“Macrocystic” pattern: Few (< 6) macrocystic locules, which are relatively large (> 2 cm)
• MR: Usually simple fluid signal (high T2; low T1), but may be slightly less T2 hyperintense due to mucin content
CLINICAL ISSUES
• Considered premalignant or frankly malignant: Rate of malignancy in different series ranges between 10-40%
• Risk factors for invasive malignancy: Older age, lesion size, mural nodularity, thick wall, patient symptoms (pain, pancreatitis), and ↑ CEA and CA 19-9
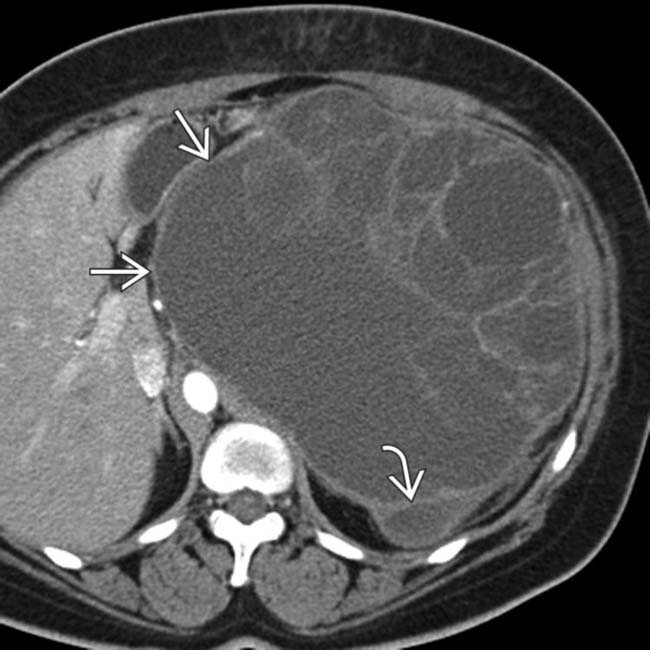
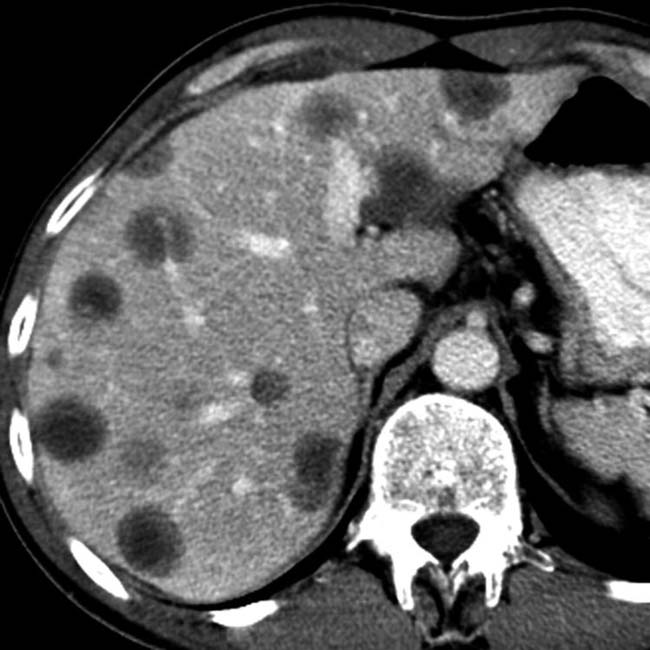
 arising from the pancreatic tail with multiple internal cystic locules and septations, some of which are thick
arising from the pancreatic tail with multiple internal cystic locules and septations, some of which are thick  . This mass was found to be a mucinous cystic neoplasm (MCN) with invasive adenocarcinoma at surgery.
. This mass was found to be a mucinous cystic neoplasm (MCN) with invasive adenocarcinoma at surgery.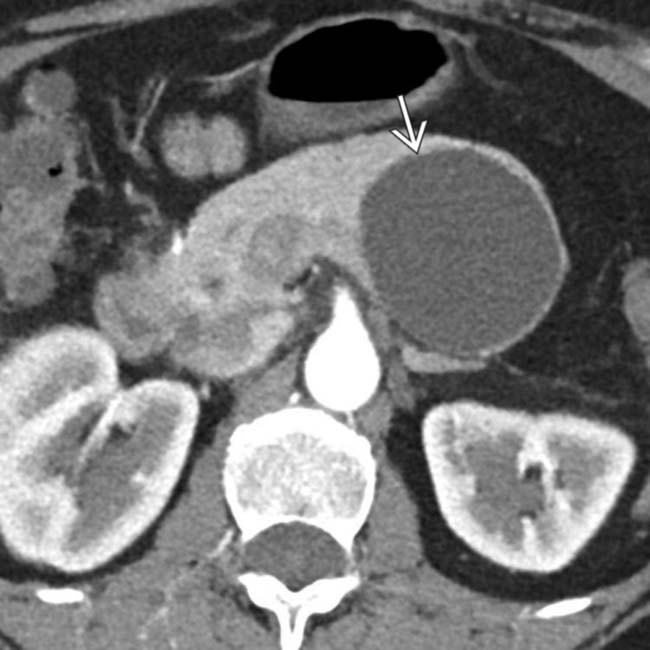
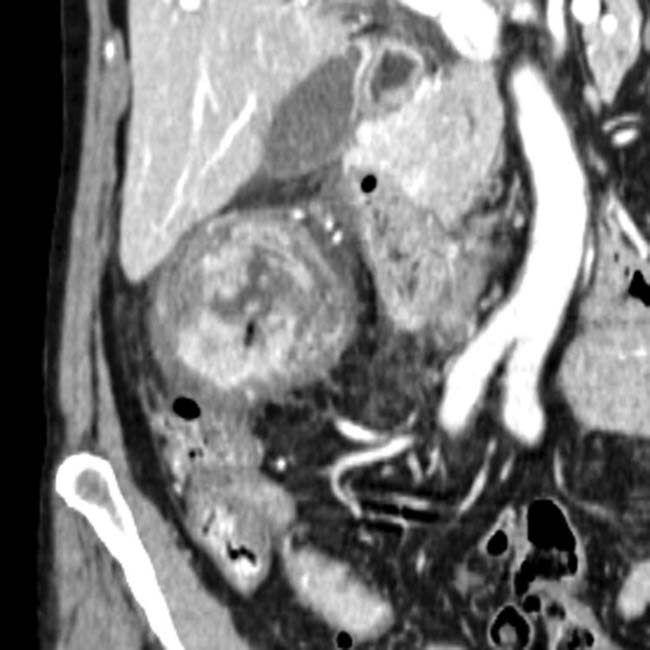
 arising from the upstream pancreatic body.
arising from the upstream pancreatic body.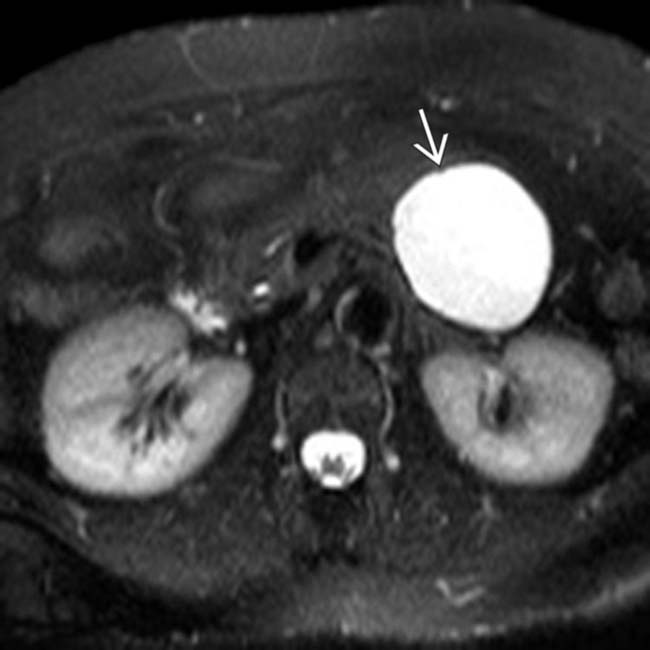
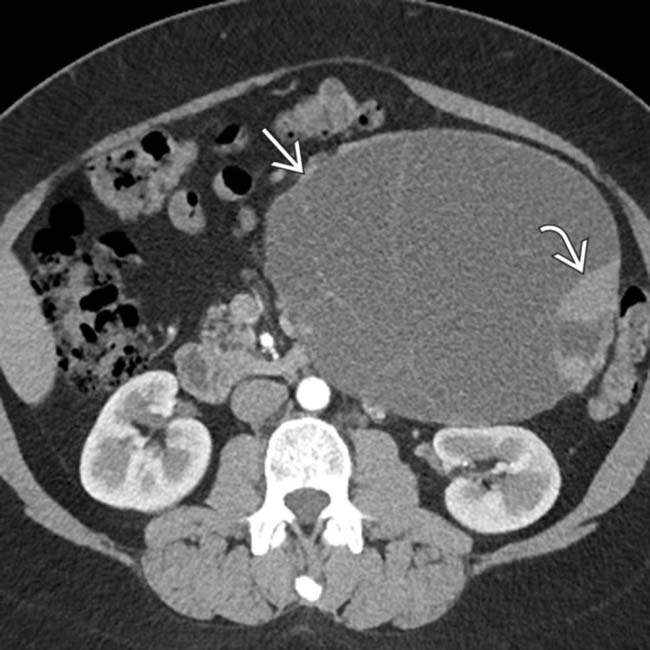
 . MR can sometimes show complexity and suspicious features that might be difficult to identify on CT. This was found to be a MCN with low-grade dysplasia at surgery.
