CHAPTER 14 MRI Evaluation of Meningiomas
INTRODUCTION
Magnetic resonance imaging (MRI) is the state of art in the preoperative diagnosis of meningiomas. The diagnostic accuracy of standard MRI is approximately 95%. Further, contrast-enhanced MRI has the highest ability to detect and characterize meningiomas radiologically.1 This high rate of diagnostic accuracy can be attributed to special properties of the MRI technique presented in this chapter that provide vast information on meningiomas.
MRI OF THE DURA MATER RELATED TO MENINGIOMAS
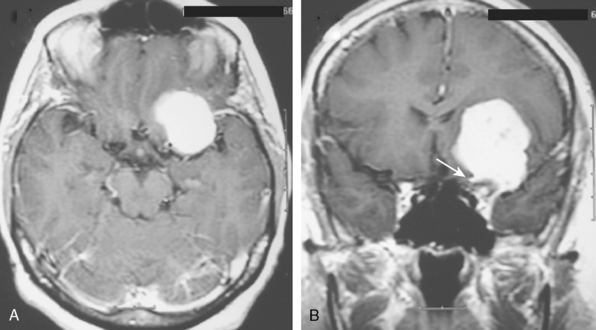
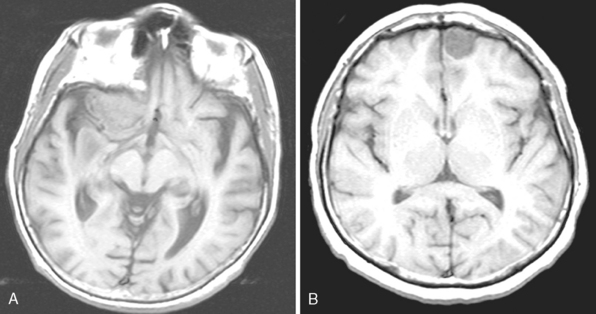
Meningiomas arise from the meningothelial cells of the arachnoid membranes, which are attached to the inner layer of the dura mater. This close relationship between the meningioma and the dura mater can be demonstrated on MR imaging. Normal dura mater is a non-neuronal connective tissue and appears as a hypointense thin layer adjacent to the hypointense inner table of bone on T1- and T2-WI (Fig. 14-1). After contrast injection, dura and the dural reflections of falx cerebri, tentorium, and cavernous sinus enhance as a thin discontinuous layer (Fig. 14-2).2 The demonstration of the dura mater and the adjacent meningioma on MRI supplies information about the extra-axial location and the origin of meningioma. In this respect, MRI is advantageous over computed tomography (CT) imaging, in which bone and enhanced dura both appear as hyperdense structures and cannot be distinguished from each other. The sensitivity of MRI is two to three times higher than that of CT for imaging dural and meningeal pathologies.3 Dural attachment of a meningioma is usually broad and the angle between the tumor and the dura is an obtuse one, indicating the extra-axial location of the meningioma (Figs. 14-2 and 14-3). Rarely the dural attachment may be narrow and a pedunculated meningioma can be demonstrated, more so in cases with large subarachnoid spaces where the peduncle becomes more conspicuous.
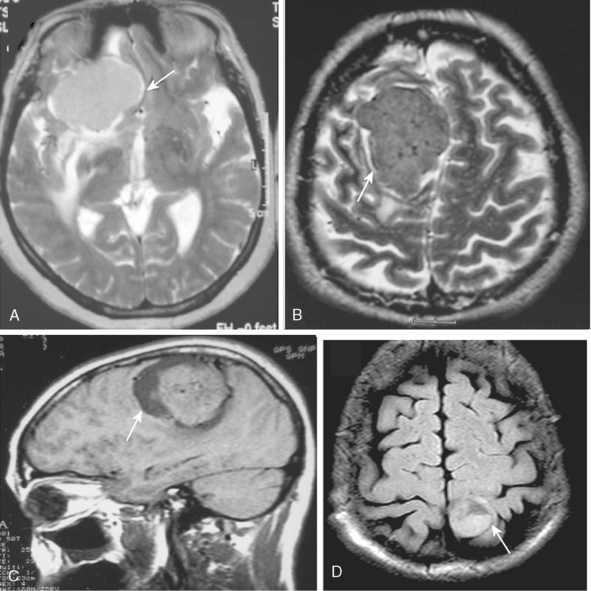
Pathologic enhancement of the dura mater and the adjacent inner table of bone, known as pachymeningeal enhancement, may be due to abnormally increased capillary permeability, increased blood volume, edema, tumor invasion of the dura mater, or as a result of surgery.2 The local pachymeningeal enhancement adjacent to a meningioma which tapers smoothly away from the tumor is designated as a dural “tail” (Fig. 14-4). The dural “tail,” which is depicted radiologically only on MRI, is reported in 52% to 72% of meningiomas. Nevertheless, it is nonspecific, and has also been associated with lymphomas, sarcoidosis, schwannomas, metastatic tumors, syphilitic gumma, and hemangiopericytomas.4–10 A dural “tail” may also be demonstrated, without contrast injection, on MR FLAIR sequences. Both meningiomas and the dural “tail” are demonstrated on FLAIR sequences, yet the dural “tail” usually has higher signal intensity than the tumor.11
MRI FINDINGS SUPPORTING EXTRA-AXIAL LOCALIZATION OF MENINGIOMAS
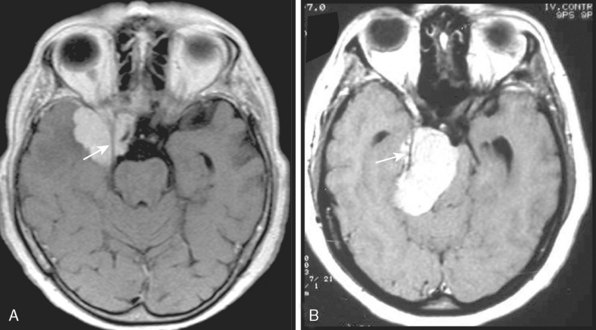
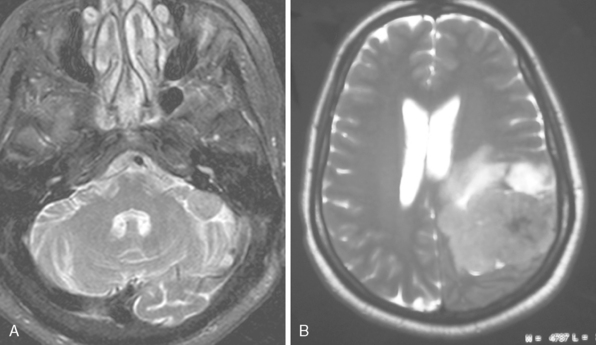
The extra-axial localization of a mass is an important diagnostic finding, albeit it is not always simple to appreciate this finding, more so when a dural relation cannot be detected (Fig. 14-5). A cerebrospinal fluid (CSF) interface between a mass and the brain surface is another finding supporting the extra-axial localization (Fig. 14-6). Occasionally the subarachnoid space around a meningioma may enlarge and acquire the form of a cyst. Another finding reflecting the extra-axial localization of a meningioma is the “buckling” or compression and displacement of adjacent brain cortex and the white matter (Fig. 14-7).
