Multiple sclerosis
After reading this chapter the student or therapist will be able to:
1. Describe the pathological processes, prevalence, and clinical presentation of people with multiple sclerosis.
2. Compare and contrast the types of multiple sclerosis and the common disease progression in each.
3. Discuss the medical management of the disease and the disease symptoms.
4. Describe how the International Classification of Functioning, Disability and Health provides a common language for describing the impact of disease on people with multiple sclerosis and how it provides a framework for rehabilitation management.
5. Describe the outcome measures that can be used to examine people with multiple sclerosis that cover body system problems (impairments), functional skill and activity limitations, and participation restrictions.
6. Develop a rehabilitation plan of care using evidence-based interventions to maximize patient function and quality of life.
Overview of multiple sclerosis
Pathophysiology
Multiple sclerosis (MS) is a chronic, inflammatory disease of the brain, optic nerve, and spinal cord mediated by the immune system.1 It is characterized by lesions of disseminated focal demyelination accompanied by variable axon damage and destruction and reactive gliosis. Initially, MS was thought to be a disease of the white matter (WM); however, recent investigations have shown that the gray matter (GM) is significantly involved. Lesions found in the GM typically contain demyelination and loss of neurons without the immune system infiltrates and inflammation characteristic of lesions in the WM. Tissue damage has been found outside the focal lesions throughout the GM that is associated with brain and spinal cord atrophy. These areas of demyelination and axonal damage interfere with normal conduction of neural signals, leading to a disruption of function.
Later in the course of the disease, inflammation becomes uncommon while demyelination and axonal loss continue, suggesting replacement by a neurodegenerative disease process. Disease progression becomes more constant with a lack of exacerbation. The motor, sensory, and cognitive disability that accumulates in the advanced stages of the disease appears to be associated with the cortical GM pathology.2 Owing to the lack of inflammation, DMAs have not been shown to be beneficial in the later stages of the disease.
Incidence and prevalence
MS is the primary cause of nontraumatic disability in young and middle-aged adults and the most common inflammatory condition of the central nervous system (CNS). It is reported that approximately 350,000 to 400,000 people in the United States and over 2.5 million people worldwide have the disease.3,4 People are most commonly diagnosed at age 20 to 50 years, with an average age of 32. However, MS can be diagnosed in people of any age. Approximately 5% of all patients with MS are diagnosed before their sixteenth birthday.5
MS is found in people who reside above the northern or below the southern 40° latitude with greater frequency than those who live closer to the equator (Figure 19-1). Given the increased sun exposure of people living closer to the equator, lack of vitamin D is being investigated as a potential factor contributing to disease development.6 Many researchers believe that exposure to an infectious agent may trigger the disease process: Epstein-Barr virus is currently considered a likely candidate.
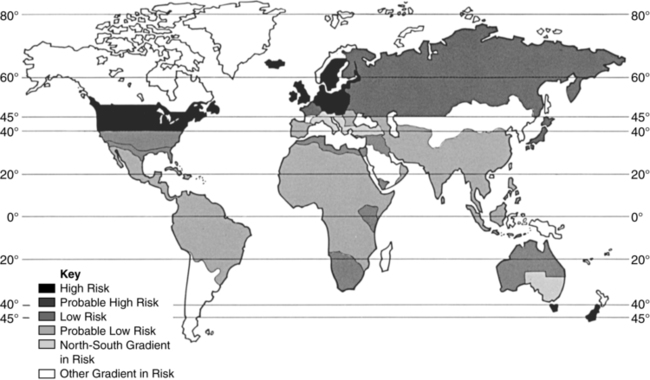
 World distribution of multiple sclerosis. (From Multiple Sclerosis Resource Center, www.msrc.co.uk.)
World distribution of multiple sclerosis. (From Multiple Sclerosis Resource Center, www.msrc.co.uk.)Women are affected two to four times more frequently than men. Even so, men are more likely to have a more aggressive disease progression and a worse prognosis.4,7 Caucasians with Northern European ancestry have the greatest incidence of MS, whereas people of Asian, African, or Hispanic ethnicity are at lower risk. African Americans have a lower incidence, but become disabled earlier than Caucasians, suggesting that tissue destruction occurs earlier and more rapidly.8 Inuits, Yakutes, Hutterites, Hungarian Romani, Norwegian Lapps, Australian Aborigines, and New Zealand Maoris do not appear to develop MS.9 Being diagnosed with MS may be related to age, gender, genetics, geography, or ethnic background. An identical twin with MS means that the other twin will have a 25% chance of diagnosis, suggesting something beyond genetics. Having a first-degree relative with MS will increase the risk of disease from 1/750 to 1/40.3
Types of multiple sclerosis and clinical characteristics
At least four types of MS have been identified (Figure 19-2). Although the course of the disease is highly variable even within a subtype of MS, there are characteristics common to each.
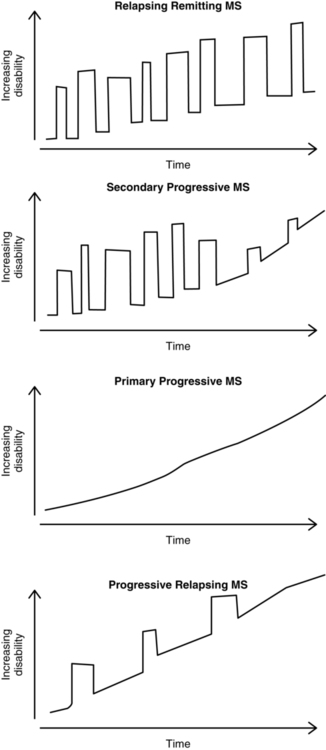
 Types of multiple sclerosis.
Types of multiple sclerosis.The initial neurological episode or attack is typically identified as clinical isolated syndrome (CIS). Symptoms must last for at least 24 hours and can be monofocal or multifocal. If there are lesions present on magnetic resonance imaging (MRI), there is a high risk of developing MS. In one group of people with CIS followed for 20 years, 63% were diagnosed with definite MS.10
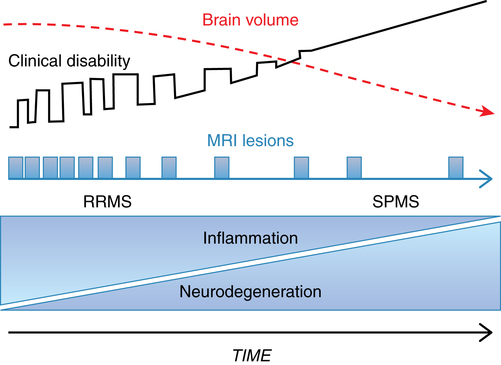
In secondary progressive MS (SPMS), relapses decrease in frequency over time and convert to a slow steady progression of increasing disability or disease severity. Relapses may occur early in SPMS but gradually lessen over time. People with RRMS eventually convert to SPMS 10 to 20 years after diagnosis.11
It is thought that the clinical disability associated with SPMS results from the neurodegeneration that occurs as a result of tissue injury that accumulates from early in the disease process. In addition to less inflammation, there is a greater amount of brain atrophy in people with SPMS compared with RRMS. Figure 19-3 shows the natural history of RRMS and SPMS, comparing the change in brain volume with increasing clinical disability and disease burden.2
Benign MS is identified when symptoms occur once and never recur. This happens in roughly 25% of cases.12 Recently, Sayao and colleagues13 reported that 52% of people with benign MS had not developed MS 20 years later. However, the remainder of people went on to develop MS, with at least 21% requiring the use of a cane. The authors could not identify any criteria associated with either developing MS or continuing to have the benign form.