. MR can sometimes show complexity and suspicious features that might be difficult to identify on CT. This was found to be a MCN with low-grade dysplasia at surgery.IMAGING
General Features
DIFFERENTIAL DIAGNOSIS
Pancreatic Pseudocyst
CLINICAL ISSUES
Presentation
• Most common signs/symptoms
Natural History & Prognosis
• Because almost all MCNs are resected, true natural history not completely certain: Rate of malignancy in different series ranges between 10-40%
• Overall 5-year survival of MCNs is 98% for benign lesions and 62% for lesions with high-grade dysplasia or invasive carcinoma
 extending exophytically downwards from the pancreatic tail with multiple septations and enhancing mural nodularity
extending exophytically downwards from the pancreatic tail with multiple septations and enhancing mural nodularity  .
.
 and mural enhancing soft tissue
and mural enhancing soft tissue  , both of which raise suspicion for malignancy. This was found to be a MCN with high-grade dysplasia at surgery.
, both of which raise suspicion for malignancy. This was found to be a MCN with high-grade dysplasia at surgery.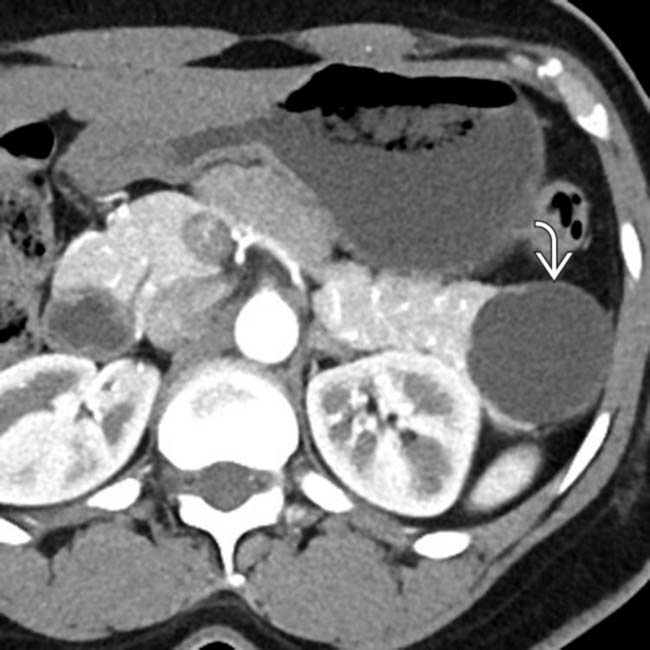
 in the pancreatic tail, found to be a MCN with low-grade dysplasia at surgery. There is no mural nodularity or a thick-wall to suggest invasive malignancy.
in the pancreatic tail, found to be a MCN with low-grade dysplasia at surgery. There is no mural nodularity or a thick-wall to suggest invasive malignancy.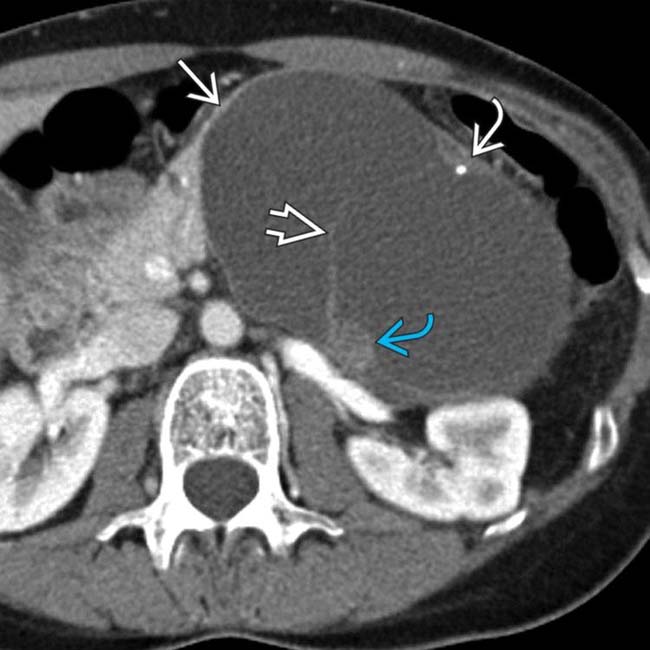
 arising from the pancreatic body. The mass has large cystic spaces separated by septa
arising from the pancreatic body. The mass has large cystic spaces separated by septa  . Also noted are peripheral calcification
. Also noted are peripheral calcification  and soft tissue mural nodularity
and soft tissue mural nodularity  . This is the classic imaging presentation of MCN.
. This is the classic imaging presentation of MCN.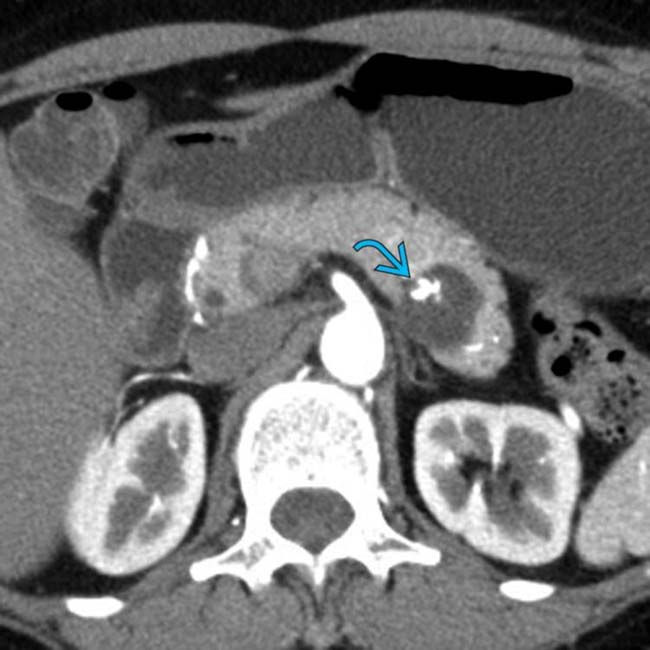
 .
.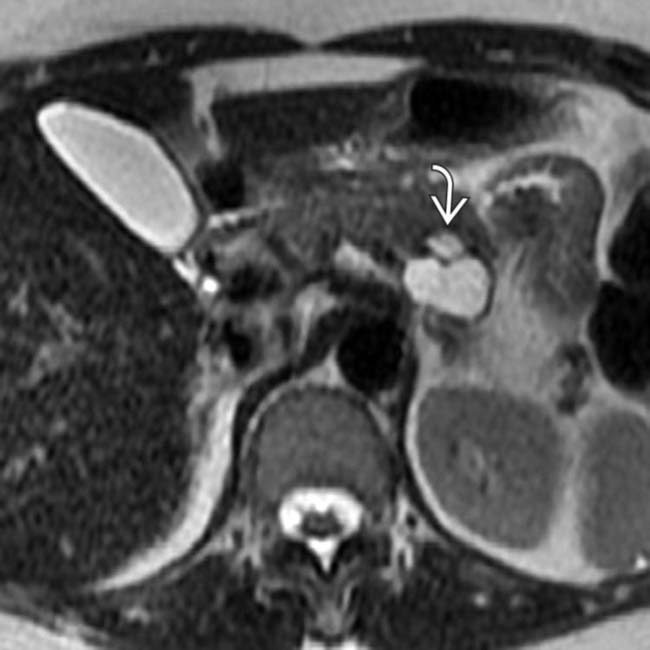
 . MCN with low-grade dysplasia was found at surgery.
