Movement dysfunction associated with cerebellar damage
Overview
Types of cerebellar damage
Cerebellar strokes are rarer than cerebral strokes, but not entirely uncommon. They account for less than 5% of all strokes.1 These strokes can involve any of the three arteries that supply the cerebellum: the superior cerebellar artery, anterior inferior cerebellar artery, and posterior inferior cerebellar artery. Depending on the territory supplied by the damaged vessel (Table 21-1), there are stereotyped patterns of cerebellar and extracerebellar motor dysfunction that result. However, there is certainly some variation in distribution from person to person. Stroke involving the superior cerebellar artery often leads to dysmetria of ipsilateral arm movements, unsteadiness in walking, dysarthric speech, and nystagmus.1 Stroke involving the anterior inferior cerebellar artery often causes both cerebellar and extracerebellar signs (owing to involvement of the pons) including dysmetria, vestibular signs, and facial sensory loss.1 Finally, stroke involving the posterior inferior cerebellar artery is usually, in the long run, the most benign, though initially it often manifests with vertigo, unsteadiness, walking ataxia, and nystagmus.1 The best predictor of recovery from cerebellar stroke is whether the deep cerebellar nuclei are involved: recovery is best when they are not damaged.2
TABLE 21-1 
TERRITORIES OF THE CEREBELLAR ARTERIES
| ARTERY | CEREBELLAR TERRITORY SUPPLIED |
| SCA | Superior or upper half (approximately) of the dorsal and upper third (approximately) of the ventral surface of the cerebellum except for the extreme lateral wing of the hemisphere |
| Portions of the vermis and nodulus | |
| Substantial upper portions of the intermediate and lateral hemispheres | |
| Portions of the deep cerebellar nuclei | |
| Superior cerebellar peduncle | |
| AICA | Middle 10%-30% of the ventral cerebellum, sometimes wrapping laterally to encompass a small portion of the most lateral aspects of the dorsal cerebellum |
| Flocculus | |
| Small portions of the lateral hemisphere | |
| Middle and inferior cerebellar peduncles | |
| PICA | Inferior or lower half (approximately) of the dorsal cerebellum and the inferior fourth to third of the ventral cerebellum |
| Portions of the nodulus, vermis, intermediate and lateral hemispheres | |
| Portions of the deep cerebellar nuclei |
Data from Amarenco P: The spectrum of cerebellar infarctions. Neurology 41:973–979, 1991; and Tatu L, Moulin T, Bogousslavsky J, Duvernoy H: Arterial territories of human brain: brainstem and cerebellum. Neurology 47:1125–1135, 1996.
Tumors in the posterior fossa (i.e., in or near the cerebellum) do occur, though they are more common in children than adults. Depending on the type and location, tumors may be treatable with surgical resection, chemotherapy, radiation therapy, or some combination of these. Children with cerebellar tumors often have a good prognosis for recovery because many of the types of tumors most common in this population are benign and can be removed. Children also typically recover very well after cerebellar damage from tumor resection and show little signs of cerebellar ataxia. Tumors in adulthood often are caused by a more aggressive form of cancer and therefore may carry a poorer prognosis. Second to tumor type, damage of the deep cerebellar nuclei is an important factor that predicts recovery, even more so than age.2
Several neurodegenerative diseases can damage the cerebellum (Table 21-2). One of the more common types of degenerative diseases is a group of hereditary, autosomal dominant diseases referred to as the spinocerebellar ataxias (SCAs). Currently there are 30 known distinct SCAs, which are named by numbers (e.g., SCA1, SCA2). Depending on the genetic abnormality, they can cause either purely cerebellar damage or combined cerebellar and extracerebellar damage.3,4 Most of the SCAs have onset in midlife and are slowly progressive, which means that children of an affected parent will likely not know if they are affected until adulthood. There are genetic tests for a subset of these diseases. Because onset of symptoms is delayed and there are no effective pharmacological treatments, genetic counseling is a must before families decide whether or not to have children undergo genetic testing. A related set of diseases is the hereditary episodic ataxias,5 which are rare autosomal dominant diseases. As the name implies, clients with episodic ataxia will have periods of ataxia lasting minutes to hours, brought on by exercise, stress, or excitement. Some of the episodic ataxias respond well to medications.6
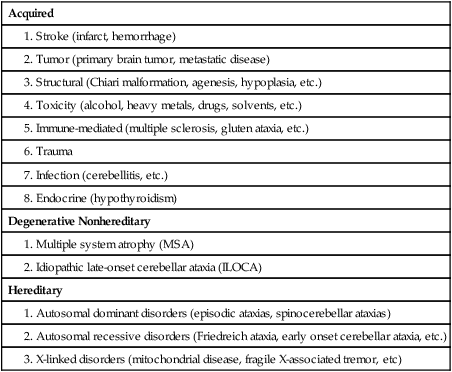
Data from Manto M, Marmolino D: Cerebellar ataxias. Curr Opin Neurol 22:419–429, 2009.
