Chapter 26 Multiple sclerosis
With contribution from Dr Lily Tomas
Introduction
Multiple sclerosis (MS) is the most common progressive, neurological disorder in most Western countries today.1 It is an auto-immune, inflammatory, demyelinating condition of the central nervous system (CNS) that has no known cure.2 Despite the incredible progress that has been made with modern medicine, the treatment of MS with pharmaceuticals is certainly not ideal. Presumably for this reason, many people with MS worldwide are actively involved in self-care, using complementary and alternative medicine (CAM) therapies to help with symptom management.3, 4
A recent review of the literature demonstrates that the primary reasons for choosing CAM include the desire to use an holistic health care approach, conventional treatment not being effective, anecdotal reports of CAM efficacy and doctor referral. It is interesting to note that many people also choose CAM because they were unsatisfied with their initial consultation with their physician lasting less than 15 minutes.5, 6 The major symptoms being treated with CAM include pain, fatigue and stress, with the major therapies being dietary modification, nutritional and herbal supplementation, exercise, cold baths and mind–body therapies.4, 7, 8
Use of CAM therapies has been associated with religiosity, functional independence, female sex, white-collar jobs and higher education. Compared with conventional treatments, CAM therapies also rarely have unwanted side-effects (9% vs 59%).5
MS is a complex disease, perhaps encompassing more than a single aetiopathological entity and very likely subject to multi-factorial aetiology9 with ample evidence revealing an intricate interplay from genetics and differing environmental factors. Current research strongly indicates that previous infection with Epstein-Barr virus, vitamin D deficiency and smoking are major risk factors for developing this most debilitating and unpredictable disorder.10–14 Furthermore, it has been shown that good nutrition, sunlight exposure, exercise, stress and social factors can all influence the rate of progression and the level of disability.1
MS has always been considered to be more common in women and in areas further from the equator. However, recent reports indicate that the female:male ratio has increased and the latitude gradient decreased in the last 5 decades.15
Lifestyle — general
It is well documented that lifestyle approaches may significantly influence the development and progression of MS, and hence offer potentially effective avenues for therapy.1
Patients with MS have frequent adverse health behaviours, such as lack of outdoor exercise, smoking and drinking alcohol, which increase the risk of other chronic diseases. Further research is required to determine the extent of such behaviours on the progression of MS.18
Mind–body medicine
As we are all aware, stress can exacerbate many pathophysiogical disease processes, including those of neurodenerative origin.19
Corticotropin-releasing hormone (CRH) plays a central role in the regulation of the hypothalamic–pituitary–adrenal (HPA) axis; that is, the common final pathway in the stress response.20 Patients with MS have also been demonstrated to have HPA axis hyperactivity, therefore stress-relieving activities are considered to be particularly beneficial in this population.21
The discipline of psychoneuroimmunology has demonstrated that the immune system most certainly interacts with the CNS.22
A recent RCT has shown that telephone counselling for those with MS significantly improves physical activity, spiritual growth, stress management, fatigue and general mental health status compared with controls. There was also greater improvement in walking speed in those who chose to exercise more.23
As previously stated, fatigue is a frequent and disabling symptom in those with MS. Underlying psychological processes may certainly be part of this aetiology.24 It has been shown that a challenging mental task can alter the pattern and increase the cerebral activation on an unrelated motor task in fatigued MS patients.25, 26
Cognitive behaviour therapy (CBT) rehabilitation in MS is in its relative infancy.27 A recent Cochrane review has indicated that there is adversity of psychological interventions that can potentially help those with MS. CBT approaches, in particular, are beneficial in the treatment of depression, and in helping people adjust to, and cope with, having MS.28 A recent Australian longitudinal assessment study of anxiety, depression and fatigue in MS has confirmed this finding.29
Sleep
Fatigue is one of the most common symptoms of MS. The mechanisms underlying such fatigue are still poorly understood and are obviously multifactorial. Eliminating sleep disorders (as well as adverse drug effects, infections, iron/vitamin B12 deficiency) is an important part of an MS work-up.30, 31, 32 The prevalence of sleep problems in MS is significantly higher than in the general population and mostly affects women with MS more than men.33
Restless legs syndrome (RLS) is also significantly associated with MS, especially in patients with severe pyramidal and sensory disability. RLS is known to have a significant impact on sleep quality in patients with MS, and as such, a thorough history regarding this should always be taken.34 Patients with RLS present with greater disability and greater levels of fatigue.35 Anecdotal evidence strongly suggests that magnesium can assist with RLS. Restoring sleep patterns may help reduce symptoms of MS.
Sunshine
Sunlight exposure has emerged as being the most likely candidate for the explanations in geographical variations in MS prevalence; that is, increased risk of MS in populations residing at higher or lower latitudes.36 This observation implies a protective effect of sunlight, which is reduced at higher or lower latitudes, contributing to insufficient levels of vitamin D that are frequently found in those with MS.37
Monthly variations in MS relapses (with more relapses occurring in colder months) have been recently documented in Australia. Relapse rates were therefore inversely associated with UV radiation and serum 25(OH)D levels and positively associated with upper respiratory tract infections.38
Australian research has also demonstrated that those individuals with fair skin/red hair have an increased risk of MS that is more evident in women. As a child, they tended to behaviourally avoid sun exposure. In contrast, increased sun exposure from ages 6–10 is associated with reduced MS risk in those individuals without red hair. It should be noted, however, that the interplay between melanocortin 1 receptor variants, red hair/fair skin phenotype, past sun exposure and MS is complex.39
Earlier studies have also shown that increased outdoor activities during summer in early life even north of the Arctic circle are associated with a reduced risk of developing MS; most pronounced age range being 16–20 years.40, 41
Adverse reports of high ambient temperatures in people with MS correlate significantly with the report of strong sunlight, apparently making MS worse. This appears to be worse with increasing age. In Australia, high temperatures are more likely to be reported as adverse in warmer, lower latitude regions. This apparent adverse factor therefore appears to be related to solar heat, not solar light.8
Environmental influences
Geographical patterns in Australia currently imply that modifiable environmental factors hold the key to preventing approximately 80% of MS cases. Genetic epidemiology demonstrates that family history has an important role. However, if these factors are held constant, the environment sets the disease threshold.42
Recent studies have shown that men develop MS at lower levels of environmental exposure than women. Women, however, appear to be more responsive to the recent changes in environmental exposure that have seen a change in the sex ratio prevalence of MS in recent decades.43
Epstein-Barr virus (EBV)
Data from studies of twins and migrants with MS certainly imply environmental factors in the development of MS.44 There is a wealth of evidence linking EBV with MS, with the viral infection having potential roles in both initiating the auto-immune process and exacerbating disease progression.12, 43, 45–48 Furthermore, there is evidence to suggest that there is infection with EBV in brain lesions of MS patients.49 There is nearly a 100% sero-prevalence to EBV antibodies in MS years before clinical onset of the disease.44, 49 It appears that individuals with EBV Nuclear antigen 1 (EBNA-1) have a 2.8 times increased risk of developing MS, independently from DR15 allele.49 Carriers of this allele who also have elevated anti-EBNA-1 titres may have a significantly increased risk of MS.50 Amongst those who develop MS, antibody titres were 2–3 times higher in those individual who were 25 years of age or higher, suggesting an age-dependent relationship between EBV and MS.51 The risk is also increased after an initial symptomatic EBV infection.44