. MCN with low-grade dysplasia was found at surgery.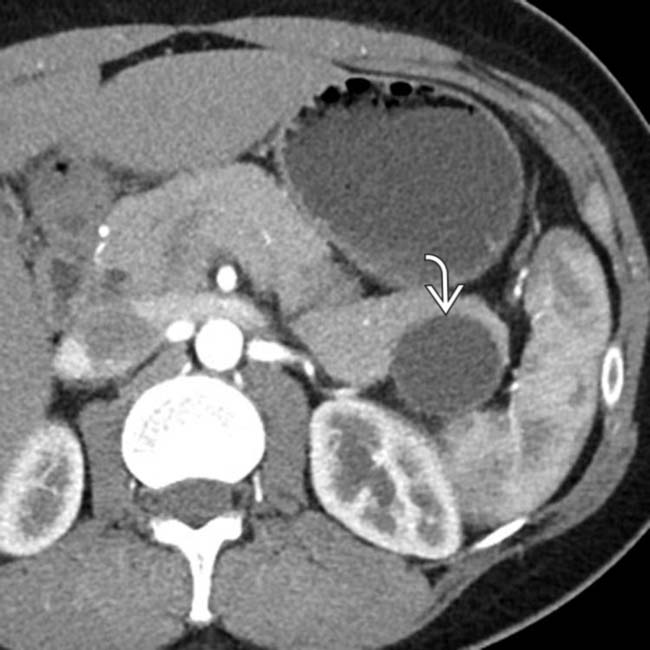
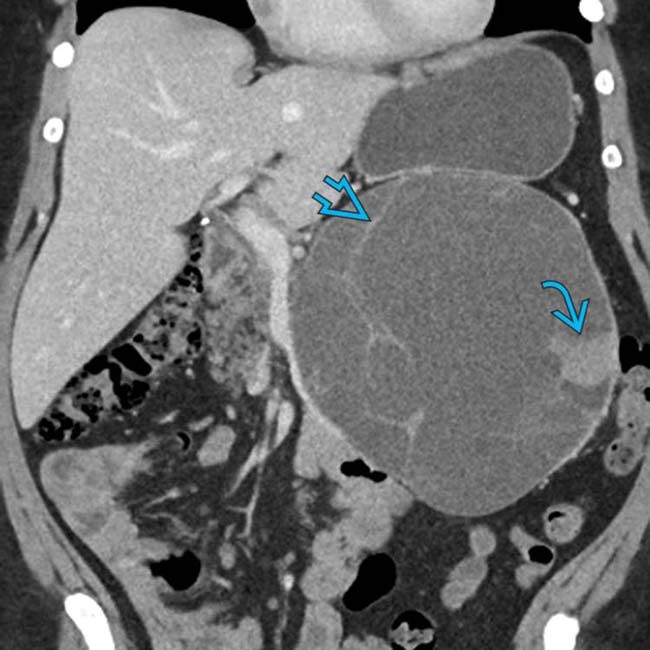
 arising from the pancreatic tail. No internal complexity is visualized.
arising from the pancreatic tail. No internal complexity is visualized.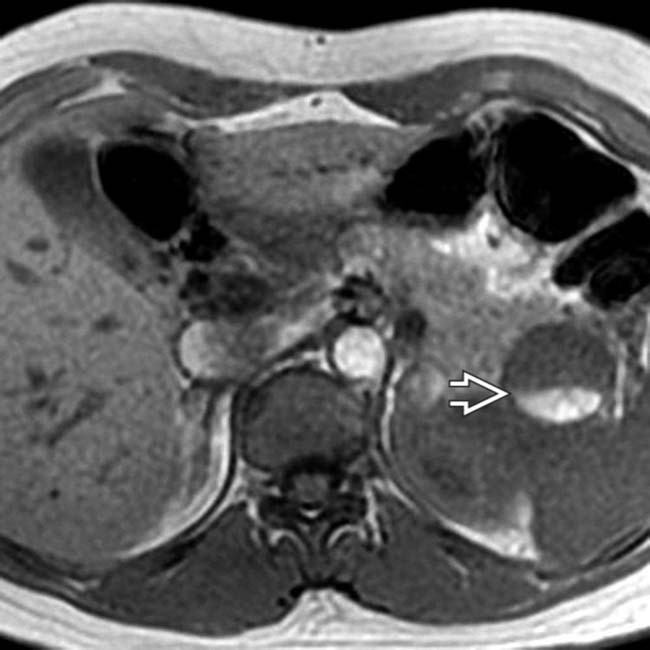
 within the cyst, representing a hematocrit level. The presence of hemorrhage within an MCN is quite unusual.
within the cyst, representing a hematocrit level. The presence of hemorrhage within an MCN is quite unusual.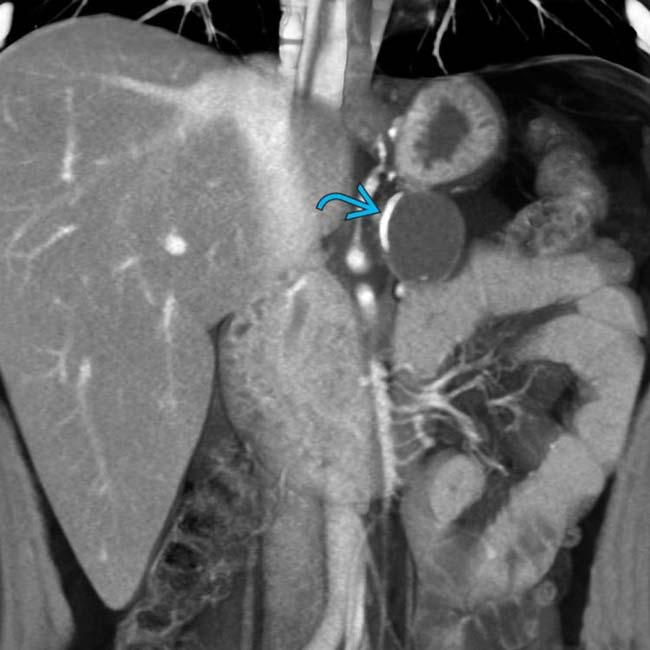
 . Calcifications are seen in roughly 16% of MCNs.
. Calcifications are seen in roughly 16% of MCNs.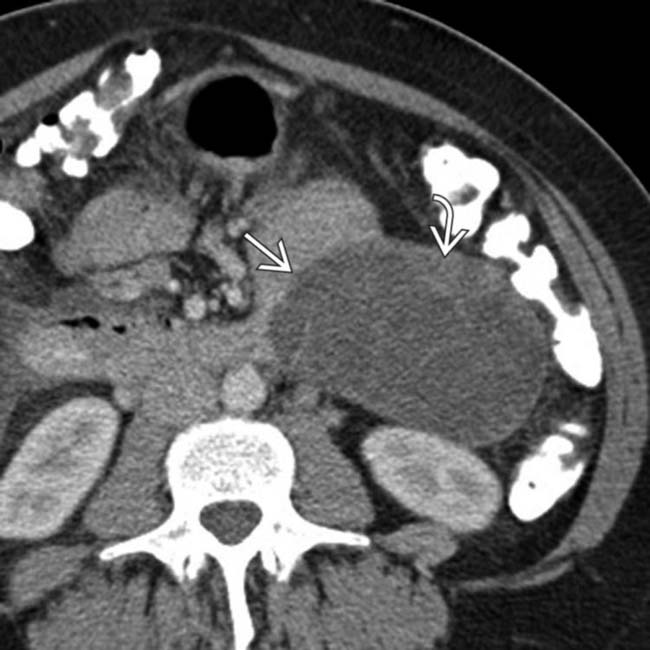
 arising from the pancreatic tail with multiple internal septations and solid mural nodularity
arising from the pancreatic tail with multiple internal septations and solid mural nodularity  . The complexity of the lesion raises suspicion for malignancy, which was confirmed after surgery where an MCN with invasive carcinoma was found.