Cerebellar damage can occur from other sources as well. In traumatic brain injury, damage of the cerebellum is almost always found in the presence of widespread brain damage and is seen as a predictor of poorer outcome.7 The cerebellum is also particularly sensitive to toxins, including certain heavy metals and alcohol. Chronic alcoholism causes cerebellar atrophy preferentially involving the anterior superior vermis.8,9 The inflammatory disorder multiple sclerosis also frequently produces lesions in the cerebellum. Finally, congenital brain abnormalities such as Chiari malformation damage the cerebellum by increased pressure and mechanical deformation. Recovery from cerebellar malformation is not understood; often these children have substantial damage to the brain stem or other neural structures, which may make therapy more challenging. A more comprehensive list of the variety of types of cerebellar damage is provided in Table 21-2.
Cerebellar anatomy and physiology
Anatomical divisions
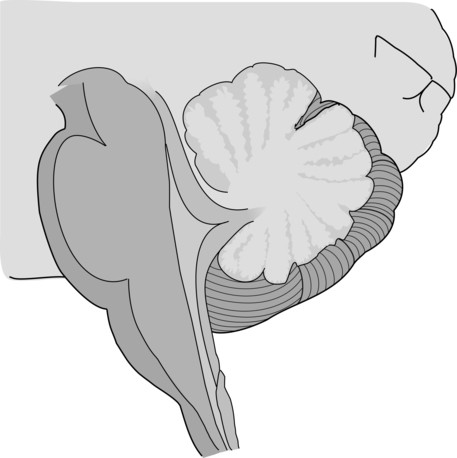
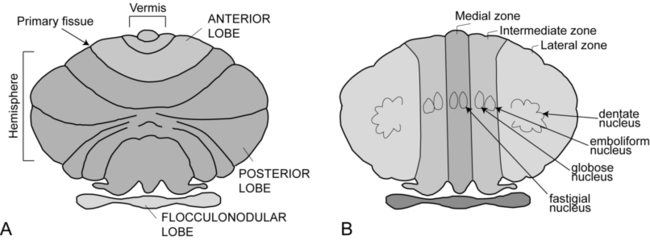
The cerebellum is part of the hindbrain and is positioned on the dorsal surface of the brain stem at approximately the level of the pons (Figure 21-1). It is connected to the brain stem by the superior, middle, and inferior cerebellar peduncles. The cerebellar peduncles contain all of the axons that transmit information to and from the cerebellum. The cerebellum can be anatomically divided into three lobes: the anterior, posterior, and flocculonodular lobes. The primary fissure divides the anterior and posterior lobes, and the posterolateral fissure divides the posterior and flocculonodular lobes (Figure 21-2).
Looking at a sagittal slice through the cerebellum, distinct cellular regions can be visualized. The most superficial region is the cerebellar cortex, which, unlike the cerebral cortex, contains only three layers. The arrangement of cells within the cortex is strikingly uniform across all cerebellar lobes and plays a vital role in determining cerebellar function, which will be described later. Deep to the cerebellar cortex is the white matter layer, which contains the axons of Purkinje cells projecting out from the cerebellar cortex and the axons of mossy and climbing fibers entering the cortex from other brain and spinal regions (see Figure 21-1). The cerebellar nuclei are the output structures of the cerebellum, and they make up the deepest region. Groups of neuronal cell bodies receive information coming into the cerebellum from a variety of brain and spinal cord regions and also from the cerebellar cortex, via Purkinje cell axons. The deep nuclei are arranged in pairs, with one nucleus of each pair on each side of the cerebellum. Most medially are the fastigial nuclei, followed by the globose and emboliform nuclei and most laterally the broad dentate nuclei (see Figure 21-2). The medial and lateral vestibular nuclei also receive input directly from the cerebellar flocculonodular lobe and are therefore considered to play a role as an additional set of cerebellar output structures.