The risk of a more rapid disease progression is correlated with older age at diagnosis; male sex; initial symptoms involving the motor, sphincter, or cerebellar systems; multifocal disease at onset; shorter time between first and second attacks; and frequent attacks in the first 5 years postdiagnosis.7,14
Clinical manifestations
Fatigue
Of people with MS, 65% to 97% report fatigue during the course of the disease; as many as 40% of people with MS state that fatigue is their most disabling symptom.15 There are two types of fatigue in people with MS: primary and secondary. Primary fatigue, often called lassitude, is caused by the effects of the demyelination and axonal destruction and its effect on nerve conduction. Restorative rehabilitation has little effect on primary fatigue from neurodegeneration. Secondary fatigue results from problems such as deconditioning, infections, sleep disturbances, poor nutrition, medication side effects, other medical conditions (such as thyroid disease), and heat intolerance. Clinicians should be extremely careful to separate the types of the fatigue in order to determine the most appropriate interventions.
Sensory impairments
Sensory impairments are among the most common symptoms associated with MS and can affect the visual, somatosensory, and vestibular systems.16 The most common problem of the visual system is optic neuritis, which can produce blurry or double vision and/or painful eye movements and nystagmus. Somatosensory or proprioception disturbances can include dysesthesias (tingling, buzzing, or vibrations) or anesthesias (complete loss of sensation in part of the body). People may experience paresthesia or anesthesia in half of the body—upper or lower or side to side—or below a certain spinal cord level. Dysesthesias may be limited to small body areas such as a patch of skin on the head or a single upper or lower extremity. Vestibular system involvement occurs in 20% of people with MS at some time during their disease course17 and may manifest as dizziness and/or vertigo.
Motor systems impairments
A broad clinical definition of spasticity is a velocity-sensitive resistance to muscle stretch or a muscle spasm during movement.18 Some people report heaviness in the limbs, difficulty moving a joint, jumping of the extremities, or involuntary painful movements. Muscle spasms or cramping are frequently experienced by people with MS. Eighty four percent of people with MS report spasticity, with 34% indicating that their spasticity is moderate to severe.19 Female sex or longer disease duration are both associated with higher prevalence of spasticity. Spasticity has been highly correlated with patient-reported disability and poorer quality of life (QOL).19 Spasticity may change according to position and may result from increased effort during activity or from the presence of a noxious stimulus such as an infection, skin lesions, fractures, renal stones, distention of bladder or colon, or other physiological stressors such as certain medications (DMAs or serotonin reuptake inhibitors) or psychological distress. Environmental factors such as tight clothing, hunger, or elevated body or air temperature may also lead to increased spasticity. Spasticity can cause muscle contractures, skin breakdown, pain, and sleep disturbances, which often lead to secondary activity limitations and participation restrictions that limit performance of activities of daily living (ADLs) and mobility.
Ataxia occurs in up to 80% of people with MS at some point in their disease progression.20 This motor deficit can occur from disturbances in the vestibular system or cerebellum or a loss of proprioception. Ataxia or a lack of coordination can manifest as difficulty with walking to difficulty with movements of the extremities such as overshooting or undershooting targets (dysmetria) or an inability to produce rapid alternating movements (dysdiadochokinesia). Occasionally, patients experience sustained body positioning (dystonia) of the extremities or head and neck. In different research studies, tremor is reported by 25% to 58% of people with MS, with the majority of people experiencing mild to moderate dysfunction.21,22 Action tremor, both postural and intention, are found in people with MS, pointing to the cerebellum as a likely source (see Chapter 21). Tremors affect the head, neck, vocal cords, limbs, and torso, with the upper extremities having the greatest occurrence.21,22
MS affects many of the systems required for postural control and balance, including sensory input (visual, somatosensory, and vestibular), central processing, and motor output. Therefore it is not surprising that over 50% of people with MS report falling one or more times in the previous 6 months.23–26
Bowel and bladder dysfunction
The incidence of bowel problems (35% to 68%) and bladder problems (52% to 97%) make them common in people with MS, as reported by two research studies.27,28 Symptoms include urinary urgency, nocturia, or retention of urine or feces.29 Incontinence of either system can also occur. Neurogenic detrusor muscle overactivity is the most common urological impairment in people with MS; 20% have detrusor muscle underactivity, and only 10% report no symptoms.28
Cognitive impairments
Cognitive dysfunction occurs in roughly 40% to 70% of people with MS, with 70% demonstrating mild to moderate impairment.32,33 Although cognitive problems can occur at anytime, abilities affected early in the course of the disease are verbal fluency and verbal memory.34 Other cognitive dysfunctions common in people with MS include impairments in memory, processing speed, executive functioning, attention, and visuospatial learning. There is a fair correlation between cognitive decline and ability to work and unemployment because of the impairments in short- and long-term memory, problems with concentration, forgetfulness, and slowed word recall.35,36 This is a likely source of frustration for both patients and caregivers alike.
Depression
Depression is two to three times more common in people with chronic health conditions than in the general population and has a greater incidence than other neurological conditions.37 From 26% to 50% of people with MS have been reported to experience depression during the course of the disease.32,38 Several factors contribute to the high incidence of depression in people with MS. The fact that MS is a chronic, progressive, and unpredictable disease that affects people in their early to middle adult years, is often invisible, and limits participation in many life roles often leads to a perceived reduction in QOL.39 Suicide is of great concern for people with depression, and rates are significantly higher in people with MS than in the general population.40 Depression is associated with a lower QOL and other symptoms of MS including fatigue, disability, pain, and cognitive impairment.41
Medical management
Diagnosis
Historically, people with MS would wait for a diagnosis for a year or more. Although there are no definitive tests that diagnose MS, the addition of MRI has accelerated diagnosis. In 2001 the International Panel on the Diagnosis of Multiple Sclerosis updated criteria to include MRI, visual evoked potentials, and cerebrospinal fluid (CSF) analysis. The 2005 Revised McDonald Criteria for MS diagnosis were designed to make the diagnostic process even more efficient and easier.42 The Poser criteria require the presence of two separate episodes over time, plus evidence of two or more lesions in separate brain or spinal cord regions identified by radiological imaging studies. Even with the improved technological measures used to facilitate diagnosis, an accurate clinical history is critical. Often patients will recall episodes of transient symptoms that did not last long enough to require attention by a primary care provider.
