Chapter 13 Multifetal Gestation and Malpresentation
 Multiple Gestation
Multiple Gestation
ETIOLOGY AND CLASSIFICATION OF TWINNING
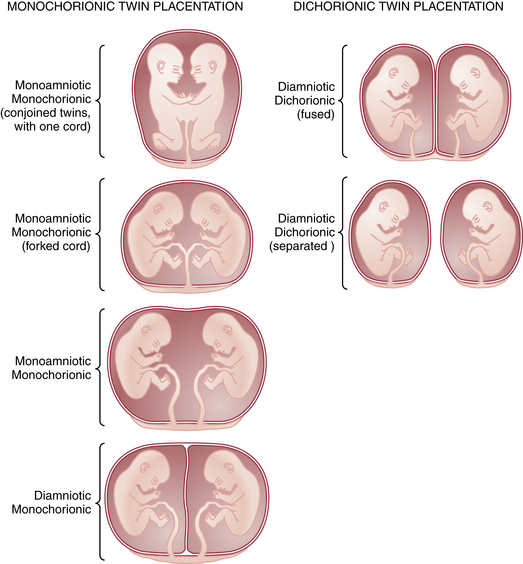
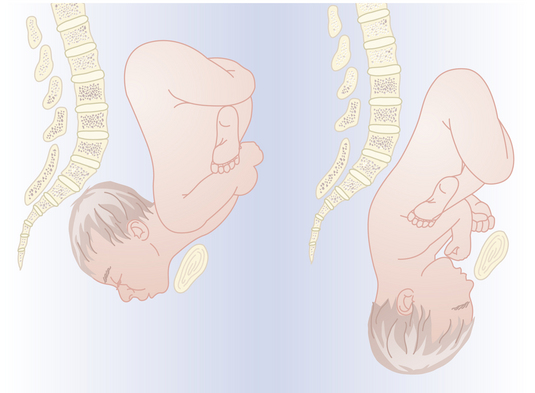
Multiple gestation occurs as the result of either the splitting of an embryo (i.e., identical or monozygotic twinning) or the fertilization of two or more eggs produced in a single menstrual cycle (i.e., fraternal or dizygotic twinning). Because dizygotic twins arise from separate eggs, they are structurally distinct pregnancies coexisting in a single uterus, each with its own amnion, chorion, and placenta. Monozygotic twins arise from cleavage of a single fertilized egg at various stages during embryogenesis, and thus the arrangement of the fetal membranes and placentas will depend on the time at which the embryo divides (Table 13-1). The earlier the embryo splits, the more separate the membranes and placentas will be. If division occurs within the first 72 hours of fertilization, the membranes will be dichorionic, diamniotic with a thick, four-layered intervening membrane. If division occurs after 4 to 8 days of development, when the chorion has already formed, monochorionic, diamniotic twins will evolve with a thin, two-layer septum. If splitting occurs after 8 days, when both amnion and chorion have already formed, the result will be monochorionic, monoamniotic twins residing in a single sac with no septum. Of all monozygotic twins, 30% are dichorionic, diamniotic, and 69% are monochorionic, diamniotic. Only 1% of twins are monoamnionic. Because twins share a sac in this type, without an intervening membrane, the risk for umbilical cord entanglement is high, resulting in a net mortality in these twins of almost 50% (Figure 13-1).