Functional divisions and their afferent and efferent projections
Probably the most useful way of thinking about the anatomy of the cerebellum is to divide it into distinct functional longitudinal “zones.”10 Each cerebellar zone consists of a region of cerebellar cortex and its own pair of deep cerebellar nuclei. Each zone also has projections to and from distinct areas of the brain and spinal cord. Thus, despite the regular arrangement of cells over the entire cerebellum, each functional longitudinal zone is uniquely positioned to control certain types of movement but not others.10–12 See Table 21-3 for a summary.
TABLE 21-3 
FUNCTIONAL LONGITUDINAL CEREBELLAR ZONES
| Functional Zone | Alternate Name | Major Afferents | Major Efferents | Role in Movement | Clinical Signs if Damaged |
| Flocculonodular lobe | Vestibulocerebellum |
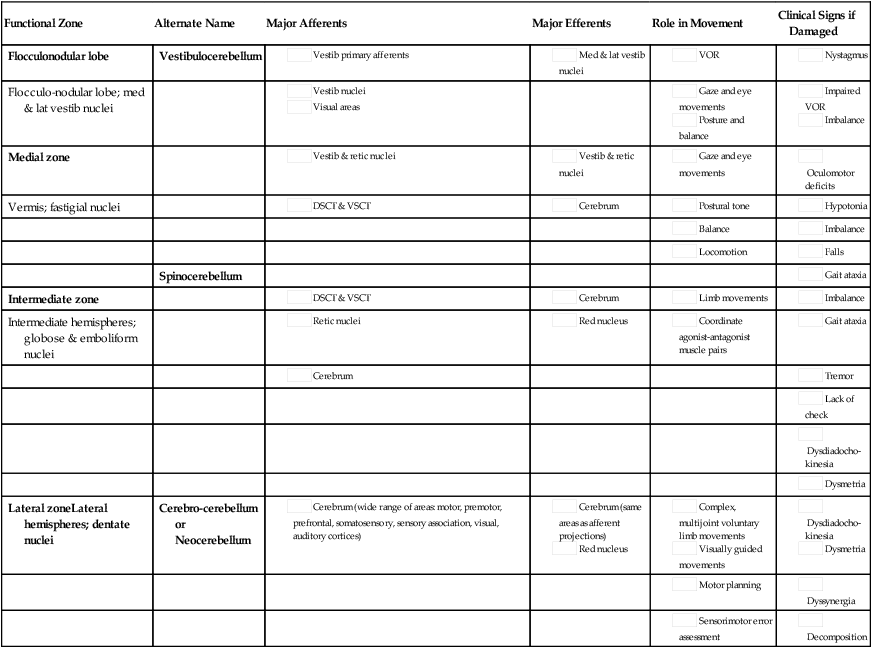
The medial zone consists of the midline structure, the vermis, and the fastigial nuclei. This region of the cerebellum predominantly receives afferent information from the brain stem vestibular and reticular nuclei and the dorsal and ventral spinocerebellar pathways,13–18 which convey important information regarding the current sensorimotor state of the trunk and limbs.19–21 In turn, its outputs, through the fastigial nuclei, are largely to reticular and vestibular nuclei that will form part of the medial descending system (reticulospinal and vestibulospinal tracts), with some additional projections to the cerebral cortex via the thalamus.22–24 The medial cerebellar zone is involved in the control of posture and muscle tone, upright stance, locomotion, and in gaze and other eye movements.
The intermediate zone is made up of the intermediate hemispheres and the globose and emboliform nuclei. This region also receives inputs from the dorsal and ventral spinocerebellar pathways and brain stem reticular nuclei, as well as some projections from the cerebral cortex that arrive via the cerebropontocerebellar pathway.11,13,14,25,26 Major projections from this cerebellar zone are to the cerebral cortex via the thalamus and to the red nucleus.23,24,27 The intermediate zone is considered to be important in controlling coordination of agonist-antagonist muscle pairs during a variety of activities including walking and voluntary limb movements. The medial and intermediate zones of the cerebellum are collectively referred to as the spinocerebellum, because these are the only cerebellar regions that receive afferents from the spinal cord.