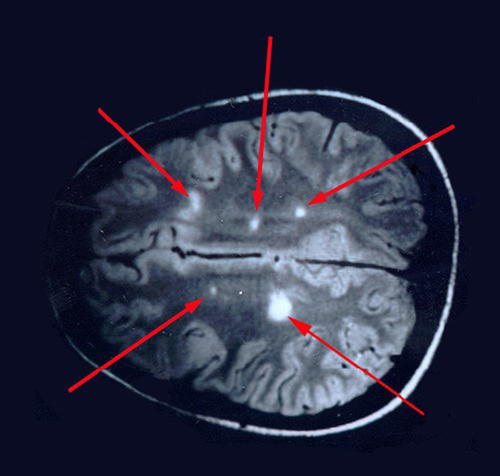
In addition to the clinical history, MRI studies have improved diagnosis of MS. Although T2-weighted MRI images show MS lesions as hyperintense and identify new or active lesions, MRI has been shown (Figure 19-4) to overestimate clinical relapses. Conventional MRI with T1 weighting identifies lesions as hypointense (black holes) and is able to identify brain atrophy. T1 imaging demonstrates a stronger correlation with clinical status and disease severity than the lesion load found with T2 weighting. Gadolinium-enhanced T1-weighted MRI images show active MS lesions as hyperintense (white).
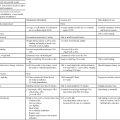
Disease severity and progression are monitored by ongoing medical checkups, MRI imaging, and the use of several outcome measures. The Kurtzke disease severity scale was developed to allow primary care providers a way to measure clinical disability and chart disease progression. It has been replaced by the Expanded Disability Status Scale (EDSS) (Table 19-1).43 The EDSS is a 10-point ordinal scale completed by a physician or physician extender, with 0 indicating no disability and 10 indicating death caused by MS. Using a cane relates to an EDSS score of 6.0. The National MS Society (NMSS) Task Force on Clinical Outcomes Assessment also recommends the Multiple Sclerosis Functional Composite (MSFC)44 as a measure of disease severity and progression. This set of outcome measures is used to chart change in physical and cognitive function and will be discussed later in this chapter. It includes three tests that measure upper-extremity function (Nine-Hole Peg Test [NHPT]), lower-extremity function and mobility (25-Foot Timed Walk [25FTW]), and cognitive function (Paced Auditory Serial Addition Test [PASAT]).
TABLE 19-1 
ABBREVIATED EXPANDED DISABILITY STATUS SCALE
| SCORE | FUNCTION |
| 1.0 | Normal neurological examination findings |
| 2.0 | Minimal disability |
| 3.0 | Moderate disability |
| 4.0 | Ambulates 12 hours without aid |
| 5.0 | Disability impairs activity (walks 1500 feet without assistance) |
| 6.0 | Intermittent or unilateral constant assistance |
| 6.5 | Bilateral support required (walker, crutches, two canes) |
| 7.0 | Unable to walk 15 feet without assistance |
| 8.0 | Basically constrained to bed |
| 9. 0 | Bedridden |
| 10.0 | Death from multiple sclerosis |
Medical management of MS has two major goals: long-term management of the disease and exacerbations and symptomatic management. Early after diagnosis with CIS, it is recommended that people take DMAs. Recent evidence suggests that as the disease progresses it becomes less inflammatory and more neurodegenerative. Therefore medications aimed at reducing inflammation will be less effective as the disease progresses. Fox2 suggests that early treatment is needed to compensate for the later stages of the disease when inflammation is less prevalent.
Medications
Disease-modifying agents
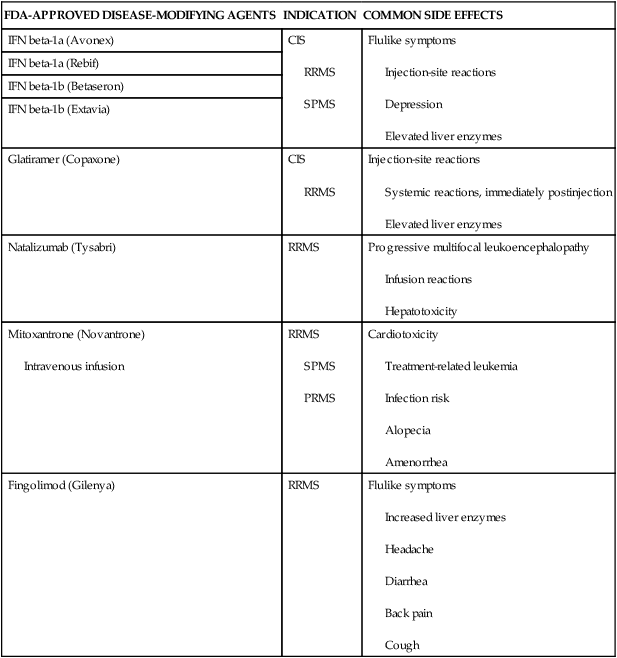
DMAs are aimed at reducing immune system dysfunction, thereby reducing damage to neural tissue and long-term disability for people with RRMS. There are several different medications that act on various components of the immune system with the intention of modifying the course of the disease (Table 19-2). In general, these drugs are approved for use with RRMS and are used off-label for other forms of MS and have been shown to reduce the number of attacks experienced. The majority of the drugs require injections; however, in 2010 the U.S. Food and Drug Administration (FDA) approved the first oral DMA, fingolimod. Measurement of therapeutic effectiveness includes relapse rate, progression of disability (EDSS), and quantitative evidence of lesions on MRI. All DMAs have side effects (see Table 19-2), but rarely are they serious. These medications are costly, and some people do not respond well or tolerate the side effects. It is common that people will try more than one type before finding the DMA they tolerate the best.
TABLE 19-2 
DISEASE-MODIFYING AGENTS: INDICATIONS AND SIDE EFFECTS
| FDA-APPROVED DISEASE-MODIFYING AGENTS | INDICATION | COMMON SIDE EFFECTS |
| IFN beta-1a (Avonex) | CIS
RRMS SPMS |
Flulike symptoms
Injection-site reactions Depression Elevated liver enzymes |
| IFN beta-1a (Rebif) | ||
| IFN beta-1b (Betaseron) | ||
| IFN beta-1b (Extavia) | ||
| Glatiramer (Copaxone) | CIS
RRMS |
Injection-site reactions
Systemic reactions, immediately postinjection Elevated liver enzymes |
| Natalizumab (Tysabri) | RRMS | Progressive multifocal leukoencephalopathy
Infusion reactions Hepatotoxicity |
| Mitoxantrone (Novantrone)
Intravenous infusion |
RRMS
SPMS PRMS |
Cardiotoxicity
Treatment-related leukemia Infection risk Alopecia Amenorrhea |
| Fingolimod (Gilenya) | RRMS | Flulike symptoms
Increased liver enzymes Headache Diarrhea Back pain Cough |
Antiinflammatory medications
High-dose corticosteroids (such as prednisone or methylprednisolone) are used to reduce inflammatory response during exacerbations for people with RRMS. Although no medications have demonstrated effectiveness in people with PPMS, anecdotal evidence suggests that intermittent pulses of intravenous methylprednisolone can help slow progression of clinical disability in some patients.2
A host of additional medications are used to manage the symptoms associated with MS. Each will be discussed as part of symptom management. Also refer to Chapter 36 for additional information.
Symptom management
Fatigue
Spasticity
Spasticity can interfere with physical function and hygiene. However, spasticity can also add support to weakened limbs, allowing more effective mobility. The goal of medical management of spasticity is to maintain full range of motion (ROM) of muscle and soft tissue structures to allow maximal physical function and proper hygiene. Haselkorn and colleagues18 describe the clinical practice guidelines for managing spasticity in people with MS written by the Multiple Sclerosis Council. A complete assessment of the spasticity and how it affects the individual’s life is required. Typically, successful management includes both pharmaceuticals and rehabilitation.