There is also recent evidence to suggest that Chlamydia pneumonia, which belongs to the rickettsial family of micro-organisms, is also linked to MS.52
Smoking
As stated previously, there is a multitude of studies demonstrating an association between smoking and MS.53, 54 Those who begin smoking at an early age are significantly more likely to develop progressive MS at an early age compared with later debut smokers or non-smokers.55
Furthermore, modestly elevated cotinine levels suggestive of passive smoking are also associated with an increased risk for MS.56, 57
Smoking during the cohort period was not associated with relapse.58
Thus far, smoking during pregnancy has not been shown to be a risk for early-onset MS amongst offspring.59
Heavy metal exposure
There is an observable difference in MS prevalence in particular regions with perceived clusters of MS throughout several countries. Such clusters have occurred around lead smelters, oil refineries and air pollutants. No direct associations have as yet been made.60–63
In contrast to immunoglobulins (IgGs) of healthy individuals, antibodies of MS patients effectively hydrolyse human myelin basic protein (MBP). Furthermore, IgG from sera of MS patients have been shown to possess metal-dependent human MBP-hydrolysing activity.64
Iron overload and the upregulation of iron-binding proteins in the brain have also been implicated in the pathogenesis of MS.65 Indeed, MRIs reveal significant and pathological iron deposition in brain MS lesions.66, 67 Urinary concentrations of iron are significantly increased in secondary progressive MS and insignificantly increased in relapsing–remitting MS (RRMS). This increased urinary iron excretion supports a role for iron dysmetabolism in MS.68 Iron imbalance is associated with pro-inflammatory cytokines and oxidative stress, suggesting that the improvement of neuronal iron metabolism may be a future target for MS therapy.65
Similarly, urinary concentrations of aluminium (Al) are also significantly increased in both secondary progressive and relapsing–remitting forms of MS. These levels of urinary Al are high enough to be compared with levels observed in individuals with Al intoxication who are undergoing specific metal chelation therapy. In accordance with this, urinary excretion of silicon is lower in MS and significantly lower in secondary progressive MS. It has since been concluded that Al may be another environmental factor associated with the aetiology of MS. If this is the case, then an increased intake of its natural antagonist, silicon, may be another therapeutic option.68
Individuals with dental amalgam have been shown to have 2–12 times more mercury in their body tissues than those without. Mercury deposits in brain and bone tissues have a half-life lasting from several years to decades and may accumulate over time of exposure.69 A recent systematic review and meta-analysis was performed in order to investigate the possible association between methyl mercury in dental amalgam and MS. The pooled odds ratios for the risk of MS amongst those with amalgams was consistent with a slight non–statistically significant increase between amalgam use and risk of MS. Further studies regarding amalgam restoration size and duration of exposure are needed to definitively rule out any link between amalgam and MS.70
Studies have also shown that Tetrathiomolybdate (TM), a potent anti-copper drug, significantly inhibits neurological damage associated with animal models of MS. TM also strongly suppresses increases in inflammatory and immune-related cytokines, reducing oxidative stress significantly.71
Physical activities
Exercise
The cumulative evidence supports the idea that exercise training is associated with an improvement in mobility amongst individuals with MS.72, 73, 74 Current studies indicate that physical activity in MS patients counteracts depression and fatigue and may improve quality of life.75, 76, 77 Aerobic treadmill activity is feasible, safe and may improve anomalies of posture and gait in early MS patients.78 Physical activity can certainly adapt and manipulate neuronal connections and synaptic activity with natural killer (NK) cells having been shown to be the most responsive immune cell to acute exercise.79, 80
Physicians should be encouraged to promote individualised strategies which enhance ‘perceived control over fatigue’ and ‘listening to your body’ in order to maximise the benefits of exercise intervention for individuals with MS-related fatigue.81 As such, it is difficult to prescribe a generalised regular exercise prescription for all those suffering with MS.82
Heat reactions are common in MS, such that exposure to heat may consequently result in the appearance of neurological signs. Exercise is one means that is known to increase basal body temperature. It is as yet not understood if or why thermal heat induces central fatigue in patients with MS, however, this is subjectively reported as a common phenomenon.83
One recent study involving MS patients who performed acute cycling has demonstrated that moderate intensity exercise was associated with reductions in anxiety and general mood disturbances. Such changes are noted to be greater in those with higher baseline anxiety.84
Exercise therapy was also noted to be beneficial for patients with MS not experiencing a relapse in a 2005 Cochrane review.85
RCTs investigating the effects of functional electrical stimulation suggest that this may also provide an orthotic benefit to those suffering with MS.86, 87
Further studies are still required as to the effects of whole body vibration therapy on muscle performance in people with MS.88
Nutritional influences
Diet
Diets and dietary supplements are widely used by people with MS in order to improve disease outcomes. Clinical data has suggested that certain dietary regimes may be beneficial in MS. However, a recent Cochrane review states that more research is needed to assess the efficacy of dietary interventions in MS.90
Caloric restriction
It is thought that caloric restriction with adequate nutrition under diligent medical supervision should be explored as a potential treatment for MS as it induces anti-inflammatory, antioxidant and neuroprotective effects that may be beneficial.91, 92
Fish intake
The risk of developing MS has been associated with an increased dietary intake of saturated fatty acids.93 A recent study has indicated that a diet rich in salmon (3–4 times weekly) may provide a protective role in demyelination.40 This is believed to be secondary to the omega-3 polyunsaturated fatty acids (PUFA) content as no protective effect was observed with a cod-liver oil-based diet.94, 95 It is important to note, however, that cod-liver oil supplementation may provide some protection.40 Norway appears to be a discrete exception to the prevalence of MS in higher latitudes. UV exposure is low in this country, however, vitamin D sufficiency is maintained through a traditional diet providing vitamin D as well as marine omega-3 PUFAs.96 Indeed, recent results suggest that a low fat diet supplemented with omega-3 PUFAs may become the standard recommended therapy for those with MS.97
Artificial sweeteners
There is currently controversy regarding the potential toxicity of low-calorie artificial sweeteners such as aspartame, acesulfame-K (ASK) and saccharin and their possible relationship to MS. Animal studies have shown that the genotoxic potential of ASK and saccharin is greater than aspartame (ASP). However, none could act as a potential mutagen alone. These findings are important as they represent a potential health risk associated with exposure to these agents.98, 99
Gluten
MS and coeliac disease are both considered to be T-cell mediated auto-immune diseases. It has been postulated that the interaction of MS and coeliac disease inflammatory processes may result in an amplification of Th1 response.100 There is some evidence that individuals with MS have highly significant increases of IgA and IgG antibodies against gliadin and gluten and significant increases against casein (cow’s milk) compared with controls. It should be noted that anti-endomycium and anti-transglutaminase antibodies were negative.101, 102 Recent studies, however, have conflicting data, showing that gluten sensitivity is not associated with MS.103, 104
Nutritional supplementation
Vitamin D
Supplementation with vitamin D is considered to be necessary in northern and southern latitudes to maintain adequate levels of 25(OH)D3 for optimal health and disease prevention.105 This is certainly understandable, however, it is imperative to note that vitamin D insufficiency is also very common across a wide latitude range in Australia.