The largest region of the cerebellum is the lateral zone, which contains the two broad lateral hemispheres and their output structures, the dentate nuclei. Afferents to the lateral zone predominantly come from the cerebrum, from a wide variety of cortical areas including motor, premotor, and prefrontal cortices, parietal somatosensory and sensory association areas, and primary visual and auditory cortices.25,26 Outputs from the dentate travel mostly back to large areas of the cerebrum (through the thalamus), to many of the same areas from which afferents arrived in the cerebellum. Again, these include vast regions of sensorimotor cortices.27–33 Other efferent fibers project to the red nucleus in the brain stem. The lateral cerebellar zone plays a major role in control of complex, multijoint voluntary limb movements, particularly those involving visual guidance, and in the planning of complex movements and the assessment of movement errors. Because this region of the cerebellum interacts predominantly with the cerebrum, it is also commonly called the cerebrocerebellum. It is also sometimes referred to as the neocerebellum because it is considered to have arisen fairly recently in the phylogenetic tree, being much more expansive in primates than in lower animals.34
The flocculonodular lobe can be considered a fourth zone of the cerebellum. It receives afferent projections directly from the vestibular primary afferents (semicircular canals and otoliths) as well as from vestibular nuclei and visual brain regions.11,13,14,16,18 Outputs from the flocculonodular lobe project directly to the medial and lateral vestibular nuclei of the brain stem, without a synapse in a deep cerebellar nucleus.12,22,35 For this reason, these vestibular nuclei are sometimes considered an additional set of deep cerebellar nuclei. This cerebellar zone helps control eye movements and balance. The well known vestibuloocular reflex (VOR), which provides gaze stabilization during head turning or walking, relies on the cerebellum for proper functioning.36,37 Because of its critical ties to the vestibular system, the flocculonodular lobe is also known as the vestibulocerebellum (see Figure 21-2).
Physiology of cerebellar neuronal circuits
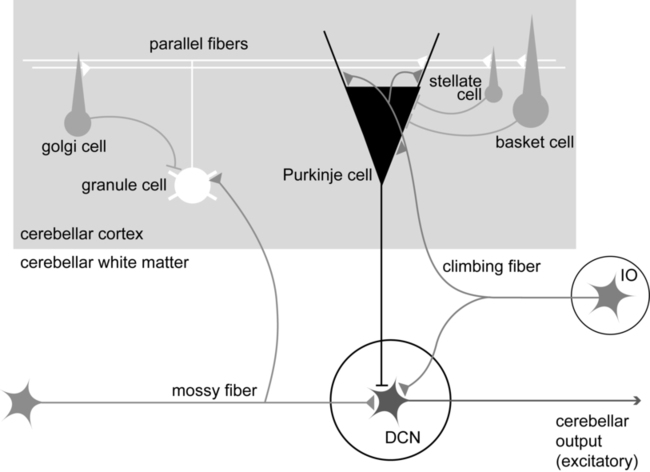
Within a longitudinal zone, thousands of microzones may exist,11 each consisting of a highly organized group of connected cerebellar cortical neurons. A microcomplex is the name given to a neural circuit made up of a single microzone plus the other connected neurons with which it communicates directly. The following section provides a very brief overview of the circuits important for cerebellar function and reviews the flow of neuronal signals into and out of cerebellar microzones (Figure 21-3).
Most afferent information enters the cerebellum through one of two pathways: the mossy fiber pathway or the climbing fiber pathway. Both have important actions on cerebellar Purkinje cells. The mossy fiber pathway affects “beams” or rows of Purkinje cells oriented along the cerebellar folia. Dense mossy fiber inputs arise from a wide variety of regions, including the cerebral cortex, several subcortical areas, the brain stem, and the spinal cord. Mossy fibers enter the cerebellar cortex and synapse onto granule cells, whose axons ascend and branch into parallel fibers. Each parallel fiber extends long distances longitudinally and synapses onto many Purkinje cells, all located along the same beam.38 Each parallel fiber has a relatively weak effect on single Purkinje cells, but the mass effect of many thousands of parallel fiber contacts with Purkinje cells drives the Purkinje cells to fire at high rates.39 In contrast, each climbing fiber arises exclusively from the inferior olive, located in the brain stem, and contacts only a few (approximately 1 to 10) Purkinje cells.12,40,41 Each Purkinje cell receives information from only one climbing fiber, yet the climbing fiber’s effect on the Purkinje cell is powerful, causing large complex spikes.