Management of focal spasticity may include local anesthetics such as lidocaine, bupivacaine, etidocaine, all of which are short acting with side effects of CNS and cardiovascular toxicity and hypersensitivity. Neurolysis treatment with phenol or alcohol is longer acting; however, these agents can have the side effects of pain, swelling, fibrosis, and dysesthesias. Focal spasticity affecting functional muscle groups can also be effectively treated with neuromuscular blocking agents including alcohol, phenol, or botulinum toxin. Botulinum toxin type A (Botox) has been shown to improve spasticity as measured by the Ashworth Scale and the hygiene score, but no changes were noted in spasm frequency score.45 Blocks last 1 to 3 months with relatively few side effects. Similarly, botulinum toxin type B was shown to reduce hip adductor spasticity.46 Clinical practice guidelines18 recommend that neuromuscular blocks be performed by appropriate specialists in conjunction with a rehabilitation program.
Refractory spasticity is defined as unsuccessful treatment with oral medications and/or rehabilitation. In this situation two other options exist: surgery or placement of an intrathecal baclofen pump (ITB). Surgical procedures include tendon lengthening or tendon transfer and are performed to maintain adequate hygiene or prevent or correct contractures and therefore preserve function. Intrathecal pumps, inserted into the spinal cord, allow adjustable drug delivery. Baclofen, the drug of choice for the intrathecal pump, can be given in higher doses; use of the pump avoids the side effects often encountered when the drug is taken orally. Relapses are more commonly reported in people on oral medications than those using ITB. People using ITB also report higher levels of satisfaction, less spasticity, and fewer painful spasms compared with those on oral medications.19
Pain
Both nociceptive and neuropathic pain can be present in people with MS. Therefore it is important to discern the type of pain in order for the most appropriate treatment to be rendered. Nociceptive pain can often be treated with analgesics (acetaminophen, nonsteroidal antiinflammatory drugs [NSAIDs], or opioids) and is more amenable to physical therapy (discussed later under rehabilitation management). Neuropathic pain generally requires pharmacological intervention, although an interdisciplinary team approach may be valuable. First-line medications for neuropathic pain that occurs in the spinal cord are calcium channel blockers (gabapentinoids) or N-methyl-d-aspartate (NMDA) antagonists (ketamine). When pain is present in the head, the primary treatment is opioid drugs such as antidepressants (tricyclics) or anticonvulsants (gabapentin or pregabalin).47 In the case of trigeminal neuralgia, the first choice is often carbamazepine. Refer to Chapter 32 on pain management for additional information.
Mobility
Physical rehabilitation is the primary intervention used to manage mobility dysfunctions. However, one medication has recently been FDA approved to improve gait. In clinical studies dalfampridine (Ampyra) demonstrated the ability to improve walking speed in people with MS.48 However, changes in the quality of gait or movement were not measured.
Tremor
Tremor management using medications such as isoniazid, carbamazepine, ondansetron, or cannabis extract has been minimally effective.49 Surgical interventions including stereotaxic thalamotomy and deep brain stimulation have been studied, but the evidence to support the effects on functional status and disability is lacking. The effectiveness of other options including physical therapy, tremor-reducing orthoses, and extremity cooling have yet to be proven beneficial in clinical trials.49
Bowel and bladder management
Behavioral modification and rehabilitation are used to help alleviate the symptoms of bladder incontinence or detrusor muscle overactivity. A few medications have been shown to be helpful: anticholinergic agents are used to manage detrusor overactivity or dyssynergia, and underactivity is treated with cholinomimetic agents.2
People with constipation are encouraged to combine adequate fluid intake with dietary fiber or bulk-forming medications.2
Depression and cognitive impairments
Depression is very common in people with MS, yet it is infrequently identified or treated.50 Therapy can include supportive psychotherapy and medication given individually or in combination. To date two pharmacological therapies have shown the most promise in reducing cognitive deficits (l-amphetamine sulfate and donepezil), and neither has serious adverse effects.51,52
Rehabilitation management
Overview
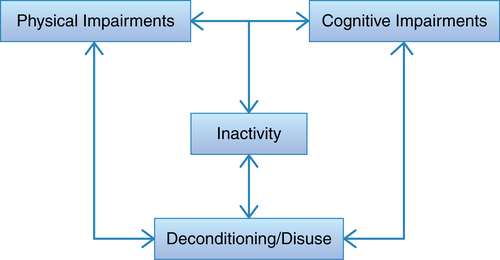
Chronic neurodegenerative conditions, such as MS, result in a loss of physical and cognitive function from the destruction of neurons and from a lack of activation of the affected systems. People with MS experience physical and cognitive impairments potentially leading to inactivity and resultant deconditioning (Figure 19-5). This often becomes a cycle that is difficult to break. One question that frames the rehabilitation strategy chosen is whether the focus should be compensation for or restoration of lost function. Compensation includes interventions such as wheelchairs or walkers to assist with mobility or braces for absent or inadequate muscle power. Restoration is aimed at increasing the capacity of the system—for example, maximizing cardiovascular endurance by increasing maximal oxygen uptake or restoring full ROM. Therefore, prescribing programs, activities, and exercises that provide an adequate stimulus to produce adaptation is critical to restore function or improve motor and cognitive performance. Although each patient case is unique, the most likely answer is that both strategies will be employed. The challenge for rehabilitation professionals is to sort out how much of a patient’s dysfunction arises from neurodegeneration, which necessitates compensation, and how much occurs from inactivity and system deconditioning, in which case system capacity can be restored to some extent. Rehabilitation professionals must choose therapeutic interventions based on whether compensation or restoration is the goal.
For rehabilitation professionals managing people with MS, the International Classification of Functioning, Disability and Health (ICF) model (refer to Chapter 1) provides an excellent framework for assessment and management regardless of the setting in which the patient or client is encountered.53 Although guided by the opening interview and chart review, the initial assessment must include how the individual with MS is functioning in home, at work, and in recreation environments and which impairments of bodily structure or function might be contributing to the identified activity limitations and participation restrictions. Rehabilitation professionals must consider how personal and environmental factors may impede or facilitate achievement of rehabilitation goals. Personal factors in people with MS may include whether the patient is heat intolerant, experiences MS-related fatigue, or has the confidence or motivation to perform certain tasks. Environmental factors that may be of particular importance for the patient with MS may be living in a hot climate or having access to cooling equipment such as air conditioning or cooling garments. It is critical to understand how the disease affects the lives of both individual patients and their caregivers. Outcome measures designed to test impairments, activity, and participation, along with assessments of environmental and personal factors, will help health care professionals understand the deficits of their patients and determine the best place to focus rehabilitation efforts and monitor the patient’s response to intervention.54
Because of the myriad CNS lesions and variable clinical presentations in people with MS, there is no one approach that is the gold standard for rehabilitation management. Whatever the approach, evidence is growing that rehabilitation is beneficial. Intensive inpatient therapy programs provide long-term improvement in a number of functional skills, participation, and QOL but may not change underlying impairments. Prospective studies have shown that intensive inpatient rehabilitation improves disability and QOL and that these benefits can be long lasting.55–58 High-intensity programs in the outpatient clinic or home environment offer evidence of short-term symptomatic changes that have translated into improved participation and QOL.56,59
Assessment
The initial interview must include a quick screen or questioning about the body systems and areas that are commonly impaired in people with MS and the problems commonly encountered: motor strength, coordination, spasticity, sensory disruption (vestibular, visual, and somatosensory), bladder control, depression, and cognition. If impairments are present, there is a strong likelihood of negative impact on the patient’s ability to perform ADLs or participate in activities related to work, home, and leisure. All patients with MS must be asked if they have fallen in the last 6 months because of the high rate of falling in people with MS.23–25,26 Results of the interview and chart review will help develop hypotheses about which potential impairments might be contributing to the patient’s or client’s physical or cognitive dysfunctions. Therefore the examination needs to be designed to observe the problematic tasks and test the hypotheses developed.
