrillator which can:
• analyse the heart rhythm and either advise a shock or the resumption of 30:2 CPR
• provide spoken or visual directions on administering a shock or deliver a shock automatically
• prompt immediate resumption of CPR after the shock. Do not stop CPR to check the patient unless he starts to breathe normally (not just agonal gasps which are common during CPR) or shows clear signs of coming back to life.
• time the two-minute CPR cycle and then prompt to assess the rhythm, respiration and pulse
AEDs have played a major role in increasing the speed of response to cardiac arrest and increasing the number of people who can now successfully perform defibrillation.
Asystole and PEA
These non-shockable rhythms are treated with CPR, initially 30:2 but once the airway is secure continuous compressions at 100 per minute without pausing during ventilation at 10 breaths per minute. Adrenaline (epinephrine) 1mg is given as soon as there is IV access and continued every 3–5 minutes of CPR. Atropine is no longer recommended for PEA.
Fine VF or asystole?
If there is doubt about whether the rhythm is asystole or fine VF manage the patient as a non-shockable rhythm. Do not attempt defibrillation – continue chest compressiion and ventilation.
Precordial thump?
A single precordial thump can be tried in a witnessed and monitored cardiac arrest but is only likely to be successful in the first few seconds after collapse particularly in VT and occasionally in VF. The bottom edge of a fist is brought down sharply from about 20 cm above the lower half of the sternum.
The uses of adrenaline (epinephrine) in the collapsed patient
Adrenaline (epinephrine) is used in cardiac resuscitation and in the treatment of acute anaphylaxis. It increases the efficiency of resuscitation by:
• increasing flow of blood in the heart muscle
• increasing flow of blood through the brain
• sustaining a falling blood pressure
There are two strengths of adrenaline (epinephrine): 1:1000 for intramuscular administration in anaphylaxis and 1:10 000, ten times weaker, for i.v. administration in cardiac resuscitation and in severe unresponsive anaphylaxis. Details of adrenaline (epinephrine) administration are given in Table 10.4. Fig. 10.14 shows the ALS algorithm.
| Strength | ||||||
|---|---|---|---|---|---|---|
| 1:10 000 | 1:1000 | |||||
| Concentration | 1g | in | 10000ml | 1g | in | 1000ml |
| 1mg | in | 10ml | 1mg | in | 1ml | |
| Indications | Cardiac arrest | Acute anaphylaxis | ||||
| Route | Intravenous | Intramuscular | ||||
| Dose | 1mg (10ml) | 0.5mg (0.5ml) | ||||
| If repeated: | Every 3–5 min | At 5 min | ||||
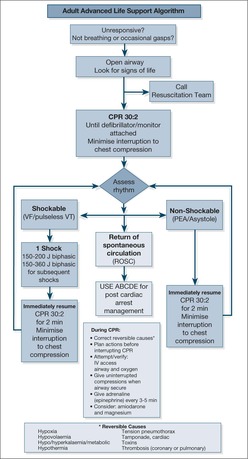 |
| Fig. 10.14 |
Treatable causes of cardiac arrest: 4H and 4T
Hypoxia. Hypoxia is the most easily preventable cause of cardiac arrest. The aim in any acutely ill patient is to monitor the oxygen saturations and keep the oxygen saturation above 90%. In the patient with COPD who has collapsed, the issue of controlled oxygen therapy is now irrelevant – in the emergency situation high concentrations of oxygen are needed as an immediate life-saving measure.
Hypovolaemia. Sudden, severe fluid loss in the form of blood (gastrointestinal bleed or massive haemoptysis) or body fluid (diarrhoea or DKA) will lead to hypotension and cardiac arrest. The key is to recognise that the low blood pressure is due to fluid depletion rather than a problem with a sick heart, and take appropriate action.
Hypothermia. Cardiac arrest can complicate severe hypothermia. Cardiopulmonary resuscitation must be continued until the patient’s temperature is brought back up above 35°C or the efforts are judged by a senior medical member of the team to be fruitless.