The Purkinje cell provides the output for the cerebellar cortex; each Purkinje cell axon projects to one of the deep cerebellar nuclei. The mossy fiber and climbing fiber pathways affect Purkinje cells differently and are thought to transmit different types of information. Mossy fibers are active at very high rates (generating action potentials at approximately 100 Hz) and are highly modulated by various sensory stimuli and motor activity. They have been speculated to relay information related to the direction, velocity, duration, or magnitude of movements or sensory stimuli.42–45 Climbing fibers, however, are active at very low rates (approximately 1 to 4 Hz) and do not appear to be as strongly modulated by sensory stimuli or motor activity.40,46,47 There is still some disagreement regarding what sort of information is encoded in the climbing fiber signals, but the frequency of discharge appears to be too low to transmit information pertaining to specific parameters of sensory or motor events. The role of the climbing fiber is clearly important, however, because its firing produces large complex spikes in the Purkinje cells and can also powerfully affect subsequent Purkinje cell firing.41,48
Cerebellar function in adapting and controlling movement
Theories of cerebellar function
One general theory states that a primary function of the cerebellum is in coordinating multiple limb segments to generate smooth and fluid multijoint movements.49–52 This “motor coordinator” theory has support from behavioral studies demonstrating that multijoint movements appear to be particularly impaired in clients with cerebellar lesions.50 Multijoint movements are inherently more complex than single joint movements because they require control of mechanical interaction torques; those occurring at one segment but caused by movement of other linked segments.53 This model suggests that the cerebellum predicts the mechanical interactions between segments based on a stored internal knowledge of limb dynamics, and helps generate the correct motor commands for appropriate multijoint movements.
A second popular theory is the timer hypothesis. This idea proposes that the cerebellum is the main site for the temporal representation of movements.54,55 Supporters of this theory suggest that cerebellar output ultimately encodes the precise temporal sequence of muscle activation with such precision that a cerebellar lesion produces obvious deficits in the spatial domain (e.g., movement direction and magnitude) as well as the temporal domain.56 Other studies have shown that individuals with cerebellar damage also have impairments in perceiving time intervals, suggesting that this could be a more general cerebellar function.57,58
A third idea is that the cerebellum acts as an internal model to allow predictive control of movement. Sensory feedback is inadequate for movements that need to be both fast and accurate: it is too slow, and as a result, motor corrections would be issued too late. Instead, the brain generates motor commands based on an internal prediction of how the command would move the body. This “feed-forward” control requires stored knowledge of the body’s dynamics, the environment, and the object to be manipulated, and it is learned from previous exposure. The neural representation of this knowledge is referred to as an internal model,59–62 as it provides the ability to reproduce the effects of motor actions in the brain. The internal model theory for cerebellar function states that the cerebellum serves as the site of an internal model for movement. Accordingly, the incoordination of movement associated with cerebellar damage is a consequence of an inaccurate internal model, which disrupts nearly all aspects of feed-forward motor control.63 This idea is appealing, as it could help explain the wide variety of motor behaviors (e.g., reaching, standing balance, eye movements) and movement parameters (e.g., force, direction) that can be impaired after cerebellar damage. Likewise, human behavioral studies have recently pointed out that cerebellar damage is frequently associated with impaired feed-forward control but relatively intact feedback mechanisms.64,65
A related theory originates from the seminal works of Marr,66 Albus,67 and Ito,68 in which the cerebellum was theorized to be a sort of “learning machine.” This theory was based on careful examination of the anatomy and physiology within cerebellar microcircuits, and today it continues to provide the basis for many of the current theories of cerebellar function (i.e., those described earlier). Central to the idea of cerebellar involvement in learning was the discovery that Purkinje cell output can be radically altered by climbing fiber induction of long-term depression (LTD) of the parallel fiber–Purkinje cell synapse.48 Hence, climbing fiber inputs onto Purkinje cells can be viewed as providing a unique type of teaching or error signal to the cerebellum. More recently, LTD, long-term potentiation (LTP), and nonsynaptic plasticity have all been shown to exist at numerous sites within the cerebellum, both in the cortex and in the deep cerebellar nuclei.69–72 Thus there are multiple avenues for activity-dependent plasticity to occur within the cerebellum over relatively short time scales. It is presumed that the plastic changes in cerebellar output are responsible for changing motor behavior during the process of learning new skills.