BORDER AND CONTOUR OF MENINGIOMAS
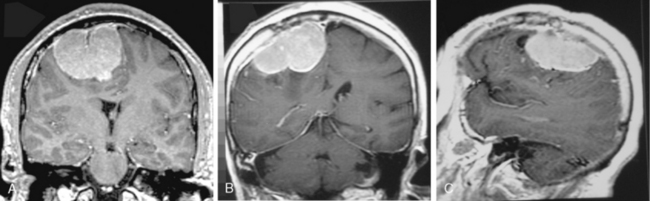
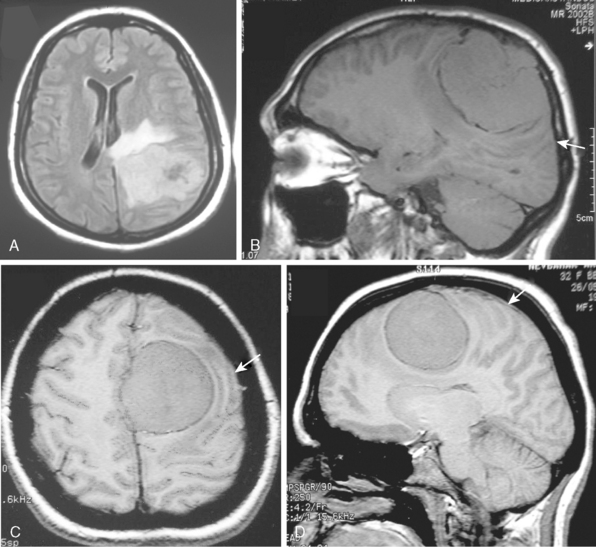
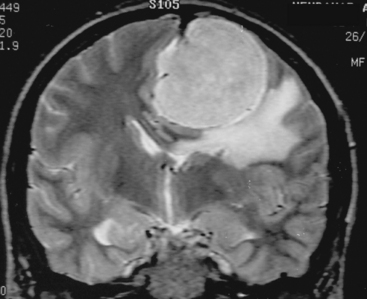
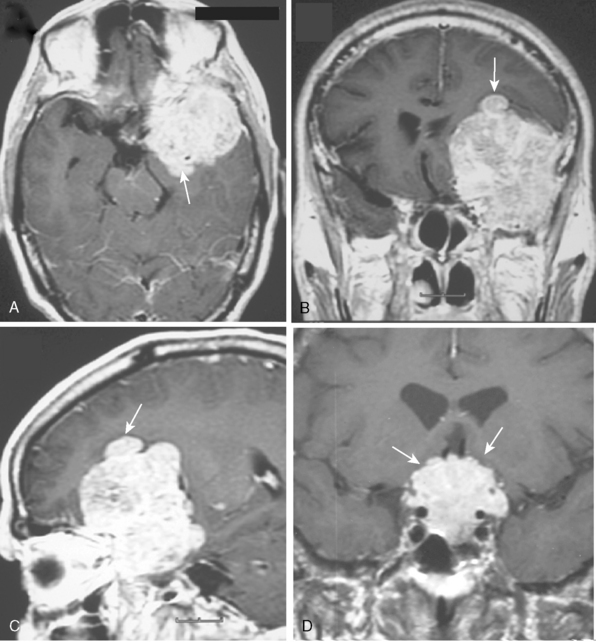
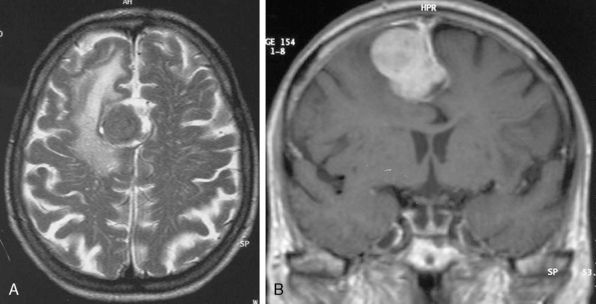
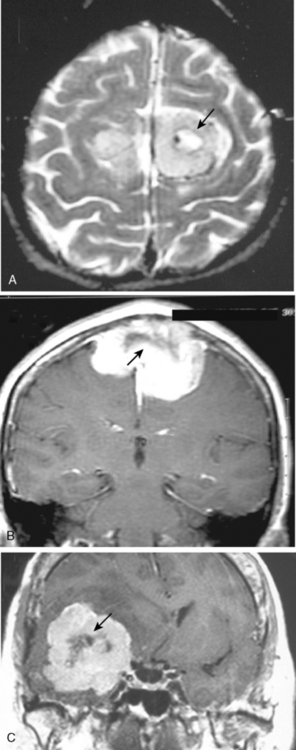
The borders and contours of meningiomas are usually regular and the margins are sharp as a result of the extra-axial location of the tumor which is surrounded by a CSF ring (Fig. 14-8). Despite the extra-axial location, meningiomas may indent into the adjacent brain cortex and so exhibit irregular margins. A meningioma that has multiple lobulated outgrowths on its contour is designated as “mushrooming” meningioma (Fig. 14-9).
CONTRAST ENHANCEMENT OF MENINGIOMAS ON MRI
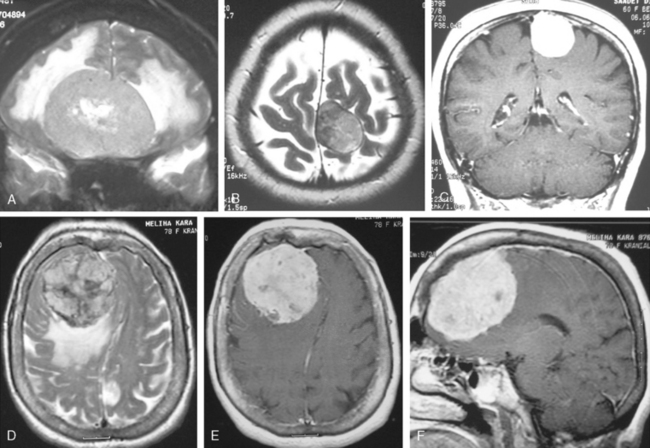
MRI contrast agents are gadolinium chelates that shorten the T1 relaxation times in certain tissues to increase signal intensity on T1-WI and produce contrast enhancement that improves the sensitivity of MRI for residual, recurrent, and multiple meningiomas.12 On early MR studies, some small meningiomas with signal intensities close to that of the brain cortex on T1- and T2-WI were not recognized if contrast medium was not used.1 Routine use of contrast media is necessary to increase the sensitivity for meningioma detection. Meningiomas that are homogeneous on T1-WI and T2-WI enhance in general intensely and in a uniform manner (Fig. 14-10). Calcified, cystic, necrotic, and hemorrhagic meningiomas enhance less intensely, and more heterogeneously (Fig. 14-11). No correlation was established between contrast enhancement and the histopathologic features of meningiomas.13 Contrast media are among strong radiodiagnostic tools; however, caution is recommended in patients with poor kidney functions, as cases of nephrogenic systemic fibrosis have been related to some ingredients of MR contrast agents.