. The complexity of the lesion raises suspicion for malignancy, which was confirmed after surgery where an MCN with invasive carcinoma was found.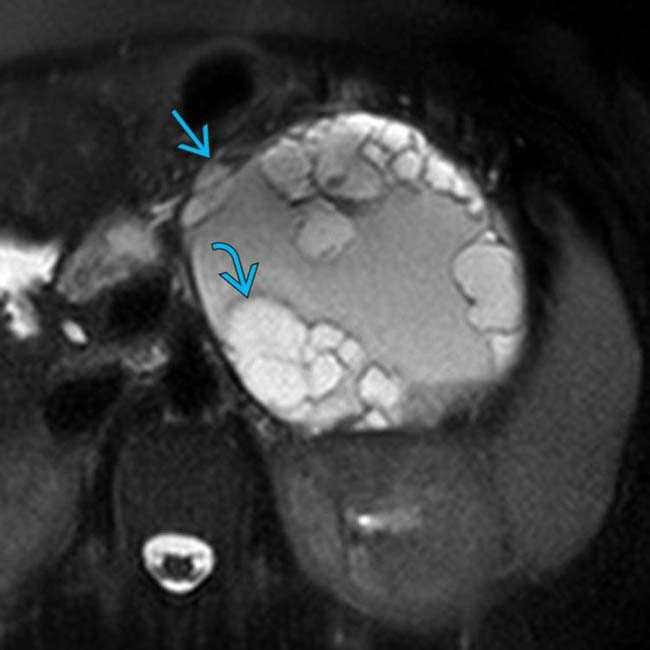
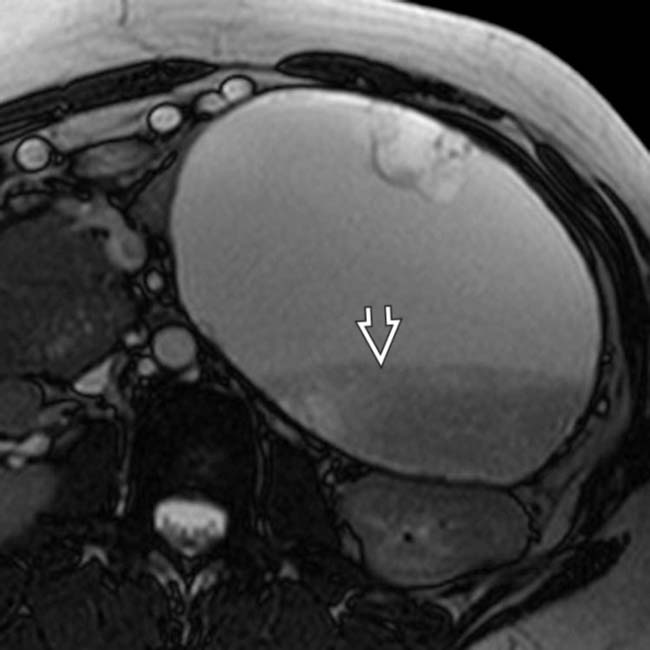
 arising from the pancreatic tail with innumerable internal T2-hyperintense cystic locules
arising from the pancreatic tail with innumerable internal T2-hyperintense cystic locules  .
.
 , probably representing old blood products or proteinaceous debris. This was found to be an MCN with invasive carcinoma at resection.
, probably representing old blood products or proteinaceous debris. This was found to be an MCN with invasive carcinoma at resection.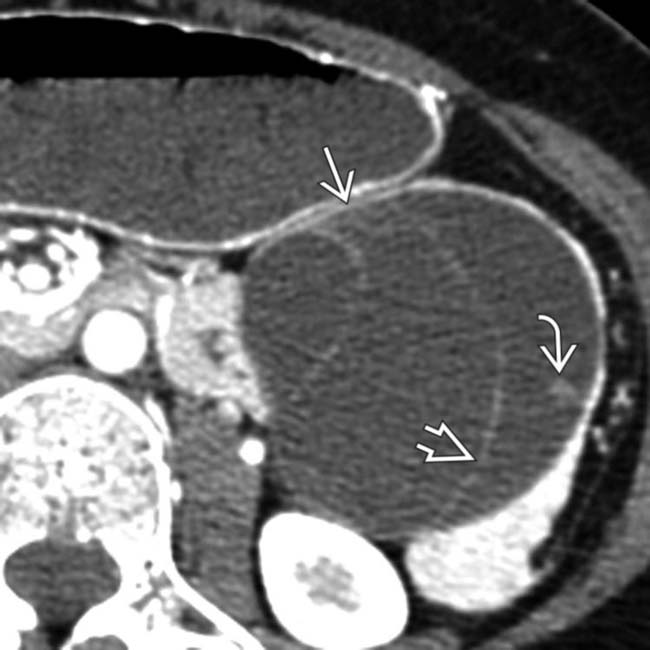
 with internal septations
with internal septations  originating from the tail of the pancreas. There is a single mural nodule
originating from the tail of the pancreas. There is a single mural nodule  . At surgery, a benign MCN was resected with no evidence of adenocarcinoma.
. At surgery, a benign MCN was resected with no evidence of adenocarcinoma.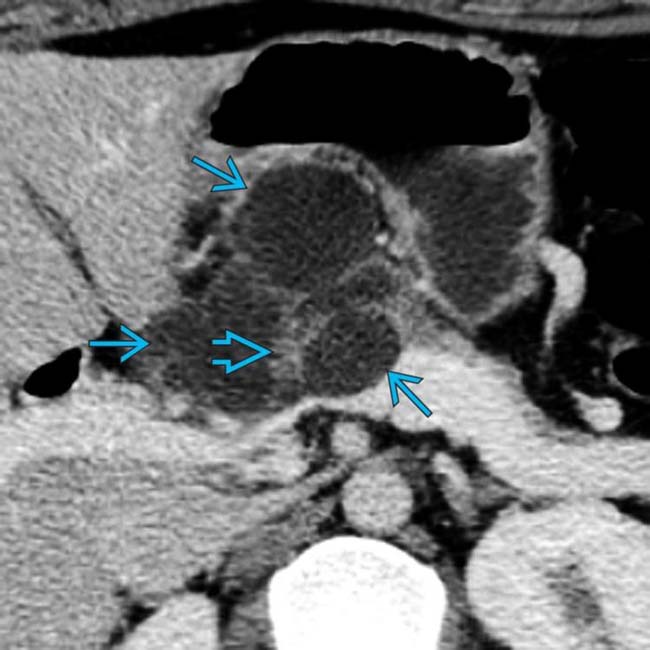
 with internally enhancing soft tissue
with internally enhancing soft tissue  . EUS-guided biopsy revealed mucinous adenocarcinoma. The presence of enhancing soft tissue within the cyst is strongly suspicious for malignancy.
. EUS-guided biopsy revealed mucinous adenocarcinoma. The presence of enhancing soft tissue within the cyst is strongly suspicious for malignancy.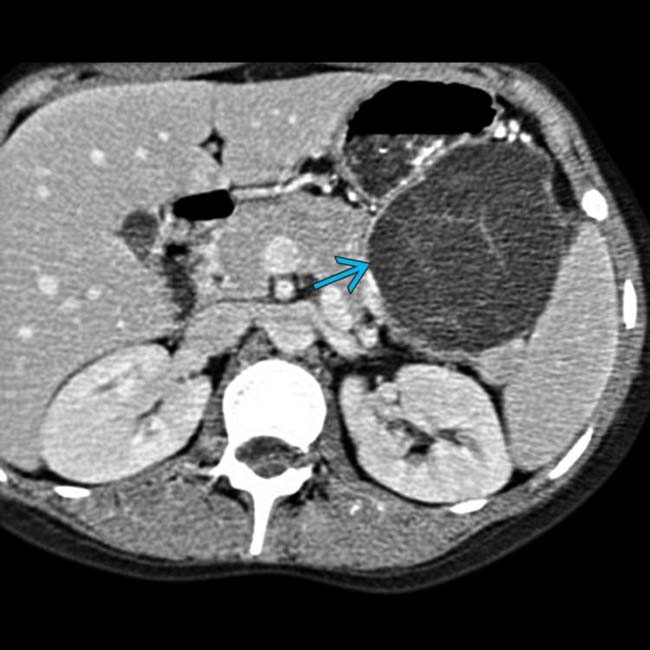
 in the body/tail segment of the pancreas. Note the septa and the large cystic spaces within the mass.