Clinical manifestations of cerebellar lesions
Ataxia is the primary sign of damage to the cerebellum or its input structures. Ataxia refers generally to uncoordinated or disordered movement, which, though most often associated with gait (gait ataxia), can also be used to describe uncoordinated arm or leg movements (limb ataxia). Ataxia is exacerbated by moving multiple joints together and by moving quickly. Because ataxia is a nonspecific term, it is important in both clinical and research settings to use more precise terminology to describe the specific aspects of motor performance that are impaired. Around the beginning of the twentieth century, Joseph Babinski and Gordon Holmes were two of the earliest investigators to describe many of these specific features we now discuss here.73–75
Dysmetria
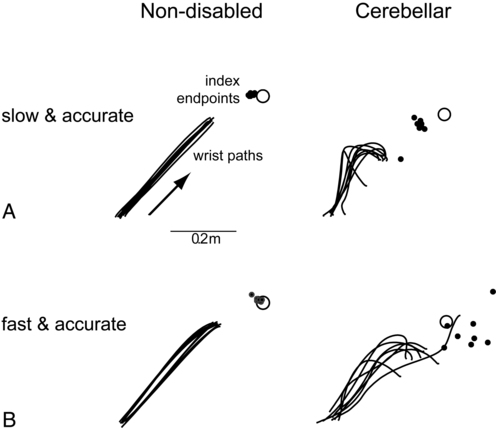
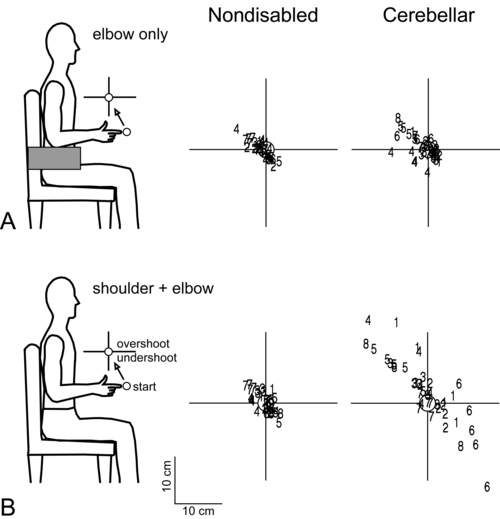
Dysmetria specifically refers to an impaired ability to properly scale movement distance. Movements are described as either hypermetric or hypometric, referring to overshooting or undershooting of targets, respectively. Many clients with cerebellar lesions will show both forms of dysmetria even during successive movements (Figure 21-4).49,50 Dysmetria can be seen in both proximal and distal joints and occurs during both single-joint and multijoint movements, though multijoint movements worsen dysmetria (Figure 21-5).49,76–78 Slow movements tend to produce hypometria, whereas fast movements almost always bring about hypermetria.49 For this reason, it has been speculated that hypometria represents more of a voluntary compensation for hypermetria than a primary impairment from cerebellar damage. Sometimes large end point errors can be reduced to some degree with visual feedback, but even the corrective movements themselves are still abnormal.79
One proposed mechanism for dysmetria is an impaired ability to predict and account for the dynamics of the limbs. In particular, clients with cerebellar lesions have been demonstrated to have a specific deficit in the ability to account for interaction torques,49,50 the rotational forces that act on a limb segment when another linked limb segment is in motion.68 When the cerebellum is intact, the central nervous system is able to predict the effects of interaction torques and appropriately counter or exploit them so as to produce a smooth, straight, and accurate reach in a feed-forward manner. When the cerebellum is damaged, an incorrect or absent accounting for interaction torques leads to an incorrect feed-forward motor plan and subsequently an uncoordinated, overly curved and hypermetric or hypometric reach that requires feedback corrections to reach the target location.