During the assessment, examiners must determine if the problems identified by the patient (or those found by the assessor) fit within their scope of practice or whether the patient requires a referral to an appropriate health care professional. A good example is identifying people with depression using a quick two-question screen. According to Mohr and colleagues,60 these questions are 98.5% sensitive for identifying major depressive disorder. The two questions are (1) “During the past 2 weeks, have you often been bothered by feeling down, depressed, or hopeless?” and (2) “During the past two weeks, have you often been bothered by having little interest or pleasure in doing things?”61 An answer of yes to either question should trigger a referral to the patient’s primary care provider for follow-up.
Comprehensive lists of standardized tests and measures for impairment, activity limitations, and participation restrictions or QOL are provided in Chapter 8. The next section of this chapter will primarily focus on the tests and measures found to be valid and reliable in the examination of individuals with MS.
Assessing body system problems contributing to activity limitations
Spasticity.
Spasticity can be measured using resistance to passive ROM and Ashworth62 and Modified Ashworth Scales.63 However, because these scales measure spasticity at rest, they may not reflect the degree to which spasticity may be interfering with function. Careful observation of the patient’s movements may also inform the clinician about how spasticity is affecting the patient’s ability to move.
Ataxia and incoordination.
Few standardized tests have been developed to specifically measure ataxia. One recent test is the Scale for the Assessment and Rating of Ataxia (SARA). Although this test has yet to be validated in people with MS, it has good reliability and validity in patients with cerebellar dysfunction, a common problem in people with MS.64,65
Tests of nonequilibrium coordination are designed to measure the presence of dysmetria or dysdiadochokinesia, both of which occur in patients with MS. However, these tests (including finger to nose, heel to shin) are somewhat subjective and are therefore difficult to use to demonstrate improvement after an intervention. However, using a stopwatch during these tests can be an important tool to record objective data. Count the number of repetitions of a given activity performed in a set amount of time (e.g., how many alternating forearm supinations and pronations can be performed in 30 seconds), or record the time it takes to complete a set number of repetitions of a given activity (e.g., how long it takes to complete five alternating supination-pronation movements). Refer to Chapter 21 for additional assessment tools.
Vestibular dysfunction.
The vestibular system is affected by MS both centrally (lesions in the vestibular nuclei or cerebellum) and at the entry site of cranial nerve VIII.66 However, benign paroxysmal positional vertigo (BPPV) can also occur.67 The techniques used to assess and treat the effects of vestibular disorder in an individual with MS are the same as those discussed in Chapter 22. When vestibular symptoms are present, Williams and colleagues68 suggest evaluation using computerized platform posturography (CPP) in people with MS with minimal to mild disability. It is important to keep in mind that the patient with MS will often have additional problems that might require modification of the vestibular intervention—for example, heat intolerance or additional visual or somatosensory deficits.
Fatigue.
Identifying if and when fatigue occurs in individuals with MS is important to assessment and the structuring of intervention. Questions should address the type of fatigue, whether mental or physical; when during the day it occurs; whether it is related to physical or mental exertion; and what the person with MS does, if anything, to relieve it. In addition, fatigue-related self-report scales can help the rehabilitation professional gain an understanding of the perceived impact that fatigue may be having on a patient with MS. Two of the commonly used scales are the Modified Fatigue Impact Scale69 and the Fatigue Severity Scale.70 These measures may also aid the therapist in determining if the intervention had any impact on the patient’s perceived level of fatigue.
Cognition.
The PASAT,71 recommended by an expert panel of the National MS Society, is a test for cognitive impairments in people with MS. A more recent test, the Audio Recorded Cognitive Screen (ARCS),72,73 appears to be a more comprehensive cognitive assessment developed for people with dementia but the psychometric properties have not yet been determined in people with MS. However, Lechner-Scott and co-workers74 found that compared with the PASAT, the ARCS was similar in detecting impairments of cognition and more sensitive at identifying problems with memory or executive impairments.
Assessing activity performance and participation
Balance.
Balance is foundational to upright movement and is produced by a complex interaction among sensory inputs, central processing, and motor responses. It can be discussed under both body structure and function or activity. In either case balance dysfunction has been identified in people with MS with minimal as well as more advanced disability.75–79 Cameron and Lord80 report the three most common problems with balance to be delayed response to postural perturbations, increased body sway while standing quietly, and an inability to move outside the base of support.
Whereas some balance tests focus on stationary or static tasks that allow observation of body sway in standing, including single-leg stance test, Romberg test with eyes open or eyes closed, tandem stance, and CPP, others add movement and challenge dynamic balance (Functional Reach Test,81 Tinetti Performance-Oriented Mobility Assessment [POMA],82 and Berg Balance Scale [BBS]83). Other tests challenge anticipatory balance (reactions to perturbations related to self-generated movement) or reactive balance (perturbation tests, CPP). Frzovic and co-workers84 found that single-leg stance, tandem stance, response to external perturbations, and the Functional Reach Test were able to distinguish people with MS from healthy controls.
Several authors have studied measures of balance in people with MS. Cattaneo and colleagues85 determined that four tests measuring balance during standing and gait and self-perception of balance had good intrarater and interrater reliability. The two tests measuring balance during standing and movement were the BBS and the Dynamic Gait Index (DGI).
CPP provides an objective assessment of sensory contributions to balance dysfunction in people with MS.86 In particular, the Sensory Organization Test is useful in identifying the relative sensory contributions (visual, vestibular, and proprioceptive) to stationary balance and response to perturbation. Understanding the sensory conditions under which the patient loses balance and falls assists the therapist in providing exercises that will challenge those conditions in a safe and controlled manner. For example, the patient who relies heavily on visual input to maintain balance (conditions with eyes closed in the Sensory Organization Test) would be provided exercises and activities that challenge the vestibular and proprioceptive systems, such as standing on foam while the eyes are closed.
Developed by Horak and colleagues,87 the Balance Evaluation Systems Test (BESTest) is an instrument examining complex balance disorders that includes the six domains that underlie orientation and postural stability: biomechanical constraints, stability limits and verticality, transitions and anticipatory postural reactions, reactive postural responses, sensory orientation, and stability in gait. Both interrater reliability in people with parkinsonism and content validity are good, but testing in other populations has not yet been completed. There is an abbreviated version of the BESTest, the mini-BESTest,88 that covers four of the six systems, focusing on dynamic balance. These promising tests may offer the clinician a better way of identifying which components of orientation and postural control are dysfunctional, which may allow more targeted interventions.