in the body/tail segment of the pancreas. Note the septa and the large cystic spaces within the mass.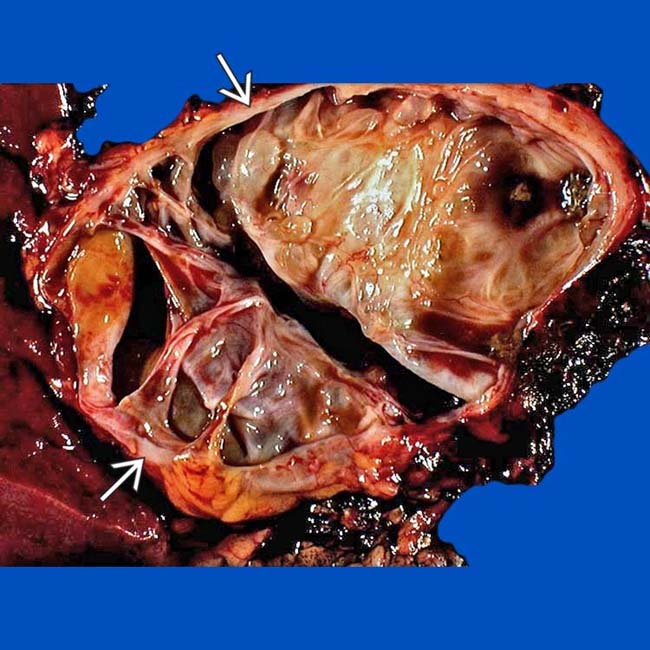
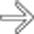 was full of mucinous fluid and had thickened septa. Histologic exam showed cellular atypia, and the lesion was considered a low-grade malignancy.
was full of mucinous fluid and had thickened septa. Histologic exam showed cellular atypia, and the lesion was considered a low-grade malignancy.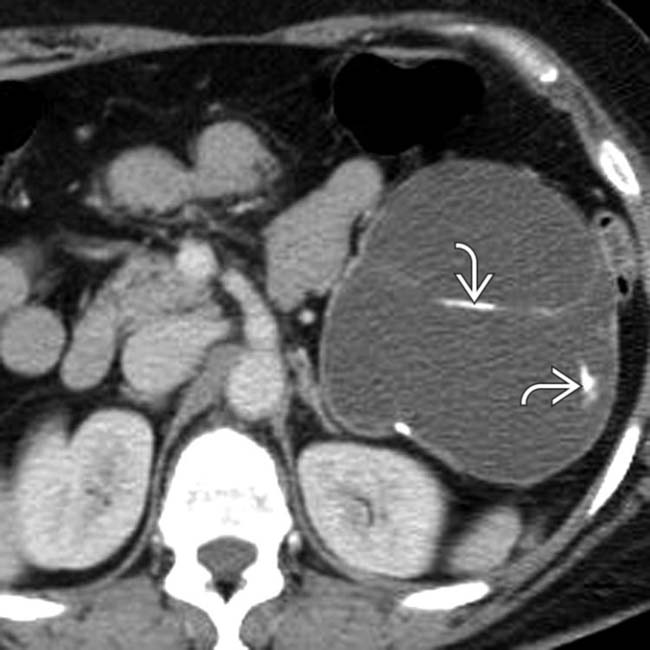
 .
.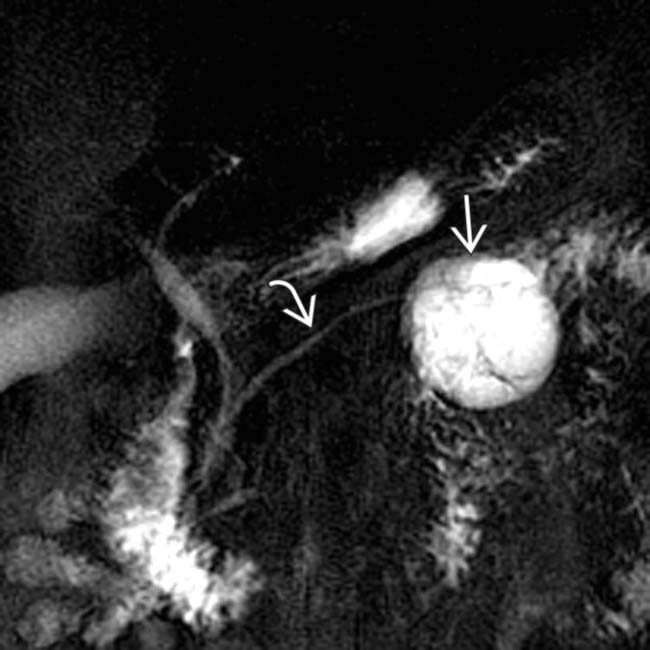
 in the pancreatic tail with a few cystic spaces and septa. The pancreatic duct
in the pancreatic tail with a few cystic spaces and septa. The pancreatic duct  is deviated but otherwise normal.
is deviated but otherwise normal.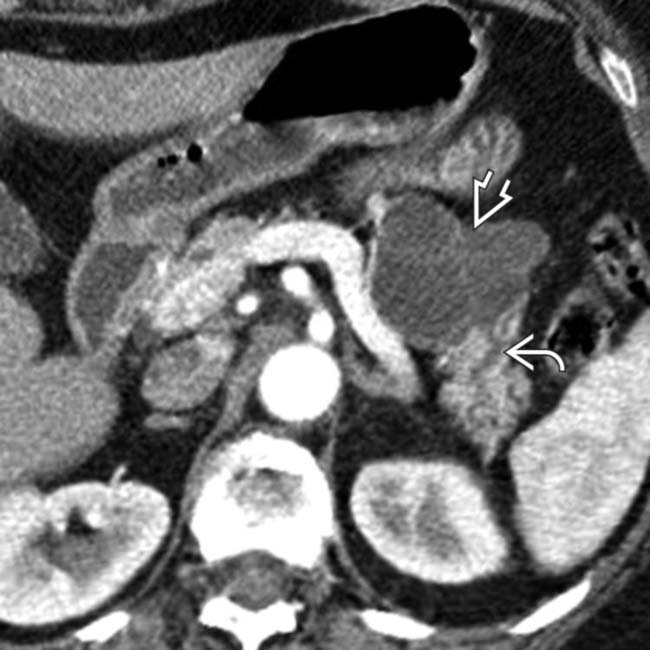
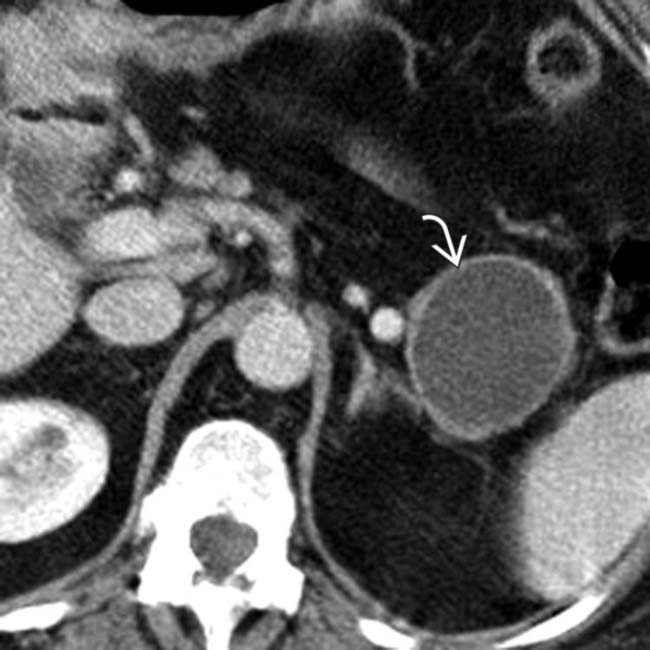
 in the pancreatic body containing a few cystic spaces and septa. The tumor compresses the pancreatic duct, causing distal dilatation
in the pancreatic body containing a few cystic spaces and septa. The tumor compresses the pancreatic duct, causing distal dilatation 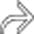 .