current Australian sun exposure guidelines do not seem to fully prevent vitamin D insufficiency, and consideration should be given to their modification or to pursuing other mean to achieve vitamin D adequacy.106
There has been a multitude of recent studies linking low levels of vitamin D with an increased risk of MS. Initial research centred around the striking prevalence of MS in populations residing at higher latitudes. The protective effect of sunlight is reduced at these latitudes, subsequently leading to insufficiencies in vitamin D. Vitamin D is a hormone now known to be involved in far more than the protection of bones. It can regulate processes of cell proliferation, differentiation and apoptosis important in cancer prevention and can profoundly affect both the innate and adaptive immune systems providing protection against certain immune-mediated disorders.107, 108, 109
Recent genetic studies have also revealed important vitamin D receptor gene polymorphisms, highlighting once again the complex interaction between genetics and environment.96, 110, 111 There is strong evidence that vitamin D is an important modifiable environmental/nutritional factor in the pathogenesis of MS with a potential role in its prevention and/or treatment.37, 107, 112
Epidemiological evidence supports the view that vitamin D metabolites have significant immune and disease-modulating effects in MS. Vitamin D mediates a shift to a more anti-inflammatory immune response and enhances regulatory T-cell functionality.113 As such, vitamin D plays an important role in T-cell homeostasis during the course of MS and correcting low levels may be useful during treatment of this disease.114
A recent hypothesis suggests that lack of sunlight exposure and viral infections such as herpes and EBV may synergistically induce a defect in IL-10-producing regulatory lymphocytes that may undermine self-tolerance mechanisms and hence enable a pathogenic autoimmune response to neural proteins.115
Low serum vitamin D3 levels correlate strongly with MS risk and have been well documented in many studies.115, 116 Lower levels are seen in RRMS compared with progressive MS patients and controls, particularly during relapses.114 Lower circulating levels of 25(OH)D have since been found to be particularly associated with higher MS-related disability in women.117
Supplementation with the active form of vitamin D to animal models of MS both suppresses disease development and leads to improvement of immune-mediated symptoms.108 Complete disease prevention only occurred with extremely high doses of vitamin D3, such that calcium levels were also significantly elevated. A combination of calcitonin and a smaller dose of vitamin D3, however, synergistically suppressed MS development in animals without causing hypercalcaemia. This finding may become extremely important in the treatment of patients with MS.118
A recent meta-analysis has demonstrated that vitamin D supplementation significantly reduces all-cause mortality, highlighting the medical, ethical and legal implications of promptly diagnosing and treating vitamin D deficiency. Treatment in otherwise healthy patients with vitamin D supplementation should be sufficient to maintain year-round 25(OH)D3 levels at least between 40–70ng/mL. Those vitamin D deficient patients with chronic diseases such as MS often need to be investigated and treated more aggressively so that levels are at least between 55–70ng/mL.116, 119 It is important to note, however, that with increasing knowledge of the importance of vitamin D to health, these reference ranges are likely to be increased in the future.
Long-term supplementation with vitamin D and cod-liver oil has been associated with a decreased incidence of developing MS. Osteoporosis is more common in patients with MS, such that prophylaxis with vitamin D and calcium is widely accepted by most.120, 121, 40 Parathyroid hormone (PTH) levels have been noted to be higher during a relapse of MS and lower during remission and in winter. MS patients also have a relative hypocalcaemia in winter, thus the endocrine circuitry regulating serum calcium is altered in MS.122
Antioxidants
Reactive oxygen species (ROS) play a major role in various events in the pathogenesis of MS. When ROS are formed in MS and animal models of MS, products such as peroxynitrite and superoxide are generated which are highly toxic to cells.123 ROS initially mediate the transendothelial migration of monocytes and induce dysfunction in the blood–brain barrier. They may also contribute to the formation and persistence of MS lesions by acting on distinct pathological processes. Extensive oxidative damage to proteins, lipids and nucleotides is well documented in active demyelinating lesions. Oxidative stress can be counteracted by endogenous antioxidant enzymes that confer protection against such damage.124 Antioxidant therapy may therefore represent an attractive treatment option for those with MS with their potential to diminish symptoms by targeting specific patho-mechanisms and support recovery.93, 125, 126
Animal studies have demonstrated beneficial effects of antioxidants such as vitamin C and E in MS, although there is limited research as yet of the effects in humans.125, 127 Clinical trials of more potent antioxidants, including lipoic acid (LA), are currently being trialled with promising results in humans.123 LA works by reducing and recycling cellular antioxidants, such as glutathione and chelating zinc, copper and other transition metal ions as well as heavy metals. It also acts as a scavenger of ROS and nitrogen species.128
Polyunsaturated fatty acids (PUFAs)
Deregulated lipid metabolism is of particular importance in CNS disorders as the brain has the highest lipid concentration after adipose tissue.2 Omega-3 PUFAs are known to play a significant role in nervous system activity, cognitive development, memory-related learning, neuroplasticity of nerve membranes, synaptogenesis and synaptic transformation.129 Deficiencies in omega-3 and omega-6 PUFAs and excess levels of monounsaturated and saturated fatty acids have been observed in patients with MS whilst omega-3 and 6 supplementation, in animal models, has been shown to decrease clinical signs of disease.93, 130