Dyssynergia
Originally, Babinski80 coined the term asynergia to mean a deficit in the coordination of movements of one body region or in one limb segment with movements of another. Today, dyssynergia is used to describe impairment of multijoint movements, wherein movements of specific segments are not properly sequenced or of the proper range or direction, resulting in uncoordinated multijoint movement. As indicated earlier, it is nearly universally true that clients with significant cerebellar damage show greater impairments during multijoint movements than single-joint movements. However, it is not fully understood whether the reason for that is because the deficits of single-joint movements are compounded during a multijoint movement or because the cerebellum plays a special and unique role in multijoint control. Dyssynergia appears to be related to dysmetria and therefore is probably also related to a deficit in predicting limb dynamics.49
Dysdiadochokinesia
Dysdiadochokinesia specifically refers to a deficit in the coordination between agonist-antagonist muscle pairs elicited during voluntary rapid alternating movements.80 Such coordination is typically tested during performance of simple, fast alternating movements such as forearm supination-pronation or hand or foot tapping. Characteristic deficits are excessive slowness along with inconsistency in the rate and range of the alternating movements, which worsen as the movement continues.74 Dysdiadochokinesia appears to be caused by poor regulation of the timing of cessation of agonist muscle activity and the initiation of antagonist muscle activity,81,82 which could be related to a deficit in predicting limb dynamics. Indeed, rapid reversals in movement are dynamically difficult to control.
Decomposition
Movement decomposition refers to the breaking down of a movement sequence or a multijoint movement into a series of separate movements, each simpler than the combined movement.75 An example of this is the well known finding that clients with cerebellar damage, when asked to reach to a target in front of and above the resting arm, will often flex the shoulder first and then, while holding the shoulder fixed, extend the elbow.49 This approach is generally slower and will produce a more curved trajectory of the finger to the target compared with nondisabled individuals, who would typically perform the shoulder flexion and elbow extension at the same time to produce a nearly straight-line finger trajectory. Most likely, decomposition reflects more of a compensatory strategy for dealing with impaired multijoint movements than it does a primary sign of cerebellar damage.49,83
Cerebellar tremor
Despite being a very common neurological sign, tremor is poorly defined and not well understood. There are several different forms of tremor, many with different causes, only some of which are related to cerebellar dysfunction, so it is important to distinguish among them. Tremor associated with damage to the cerebellum is typically called action tremor, reflecting the fact that it is absent at rest and elicited during muscle activation and distinguishing it from the resting tremor associated with Parkinson disease. Action tremor can be classified as postural or kinetic tremor.84 Postural tremor occurs in muscles maintaining a static position against gravity (e.g., holding arms out in front of the body or standing in place), whereas kinetic tremor occurs in muscles producing an active voluntary movement. Therefore the movement oscillations are most visible in the same plane as the voluntary movement. Kinetic tremor typically occurs at relatively low frequencies (approximately 2 to 5 Hz) and can be observed during simple nontarget-directed movements such as forearm pronation and supination or foot tapping, or during targeted movements such as pointing during the finger-to-nose test. Intention tremor is a specific form of kinetic tremor that occurs during the terminal portions of visually guided movements toward a target. It may actually represent the multiple corrective movements, driven by visual feedback, to reach the target. As such, intention tremor can be tested by repeating the test movement with eyes closed; if the tremor decreases substantially or disappears, it is an intention tremor.79
Classic cerebellar tremor is kinetic tremor with intention tremor at movement termination. In general, cerebellar tremor is thought to be caused by an insufficient ability to anticipate the effects of movement and excessive reliance on sensory feedback loops.85 Cerebellar tremor is highly influenced by sensory conditions and has a strong mechanical component; it is significantly reduced during isometric conditions or when vision is removed. It also can be decreased in some clients by adding an inertial load to the limb,86 though that strategy may also increase dysmetria.87 There may also be a significant central component to cerebellar tremor, possibly related to influences from the thalamus or the inferior olive.81,88