EDEMA RELATED TO MENINGIOMAS ON MRI
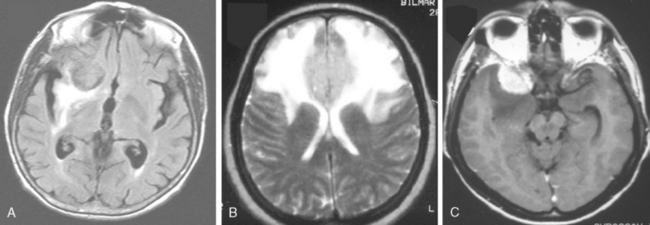
Despite their extra-axial location, meningiomas can cause mild to extensive vasogenic white-mater edema in up to 75% of cases.14 Calcified meningiomas exhibit less vasogenic edema.15 Many factors were associated with the development of edema. These include shape of meningioma, mechanical injury to the adjacent brain tissue, compression of nearby vasculature, angiogenic factors, large surface area of the tumor, invasive pattern of brain–tumor interface and hyperintensity of meningioma on T2-WI.14–16 Nevertheless, the exact cause of edema is still unknown. Newer techniques such as diffusion tensor imaging may provide additional information. Vasogenic edema in the white matter presents in the form of “finger edema” and is hypointense on T1-WI, and of hyperintense and of homogeneous signal intensity on FLAIR and T2-WI (Fig. 14-12). Small and isointense meningiomas amid edema can be depicted only after contrast medium administration.
MR SIGNAL INTENSITY CHARACTERISTICS OF MENINGIOMAS
Signal intensity characteristics of meningiomas have been studied extensively and correlated with the histologic subtypes of meningiomas. According to Elster and colleagues, in 62% of meningiomas, the signal was isointense with cortical gray matter, and in the remaining 38% hypointense to gray matter intensity on T1-WI so that no correlation could be established concerning the histologic type on T1-WI (Fig. 14-13).17 On proton density (PD) and T2-WI meningiomas are isointense in 50% and mildly hyperintense to cortex in 40% of cases (Fig. 14-14).18 Signal intensity of meningiomas on T2-WI could be correlated to histologic subtypes in 75% of cases, so that varied MR findings may be explained on a histologic basis.17 Fibroblastic meningiomas with a dense collagenous matrix produce hypointense signal on T2-WI (Fig. 14-15). Transitional meningiomas with abundant psammoma bodies have relatively low signal intensity on T2-WI (Fig. 14-16). Syncytial meningiomas with microcystic elements exhibit high signal intensity on T2-WI (see Fig. 14-11E, F).13 Angioblastic meningiomas with high cellularity and dilated blood vessels show hyperdense signal with a mottled appearance on T2-WI (Fig. 14-17).13
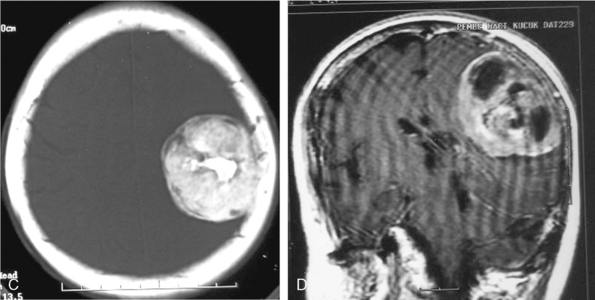
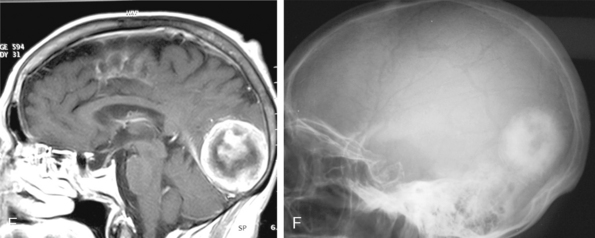