.
 in the pancreatic tail. Note the visible noncalcified wall.
in the pancreatic tail. Note the visible noncalcified wall.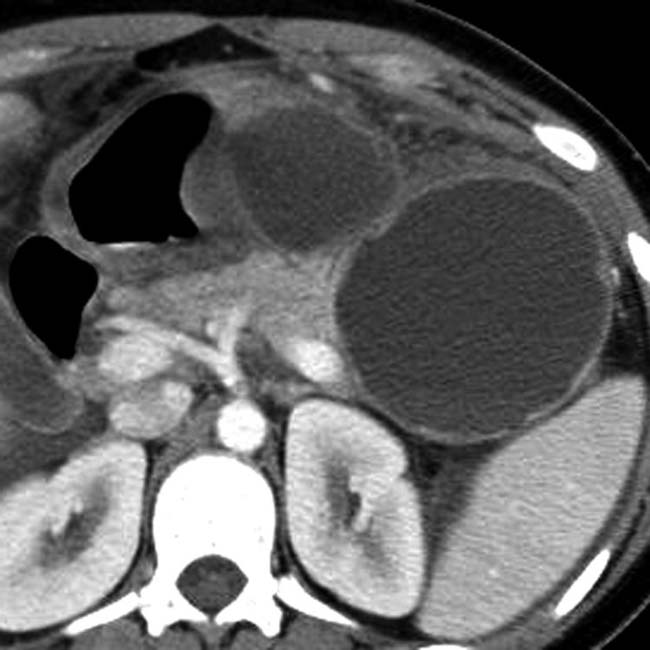
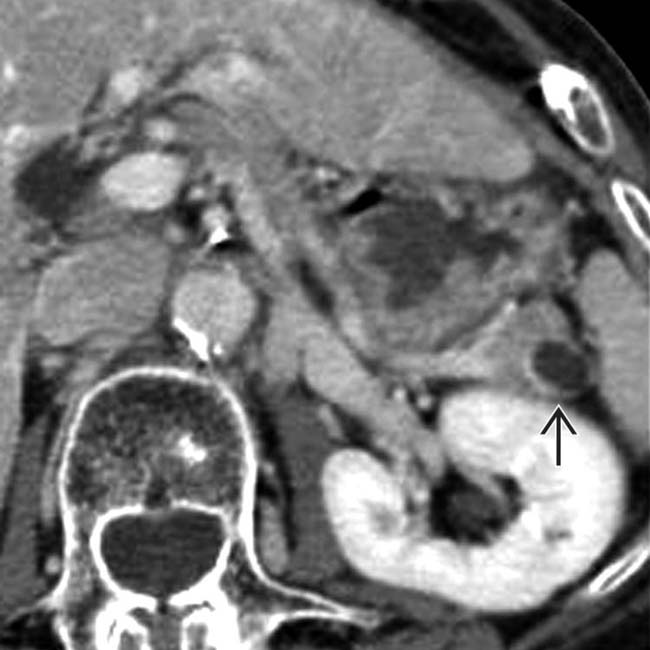
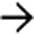 in the pancreatic tail. Mucinous cystic tumor was confirmed at surgical resection.
in the pancreatic tail. Mucinous cystic tumor was confirmed at surgical resection.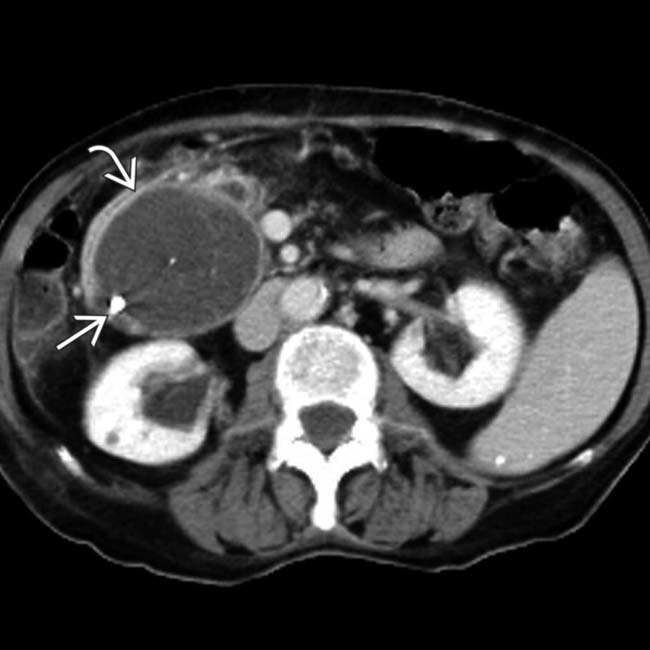
 in the head of the pancreas that causes obstruction of the pancreatic duct and common bile duct (not shown). The mass has peripheral wall calcification
in the head of the pancreas that causes obstruction of the pancreatic duct and common bile duct (not shown). The mass has peripheral wall calcification  and several internal septations. The location of this MCN in the head, and the ductal obstruction are unusual features of this tumor.
and several internal septations. The location of this MCN in the head, and the ductal obstruction are unusual features of this tumor.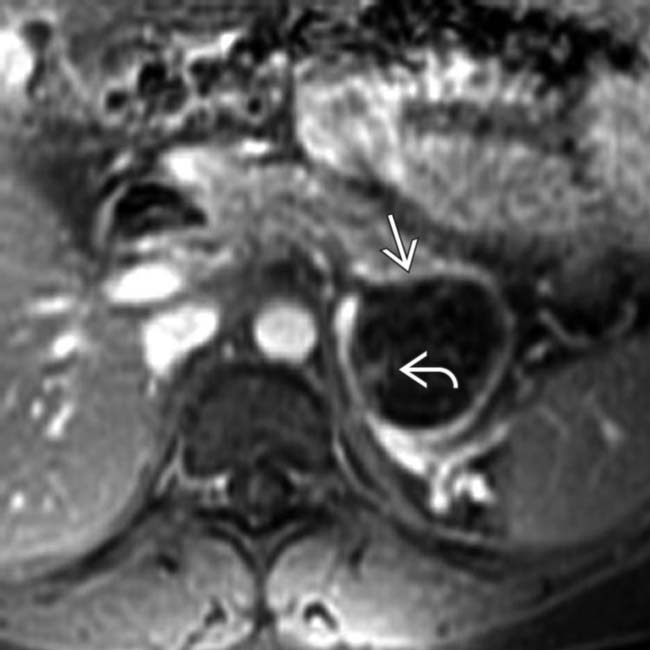
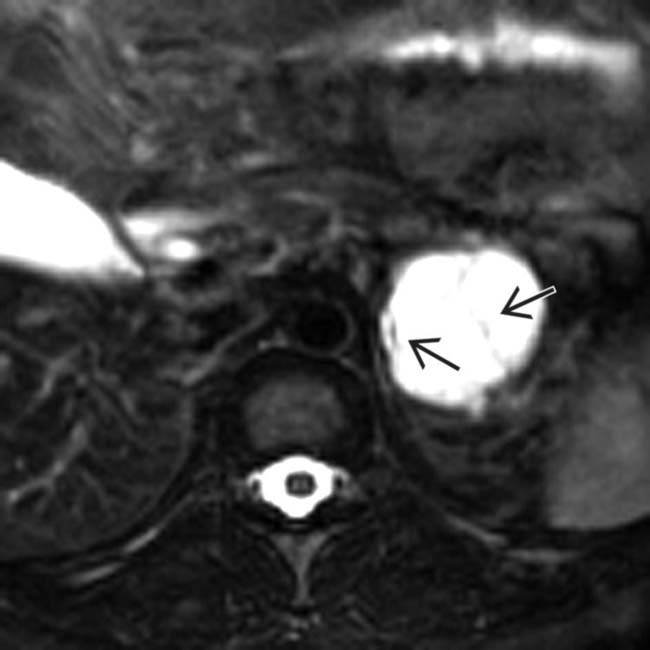
 in the pancreatic tail. Note the enhancing wall and septa
in the pancreatic tail. Note the enhancing wall and septa  .