Hyperkalaemia and hypokalaemia. A very high serum potassium, characteristically complicating acute renal failure, and a very low potassium, as may be seen in excessive diuretic treatment, can lead to cardiac arrest and must be addressed urgently. Intravenous calcium chloride is used for hyperkalaemia, hypocalcaemia and in overdose with calcium channel blockers (eg nifedipine). Intravenous magnesium can be life saving in serious arrythmias due to magnesium deficiency – seen in over-diuresis and with some chemotherapy regimes.
Tension pneumothorax. Characteristically, this gives a picture of PEA: a rhythm on the monitor but no palpable pulse. Unless the pneumothorax is recognised and treated, resuscitation will be unsuccessful.
Therapeutic accident. The common cause in this category is tricyclic poisoning after self-harm. Even very protracted resuscitation can be successful, and it is one of the few situations in which i.v. sodium bicarbonate can have a life-saving effect.
Tamponade. Cardiac tamponade occurs when there is a build-up of fluid under pressure in the pericardial sac – it compresses the heart and drastically reduces the cardiac output to an extent that it can lead to sudden PEA. Resuscitation will be unsuccessful unless the fluid is also drained off.
When to stop the resuscitation procedure
Legally, the most senior doctor present has this responsibility. However, he or she should consider other views within the team. Survival becomes less likely with time. but many patients have recovered from prolonged resuscitation, particularly after:
• drowning
• electrocution
• hypothermia
• tricyclic drug overdose
It is almost impossible to predict brain damage from the state of the patient during the arrest and, in particular, fixed dilated pupils are not a reliable guide. If the patient is oxygenated and undergoing efficient BLS, the repeated failure of defibrillation and appropriate cardiac drugs will eventually form the basis of the decision to stop resuscitation.
Dealing With the Relatives of a Victim of Cardiac Arrest
This is a task for an experienced member of the ward team, preferably the nurse who was with the patient nearest to the time of the arrest. No matter how distressed and distracted the relatives appear, it is likely that every detail of the day’s events will be etched on their minds forever.
Find out who you are addressing and if any key member such as a spouse is missing. You may need to gather clinical information from the relatives as well as providing them with news. Explain the situation in straightforward terms and do not be embarrassed if your comments sound like platitudes – it is far better to use everyday language that is clear and unambiguous than revert to medical jargon:
Thirty minutes ago John’s heart suddenly stopped and we put out an emergency call. The doctors and nurses are doing everything they can to try and get it started again. The situation is critical and we do not know if he is going to survive.
I am really sorry to have to give you this terrible news: John’s heart stopped suddenly at 5pm. We were there within seconds and tried everything we could to get it started again. We worked on him for an hour, but it was absolutely hopeless; we could not get a heart beat back and he died at 6.15pm. It happened with no warning and from the moment his heart stopped John will have known nothing about it. At the end it was very peaceful, and I am sure that he did not suffer.
You must let and encourage the family to see the body. Try not to have the family, however well intentioned, ‘protect’ a wife or husband from seeing the body – it may be their last chance to say goodbye and they will come to value that moment. They must be allowed the chance to say an unhurried goodbye, to touch the body, to show their distress and to express their grief.
It is unlikely that you will know what to say, and silence is often as good as anything. Keep in mind that, to the ward staff, this is another episode in a very crowded day – but to the family it is a life-changing event.
Answering Relatives’ Questions in Cardiac Arrest
What has happened? The heart suddenly stopped beating, but we have been able to ‘kick start’ it using a small electric shock. The episode was short-lived and we were able to restore a normal heartbeat, probably before any further damage could be caused. The bruising or red marks on the chest are due to manoeuvres we had to perform to keep the blood pumping during the period when the heart had stopped. There will be pain and tenderness in this area for a few days.