Central necrosis in meningioma, which is encountered quite often (14% in the series of Russel and colleagues),19 will appear as a heterogenous hyperintense signal on T2-WI, whereas cysts show a homogeneous hyperintense signal on the same images (Fig. 14-18). Signal intensities of atypical and malignant meningiomas are not significantly different from those of benign meningiomas.13 Clear-cell meningioma is recently defined as World Health Organization (WHO) grade II subtype, with high spinal prevalence and recurrence rates. If a meningioma is accompanied with extensive leptomeningeal enhancement, a clear-cell meningioma should be suspected and followed up for aggressive behavior.20 Fifteen anaplastic rhabdoid meningiomas studied by Kim and colleagues21 displayed cystic changes in 38%, extensive edema in 75%, hyperostosis in 50%, and bone destruction in 25% of cases. The authors concluded that the aforementioned radiologic features do not seem to be useful in distinguishing rhabdoid meningiomas from other high-grade tumors or even from benign meningiomas.20
UNUSUAL FINDINGS OF MENINGIOMAS ON MRI
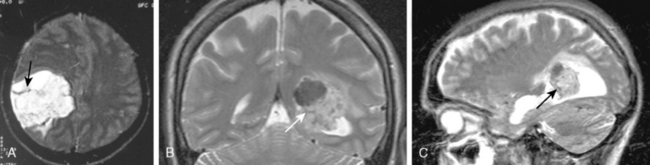
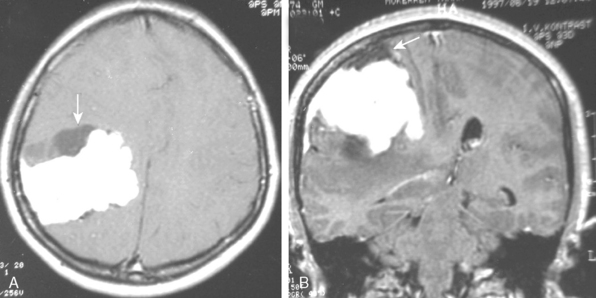
Meningiomas may manifest unusual radiologic features in 15% of cases that may complicate or mislead the diagnosis. Hemorrhage associated with meningiomas is a well known finding;21 however, blood products display variable signal intensities on MRI and a definitive diagnosis is not simple. Intratumoral hemorrhage is usually accompanied by tumor necrosis and the two findings cannot be differentiated on T1- and T2-WI in the acute phase of the bleeding, except on 3T. Chronic blood products are hypointense whereas necrosis is hyperintense on T2-WI. Inratumoral microhemorrhage can be depicted on T2-weighted gradient-echo (GE) images as punctate hypointense structures. Unusual presentations of meningiomas related with a subdural, a subarachnoid, or intracerebral hemorrhage are possible; however, the presence of extratumoral hemorrhage may be diagnostically misleading, especially if contrast medium is not used to highlight the meningioma adjacent to a subdural or subarachnoid hemorrhage. Subarachnoid hemorrhage is detected as hyperintensities in the sulci on FLAIR images. A review of the literature disclosed 33 cases of ipsilateral subdural hematomas associated with meningiomas.22
Fat storage in meningioma cells or lipomatous transformation has been described and such cases are called “lipoblastic” meningioma that appear hyperintense on T1-WI and mildly hypointense on T2-WI.21
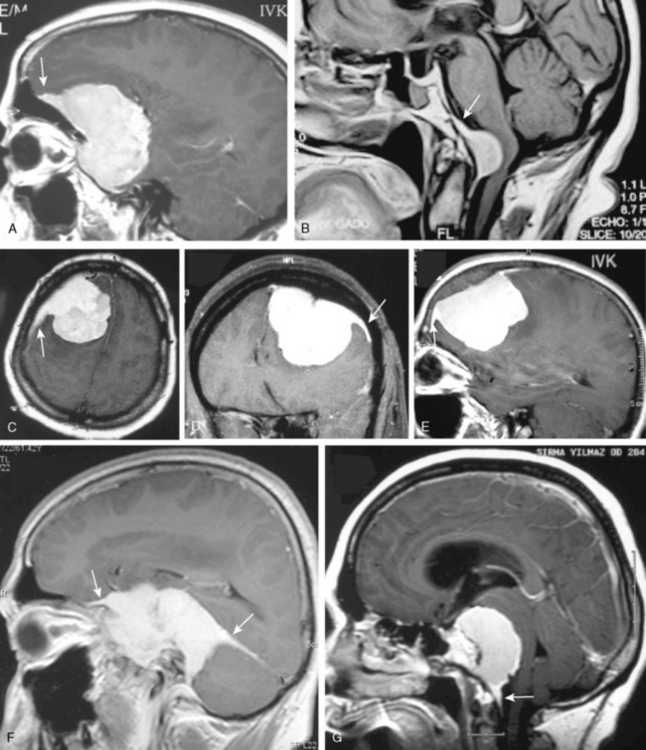
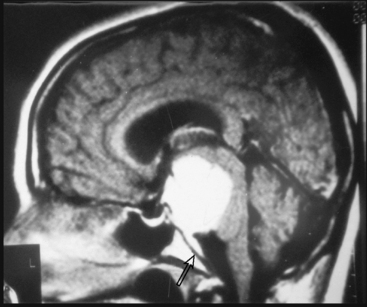
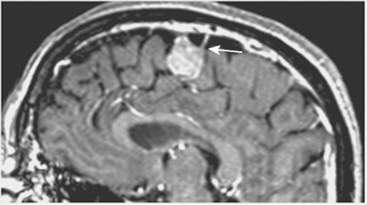
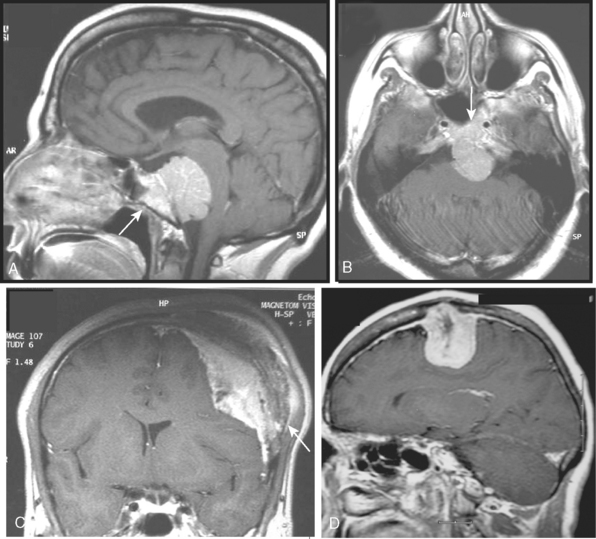
Most meningiomas have a broad dural base; rarely a meningioma may have a thin attachment to the dura mater, which is known as “pedunculated meningioma” (Fig. 14-19). Meningiomas without any detectable dural attachment may also be observed, and this finding is more common in the pediatric population.