The Activities-specific Balance Scale (ABC)89 is a questionnaire that rates people’s self-perception of how confident they are to perform activities that challenge their balance. The Dizziness Handicap Inventory (DHI)90 assesses three domains of disability related to dizziness: physical, emotional, and functional. The sum score or each subscale score can be reported. Higher scores mean greater levels of handicap and disability. Cattaneo and colleagues91 found that both the ABC and DHI tools discriminated between fallers and nonfallers and were therefore good predictors of fall status in people with MS. Refer to Chapter 22 for additional information on balance.
Gait.
Gait can be measured in myriad ways depending on the goal of the assessment. Speed, distance, and quality may all be important to the patient and therapist. Observational gait analysis is the gold standard for clinical measurement of gait quality. Although motion-analysis laboratories are able to provide detailed kinetic and kinematic assessment of joint angles and gait cycle, it is costly and typically not available in most clinical settings. Instrumented mats such as the GaitRit can provide clinicians with temporal and spatial gait parameters such as step length, step width, cadence, and single-leg support and double-leg support times. Although this is less costly than motion analysis, it may still be out of reach for many clinics. Gait speed and velocity can also be measured by having the patient walk a given distance while being timed. These walks can occur at a self-selected pace or as fast as the person can walk safely. Several short-distance timed tests exist, the 25FTW and the timed 10-meter gait test,92 both of which have been shown to have good reliability and sensitivity to change.93,94 The 6-minute walk test (6MWT) measures walking endurance and is recommended by the NMSS Task Force on Clinical Outcome Measures as a measure of walking ability that is sensitive to change. Gijbels and co-workers95 report that the 6MWT was better at predicting habitual walking in people with mild to moderate MS than the 25FTW. However, the 25FTW may be more sensitive to change when compared with the EDSS.96 The 6MWT distance was reduced in people with MS compared with healthy controls and was inversely related to disability.97
Two additional performance-based tests, the DGI and the Timed Up-and-Go Test (TUG), combine walking with other functional tasks. The DGI measures the ability of an individual to walk while adding various challenges such as slowing down or speeding up, head turning, stepping over or around obstacles, and stair climbing. It was developed to assess gait dysfunction associated with peripheral vestibular disease.98 McConvey and Bennett99 found the DGI to be a reliable and valid tool for use in people with MS. The TUG test combines walking with transfers and turning. It is frequently used in both clinical and research settings and has been shown to be reliable in measuring function in people with MS.93
The Multiple Sclerosis Walking Scale–12 (MSWS-12) is a 12-item patient-rated questionnaire that measures the perception of the impact of MS on walking ability. This scale has good reliability and validity and may be very useful to document patient perceived change in walking ability before and after intervention.100,101
Upper-extremity tests of function.
Movement impairments of the upper extremities can result in decreased ability to perform ADLs and other functional activities. Standardized tests such as the Box and Block Test (BBT)102 or the NHPT103 provide objective data about unilateral manual dexterity or the ability to manipulate objects. Both tests are inexpensive but do require some equipment and a stopwatch. The NHPT is part of the MSFC and therefore has been used extensively in evaluating people with MS.
Composite tests.
An expert panel of the NMSS recommended the use of the MSFC,44,104 including the 25FTW, the NHPT, and the PASAT. The MSFC has been tested against lesion load as measured via MRI, EDSS scores, and QOL measures, showing that it has good validity and reliability and is sensitive to change.104–106 Each component scale of the MSFC can also be used independently to monitor physical and cognitive function as written previously.
Assessing quality of life
QOL measures are patient-report tools that evaluate the value a person places on his or her abilities and limitations and how these affect the individual’s social, emotional, and physical well-being. Many of these tools include questions that address an individual’s perception of how well he or she is able to fulfill life roles and how the disease affects this participation. In a meta-analysis of exercise training on QOL in people with MS, Motl and Gosney107 found that disease-specific measures of QOL detected larger changes than generic QOL measures. Several measures have been commonly used to evaluate people with MS: the Multiple Sclerosis Quality of Life–54 (MSQOL-54)113 and the Multiple Sclerosis Quality of Life Inventory (MSQLI).114 The multidimensional MSQOL-54 was based on the Health Status Questionnaire (SF-36), with 18 additional items specific to MS covering fatigue, and cognitive and sexual functioning. There are 12 subscales that cover physical function, role limitations—physical, role limitations—emotional, pain, emotional well-being, energy, health perceptions, social function, cognitive function, health distress, overall QOL and function, and change in health. The measure takes about 15 minutes to complete and requires 15 to 20 minutes to score. Reliability is good to excellent in people with MS.113
The MSQLI was developed by the Consortium of Multiple Sclerosis Centers Health Research Subcommittee in 1997. It is composed of 10 components covering issues important in MS. It includes the Health Status Questionnaire, Modified Fatigue Impact Scale, MOS Pain Effects Scale, Sexual Satisfaction Survey, Bladder Control Scale, Bowel Control Scale, Impact of Visual Impairment Scale, Perceived Deficits Questionnaire, Mental Health Inventory, and MOS Modified Social Support Survey. It takes about 45 minutes to administer the complete set of questionnaires and does not provide a sum score for all tests. There is good test-retest reliability for the MSQLI even in people with MS and cognitive dysfunction.108 A shortened version of the tool exists, but the psychometric properties have not been thoroughly tested.
Disease severity measures
Disease severity is a measure of disablement. Interventions that change function (e.g., improve walking distances or decrease reliance on assistive devices to move) can reduce disability. There is also compelling evidence that exercise may actually modify disease progression in people with MS. Therefore disease progression may be used to assess the impact of an intervention on the patient’s perceived level of disability. Although the EDSS43 is the gold standard for assessing disease severity, it requires a trained primary care provider to administer. Disease Steps111,112 and Guy’s Neurological Disability Scale (GNDS)109 are two additional disability scales that have demonstrated good correlation with the EDSS. Whereas Disease Steps must be administered by a professional, GNDS can be given to patients to complete on their own.110
Interventions
The National Clinical Advisory Board of the National MS Society recommends that rehabilitation occur whenever there is a sudden or gradual decline in function or an increase in impairment that has a negative impact on an individual’s safety, independence, mobility, or QOL. In addition, it is recommended that rehabilitation be a part of a comprehensive health care plan at all stages of the disease.115
Regardless of the type of intervention chosen, evidence is growing that increased activity, whether cognitive or physical, may have a neuroprotective effect on the brains of people with neurological insults. In fact, Golzari and colleagues116 demonstrated that an 8-week, 24-session, combined exercise program improved muscle strength and balance and reduced disability in people with MS. In this study, levels of proinflammatory immune system mediators were measured before and after the intervention. The authors demonstrated that this dosage of exercise reduced markers of inflammation in the blood. This is one of the first studies in people with MS showing that inflammation and therefore the disease process may be altered by the application of an exercise intervention, suggesting a role for rehabilitation in neuroprotection and not simply symptom management. This also implies that rehabilitation, specifically exercise, should occur early in the course of the disease and not only after clinical disability has occurred. However, the exact dosage, intensity, or type of exercise required to produce activity-dependent neuroplasticity is not yet known. At least one study in an animal model of MS, experimental allergic encephalomyelitis, has shown the beneficial effects of exercise.117
Rehabilitation can occur in a variety of locations: inpatient, outpatient, home, and the community. Figure 19-6 shows a physical therapy–led community-based exercise program for people with MS in which group activities addressing strength, balance, and endurance are modified for each individual. In addition, a number of health providers can be members of the rehabilitation team, including nurses, occupational therapists, physical therapists, speech-language pathologists, psychologists, neuropsychologists, and physicians.