Has the heart been damaged? It is too early to judge the extent of the damage. Good signs will be a return of the blood pressure to normal and successful withdrawal of all the intravenous drips and drugs.
Has there been a heart attack? We will only know from tests on the heart and blood, which we will get back once he has recovered. It is possible to have a cardiac arrest from a sudden electrical fault without having an actual heart attack.
Has there been any brain damage? We cannot tell at present. The confusion/drowsiness/coma is not uncommon after a cardiac arrest and does not necessarily mean there is any permanent damage. The brain can go through a short-lived period of ‘hibernation’ after a cardiac arrest and still recover completely. We will only know if this is the case here after a period of time has elapsed (usually at least 24 and often up to 72 h).
Is he on a life-support machine? The mask gives extra oxygen and must be kept in position for the next 24h. If the condition deteriorates and the oxygen mask alone is insufficient, we can use a life-support machine. This will take over all the breathing functions and pump air directly into the patient’s lungs through a tube. If this is needed, the patients are moved to the ITU, put to sleep, and then kept heavily sedated while they are on the machine.
The Aftercare of Patients Who Undergo Successful CPR
Some patients are traumatised by the events associated with successful cardiopulmonary resuscitation. The physical effects – chest bruising, defibrillation burns, and even rib fractures – require simple explanation and symptomatic treatment. The psychological effects are more enduring:
• ‘near death experiences’
• acute anxiety
• loss of confidence
• fear of a second arrest
• recurrent nightmares
For the first time in their life, the patient has become aware of his own mortality. The key to their immediate management is:
• give an honest account and explanation of the events that took place
• listen to the patient’s concerns
• give firm reassurance
• consider offering other forms of help – discussion with the doctors, the priest and the clinical psychologist
‘Do Not Attempt Resuscitation’ – DNAR
It is important to try and differentiate between the natural process of dying and a sudden cardiorespiratory arrest from which a patient can be rescued. If the patient has a physical illness from which there is no likelihood of recovery and if it is clear that before admission the patient’s life was nearing its end – then resuscitation would seem futile. Common examples of the latter include patients with cancer who are bed-bound and can no longer take oral medication and patients with worsening heart failure in spite of repeated admissions and optimisation of their drug regimen. Conversely, cardiac arrest as an early complication of an acute myocardial infarction is worth an attempt at resuscitation in virtually all situations.
The distinction may not be obvious in every case, and so it is important that decisions concerning resuscitation are made by a senior member of the medical team – ideally, but not always, the consultant. It is also essential to understand that a mentally competent adult can refuse consent to medical treatment (including resuscitation), even if that decision would lead to the person’s death.
Communication and explanation
Communication is of paramount importance in this area. Discussions must be held with all the relevant parties, which should also involve the patient. It is important to provide patients with a realistic assessment of the likely outcome of a cardiac arrest (the benefits versus the burdens). Many patients, for example, would decline resuscitation if they were given a 50% chance that the outcome would include severe cognitive or functional impairment. DNAR decisions must be clearly documented, with the underlying reasons, in the nursing and medical notes, and the status of each patient on the ward communicated at handover (→Case Study 10.8).
Case Study 10.8
A 55-year-old man with lung cancer had undergone palliative chemotherapy and came in with marrow failure and septicaemia. In spite of antibiotics, he deteriorated. He asked the staff, in the presence of his family, to have no more tests or any ‘messing about’. Although weak and drowsy he understood the situation exactly. Subcutaneous morphine and haloperidol were used to control his pain and breathlessness.The decision not to resuscitate was formally posted in his nursing and medical notes. He died peacefully.
The on-call team must be included in the communication. There is little point in having a detailed discussion at 17.00h, only for the arrest team to attend at 03.00h and be faced with a ward team who are not aware of the resuscitation status of their patients.
When in doubt: resuscitate.
A DNAR protocol
It is important to work to a protocol
There should be a standard way to review and record DNAR decisions in the notes. Any DNAR notice that has been posted should be kept under regular review, as the status of the patient may alter as their medical condition changes.