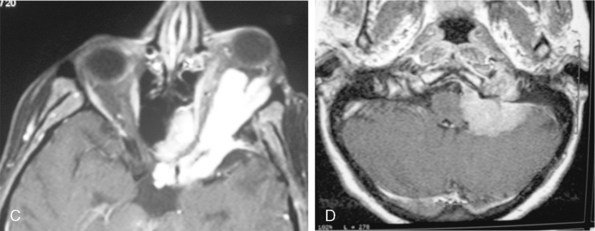
A few cases of meningiomas have been described in the literature as having both intra- and extracranial components, and possessing different MR signal characteristics (Fig. 14-20). In a jugular foramen meningioma the parapharyngeal component displayed lower signal intensity and less enhancement, which may be related to the histologic composition and its collagen content. The increased collagen content has been explained as being the result of fibroblast migration into the extracranial component of the meningioma.23
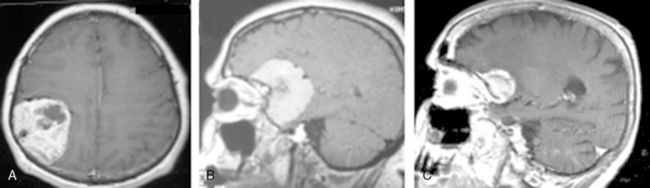
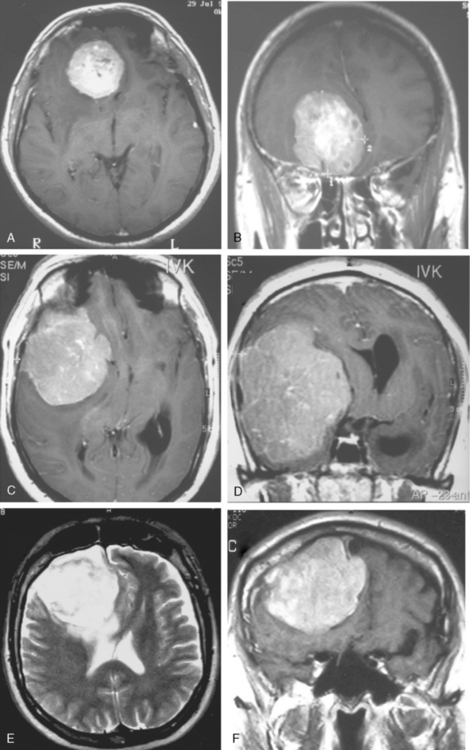
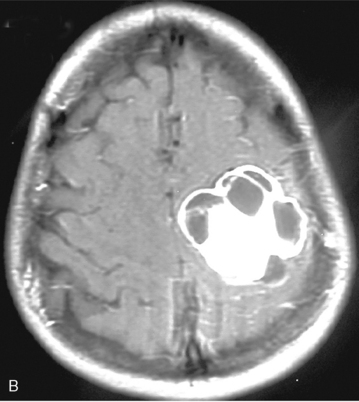
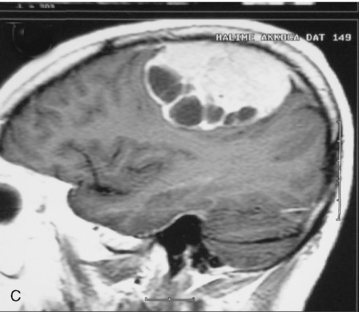
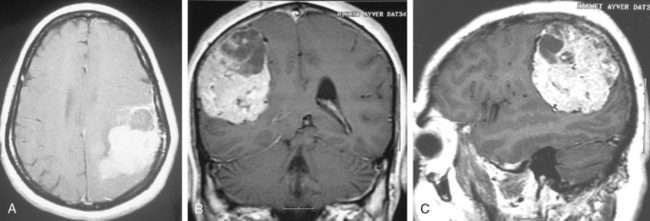
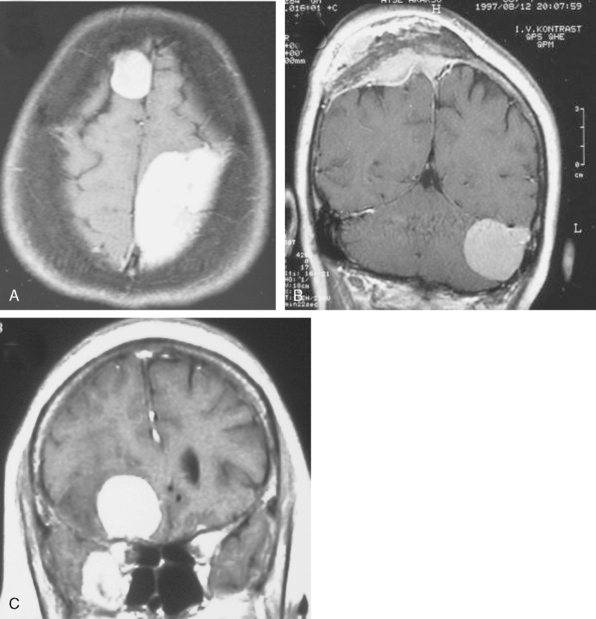
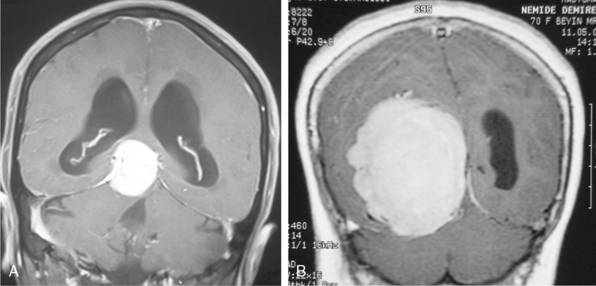
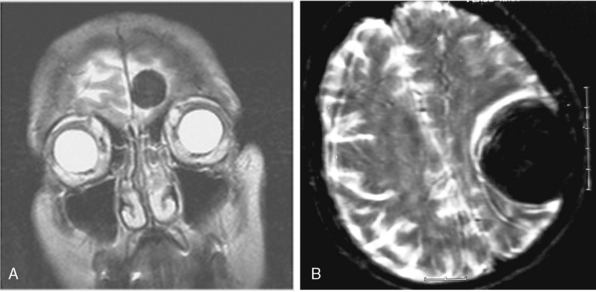
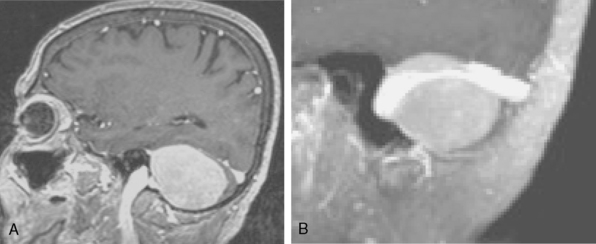
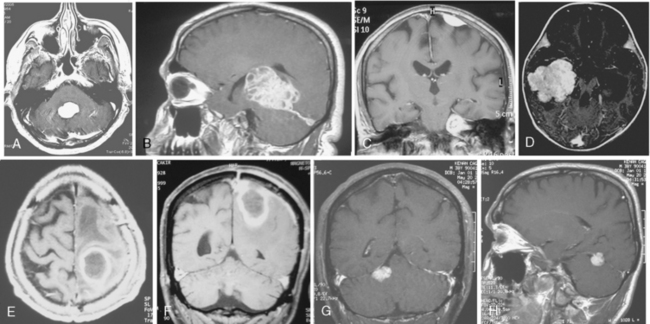
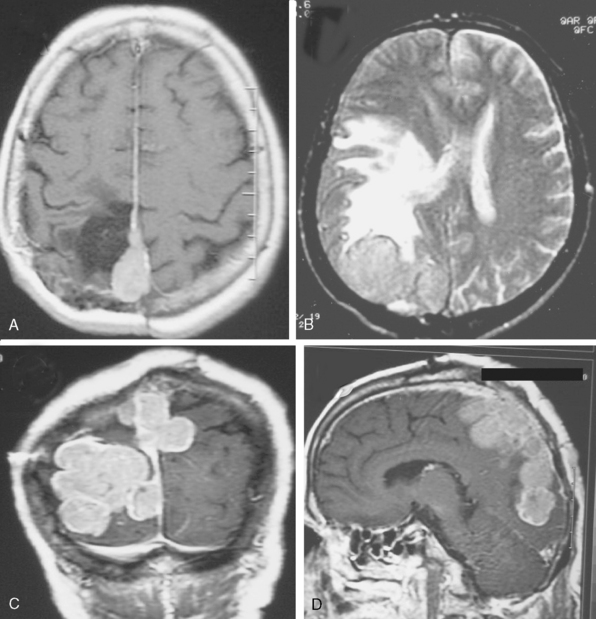
Cystic meningiomas are not uncommon, and their detailed radiologic description will assist in surgical planning. Three types of intratumoral cysts have been described in meningiomas. True intratumoral cysts have homogeneous hyperintense signal on T2-WI and show an enhancing cyst wall. These cases warrant differential diagnosis from schwannomas and even cystic astrocytomas (Fig. 14-21). The second type may arise due to tumoral degeneration or necrosis, which produces cystic cavities with irregular walls, and heterogeneous content of hyperintensity on T2-WI (Fig. 14-22). The third and most common type are meningiomas with extratumoral cysts. In this variant the cyst may be a true arachnoid cyst or a cerebrospinal fluid loculation due to arachnoid adhesions (Fig. 14-23).24 FLAIR sequences may be used to differentiate between water-like cystic lesions and CSF, which is especially useful to study extratumoral cystic components of meningiomas.25
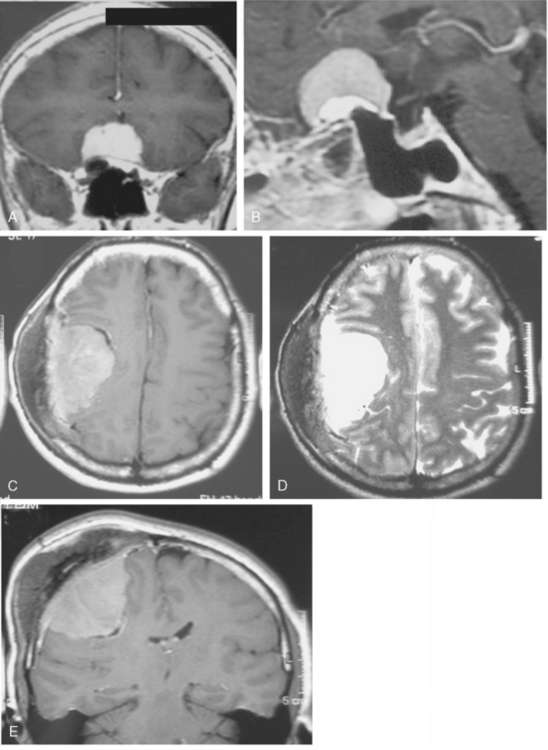
Multiple meningiomas are reported in 9% of cases.26 Although multiple meningiomas are known to be associated with neurofibromatosis type II, most patients do not harbor other features of this disease. Multiplanar imaging with contrast injection is mandatory to rule out possible additional small meningiomas (Fig. 14-24).1 In general, meningiomas may arise at any site where meningothelial cells are found, such as in arachnoid associated with cranial nerves, spinal and intraventricular