Exercise
Historically, exercise was thought to worsen disability and bring on exacerbations. Medical advice warned patients that overexertion could hasten relapse and progression. There now exists clear evidence that this is not the case. Regular, appropriate exercise has been shown to increase strength, aerobic capacity, overall function, and QOL. In 1996 Petajan and colleagues published a seminal study in which a 60% V˙o2max aerobic ergometer exercise program was well tolerated in people with MS and did not provoke remission.118 After 10 weeks, participants had improvements in V˙o2max, work capacity, isometric strength, and blood lipids and reduced depression, anger, and fatigue. In a 2009 systematic review of the literature,119 exercise was shown to be an effective intervention for people with MS to improve muscle strength, endurance, mobility-related actions, and to a lesser extent mood compared with control conditions. This evidence did not suggest the superiority of one particular type of exercise program over others. It is very important to note that adverse effects were rarely seen in any of the exercise studies, and when they did occur they did not last for longer than 24 hours, indicating that exercise is safe for people with MS.
In a review of the exercise literature, White and Dressendorfer120 recommend that endurance exercise programs for people with MS with mild to moderate disability use the following guideline: perform regularly, two or three sessions per week, at an intensity of 65% to 75% heart rate maximum, and last 20 to 30 minutes per session. Resistance exercise should include 15 to 18 repetitions for one to three sets initially with a goal of increasing to three to four sets. Training should last at least 12 weeks.121 Owing to heat intolerance, exercise should incorporate intermittent rest periods that allow heat to dissipate.120 Heesen and colleagues122 developed a guideline for exercise prescription for people with MS for all levels of disability (Table 19-3).
TABLE 19-3 
GENERAL EXERCISE GUIDELINES FOR LEVELS OF DISABILITY
| LEVEL OF DISABILITY | EDSS LEVEL | TRAINING PROGRAM |
| None: no fatigue or thermosensitivity | 0 | Full exertion, aerobic and resistance exercise, no extreme sports |
| Minimal: limited fatigue and heat sensitivity; minor balance or gait problems | 1-2 | Monitored exercise program including strengthening and endurance using a variety of exercise types, precooling if heat-sensitive, avoid overtraining |
| Moderate: limited gait; may have spasticity, weakness, ataxia, balance problems | 3-5 | Deficit-driven exercise protocols including strengthening and endurance training using methods tolerated, walking, cycle ergometry, precooling if needed |
| Severe: cannot participate in all daily activities; short-distance, aided walking only | 6-7 | Movement preservation, stretching, targeted strengthening needed for task-specific training |
| Bedridden | 8-9 | Primarily passive movements to maintain motion, breathing exercises |
EDSS, Expanded Disease Severity Scale.
Modified from Heesen C, Romberg A, Gold S, Schulz KH: Physical exercise in multiple sclerosis: supportive care of a putative disease-modifying treatment. Expert Rev Neurother 6:347–355, 2006.
Evidence-based interventions for specific problems
Fatigue
One study found that the cooling suit was shown to improve all dimensions of fatigue on the Fatigue Impairment Scale (physical, cognitive, and psychosocial) in a small multiple-case study.123 Although recommended in the clinical practice guidelines on fatigue and MS by expert opinion and anecdotal reports of people with MS, little additional evidence exists to support cooling as a therapeutic intervention. Two additional studies have shown that cooling garments can reduce symptoms of fatigue and improve ambulatory ability.124,125
Exercise shows promise as an intervention that can improve fatigue for people with MS that may improve muscle weakness caused by disuse and deconditioning. However, no one type of exercise, resistance or aerobic, or program has been proven most effective. One program included a 5-day-per-week, 30-minute bicycle aerobic training program for 4 weeks that improved fitness and showed a tendency for reduced fatigue. This study had an age, sex, and activity level control group.126 Di Fabio58 showed that a prolonged outpatient rehabilitation program in patients with progressive MS led to a decrease in MS-related symptoms, including fatigue. However, there was no control group. A randomized study comparing bicycle training with yoga found that fatigue improved in both groups, with neither group shown to be better than the other.127
Energy conservation is defined by the fatigue and MS guidelines of the Multiple Sclerosis Council for Clinical Practice Guidelines128 as energy effectiveness and includes an analysis of individuals’ home, work, and leisure activities and the environments in which they occur in order to develop activity modifications designed to reduce fatigue. This can include a variety of strategies such as reducing energy expenditure through activity and modification, workspace organization and improving efficiency of movements; balancing work and rest periods; delegating tasks; evaluating standards and prioritizing activities; and using assistive technologies that conserve energy usage.129,130 In a randomized controlled trial, a 6-week community-based energy conservation class using the strategies listed previously was compared with a wait-list control group. Immediate postcourse improvements in fatigue were noted129 and were present after a 1-year follow-up period.131
The multidimensional fatigue management class “Fatigue: Take Control” was developed based on the recommendations of the Fatigue Management Guidelines of the NMSS from 1998.132 The content of the 6-week class includes many of the aspects of fatigue management education and training that were described previously. The pilot study found that participants had less fatigue compared with a wait-list control group.132 These classes are often offered by local chapters of the NMSS.
Patients may need to be prescribed assistive devices for ADLs. People with MS who have spasticity have a greater cost of walking.133 Using wheeled mobility for longer-distance outings (to the shopping mall, an extended event, on vacation) can conserve energy and extend the time a person can participate in activities of importance to him or her. However, therapists should be aware that using assistive devices such as walkers or crutches actually increases energy expenditure for elderly people,134 and therefore the need for improved support must be balanced with the increased energy burden an assistive device might add.
Spasticity
Several rehabilitation strategies to manage spasticity are available, including ROM, stretching, light pressure or stroking,135 cold therapy, electrical stimulation, and education. Although none of these interventions is supported by strong research evidence, many are used routinely in clinical practice (ROM, stretching). Other approaches (cold therapy, light pressure or stroking) are recommended for use in conjunction with stretching or ROM programs. Regardless of the technique employed, educating individuals and caregivers about the importance of adhering to a spasticity management program is essential. The Multiple Sclerosis Council for Clinical Practice Guidelines18 recommends, based on expert opinion, stretching a muscle with spasticity for 60 seconds or longer or using a prolonged stretch, lasting hours, with braces or splints.
Cold can be applied in a number of ways: baths, towels, or cooling garments. There are multiple quasi-experimental research studies that suggest an improvement in spasticity for a brief period after cooling18; however, the number of subjects and study methods make these results equivocal. Nilsagård and co-workers136 found subjective reports of improved spasticity after a single session of cooling, although no statistically significant differences in spasticity measures were found.