TABLE 13-1 RELATIONSHIP BETWEEN TIMING OF CLEAVAGE AND NATURE OF MEMBRANES IN TWIN GESTATIONS
| Time of Cleavage∗ | Nature of Membranes |
|---|---|
| 0-72 hr | Dichorionic, diamniotic |
| 4-8 days | Monochorionic, diamniotic |
| 9-12 days | Monochorionic, monoamniotic |
DETERMINATION OF ZYGOSITY
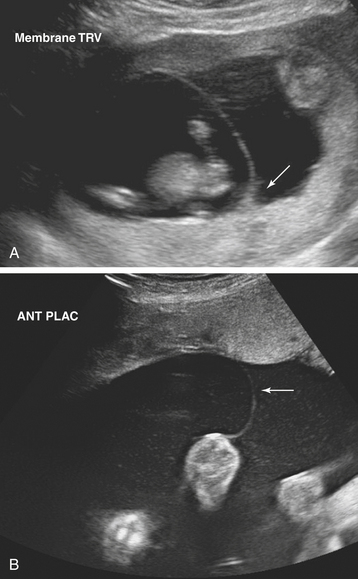
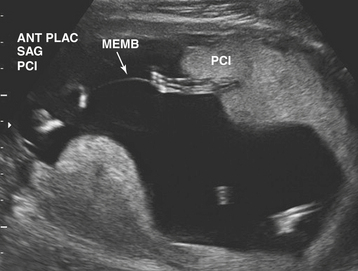
Ultrasonographic evaluation of the pregnancy is frequently very helpful in determining zygosity. Imaging of discordant fetal gender confirms a dizygotic gestation. Visualization of a thick amnion-chorion septum is suggestive of dizygotic twins, as is the presence of a “peak” or inverted “V” at the base of the membrane septum (Figure 13-2A). Conversely, in monochorionic gestation, the dividing membrane is fairly thin (Figure 13-2B). Because an early embryonic split can infrequently result in dichorionic, diamniotic twins with separate placentas, these findings are not definitive. Similarly, in rare cases of postzygotic genetic events, monochorionic twins may be gender discordant. Thus, confident diagnosis of zygosity may require detailed examination of the placenta after delivery. Thirty percent of twins will be of different sex and are, therefore, dizygotic. Twenty-three percent have monochorionic placentas and are, therefore, monozygotic. Twenty-seven percent have the same sex, dichorionic placentas, but different blood groupings, and must be, therefore, dizygotic. Twenty percent have the same sex, dichorionic placentas, and identical blood groupings. For the latter group, further studies, such as human leukocyte antigen (HLA) typing or DNA analysis, allow determination of zygosity.
ABNORMALITIES OF THE TWINNING PROCESS
Twin-Twin Transfusion Syndrome
TTTS is diagnosed using ultrasound. Typically the donor twin is smaller and may have oligohydramnios, absent bladder, and anemia. The recipient, on the other hand, is larger with possible polyhydramnios, cardiomegaly, and ascites or hydrops (Figure 13-3).
ANTEPARTUM MANAGEMENT
Because of the high risk for preterm birth, intensive antepartum management schemes are directed at prolonging gestation and increasing birth weight in order to decrease perinatal morbidity and mortality. The complications of multiple gestation are shown in Box 13-1.
Intrapartum Management
TREATMENT OF PRETERM LABOR
The treatment of preterm labor is discussed elsewhere, but multiple gestations present special challenges. Relative contraindications to tocolysis in these pregnancies include a gestational age of 34 weeks or more, growth failure of one or more fetuses, concerning fetal status on biophysical monitoring, and preeclampsia. Aggressive tocolysis typically involves use of agents with adverse cardiovascular effects in the mother, such as β-mimetics and magnesium sulfate and calcium channel blockers. These agents, particularly when combined with antenatal corticosteroid therapy, have been associated with maternal volume overload and congestive heart failure. Box 13-2 provides a list of necessary prerequisites for the management of labor in pregnancies complicated by multiple gestation.
BOX 13-2 Prerequisites for the Intrapartum Management of Multiple Gestation.
PERINATAL OUTCOME
The high perinatal mortality rate in twin gestations (30 to 50 per 1000 births), which is about 5 times that in singleton gestations, is largely attributable to prematurity and congenital anomalies (Box 13-3). Birth asphyxia is also a significant factor, and thus it is not surprising that second twins have twice the perinatal mortality of first-born twins. Compared with singletons, death from complications of birth trauma is 4 times more frequent with second-born twins and twice as frequent in first-born twins. Congenital anomalies and stillbirths account for about one third of the perinatal mortality rate. Stillbirths occur twice as frequently in twins as in singletons. Cerebral hemorrhage, asphyxia, and anoxia account for one tenth of the overall perinatal mortality rate.
Fetal Malpresentation
BREECH PRESENTATION
Classification
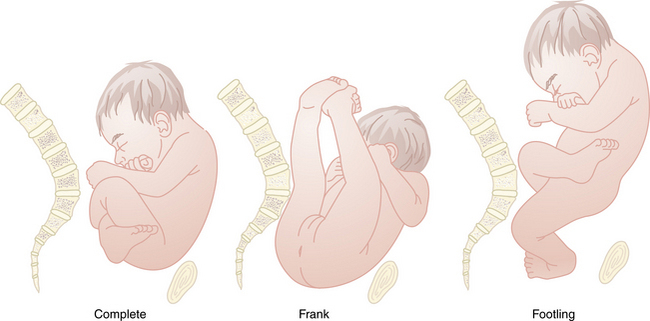
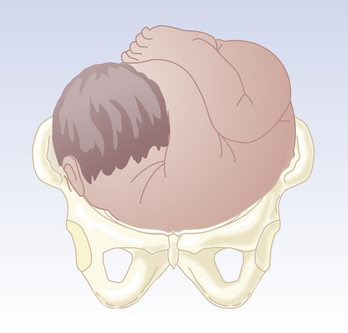
There are three types of breech presentation: frank, complete, and incomplete or footling (Figure 13-4). Frank breech occurs when both fetal thighs are flexed and both lower extremities are extended at the knees. A complete breech has both thighs flexed and one or both knees flexed (sitting in a “squat” position). An incomplete (or footling) breech has one or both thighs extended and one or both knees or feet lying below the buttocks. At term, 65% of breech fetuses are frank, 25% are complete, and 10% are incomplete.