As such, considerable interest has been shown in the potential anti-inflammatory effects of PUFAs in MS and other auto-immune and neurodegenerative disorders. There is good evidence that both omega-3 and omega-6 supplementation can reduce immune-cell activation by various pathways.131 In particular, PUFA supplementation has been shown to dose-dependently inhibit the lipopolysaccharide-induced production of the myelinotoxic factor, MMP-9, from microglial cells. Such results suggest that supplementation with omega-3 may become recommended for the wellbeing of all MS patients under therapy.97, 120, 132
A recent small study of RRMS patients receiving 9.6g/day fish oil has confirmed that immune cell secretion of MMP-9 was decreased by 58% after 3 months supplementation when compared with baseline levels. This effect was coupled with a significant increase in omega-3 fatty acid levels in red blood cell (RBC) membranes. Omega-3 PUFAs may therefore act as immune modulators with potentially beneficial results in individuals with MS.133
Epidemiological, biochemical, animal model and clinical trial data strongly suggest that omega-6 PUFAs also play a significant role in the pathogenesis and treatment of MS. In another double-blind, placebo-controlled RCT, patients with MS were given either high-dose GLA (omega-6), low-dose GLA or placebo. High dose GLA was shown to have a marked clinical effect in RRMS, significantly decreasing the relapse rate and disease progression. Such improvements in disability suggest a beneficial effect on neuronal lipids and neural function in MS.134
It should be noted, however, that clinical trials in MS patients provide mixed results. There is a need for larger good quality trials, preferably with MRI investigations.131
B vitamins and iron
Myelin is continually regenerated in the human body. For this process to occur, adequate iron and a functional folate/vitamin B12 methylation pathway is required.135 Homocysteine levels are often increased in those with MS and are associated with cognitive impairment, depression and abnormal electrophysiological parameters in this disease.136–139
In Caucasian females with RRMS, serum iron and ferritin concentrations have been found to be significantly lower than in matched controls. A small 6-month study of RRMS patients taking nutritional supplements specifically designed to promote demyelination, has shown a significant neurological improvement when compared to a control group taking multivitamins. Both groups had significantly reduced homocysteine levels at 6 months, suggesting that methylation is necessary but not sufficient for myelin regeneration.135
Herbal medicines
Cannabis
Endocannabinoids (eCBs) play a role in the modulation of neuro-inflammation, and experimental findings suggest that they may be directly involved in the pathogenesis of MS. Significantly reduced levels of eCBs have been found in the CSF of MS patients compared to controls. These findings support the development of drugs targeting eCBs to reduce symptoms and slow disease progression in MS.140, 141
A recent internet-based survey for Australian residents with MS has shown that cannabis was beneficial in reducing symptoms of MS.8 Indeed, world-wide, many patients use cannabis to alleviate spasticity and pain. Small scale studies do indicate positive effects, however, larger RCTs are thus far negative for improvements in spasticity. It should be noted that most patients report a subjective benefit even if their objective parameters are unchanged.120 Recently cannabis has been analysed in more controlled studies which have provided evidence that it may have some benefit.4, 7
It should be noted, however, that inhaled cannabis is associated with impaired mentation in MS patients, particularly in respect to cognition.142
Other supplements
Hormones
Mean DHEA levels have been found to be lower in MS patients than controls, with no significant difference between the different subgroups.145 Those MS patients with fatigue displayed even lower levels of DHEA therefore hormone replacement is a possible option for treating fatigue-related MS.146
Testosterone treatment has been shown to ameliorate symptoms in animal models of MS. A small pilot study involved administering 100mg testosterone gel daily to a group of men with RRMS for 1 year. An improvement in cognitive performance and a slowing of brain atrophy was demonstrated, however, there was no significant effect on MS brain lesions. Thus, it was concluded that testosterone treatment is safe, well tolerated and has potential neuroprotective effects in men with RRMS.147
Conclusion
Recently additional patterns have emerged as to why chronically ill people include CAM therapies and this is to complement conventional medicine.148 Individuals do not tend to give up their conventional health care providers in lieu of CAM treatment. The trend is to use CAM as an adjunct to the treatment being received from a conventional clinician. Another pattern that emerged was the use of CAM to treat or manage MS symptoms. Although a small percentage of patients do seek CAM for disease-modifying purposes, research reports that significantly more patients use CAM to treat or manage the daily symptoms.149
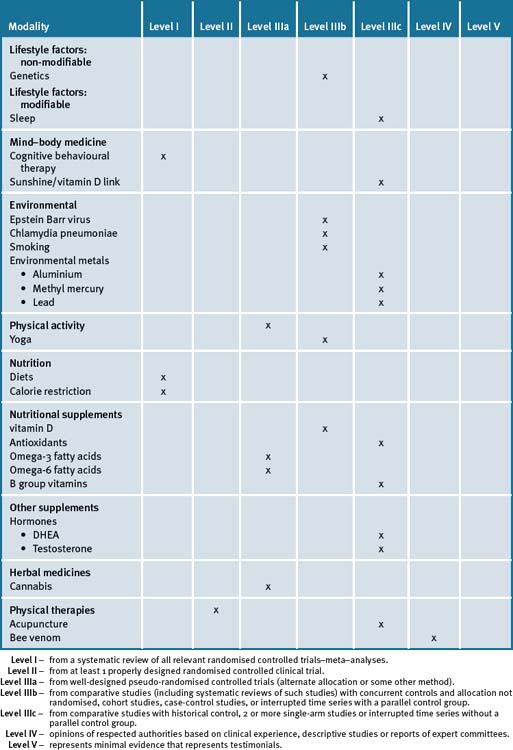
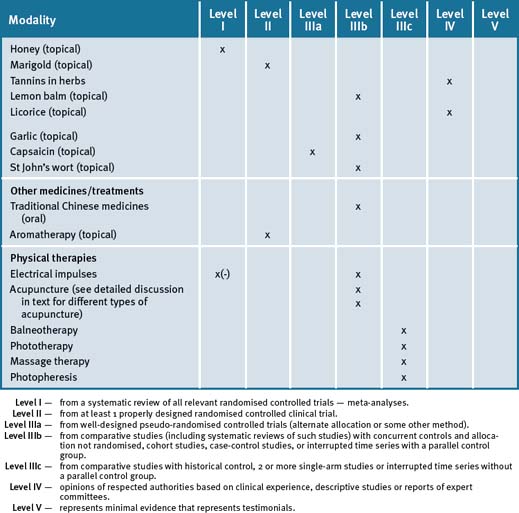
The most frequently used CAM therapies include massage, acupuncture, chiropractic, vitamins/herbal medicines and nutrition, and hence this trend lends itself significantly to an integrative management approach. Lack of sunshine exposure and vitamin B deficiency are significant risk factors for MS. Table 26.1 summarises the current evidence for CAM treatments.