Balance and postural control
Balance is foundational to the ability to stay upright and perform dynamic movements. It is a frequent problem in people with MS and results in a person limiting his or her participation in home, work, and leisure activities. Abnormalities of balance along with cane use and poor performance on tests of balance and ambulation can increase the risk of falling.26 Other fall risk factors that have been identified include fear of falling, male sex, poor concentration or forgetfulness, and urinary incontinence.25 Rehabilitation programs must be based on a thorough understanding of the impairments and personal and environmental factors that may be contributing to the balance dysfunction. Cattaneo and co-workers137 compared the effects of three balance interventions on falling and other measures of balance. Three rehabilitation groups were included: one in which motor and sensory strategies were targeted, the second focusing on motor strategies alone, and the third group not receiving balance-specific training. The greatest reduction in falls and improvement on the BBS were associated with group one, and the least with group three. Hayes138 compared 12 weeks of standard physical therapy with high-intensity resistance exercise (60% to 80% maximal contraction) added to standard therapy and found that standard therapy produced better balance outcomes. In addition, strength and the ability to ascend and descend stairs were all better in the standard therapy group. Importantly, people with MS tolerated the high intensity resistance exercise without problems. One pilot study found that a 12-week, biweekly aerobic exercise program did not improve balance as measured by the Functional Reach Test but did result in an improvement in walking distance.139 For additional intervention strategies on balance, refer to Chapter 22.
Mobility
People with MS rate gait as one of the most important bodily functions122; gait is often adversely affected in people with MS. Gait disturbances have been observed in people with MS even before disability is measured on the EDSS scores.140 Lesions in the brain and spinal cord produce a wide variety of potential impairments that can adversely affect gait. In a review article by Kelleher and colleagues,141 imbalance, fatigue, spasticity, incoordination, muscle weakness, and sensory system impairments were all reported to negatively affect ambulation ability. Therefore addressing each of these impairments has the potential to improve gait. A recent literature review of therapeutic interventions for mobility problems suggests that a variety of different methods can be used to improve ambulation.142 Snook and Motl143 performed a meta-analysis of exercise studies aimed at improving walking mobility in people with MS and found that greater effects were associated with supervised exercise training, programs of less than 3 months’ duration, in mixed samples of people with RRMS and progressive MS.
Task-specific gait training has been evaluated in people with MS. A randomized controlled trial compared two different treatment groups—facilitation and task-specific training—that each received 15 to 19 1-hour treatment sessions over 5 to 7 weeks and found that both improved 10-m gait speed, stride length, and balance; however, there was no control group.144 Treadmill training has been investigated in several small, pilot or case studies with promising results of improved QOL, energy expenditure, and gait parameters.145–147
Several exercise studies have an association with improved gait. A combined resistance and aerobic home program lasting 23 weeks improved gait speed for short and longer distances in exercise compared with a control group.148 Rampello and co-workers149 compared a neurorehabilitation program with an aerobic training program of similar duration (three times per week for 8 weeks). The authors found that aerobic training improved walking distances and speeds and measures of aerobic capacity over the neurorehabilitation group. Both groups had QOL improvements in emotional well-being and health distress; the neurorehabilitation group demonstrated improved mental health.
An additional technique that shows promise for improving mobility in people with MS is an evaluation and intervention approach that uses small amounts of weight placed on the torso in response to identified balance dysfunction. Balance-Based Torso-Weighting (BBTW) is an intervention that uses directional loss of balance in both static and dynamic assessment to determine where small amounts of weight (generally less than 1% to 1.5% of body weight) are placed in a treatment orthotic called BalanceWear. The BalanceWear orthotic can be worn during the performance of activities in therapy or daily for home, work, or leisure activities. A recent randomized controlled trial in people with MS who reported gait abnormalities showed that when wearing the weighted BalanceWear orthotic participants increased their gait speed compared with no weight controls, and improved TUG scores compared with a standard weighted control.150
When people with MS do not respond to therapeutic interventions to restore function, mobility assistive devices such as canes, crutches, walkers, wheelchairs, and scooters are used to enhance mobility through compensation. Mobility-assisted technology (MAT) can improve function in people with moderate to severe impairments of ambulation and may reduce activity limitations and participation restrictions by reducing fatigue and enhancing energy conservation to allow greater involvement in work, family, social, vocational, and leisure activities. Other MAT technologies include functional electrical stimulation (FES), neuroprostheses, and orthotics. FES is applied to specific muscles or muscle groups to activate weak muscles. Some of these stimulators can be built into a neuroprosthesis that can be set up for use during exercising or walking.151 Orthotics such as the ankle-foot orthosis (AFO) or hip flexion assist orthosis (HFAO)152 can compensate for muscle weakness in the lower extremity, improve foot and knee positioning, and reduce energy expenditure. Therapists often work cooperatively with orthotists to ensure proper fit. Use of wheeled mobility devices such as a manual wheelchair, power wheelchair, or scooter requires a formal evaluation by an occupational or physical therapist with justification that it is required for mobility at home at least on a part-time basis. Therapists must take a long-term view of the projected needs of the patient when prescribing wheeled mobility, as most insurance companies will replace this equipment only every 5 years.
Pain and dysesthesias
The occurrence of pain in people with MS is often underestimated. Pain can be acute, as in optic neuritis or Lhermitte syndrome, or chronic, as in dysesthesias in the limbs or joints or mechanical pain related to abnormal positions or repeated movements that cause abnormal wear and tear on the musculoskeletal system. Occupational and physical therapists can address poor body mechanics and weakness and poor movement patterns with retraining, and soft collars may help reduce Lhermitte syndrome. However, little evidence supports these interventions.153 Transcutaneous electrical nerve stimulation has been suggested anecdotally by Kassirer154 as beneficial for reducing pain. Cognitive-behavioral therapy has been researched for managing chronic pain155; however, little evidence exists for using it in people with MS.
Bladder dysfunction
Urinary incontinence and retention are common and often embarrassing problems for people with MS. Patients may be advised to avoid bladder irritants including caffeine, alcohol, concentrated urine, and infection. Physical therapists may work with patients to assess the factors contributing to bladder dysfunction by retraining hyperactive or weak pelvic floor muscles using biofeedback techniques and exercise. Nurses may need to teach patients with urinary retention intermittent catheterization. Refer to Chapter 29 for additional information on pelvic floor dysfunction and its treatment.
Cognition
There is growing evidence to support psychological interventions for people with mild to severe MS-related cognitive deficits, aimed at alleviating depressive symptoms and helping people cope with and adjust to their impairments.156,157 However, the evidence is not yet convincing for specific programs addressing attention and executive functioning. O’Brien156 was able to recommend the use of a modified story technique to address learning and memory deficits in people with MS. In a systematic review Maitra158 found that cognitive behavioral therapy programs performed by occupational therapists were positively correlated with improvement in Functional Independence Measure (FIM) scores. Refer to Chapter 27 for additional information regarding interventions with individuals with cognitive problems.
Dysphagia and dysarthria
Dysphagia or difficulty with chewing and swallowing becomes more prevalent in people with MS as the disease progresses.159 Therapists facilitate proper swallowing with exercises that will improve posture to prevent aspiration and strengthen muscles of mastication. Other interventions may include diet modifications and education for the patient and his or her family or caregivers. Dieticians may be consulted to facilitate proper food choices.
Dysarthria from the disruption of muscular control in the central and peripheral speech mechanisms leads to abnormalities of speed, range, timing, strength, sound, and accuracy of speech movements. Speech-language pathologists determine therapy programs that take into consideration the stage of the disease and speech quality. Typical programs may include exaggerating articulation, increasing voice volume, and increasing strength of oral musculature. Exercise programs designed to increase respiratory muscle strength have not been successful in improving voice quality or production.160