.
 within the cystic mass. Surgical resection was performed and a benign mucinous cystic neoplasm was found.
within the cystic mass. Surgical resection was performed and a benign mucinous cystic neoplasm was found.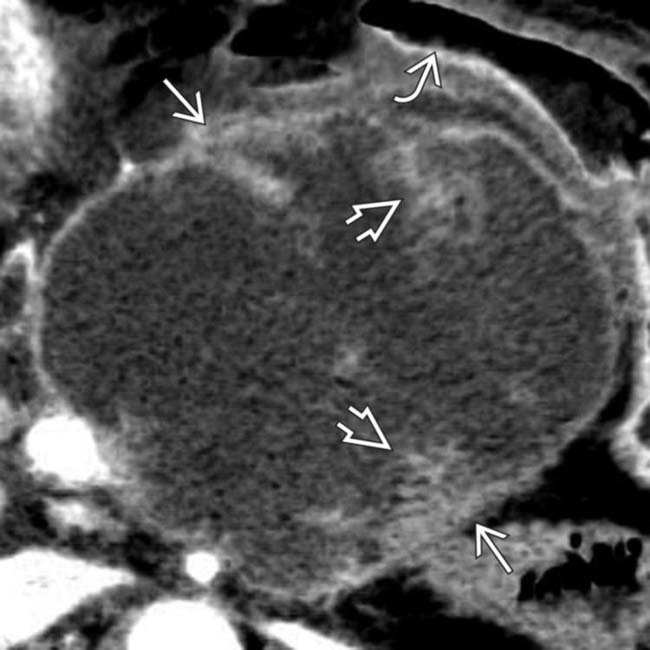
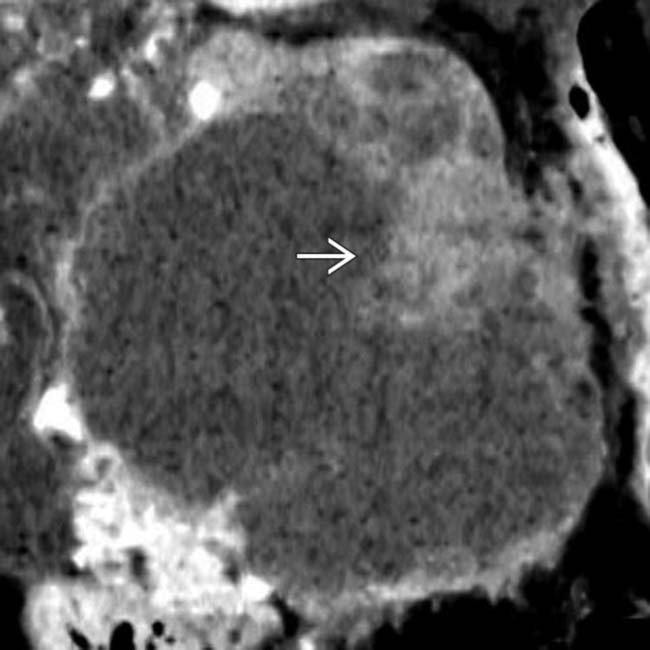
 arising from the body of the pancreas. Note the soft tissue mural nodules
arising from the body of the pancreas. Note the soft tissue mural nodules  , as well as gastric invasion
, as well as gastric invasion  .
.
 strongly favoring a malignant lesion. EUS-guided biopsy revealed mucinous cystadenocarcinoma.
strongly favoring a malignant lesion. EUS-guided biopsy revealed mucinous cystadenocarcinoma.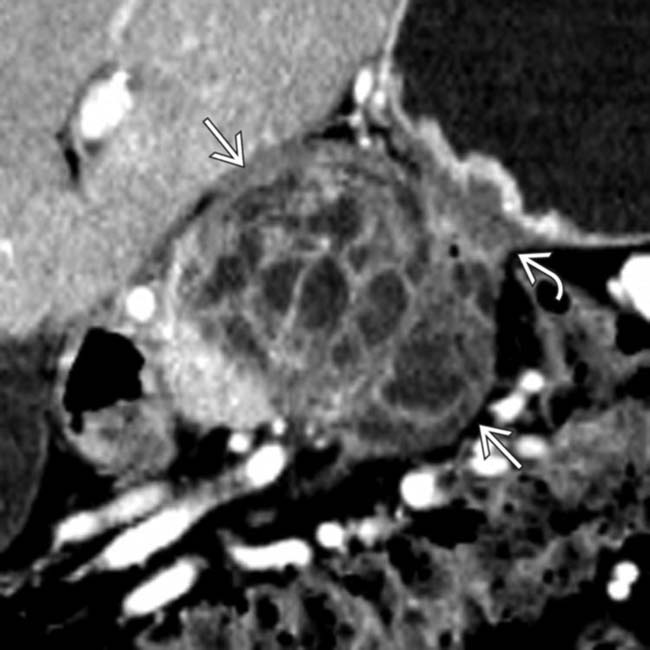
 in the body of the pancreas containing both solid and cystic elements. Note that the mass invades into the adjacent serosa of the stomach
in the body of the pancreas containing both solid and cystic elements. Note that the mass invades into the adjacent serosa of the stomach  causing focal mural thickening.
causing focal mural thickening.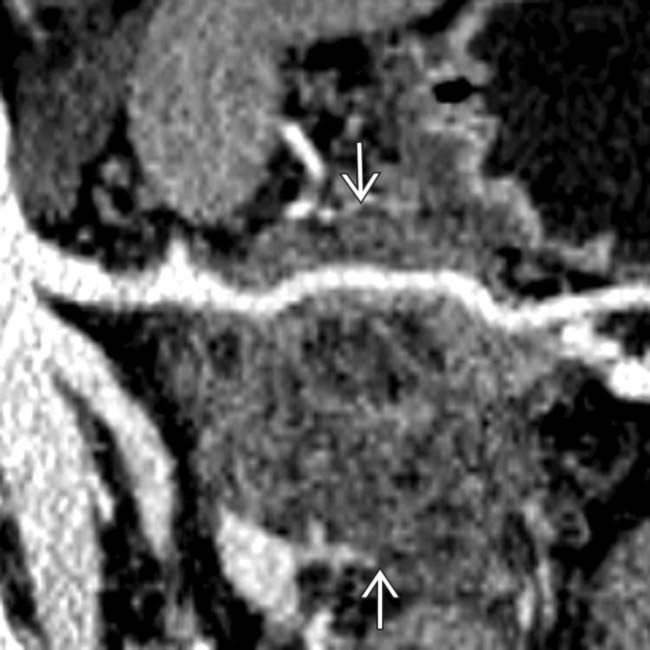
 confirming that this is a mucinous cystadenocarcinoma. Gastric invasion and splenic artery encasement make this unresectable for cure.
confirming that this is a mucinous cystadenocarcinoma. Gastric invasion and splenic artery encasement make this unresectable for cure.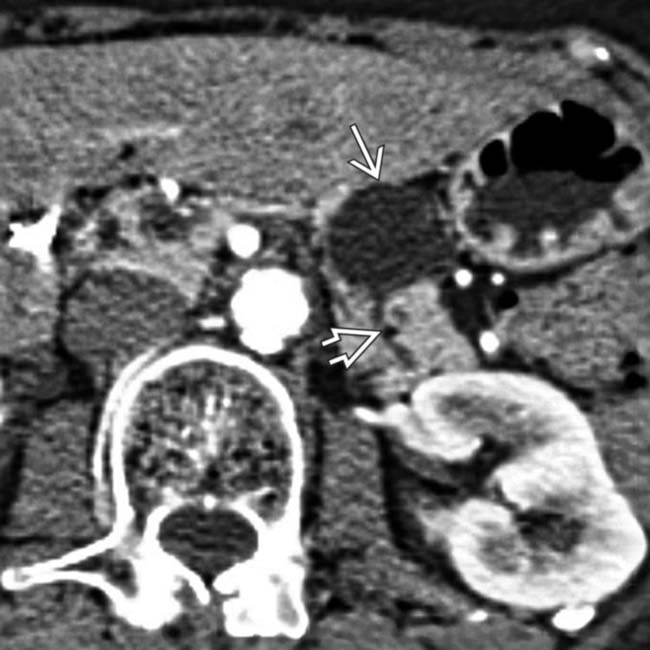
 in the body of the pancreas. The pancreatic duct
in the body of the pancreas. The pancreatic duct  is only mildly dilated, and there is no glandular atrophy “upstream” from the mass.