Clinical tips handout for patients — multiple sclerosis
1 Lifestyle advice
Sleep
2 Physical activity/exercise
3 Mind-body medicine
4 Environment
5 Dietary modification
7 Supplementation
Vitamin D3 (cholecalciferol)
Cod-liver oil
Calcium/magnesium
Antioxidants
1 Jelinek G.A., Hassed C.S. Managing MS in primary care: are we forgetting something? Qual Prim care. 2009;17(1):55-61.
2 Adibhatia R.M., Hatcher J.F. Altered lipid metabolism in brain injury and disorders. Subcell Biocem. 2008;49:241-268.
3 Fryze W., Mirowski-Guzel D., Wiszniewska M., et al. Alternative methods of treatment used by MS patients in Poland. Neurol Neurochir Pol. 2009;40(5):386-390.
4 Olsen S.A. A review of CAM by people with MS. Occup Ther Int. 2009;16(1):57-70.
5 Schwartz S., Knorr C., Geiger H., et al. CAM for MS. Mult Scler. 2008;14(8):1113-1119.
6 Nayak S., Matheis R.J., Schoenberger N.E., et al. Use of unconventional therapies by individuals with MS. Clin Rehabil. 2003;17(2):181-191.
7 Yadav V., Bourdette D. CAM Medicine: is there a role in MS? Curr Neurol Neurosci Rep. 2006;6(3):259-267.
8 Simmons R.D., Ponsonby A.L., van der Mei I.A., et al. What effects you MS? Responses to an anonymous Internet-based epidemiological survey. Mult Scler. 2004;10(2):202-211.
9 Willer C.J., Ebers G.C. Susceptibility to multiple sclerosis: interplay between genes and environment. Curr Opin Neurol. 2000;13:241-247.
10 Ascherio A., Munger K. Epidemiology of MS: from risk factors to prevention. Semin Neurol. 2008;28(1):17-28.
11 Fujihara K. Update on the aetiology and pathogenesis of MS and neuromyelitis optica. Nippon Rinsho. 2008;66(6):1087-1091.
12 Holmey T., Hestvik A.L. MS: Immunopathogenesis and controversies in defining the cause. Curr Opin Infect Dis. 2008;21(3):271-278.
13 Pugliatti M., Harbo H.F., Holmey T., et al. Environmental risk factors in MS. Acta Neurol Scand Suppl. 2008;188:34-40.
14 Giovannoni G., Ebers G. MS: the environment and causation. Curr Opin Neurol. 2007;20(3):261-268.
15 Alonso A., Hernan M.A. Temporal trends in the incidence of MS: a systematic review. Neurology. 2008;71(2):129-135.
16 Anderson D.W., Ellenberg J.H., Leventhal C.M., et al. Revised estimate of the prevalence of multiple sclerosis in the United States. Am Neurol. 1992;31:333-336.
17 Hirtz D., Thurman D.J., Gwinn-Hardy K., et al. How common are the ‘common’ neurologic disorders? Neurology. 2007;68:326-337.
18 Marrie R., Horwitz R., Cutter G., et al. High frequency of adverse health behaviours in MS. Mult Scler. 2009;15(1):105-113.
19 Esch T., Stafano G.B., Fricchione G.L., et al. The role of stress in neurodegenerative diseases and mental disorders. Neuro Endocrinol Lett. 2002;23(3):199-208.
20 Swaab D.F., Bao A.M., Lucassen P.J. The stress system in the human brain in depression and neurodegenration. Ageing Res Rev. 2005;4(2):141-194.
21 Ysrraelit M.C., Gaitan M.I., Lopez A.S., et al. Impaired HPA-axis activity in patients with MS. Neurology. 2008;71(24):1948-1954.
22 Torem Ms. MB Hypnotic imagery in the treatment of AI disorders. Am J clin Hypnosis. 2007;50(2):157-170.
23 Bombardier C.H., Cunniffe M., Wadwani R., et al. The efficacy of telephone counseling for health promotion in people with MS; a RCT. Arch Phys Med Rehabil. 2008;89(10):1849-1856.
24 Bol Y., Duits A.A., Hupperts R.M., et al. The psychology of fatigue in patients with MS: a review. J Psychosom Res. 2009;66(1):3-11.
25 Tartaglia M.C., Narayanan S., Arnold D.L. Mental fatigue alters the pattern and increases the volume of cerebral activation required for a motor task in MS patients with fatigue. Eur J Neurol. 2008;15(4):413-419.
26 White A., Lee J., Light A., et al. Brain activation in MS: a BOLD fMRI study of the effects of fatiguing hand exercise. Mult Scler. 2009 Mar 19.
27 O’Brien A.R., Chiaravalotti N., Goverover Y., et al. Evidenced-based cognitive rehabilitation for persons with MS: a review of the literature. Arch Phys Med Rehabil. 2008;89(4):761-769.
28 Thomas P.W., Thomas S., Hillier C., et al. Psychological interventions for MS. Cochrane Database Syst rev. (1):2006. CD004431
29 Brown R.F., Valpiani E.M., Tennant C.C., et al. Longitudinal assessment study of anxiety, depression and fatigue with MS. Psycholog Psychother. 2009;82(Pt 1):41-56.
30 Dworzanska E., Mitosek-Szewczyk K., Stelmasiak Z. Fatigue in MS. Neurol Neurochir Pol. 2009;43(1):71-76.
31 Attarian H. Importance os sleep in the QOL of MS patients:a long under-recognised issue. Sleep Med. 2009;10(1):7-8.
32 Merlino G., Frattici L., Lenchig C., et al. Prevalence of poor sleep amomg patients with MS: an independent predictor of mental and physical status. Sleep Med. 2009;10(1):26-34.
33 Bamer A.M., Johnson K.L., Amtmann D., et al. Prevalence of sleep problems in individuals with MS. Mult Scler. 2008;14(8):1127-1130.
34 Manconi M., Ferini-Strambi L., Filipi M., et al. Multi-centre case-control study on RLS in MS: the REMS study. Sleep. 2008;31(7):944-952.
35 Moreira N.C., Damasceno R.S., Medieras C.A., et al. RLS, sleep quality and fatigue in MS. Braz J Med Biol Res. 2008;41(10):932-937.
36 Ascherio A., Munger K.L. Environmental risk factors for MS. Part II: Non-infectious factors. Ann Neurol. 2007;6196):504-513.
37 Niino M., Fukazawa T., Kikuchi S., et al. Therapeutic potential of Vit D for MS. Curr Med Chem. 2008;15(5):499-505.
38 Tremiett H., vander Mei I.A., Pittas F., et al. Monthly ambient sunlight, infections and relapse rates in MS. Neuroepidemiology. 2008;31(4):271-279.