When a DNAR decision is appropriate
• When resuscitation would not be successful
• When resuscitation is against the patient’s wishes (expressed when the patient was competent to do so)
• When resuscitation would not be in accord with an Advanced Directive
Important points about DNAR
• In any discussions, do not exclude close friends and family members. Particularly in the case of patients with learning disorders and the young chronic sick, the professional carers from long-stay institutions and nursing homes may consider themselves as ‘family’ and must be included in the discussions.
• It can be helpful to ‘anticipate’ cardiac arrests or sudden deterioration so that some early discussion can be had with relatives. Patients with advanced disease, say with COPD or severe cardiac failure who survive ‘near misses’, can be asked if they want to be involved in future discussions concerning resuscitation should the same thing happen again.
• It should be made clear to everyone –staff, patient and relatives –that a DNAR decision is NOT the same as withdrawing all forms of care. The priority in these patients remains medical treatment, nursing care and, if the situation deteriorates, ensuring the patient is kept comfortable and free of pain.
Answering Relatives’ Questions About ‘Do Not Resuscitate’ Decisions
Does this decision mean that all treatment will now be withdrawn? No, it does not mean this at all. Resuscitation means starting urgent life-support measures if the heart stops – the aim is to restore a normal heart beat. We do this if there is a reasonable chance of restoring the patient to his previous health without suffering side-effects or damage as a result of the cardiac arrest and subsequent resuscitation. After considering this, and as a result of our discussions with you, we have decided that either resuscitation would not work or it would leave the patient in an intolerable situation. However, we can still treat other complications apart from a cardiac arrest – infection, congested lungs, low blood pressure – and we can still ensure effective symptom control if there is pain, distress or breathlessness.
Bereavement on the Acute Medical Unit
Bereavement on the Acute Medical Unit is an important and difficult problem:
• the deaths will be sudden
• the relatives will have had little warning
• the ward will be busy and staff time will be at a premium
However, if bereavements are handled sensitively, relatives are more likely to come to terms with their loss and the risks of subsequent abnormal grief reactions will be reduced. Two examples of the common problems encountered after bereavement are given in Case Studies 10.9.
Case Studies 10.9
CASE 1
A 75-year-old woman with known ischaemic heart disease, severe airflow obstruction and myelodysplasia was diagnosed as having an advanced squamous carcinoma of the lung.There had been full involvement by a consultant chest physician and the lung cancer specialist nurse.The patient had her own patient-held record.
The patient was admitted after a period of deterioration with dehydration and sepsis. She initially responded to i.v. antibiotics. Unfortunately, at 03.00h the patient became acutely distressed, hypotensive and confused. She died over a period of 20 min.The relatives came to the ward after the patient had died.
Several months after the event, despite extensive counselling, the daughter was still extremely distressed. She felt guilty, having promised that her mother would die at home and that, if it was necessary, she would take her out of hospital. Previous members of the family had died in unexpected circumstances on their own. In addition, she had ‘chivvied’ her mother along during her final illness to encourage her not to give up. She felt guilty about this.
CASE 2
A 55-year-old woman with known advanced breast cancer previously treated with chemotherapy and radiotherapy was admitted for ‘terminal care’.The lesion was fungating, she had a pleural effusion and could not move at all in bed without the help of two nurses. Her husband did not leave her side. She was started on subcutaneous opiates and moved onto another ward which, in the information sheet for the Acute Medical Unit, is described as a ‘recovery ward’. She died suddenly from a pulmonary embolus at 05.00h a few days later. weeks later, her husband came back to the hospital with a number of complaints.
• He had not realised that she was dying
• If she was dying, why had she been moved to a ‘recovery ward’?
• When was she last seen alive and by whom?
• Why was her usual medication cut down?
• Did she die alone, and why was he not called in?