locations. Surgical decision is also a function of the anatomic location of the tumor, so that knowledge of the typical and unusual locations where meningiomas arise is essential. Intracranial meningioma locations are, according to descending order of frequency, as follows: convexity, parasagittal, sphenoid, fronto-basal, posterior fossa, cerebellopontine angle, petroclival, clivus, intraventricular, and orbital (Fig. 14-25). However, 1% of meningiomas are extradural and ectopic so that they may be encountered in the diploe, the skin of the skull, the paranasal sinuses, the parapharyngeal space, and even the mediastinum.27
MRI CHARACTERISTICS OF CALCIFICATION AND BONE CHANGES ASSOCIATED WITH MENINGIOMAS
The ability to detect calcification within meningioma on MR images is variable. A mild or moderate degree of calcification is usually not discernible on MRI on T1- and T2-WI, although heavily calcified meningiomas exhibit hypointense signal on T2-WI (Fig. 14-26). Psammoma bodies appear isointense on T1- and T2-WI. Intratumoral vasculature is also demonstrated as punctate or linear hypointensities or as “signal void” structures on conventional MR images. If the vessel is not demonstrated in its typical morphology, a signal void structure may reflect calcification, blood, or vasculature on routine sequences. Recently GRE T2* and SWI sequences have gained wide acceptance to detect subtle calcifications.
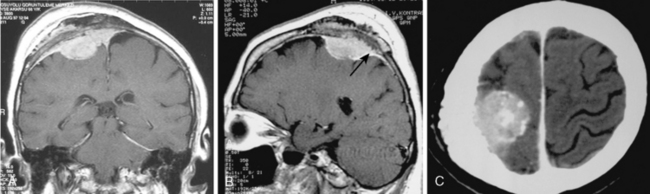
Bone changes occur in approximately 25% to 48% of all meningiomas.28–30 These changes may take the form of hyperostosis, osteolysis, and bone rarefaction which can exist independently or in combination with each other. The radiologic gold standard is high-resolution CT to demonstrate and diagnose these bony changes associated with meningiomas. Nevertheless all bone should be evaluated on standard MR images. On T1- and T2-WI the normal inner and outer tables of the skull are displayed as a hypointense layer without structure, and the diploe is seen as hyperintense on T1-WI due to its fat content. Hyperostosis is seen usually as widening of the layer of hypointense signal, and sometimes MR reveals inhomogeneous areas of increased signal intensity in the bone on conventional sequences (Fig. 14-27). However, because the cortical and trabecular detail cannot be well discerned on MRI, osteolysis or rarefication of bone cannot be easily appreciated. Bone invasion by meningioma can be diagnosed on MRI if increased signal is seen in the involved bone or if on fat suppressed-contrast enhanced studies, enhancement of bone is displayed (Figs. 14-28 and 14-29).