39 Dwyer T., van der Mei I., Ponsonby A.L., et al. MC1R genotype, past environmental sun exposure and risk of MS. Neurology. 2008;71(8):583-589.
40 Kampman M.T., Wilsgaard T., Mellgren S.I. Outdoor activities and diet in childhood and adolescence relate to MS risk above the Arctic circle. J Neurol. 2007;254(4):471-477.
41 Ascherio A., Munger K.L. Environmental risk factors for MS. part i:the role of infection. Ann Neurol. 2007;61(4):288-299.
42 Ebers G.C. Environmental factors and MS. Lancet Neurol. 2008;7(3):268-277.
43 Goodin D.S. The causal cascade to MS: a model for MS pathogenesis. PLoS ONE. 2009;4(2):e4565. Epub 2009 Feb 26
44 Ruprecht K. MS and EBV: new developments and perspectives. Nervenartz. 2008;79(4):399-407.
45 Jilek S., Schluep M., Meylan P., et al. Strong EBV-specific CD8+ T cell response in patients with early MS. Brain. 2008;131(Pt 7):1712-1721.
46 Posnett D.N. Herpes viruses and auti-immunity. Curr Opin Investig drugs. 2008;9(5):505-514.
47 Niller H.H., Wolf H., Minarovits J. Regulation and dysregulation of EBV latency: implications for the development of Auto-immune Disease. Autoimmunity. 2008;41(4):298-328.
48 Marrie R.A. When one and one make three: HLA and EBV infection in MS. Neurology. 2008;70(13 part 2):1067-1068.
49 Haahr S., Hollsberg P. MS is linked to EBV infection. Rev Med Virol. 2006;16(5):297-310.
50 De Jager P.L., Simon K.C., Munger K.L. Integrating Risk factors:HLA-DRB1∗1501 and EBV in MS. Neurology. 2008;70(13 Pt 2):1113-1118.
51 Levin L.I., Munger K.L., Rubertone M.V., et al. Temporal relationship between elevation of EBV antibody titres and initial onset of neurological symptoms in MS. JAMA. 2005;293(20):2496-2500.
52 Frykholm B. On the question of infectious aetiologies for MS, schizophrenia and CFS and their treatment with antibiotics. Med Hypotheses. 2009;72(6):736-739.
53 Marrie R.A., Cutter G., Tyry T., et al. Smoking status over 2 years in patients with MS. Neuroepidemiology. 2009;32(1):72-79.
54 Di Pauli F., Reindl M., Ehling R., et al. Smoking is a risk factor for early conversion to clinically definite MS. Mult Scler. 2008;14(8):1026-1030.
55 Sundstrom P., Nystrom L. Smoking worsens the prognosis in MS. Mult Scler. 2008;14(8):1031-1035.
56 Sundstrom P., Nystrom L., Hallmans G. Smoke exposure increases the risk for MS. Eur J Neurol. 2008;15(6):579-583.
57 Tardieu M., Mikaeloff Y. MS in children: Environmental risk factors. Bull acad Natl Med. 2008;192(3):507-509.
58 Pittas F., Ponsonby A.L., van der Mei I.A., et al. Smoking is associated with progressive disease course and increased progression in clinical disability in a prospective cohort of people with MS. J Neurol. 2009 Apr 9. Epub ahead of print
59 Montgomery S.M., Bahmanyar S., Hillert J., et al. Maternal smoking during pregnancy and MS amongst offspring. Eur J Neurol. 2008;15(12):1395-1399.
60 Williamson D.M. Studies of MS in communities concerned about environmental exposures. J Womens Health. 2006;15(7):810-814.
61 Henry J.P., Willialson D.M., Schiffer D.M., et al. Investigation of a cluster of MS in 2 elementary school cohorts. J Environ Health. 2007;69(10):34-38.
62 Neuberger J.S., Lynch S.G., Sutton M.L., et al. Prevalence of MS in a residential area bordering an oil refinery. Neurology. 2004;63(10):1796-1802.
63 Turabelidze G., Schootman M., Zhu B.P., et al. MS Prevalence and possible lead exposure. J Neurol Sci. 2008;269(1-2):158-162.
64 Polosukhini D.I., Kanyshkova T.G., Doronin B.M., et al. Metal-dependent hydrolysis of MBP by IgGs from the sera of patients with MS. Immunol lett. 2006;103(1):75-81.
65 Abo-Krysha N., Rashed L. The role of iron dysregulation in the pathogenesis of MS: an Egyptian study. Mult Scler. 2008;14(5):602-608.
66 Haacke E.M., Makki M., Ge Y. Characterising iron deposition in MS lesions using susceptibility weighted imaging. J Magn Reson Imaging. 2009;29(3):537-544.
67 Hammond K.E., Metcalf M., Carvajal L., et al. Quantitative in vivo MRI of MS at 7 Tesla with sensitivity to iron. Ann Neurol. 2008;64(6):707-713.
68 Exley C., Mamutse G., Korchazhkina O., et al. Elevated urinary excretion of Al and Fe in MS. Mult Scler. 2006;12(5):533-540.
69 Mutter J.J., Naumann J., Guethlin C. Comments on the article ‘the toxicology of mercury and its chemical compounds’ by Clarkson and Magos (2006). Crit Rev Toxicol. 2007;37(6):537-549.
70 Aminzadeh K.K., Etminan M. Dental amalgum and MS: a SR and meta-analysis. J Public Health Dent. 2007;67(1):64-66.
71 Hou G., Abrams G.D., Dick R., et al. Efficacy of TM in a mouse model of MS. Trans Res. 2008;152(5):239-244.
72 Snook E.M., Motl R.W. Effect of exercise training on walking mobility in MS;23(2):108-16. Neurorehabil Neural Repair. 2009;23(2):108-116.
73 Dettmers C., Sulzmann M., Ruchav-Plossl A., et al. Endurance exercise improves walking distance in MS patients with fatigue. Acta Neurol Scand. 2009 Jan 19. Epub ahead of print
74 Motl R.W., Snook E.M., Wynn D.R., et al. Physical activity correlates with neurological impairment and disability in MS. J Nerv Ment Dis. 2008;196(6):492-495.
75 Waschbisch A., Tallner A., Pfeifer K., et al. MS and exercise: Effects of physical activity on the immune system. Nervenartz. 2009 Jan 23. Epub ahead of print
76 Fragaso Y.D., Santana D.L., Pinto R.C. The positive effects of a physical activity program for MS patients with fatigue. NeuroRehabilitation. 2008;23(2):153-157.