We had talked to this man on several occasions at the bedside.We had not appreciated that, in his distress, he had not taken in the most fundamental issue about his wife’s illness. He needed to know everything about the final few hours when he was not there with her. He was still inconsolable months later, because of the way in which we had failed to communicate effectively with him.
There are a number of problems surrounding sudden death in hospital, particularly regarding the actual sequence of events. Relatives need to know what went on, who was present, whether the patient asked for his next of kin, whether there were any nurses present and whether the doctors had been called. It is extremely important that the actual events are covered in detail at the time of the death, because only then can the relatives start to come to terms with the situation. It is common for relatives to mull over the events surrounding the death. Even months later, they may have several unanswered questions.
Critical nursing tasks when dealing with the bereaved
Ensure effective communication with relatives
Ensure that communication with the relatives of very sick patients remains the utmost priority on the ward.
Make sure the facts are correct
• Make absolutely sure that you have the facts correct:
— that the patient in question has died
— that you are dealing with that patient’s relatives
— that you know their names and relationships
— that the relatives you are talking to include the next of kin.
Find out the likely cause and mode of death
• Find out from the doctors the likely cause of death.
• Speak to those who were with the patient in his final moments: the relatives will need to know these details. Sometimes relatives will ask to speak to the night shift who were involved.
Give the relatives time with the deceased
• Ensure the relatives have an unhurried and private time with the deceased.
Ensure liaison with the patient’s GP
Ensure that the ward lets the patient’s GP know of the death.
Important nursing tasks when dealing with the bereaved
Establish the need for coroner involvement or post-mortem
Establish from the doctors whether they:
• will need to inform the coroner/procurator fiscal
• would like to seek permission for a post-mortem
Help the relatives with practical details
Ensure the relatives understand the process of:
• death certification
• handling the deceased patient’s possessions
• the help that bereavement officers and the undertakers will give
Understand the role of the coroner and death certification
Make sure you understand the role of the coroner and know before dealing with the relatives whether (and when) a death certificate can be issued.
Be sensitive to distress among staff
Be sensitive to distress among junior members of the nursing and medical team after a bereavement. For staff, a series of deaths and the associated feelings of inadequacy/failure that they engender may leave them vulnerable and stressed.
▪ Establish the need for coroner involvement or post-mortem
▪ Help the relatives with practical details
▪ Understand the role of the coroner and death certification
Further Reading
Resuscitation
Field, D.; Kitson, C., Coping with death: the practical reality, Nursing Times 19 (March 1986) 33–34.
Featherstone, P.; Chalmers, T.; Smith, G.B., RSVP: a system for communication of deterioration in hospital patients, British Journal of Nursing 17 (2008) 860–864.
Transfusion
British Committee for Standards in Haematology, Blood Transfusion Task Force, Guideline: the administration of blood and blood components and the management of transfused patients, Transfusion Medicine 9 (1999) 227–238S.
Oxygen Therapy
Bateman, N.T.; Leach, R.M., ABC of oxygen: acute oxygen therapy, British Medical Journal 317 (1998) 798–801.
Murphy, R.; Mackway-Jones, K.; Sammy, I.; et al., Emergency oxygen therapy for the breathless patient. Guidelines prepared by the North West Oxygen Group, Emergency Medicine Journal 18 (2001) 421–423.
O’Driscoll, B.R.; Howard, L.S.; Davison, A.G., BTS guideline for emergency oxygen use in adult patients, Thorax 63 (Suppl 6) (2008) 1–68.
Woodrow, P., Pulse oximetry, Nursing Standard 13 (42) (1999) 42–47.
Sepsis
Dellinger, P.R.; Carlet, J.M.; Masur, H.; et al., Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock [Review], (Erratum in: Crit Care Med 32 (6) (2004) 1448; 2004 Correction of dosage error in text.) Crit Care Med 32: 858–873.
Websites
Hoste, E.A.; Clemont, G.; Kerstein, A.; et al., RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis, Critical Care 10 (3) (2006) R73.