MRI OF VASCULATURE ADJACENT TO A MENINGIOMA
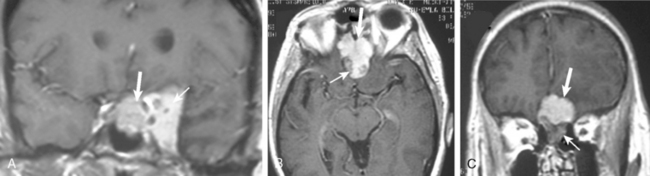
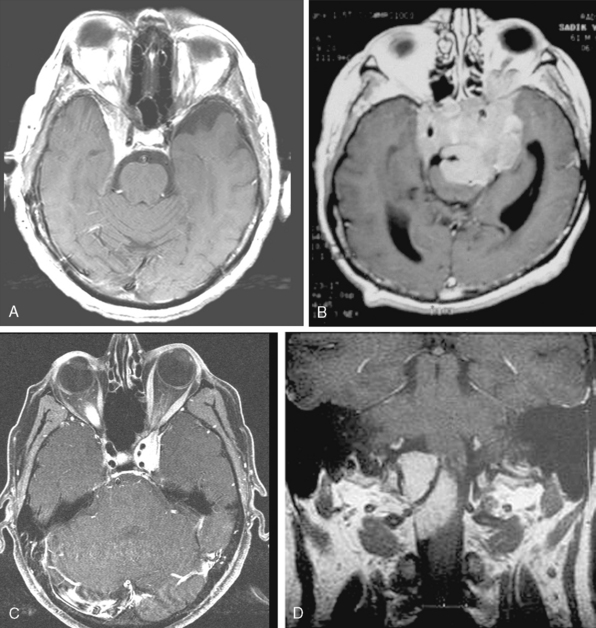
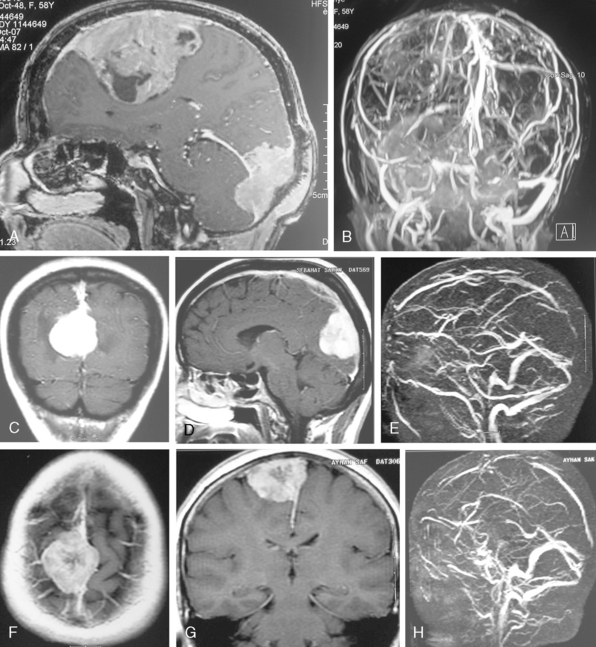
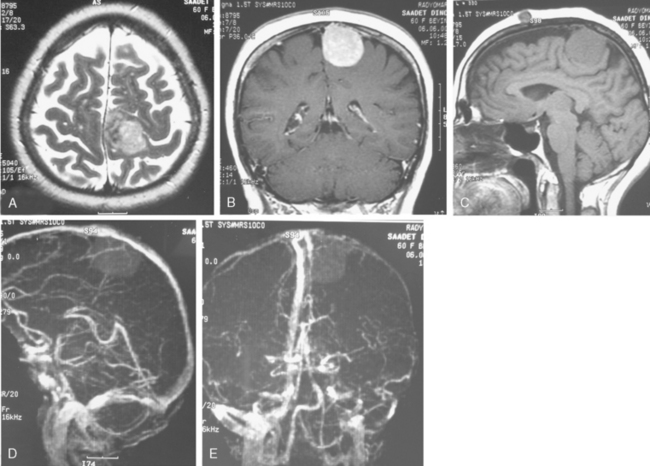
Conventional MRI has the ability to display arteries as signal void structures on T1- and T2-WI. Major arteries adjacent to meningiomas can be scrutinized for displacement, encasement, or stenosis without angiography (Fig. 14-30). In cases where the meningioma is located near a venous sinus, on conventional images blood flow in the sinus may not be appreciated if the flow is low. Such cases should be studied by MR venography to depict a narrowing or occlusion of the sinus by meningioma (Figs. 14-31, 14-32, and 14-33).
RADIOLOGIC DIFFERENTIAL DIAGNOSIS OF MENINGIOMAS
Variable neoplastic and infectious mass lesions come into consideration for the differential diagnosis of meningiomas (Fig. 14-34). Radiologically hemangiopericytomas strongly resemble meningiomas, because they are dural-based and have a falx predilection. They may also exhibit the same MR signal characteristics as meningiomas; they may show intratumoral hemorrhage, a mushrooming configuration, rich vasculature, and bony erosion. A definite diagnosis may necessitate angiographic investigation.10 Schwannomas are the second most frequent extra-axial tumors that come into differential consideration. Cystic and hemorrhagic schwannomas are common, but calcification and hyperostosis are not features of this tumor. A dural tail may accompany schwannomas if they abut the dura. Schwannomas are usually hypointense on T1-WI and hyperintense on T2-WI, whereas meningiomas are more often isointense to cortex. Granulomatous diseases such as sarcoidosis, tuberculosis, plasma cell granulomas, and Erdheim Chester, Rosai-Dorfman disease may exhibit dural based mass lesions and mimic meningiomas. Ganglioglioma or pleomorphic xanthoastrocytoma (PXA) if located near the tentorium may mimic a cystic meningioma. Meningeal metastases, plasmacytomas, lymphoma, and leukemic infiltrations may all resemble meningioma, apart from being usually multiple lesions. Vascular lesions adjacent to the tentorium such as cavernous angiomas may also be mistaken for meningiomas. Intraventricular meningiomas may be mimicked by choroid plexus papillomas, but are not associated with hydrocephalus like papillomas. Meningiomas that invaginate the brain may be mistaken for an intra-axial tumor, more so if the meningioma harbors atypical features. In such challenging cases one needs to remember that neoplastic tissue is rich in water and is hyperintense on T2-WI; yet meningiomas are commonly isointense to brain cortex. In difficult cases, differential diagnosis of meningiomas necessitates the application of advanced MR techniques that are presented in a separate chapter.
MRI OF RESIDUAL AND RECURRENT MENINGIOMAS
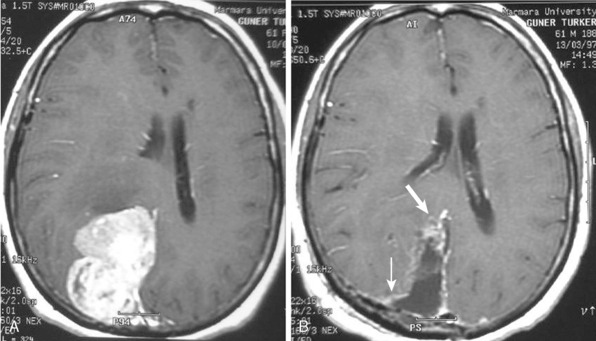
A long-term recurrence-free survival can be obtained in meningioma patients after gross total surgical resection of the tumor involving dura and bone. This, however, is seldom possible and a large proportion meningiomas do recur after surgical resection. The recurrence rate for meningiomas is variable; for benign meningiomas it is 7% to 20%, for atypical meningiomas 29% to 40%, and for malignant meningiomas 50% to 78%.21 The degree of surgical removal is one of the major determinants of recurrence and important in shaping a postsurgical treatment strategy. Routine contrast-enhanced (CE) MRI sequences are very sensitive in detection of residual tumor. In our experience, early postoperative MRI, performed within the first 48 hours after surgery, has been very effective in objectively determining the degree of residuals in most neuro-oncologic surgeries.31–33 The tissue resolution of MRI is very high for complex localizations such as the cavernous sinus, where MRI is far superior to CT in demonstrating the presence of residual tumor. On early postoperative imaging, anatomic distortion and the presence of CSF mixed with blood may obscure a small residual tumor so that contrast enhancement is necessary to highlight a tumor (Fig. 14-35).