77 Motl R.W., McAuley E., Snook E.M., et al. Physical activity and QOL in MS: Intermediary roles of disability, fatigue, mood, pain, self-efficacy and social support. Psychol Health Med. 2009;14(1):111-124.
78 Benedetti M.G., Gasparroni V., Stecchi S., et al. Treadmill exercise in early MS: a case series study. Eur J Phys Rehab Med, 2009 Jan 21, Epub ahead of print
79 Achiron A., Kalron A. Physical activity: positive impact on brain plasticity. Harefuah. 2008;147(3):252-255.
80 Timmons B.W., Cieslak T. Human NK cell subsets and acute exercise: a brief review. Exerc Immunol Rev. 2008;14:8-23.
81 Smith C., Hale L., Dison K., et al. How does exercise influence fatigue in people with MS? Disabil rehabil. 2008:1-8.
82 Sano M., Dawes D.J., Arafah A., et al. What does a structured review of the effectiveness of exercise interventions for persons with MS tell us about the challenges of designing trials? Mult Scler. 2009;15(4):412-421.
83 Marino F.E. Heat reactions in MS: an overlooked paradigm in the study of comparative fatigue. Int J Hyperthermia. 2009;25(1):34-40.
84 Petruzzello S.J., Snook E.M., Gliotoni R.C., et al. Anxiety and mood changes associated with acute cycling in persons with MS. Anxiety Stress Coping. 2009;22(3):297-307.
85 Rietbrg M.B., Brooks D., Uitdehaag B.M., et al. Exercise therapy for MS. Cochrane Database syst Rev. (1):2005. CD003980
86 Paul L., rafferty D., Young S., et al. The effect of FES on the physiological cost of gait in people with MS. Mult Scler. 2008;14(7):954-961.
87 Barrett C., Mann G., Taylor P., et al. A RCT to investigate the effects of FES and therapeutic exercise on walking performance for people with MS. Mult Scler. 2009;15(4):493-504.
88 Jackson K.J., Merriman H.L., Vanderburgh P.M., et al. Acute effects of whole-body vibration on lower extremity muscle performance in persons with MS. J Neurol Phys Ther. 2008;32(4):171-176.
89 Esmonde L., Long A.F. Comp therapy use by persons with MS: benefits and research priorities. Complement Ther Clin Pract. 2008;14(3):176-184.
90 Farinotti M., Simi S., Di Pietrantonj C., et al. Dietary interventions for MS. Cochrane Database Syst Rev. (1):2007. CD004192
91 Piccio L., Stark J.L., Cross A.H. Chronic caloric restriction attenuates experimental AI encephalomyelitis. J Leukoc Biol. 2008;84(4):940-948.
92 Fernandes G. Progress in nutritional immunology. Immunol Res. 2008;40(3):244-261.
93 van meeteren M.E., Teunissen C.E., Dijkstra C.D., et al. Antiox and PUFAs in MS. Eur J Clin Nutr. 2005;59(12):1347-1361.
94 Torkildsen O., Brunberg L.A., Thorson F., et al. Effects of dietary interevntion on MRI activity, de and remyelination in the cuprizone model for demyelination. Exp Neurol. 2009;215(1):160-166.
95 Torkildsen O., Brunborg L.A., Milde A.M., et al. A salmon-based diet protects mice from behavioural changes in the cuprizone model for demyelination. Clin Nutr. 2009;28(1):83-87.
96 Kampman M.T., Brustad M., Vit D. a candidate for the environmental effect in MS; observations from Norway. Neuroepidemiology. 2008;30(3):140-146.
97 Liuzzi G.M., Latronico T., Rossano R., et al. Inhibitory effect of PUFAs on MMP-9 from microglial cells: implications for complementary MS treatment. Neurochem Res. 2007;32(12):2184-2193.
98 Whitehouse CR, Boullata J, McCauley LA. The potential toxicity of artificial sweeteners. AAOHN J;56(6):251-9
99 Bandyopadhyay A., Ghoshal S., Mukherjee A. Genotoxicity testing of low-calorie sweeteners: aspartame, ASK and saccharin. Drug Chem Toxicol. 2008;31(4):447-457.
100 Frisullo G., Nociti V., Iorio R., et al. Increased expression of T-bet in circulating B cells from a patient with MS and CD. Hum Immunol. 2008;69(12):837-839.
101 Reichelt K.L., Jensen D. IgA antibodies against gliadin and gluten in MS. Acta Neurol Scand. 2004;110(4):239-241.
102 Pengiran Tengah C.D., Lock R.J., Unsworth D.J., et al. MS and occult gluten sensitivity. Neurology. 2004;62(12):2326-2327.
103 Borhani Haghighi A., Ansari N., Mokhtari M., et al. MS and gluten sensitivity. Clin Neurol Neurosurg. 2007;109(8):651-653.
104 Nicoletti A., Patti F., Lo Fermo S., et al. Frequency of coeliac disease is not increased among MS patients. Mult Scler. 2008;14(5):698-700.
105 Huotari A., Herzig K.H. vitamin D and living in the Northern latitudes – an endemic risk area for Vit D deficiency. Int J Circumpolar Health. 2008;67(2-3):164-178.
106 van der Mei I.A., Ponsonby A.L., Engelson O., et al. The high prevalence of Vit D insufficiency across australian populations is only partly explained by season and latitude. Environ Health Perspect. 2007;115(8):1132-1139.
107 Raghuwanshi A., Joshi S.S., Christakos S., J Cell Biocem. Vit D and MS. 2008;105(2):338-343.
108 Szodoray P., Nakken B., Gaal J., et al. The complex role of Vit D in AI diseases. Scand J Immunol. 2008;68(3):261-269.
109 Holick M.F. Vit D and sunlight: strategies for cancer prevention and other health benefits. Clin J Am Soc Nephrol. 2008;3(5):1548-1554.
110 Ramagopalan S.V., Maugeri N.J., Handunnetthi L., et al. Expression of the MS associated MHC class II Allele HLA-DRB1∗1501 is regulated by Vit D. PLoS Genet. 2009;5(2):e1000369. Epub 2009 Feb 6
111 Smolders J., Damoiseaux J., Menheere P., et al. Fok-1 Vit D receptor gene polymorphism (rs10735810) and Vit D metabolism in MS. J Neuroimmunol. 2009;207(1-2):117-121.
112 Cantorna M.T. Vit D and MS: an update. Nutr Rev. 2008;66(10 Suppl. 2):S135-S138.
113 Smolders J., Damoiseaux J., Menheere P., et al. Vit D as an immune modulator in MS: a review. Neuroimmunol. 2008;194(1-2):7-17.