Diagnosis
The diagnosis of breech presentation can often be made by the Leopold maneuvers (see Chapter 7, pg 86),in which the firm fetal head is palpated in the fundal region and the softer, smaller breech occupies the lower uterine segment above the symphysis pubis. In a frank breech in labor, the fetal buttocks, anus, sacrum, and ischial tuberosities can be palpated on vaginal examination. With a complete breech, the feet, ankles, and often the buttocks are palpable through the dilated cervix. Vaginal examination of an incomplete breech reveals one or both fetal feet but may require ultrasound for definitive diagnosis.
Labor Management
VAGINAL DELIVERY
Until the publication of randomized trials demonstrating that vaginal breech delivery is associated with increased perinatal mortality compared with planned cesarean birth, vaginal breech deliveries were performed in selected centers in patients who met strict criteria. These criteria are summarized in Box 13-4. The standard of care now in most practices is to deliver all breeches by cesarean birth to avoid the potential morbidities of umbilical cord prolapse, head entrapment, birth asphyxia, and birth trauma.
ASSISTED BREECH DELIVERY
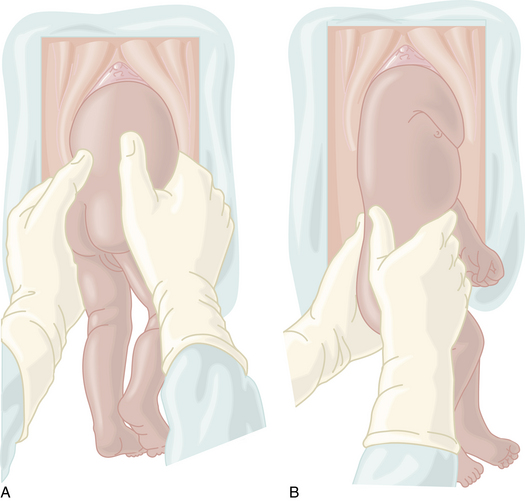
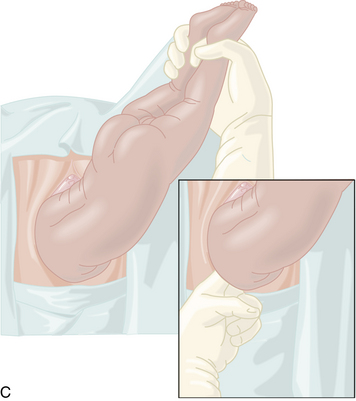
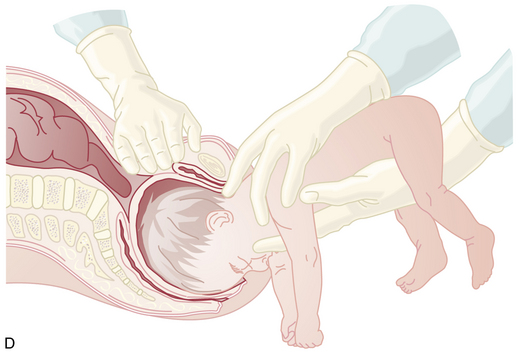
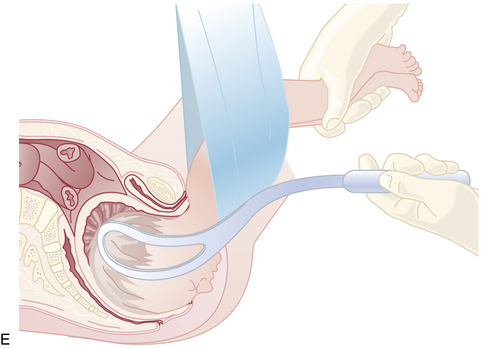
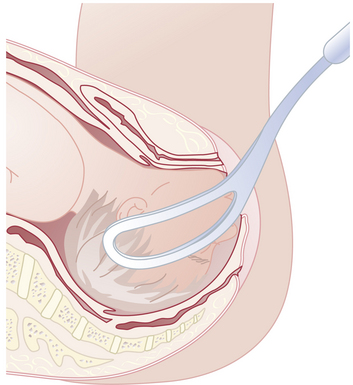
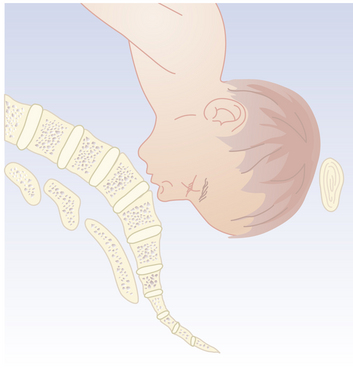
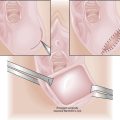
Because the breech presentation can present in a setting in which cesarean birth is impossible or unsafe, vaginal delivery of the breech continues to be an important practitioner skill. Once the fetus has delivered spontaneously to the umbilicus (Figure 13-5A), gentle downward traction is exerted until the scapulae appear at the introitus (see Figure 13-5B). After delivery of the scapulae, the shoulders are delivered by sweeping each arm in turn across the fetal chest until only the fetal head remains undelivered (see Figure 13-5C). Once the shoulders have been delivered, the head is delivered by manual flexion of the fetal head with one hand flexing the head at the base of the skull while the operator’s other hand is applied to the fetal maxilla for downward flexion (see Figure 13-5D). Some obstetricians use Piper forceps routinely because this method has been shown to result in delivery of the head with the least amount of trauma to the fetus (see Figure 13-5E).
FACE PRESENTATION
Mechanism of Labor