Radiologic follow-up interval for MRI is set between 3 months and 1 year for malignant and benign meningiomas, respectively. Multiplanar imaging in any desired plane with thin sections and contrast medium administration provides exquisite detail of the morphology of pathologic lesions and is the modality of choice for recurrent and residual lesions. Residual meningiomas, especially those after radiotherapy, usually show a milder contrast enhancement than the primary lesion, probably due to increased fibrosis in older tumors (Fig. 14-36).
[1] Zimmerman R.D. MRI of intracranial meningiomas. In: Al-Mefty O., editor. Meningiomas. New York: Raven Press; 1991:209-223.
[2] Smirniopoulos J.G., Murphy M.F., Rushing E.J., et al. From the archives of AFIP: patterns of contrast enhancement in the brain and meninges. Radiographics. 2007;27:525-551.
[3] Kreuzberg B., Kastner J., Ferda J. The contribution of MRI to the diagnosis of diffuse meningeal lesions. Neuroradiology. 2004;46:198-220.
[4] Bourekas E.C., Wildenhain P., Lewin J.S., et al. The dural sign revisited. Am J Neuroradiol. 1995;16:1514-1516.
[5] Aoki S., Sasaki Y., Machida T., Tanioka H. Contrast enhanced images in patients with meningiomas: importance of enhancement of the dura adjacent to the tumor. Am J Neuroradiol. 1990;11:935-938.
[6] Wallace E.W. The dural tail sign. Radiology. 2004;233:56-57.
[7] Wiggins R.H.III, Harnsberger H.R., Salzman K.L., et al. The many faces of facial nerve schwannoma. Am J Neuroradiol. 2006;27:694-699.
[8] Tokumaro A., Toshihiro O., Tsuneyoshi E., et al. Prominent meningeal enhancement adjacent to meningioma on Gd-DTPA-enhanced MR images: histopathologic correlation. Radiology. 1990;175:431-433.
[9] Goldsher D., Litt A.W., Pinto R.S. Dural “tail” associated with meningiomas on Gd-DTPA- enhanced MR images: characteristics, differential diagnostic value, and possible implications for treatment. Radiology. 1990;176:447-450.
[10] Sibtain N.A., Butt S., Connar S.E.J. İmaging features of central nervous system haemangiopericytomas. Eur Radiology. 2007;17:1685-1693.
[11] Takeguchi T., Miki H., Shimizu T., et al. The dural “tail” of intracranial meningiomas on fluid attenuated inversion-recovery images. Neuroradiology. 2004;46:130-135.
[12] Haughton V.M., Rim A.A., Czervionke L.F., et al. Sensitivity of Gd-DTPA-enhanced MR imaging of benign extraaxial tumors. Radiology. 1988;166:829-833.
[13] Maiuri F., İaconetta G., de Divitiis O., et al. Intracranial meningiomas: correlations between MR imaging ant histology. Eur J Radiol. 1999;31:69-75.
[14] Bradac G.B., Ferszt R., Bender A., Schorner W. Peritumoral edema in meningioma: a radiological and histological study. Neuroradiology. 1986;28:304-312.
[15] Stevens J.M., Ruiz J.S., Kendall B.E. Observations on peritumoral edema in meningioma. II. Mechanisms of edema production. Neuroradiology. 1983;25:125-131.
[16] Nakano T., Asano K., Miura H., Itoh S., Suzuki S. Meningiomas with brain edema: radiological characteristics on MRI and review of the literature. Clin Imaging. 2002;26:243-249.
[17] Elster A.D., Challa V.C., Gilbert T.H., et al. Meningiomas: MR and histopathologic features. Radiology. 1989;1170:857-862.
[18] Zimmerman R.D., Fleming C.A., Saint-Luis L.A., et al. Magnetic resonance imaging of meningiomas. Am J Neuroradiology. 1985;6:149-157.
[19] Russel E.J., Geoerge A.G., Kircheff II, et al. A typical computed tomographic features of intracranial meningioma. Radiology. 1980;135:673-682.
[20] Lee W., Chang K.-H., Choe G., et al. MR imaging features of clear-cell meningiomas with diffuse leptomeningeal seeding. Am J Neuroradiol. 2000;21:130-132.
[21] Kim E.Y., Weon Y.C., Kim S.T., Kim H.-J., Byun H.S., Lee J.I., Kim J.H. Rhabdoid meningioma: clinical features and MR imaging findings in 15 patients. Am J Neuroradiol. 2007;28:1462-1465.
[22] Lefranc F., Nagy N., Dewitte O., Baleriaux D., Brotchi J. Intracranial meningiomas revealed by non-traumatic subdural haematomas; a series of four cases. Acta Neurochirur (Wien). 2001;143:977-983.
[23] Shimono T., Fumiharu A., Yamamoto A., et al. Different signal intensities between intra-and extracranial components in jugular foramen meningioma: an enigma. Am J Neuroradiol. 2005;26:1122-1127.
[24] Wasenko J.J., Hochhauser L., Stopa E.G., Winfield J.A. Cystic meningiomas: MR characteristics and surgical correlations. Am J Neuroradiol. 1994;15:1959-1965.
[25] Aprile I., Iaiza F., Lavaroni A., et al. Analysis of cystic intracranial lesions performed with fluid attenuated inversion recovery MR imaging. Am J Neuroradiol. 1999;20:1259-1267.
[26] Lusin J.O., Nakagawa H. Multiple meningiomas evaluated by computed tomography. Neurosurgery. 1981;9:137-141.
[27] Buetow M.P., Buetow P.C., Smirniotopoulos J.G. From the archives of the AFIP: typical, atypical and misleading features in meningioma. Radiographics. 1991;11:1087-1106.
[28] Terstegge K., Schorner W., Henkes H., et al. Hyperostosis in meningiomas: MR findings in patienys with recurrent meningioma of the sphenoid wings. Am J Neuroradiol. 1994;15:555-560.
[29] Pieper D.R., Al-Mefty O., Hanada Y., Buechner D. Hyperostosis associated with meningioma of the cranial base: secondary changes or tumor invasion. Neurosurgery. 1999;44:742-747.
[30] Sheporaitis L.A., Osborn A.G., Smirniotoupolos J.G., et al. Intrcranial meningioma. Am J Roentgenol. 1992;13:37-39.
[31] Ekinci G., Akpinar I.N., Baltacioglu F., et al. Early-postoperative magnetic resonance imaging in glial tumors: prediction of tumor regrowth and recurrence. Eur J Radiol. 2003;45:99-107.
[32] Kilic T., Ekinci G., Seker A., Elmaci I., Erzen C., Pamir M.N. Determining optimal MRI follow-up after transsphenoidal surgery for pituitary adenoma: scan at 24 hours postsurgery provides reliable information. Acta Neurochir (Wien). 2001;143:1103-1126.
[33] Pamir M.N., Kilic T., Ture U., Ozek M.M. Multimodality management of 26 skull-base chordomas with 4–year mean follow-up: experience at a single institution. Acta Neurochir (Wien). 2004;146:343-354.