114 Correale J., Ysrraelit M.C., Gaitan M.I. Immunomodulatory effects of vitamin D in MS. Brain. 2009 Mar 24. Epub ahead of print
115 Hayes C.E., Donald Acheson E. A unifying MS aetiology linking virus infection, sunlight and vitamin D through viral IL-10. Med Hypotheses. 2008;71(1):85-90.
116 van der Mei I.A., Ponsonby A.L., Dwyer T., et al. Vit D levels in people with MS and community controls in Tasmania, Australia. J Neurol. 2007;254(5):581-590.
117 Kragt J., van Amerongen B., Killestein J., et al. Higher levels of 25(OH)D are assoc with a lower incidence of MS only in women. Mult Scler. 2009;15(1):9-15.
118 Becklund B.R., Hansen D.W.Jr., Deluca H.F. Enhancement of 1,25(OH)D3-mediated suppression of EAE by calcitonin. Proc Natl Acad Sci USA. 2009;106(13):5276-5281.
119 Cannell J.J., Hollis B.W. Use of vitamin D in clinical practice. Altern Med Rev. 2008;13(1):6-20.
120 Schwartz S., Leweling H., Meinck H.M. Alternative and Complementary therapies in MS. Fortschr Neurol Psychiatr. 2005;73(8):451-462.
121 Namaka M., Crook A., Doupe A., et al. Examining the evidence: complementary adjunctive therapies for MS. Neurol Res. 2008;30(7):710-719.
122 Soilu-Hanninen M., Laaksonen M., Laitinen I., et al. A longitudinal study of serum 25(OH)D and intact PTH levels indicate the importance of Vit D and calcium homeostasis regulation in MS. J Neurol Neurosug Psychiatry. 2008;79(2):152-157.
123 Carlson N.G., Rose J.W. Antioxidants in MS: do they have a role in therapy? CNS Drugs. 2006;20(6):433-441.
124 van Horssen J., Schreibelt G., Drexhage J., et al. Severe ox damage in MS lesions coincides with enhanced antiox enzyme expression. Free radic Biol Med. 2008;45(12):1729-1737.
125 Mirshafiey A., Mohsenzadegan M. Antioxidant therapy in MS. Immunopharmacol Immunotoxicol. 2009;31(1):13-29.
126 Schreibeit G., van Horssen J., van Rossum S., et al. Therapeutic potential and biological role of endogenous antioxidant enzymes in MS pathology. Brain Res Rev. 2007;56(2):322-330.
127 Gilgun-Sherki Y., Melamed E., Offen D. The role of ox stress in the pathogenesis of MS: the need for effective antioxidant therapy. J Neurol. 2004;251(3):261-268.
128 Salinthone S., Yadav V., Bourdette D.N., et al. Lipoic acid: a novel therapeutic approach for MS and other chronic inflammatory diseases of the CNS. Endocr Metab Immun Disorder Drug Targets. 2008;8(2):132-142.
129 Mazza M., Pomponi M., Janiri L., et al. Omega 3 FAs and antioxidants in neurological and psychiatric diseases: an overview. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(1):12-26.
130 Aupperle R.P., Denney D.R., Lynch S.G., et al. Omega-3 FAs and MS: relationship to depression. J Behav Med. 2008;31(2):127-135.
131 Mehta L.R., Dworkin R.H., Schwid S.R. PUFAs and their potential therapeutic role in MS. Nat Clin Pract Neurol. 2009;5(2):82-92.
132 Salvati S., Attorri L., Di Benedetto R., et al. PUFAs and neurological diseases. Mini Rev Med Chem. 2006;6(11):1201-1211.
133 Shinto L., Marracci G., Baldauf-Wagner S., et al. Omega-3 FA supp decreases MMP-9 production in RRMS. Prostaglandins Leukotr Essent Fatty Acids. 2009;80(2-3):131-136.
134 Harbige L.S., Sharief M.K. PUFAs in the pathogenesis and treatment of MS. Br J Nutr. 2007;98(Suppl. 1):S46-S53.
135 van Rensburg S.J., Kotze M.J., Hon D., et al. Fe and the folate/vitamin B12 methylation pathway in MS. Metab Brain Dis. 2006;21(2-3):121-137.
136 Russo C., Morabito F., Luise F., et al. Hyperhomocysteinaemia is assoc with cognitive impairment in MS. J Neurol. 2008;255(1):64-69.
137 Obeid R., McCaddon A., Herrmann W. The role of hyperhomocysteinemia and B vitamin deficiency in neuro and psychiatric diseases. Clin Chem Lab Med. 2007;45(12):1590-1606.
138 Kocer B., Engur S., Ak F., et al. Serum B12, folate and HC levels and their association with clinical and electrophysiological parameters in MS. J Clin Neurosci. 2009;16(3):399-403.
139 Triantafyllou N., Evangelopoulos M.E., Kimiskidis V.K., et al. Increased plasma HC in patients with MS and depression. Ann Gen Psychiatry. 2008;7:17.
140 Di Filippo M., Pini L.A., Pelliccioli G.P., et al. Abnormalities in the CSF levels of eCBs in MS. J neurol Neurosurg Psychiatry. 2008;79(11):1224-1229.
141 Ashton J. Pro-drugs for indirect cannabinoids as therapeutic agents. Curr drug deliv. 2008;5(4):243-247.
142 Ghaffar O., Feinstein A. MS and cannibis: a cognitive and psychiatric study. Neurology. 2008;71(3):164-169.
143 Donnellan C.P., Shanley J. Comparison of the effect of 2 types of acupuncture on QOL in SPMS: a preliminary single-blind RCT. Clin rehab. 2008;22(3):195-205.
144 Lee H., Park H.J., Park J., et al. Acupuncture application for neurological disorders. Neurol Res. 2007;29(Suppl. 1):S49-S54.
145 Ramsaransing G.S., Heersema D.J., De Keyser J. Serum uric acid, DHEA and Apolipoprotein E genetype in benign vs progressive MS. Eur J Neurol. 2005;12(7):514-518.
146 Tellez N., Comabella M., Julia E., et al. Fatigue in progressive MS is associated with low levels of DHEA. Mult Scler. 2006;12(4):487-494.
147 Sicotte N.L., Giesser B.S., Tandon V., et al. Testosterone treatment in MS: a pilot study. Arch Neurol. 2007;64(5):683-688.
148 Statistics, National Center for Health. National Health Interview Survey 2008. Online. Available: http://www.cdc.gov/nchs/about/major/nhis/quest_data_related_1997_forward.htm (accessd May 2009).
149 Pucci E., Cartechini E., Taus C., et al. Why physicians need to look more closely at the use of complementary and alternative medicine by multiple sclerosis patients. European Journal of Neurology. 2004;11:263-267.