95 Multiple system atrophy
Salient features
Examination
Advanced-level questions
What are the types of multisystem atrophy?
Striatonigral degeneration. Clinical picture resembles Parkinson’s disease but without tremor. These patients do not respond to antiparkinsonian medications and often develop adverse reactions to these agents.
Shy–Drager syndrome. Clinical picture consists of Parkinson’s disease combined with severe autonomic neuropathy (particularly postural hypotension). Other important clinical features are impotence and bladder disturbances.
Olivopontocerebellar atrophy. Combination of extrapyramidal manifestations and cerebellar ataxia. Patients may also have autonomic neuropathy and anterior horn cell degeneration.
What factors can lower blood pressure in these patients?
Standing up: orthostatic hypotension, a hallmark of this condition.
What is the morbidity of this condition?
It is tends to disable most patients severely by the end of 5–7 years.
What are the radiological signs of multisystem atrophy?
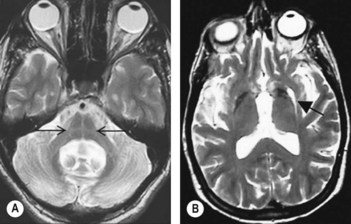
• The more common typical radiological findings in multisystem atrophy include atrophy of the cerebellum, most prominently in the vermis, middle cerebellar peduncles, pons and lower brainstem (Neuroimag Clin North Am 2010;20: Issue 1; on imaging of movement disorders).
• In addition to putaminal atrophy, a characteristic hypointense signal in T2 with hyperintense rim, corresponding to reactive gliosis and astrogliosis, can be observed in the external putamen and is termed the ‘slit-like void sign’ (Fig. 95.1B). This combination of hypointense and hyperintense putaminal signal change is specific for multisystem atrophy and its finding can be used to differentiate multisystem atrophy from progressive supranuclear palsy and Parkinson’s disease.
• The ‘hot-cross bun’ sign is characterized by cruciform signal hyperintensity on T2-weighted images in mid pons (Fig. 95.1A). This finding is said to correspond to the loss of pontine neurons and myelinated transverse cerebellar fibres with preservation of the corticospinal tracts. However, this sign is not specific to multisystem atrophy and has been reported in other conditions such as spinocerebellar ataxia.
• Hypointensity alone without hyperintense rim is a sensitive radiological feature but non-specific for multisystem atrophy.
What are the diagnostic criteria for probable multisystem atrophy?
A sporadic, progressive, adult-onset (>30 years) disease characterized by:
• autonomic failure involving urinary incontinence (with erectile dysfunction in males) or
• orthostatic decrease of BP within 3 min of standing by at least 30 mmHg systolic or 15 mmHg diastolic and either
• poorly levodopa-responsive parkinsonism (bradykinesia with rigidity, tremor, or postural instability) or
• a cerebellar syndrome (gait ataxia with cerebellar dysarthria, limb ataxia or cerebellar oculomotor dysfunction).
What are the diagnostic criteria for possible multisystem atrophy?
A sporadic, progressive, adult-onset (>30 years) disease characterized by:
• parkinsonism (bradykinesia with rigidity, tremor, or postural instability) or
• a cerebellar syndrome (gait ataxia with cerebellar dysarthria, limb ataxia or cerebellar oculomotor dysfunction) and
• at least one feature suggesting autonomic dysfunction (otherwise unexplained urinary urgency, frequency or incomplete bladder emptying, erectile dysfunction in males, or significant orthostatic BP decline that does not meet the level required in probable MSA) and
Possible MSA-P is indicated by:
• rapidly progressive parkinsonism
• postural instability within 3 years of motor onset
• gait ataxia, cerebellar dysarthria, limb ataxia or cerebellar oculomotor dysfunction
• dysphagia within 5 years of motor onset
• atrophy on MRI of putamen, middle cerebellar peduncle, pons or cerebellum
• hypometabolism on fludeoxyglucose PET (FDG-PET) in putamen, brainstem or cerebellum.
What are features supporting a diagnosis of multisystem atrophy?
What are features that do not support a diagnosis of multisystem atrophy?
What do you know about the pathogenesis of multisystem atrophy?
• Inclusions could trigger microglial activation, which causes chronic oxidative stress and ultimately leads to neuronal cell death
• Inclusions could exacerbate susceptibility to exogenous oxidative stress and lead to neuronal cell death in striatonigral and olivopontocerebellar systems
• Inclusions could lead to secondary axonal α-synuclein aggregation or oligodendroglial mitochondrial dysfunction, which eventually lead to neuronal cell death.