is only mildly dilated, and there is no glandular atrophy “upstream” from the mass.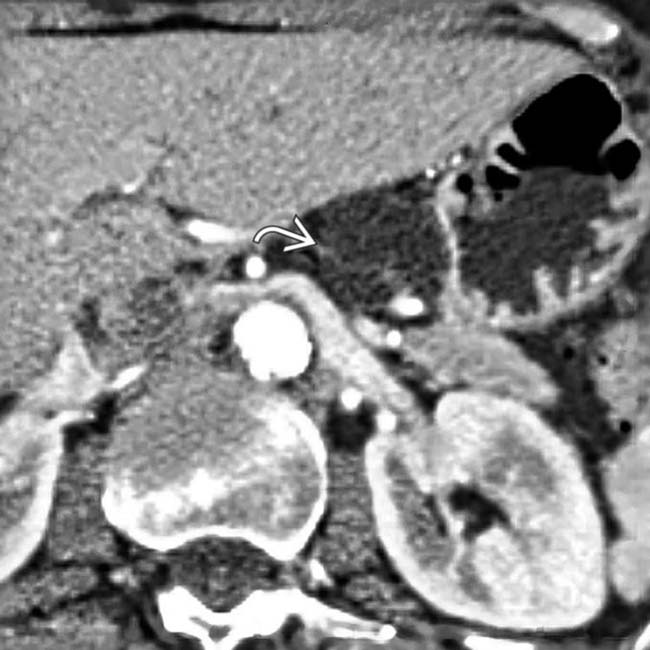
 visible within the mass. Endoscopic US confirmed complex cystic features of the mass, and aspiration of cyst contents revealed thick, mucinous fluid with elevated CEA and CA 19-9 markers. Surgical resection confirmed mucinous cystic tumor with dysplastic cells in cyst lining.
visible within the mass. Endoscopic US confirmed complex cystic features of the mass, and aspiration of cyst contents revealed thick, mucinous fluid with elevated CEA and CA 19-9 markers. Surgical resection confirmed mucinous cystic tumor with dysplastic cells in cyst lining.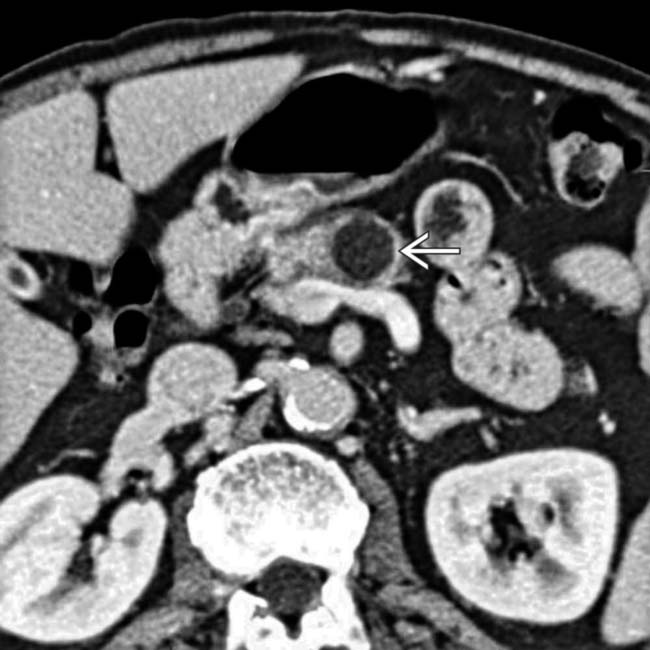
 that appears relatively “simple,” although it has a visible wall.
that appears relatively “simple,” although it has a visible wall.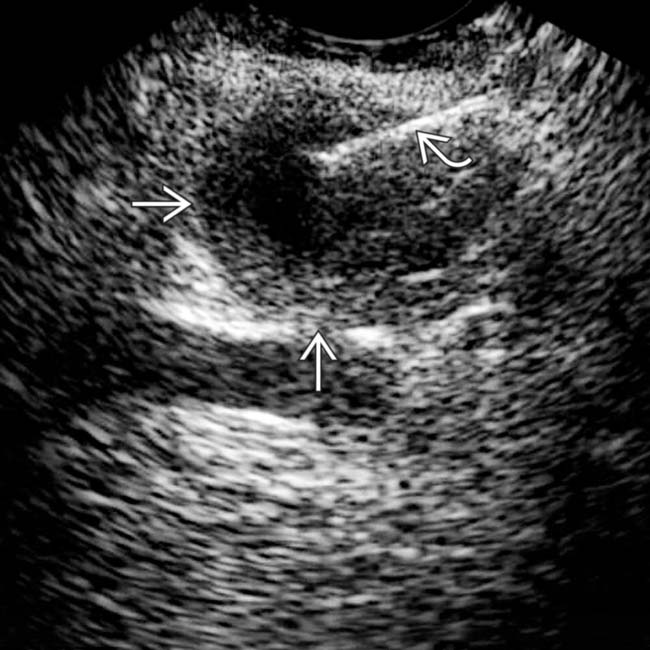
 . Needle aspiration
. Needle aspiration  yielded mucinous fluid. Resection showed a mucinous cystic neoplasm with malignant foci in the wall.
yielded mucinous fluid. Resection showed a mucinous cystic neoplasm with malignant foci in the wall.






















































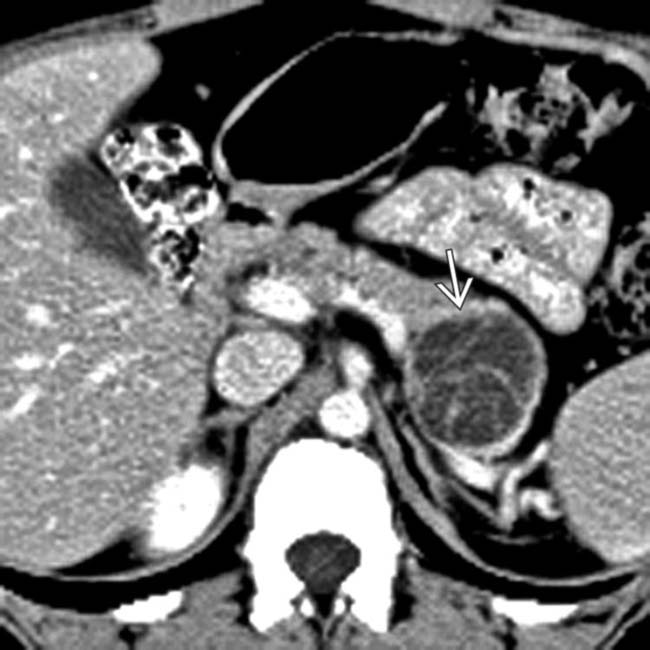
 in the pancreatic tail with multiple enhancing septa and displacement of the pancreatic duct.
in the pancreatic tail with multiple enhancing septa and displacement of the pancreatic duct.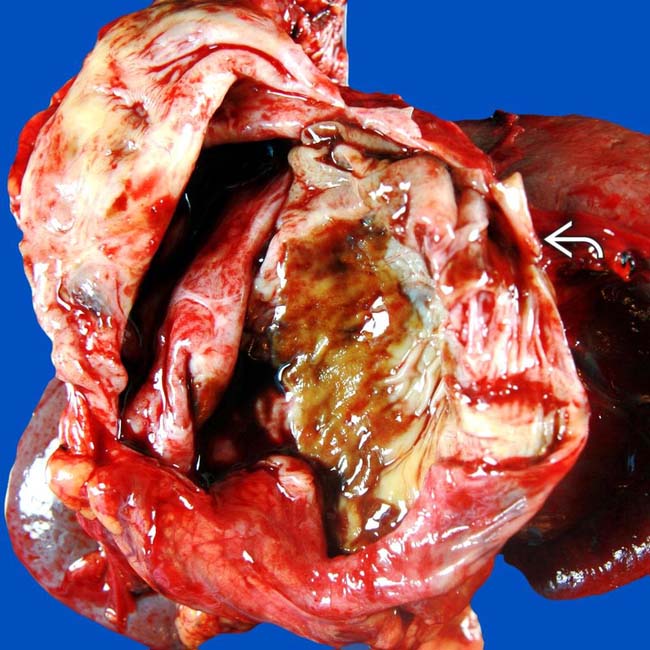
 located in the tail of the pancreas.
located in the tail of the pancreas.