The position of the presenting face is classified according to the location of the fetal chin (mentum). About 60% of face presentations are mentum anterior at the time of diagnosis, whereas 15% are mentum transverse and 25% mentum posterior. The mechanism of labor with a face presentation is similar to the vertex presentation in that the longest diameter (mentum to brow) enters the pelvis transversely. As labor proceeds and the face descends to the midplane, internal rotation occurs into the vertical axis. If the mentum rotates anteriorly under the symphysis pubis, vaginal delivery should be expected. Forceps, but not vacuum, can be applied to assist if prerequisites are met. However, if the mentum rotates posteriorly, the fetal head will be unable to extend farther to complete the expulsive process. Thus, mentum posterior cases and those with persistent mentum transverse must be delivered by cesarean birth. However, because final rotation from mentum transverse may occur only after a significant period of maternal pushing, patience is necessary. About half of the mentoposterior and mentotransverse presentations spontaneously rotate to a mentoanterior position. When delivered by spontaneous vaginal delivery (Figure 13-6) or low forceps (Figure 13-7), perinatal morbidity and mortality for face presentations are similar to those for vertex presentations.
Other Presentations
Brow presentation occurs when the presenting part of the fetus is between the facial orbits and anterior fontanelle (Figure 13-8). This type of presentation arises as the result of extension of the fetal head such that it is midway between flexion (vertex presentation) and hyperextension (face presentation). The incidence is about 1 in 1400 deliveries. With a brow presentation, the presenting diameter is the supraoccipitomental diameter, which is much longer than the presenting diameter for a face or a vertex presentation.
A compound presentation occurs when a fetal extremity (usually the hand) prolapses alongside the presenting part (the head) and both parts enter the maternal pelvis at the same time. This presentation occurs more frequently with premature gestations. The incidence of a hand or arm prolapsing alongside the presenting fetal head is 1 in 700 deliveries, and management is expectant. Usually, the prolapsed part of the fetus does not interfere with labor. If the arm prolapses, it is best to wait to see if it moves out of the way as the head descends. If it does not, the arm may be gently pushed upward while the head is simultaneously pushed downward by fundal pressure. If the complete extremity prolapses and the fetus then converts to a shoulder presentation (Figure 13-9), delivery must be accomplished by cesarean birth.
American College of Obstetricians and Gynecologists. Committee Opinion No. 265: Mode of term single breech delivery. Obstet Gynecol. 2001;98:1189-1190.
American College of Obstetricians and Gynecologists. Multiple gestation: Complicated twin, triplet, and high-order multifetal pregnancy. ACOG Practice Bulletin No. 56. Obstet Gynecol. 2004;104:869-883.
Cruikshank D.P. Intrapartum management of twin gestations. Obstet Gynecol. 2007;109:1167-1176.
Schmitz T., Carnavalet C., de C., Azria E., et al. Neonatal outcomes of twin pregnancy according to the planned mode of delivery. Obstet Gynecol. 2008;111:695-703.