Hypotonia
Hypotonia in clients with cerebellar damage was first described by Holmes.75 It appears to arise from decreased excitatory drive to vestibulospinal and reticulospinal pathways, two major output pathways from the cerebellar vermis and flocculonodular lobe. The hypotonia usually manifests as a decrease in the extensor tone necessary for holding the body upright against gravity. In cats, lesions of either the vestibular or fastigial nuclei cause this sort of postural hypotonia.51,89–91 More recent observations in humans indicate that hypotonia is typically most problematic in cases of severe cerebellar hypoplasias affecting the vermis, such as Joubert syndrome,92 or in adults during the acute stage of cerebellar injury only. In cases of adult-onset acute injury, hypotonia usually resolves naturally over time and clients recover normal passive muscle tone and normal reflexes quickly. Thus hypotonia typically presents minimal to no problems for physical function.81
Imbalance
Classically, cerebellar imbalance during stance was considered to be of a similar magnitude whether or not the eyes are open; that is, little improvement noted with visual feedback73,75 and a negative Romberg test result. However, more recently, investigators using posturography measures have been able to distinguish several different categories of cerebellar imbalance during quiet standing, some of which do show improvement with visual stabilization.93,94 For instance, clients with cerebellar damage relatively isolated to the anterior lobe typically show increased postural sway, which is of a high velocity and low amplitude and occurs mainly in the anterior-posterior dimension. These individuals also tend to have associated postural tremor and increased intersegmental movements of the head, trunk, and legs and tend to improve when allowed visual information. On the other hand, localized damage to the vestibulocerebellum more often leads to increased postural sway that consists of low-frequency and high-amplitude movements without a preferred direction and without increased intersegmental movements. These individuals typically show no improvement with visual information. Clients with damage limited to the lateral cerebellum tend to have only slight or even no postural instability at all.93–95
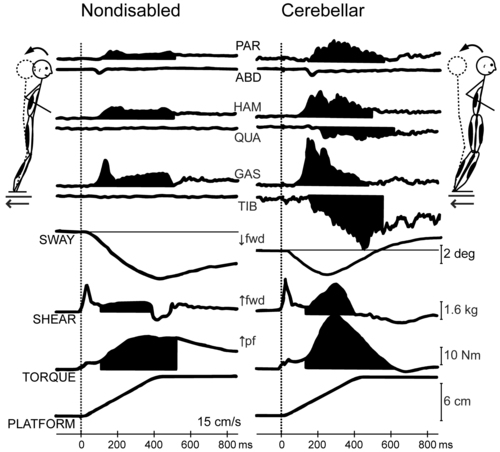
Human cerebellar damage is also associated with hypermetric postural responses to surface displacements or during step initiation; that is, dynamic instability.96,97 Specifically, clients tend to produce larger-than-normal surface-reactive torque responses and exaggerated and prolonged muscle activity, thereby overshooting the initial posture during the return phase of the recovery from a perturbation (Figure 21-6).
Gait ataxia
Probably the greatest complaint and the most obvious sign of cerebellar damage is gait ataxia. This abnormal pattern of walking is often described as a “drunken” gait because clients often stagger and lose balance as if intoxicated. Early work of Holmes showed that patients with cerebellar lesions have severe difficulty maintaining balance during walking, which often leads to falls, typically directed backward and toward the side of the lesion. Holmes reported specifically that walking is slowed, with steps that are short, irregular in timing, and unequal in length. The legs sometimes lift overly high during the swing phase by excessive flexion at the hip and knee and then lower abruptly and with uncontrolled force. The trajectory of walking often veers erratically and patients have difficulty with stops or turns, especially if performed quickly.75
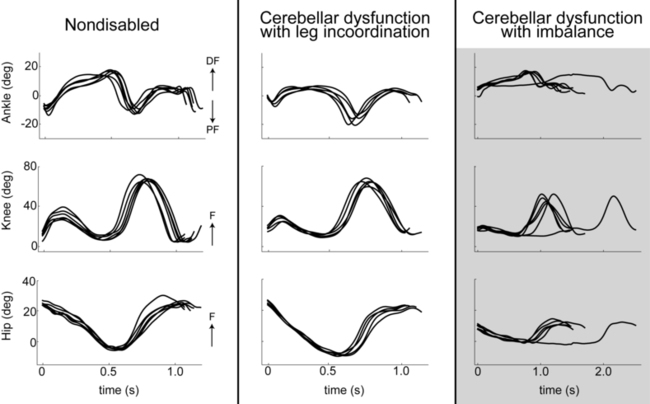
Those initial reports have now been confirmed numerous times; clients with cerebellar damage walk without the consistency in timing, length, and direction of steps typical of healthy adults.77,98 In some cases gait appears wide based. There is also increased variability in both the timing and movement excursion at the hip, knee, and ankle joints and irregularities in the resulting path of the foot during swing. Coordination between joints of one leg and between legs (intralimb and interlimb coordination, respectively) is also abnormal.77,98,99 As an example, the timing of peak flexion at one joint with respect to other joints’ positions may be altered or inconsistent. Often decomposition is also observed between hip and knee, knee and ankle, and/or hip and ankle joints.99,100
A critical component of locomotor control is the requirement for stability and dynamic balance while maintaining forward propulsion. Thus imbalance, described previously, is also a major contributor to many features of gait ataxia. In fact, it has been shown that clients with cerebellar damage and significant balance deficits also typically demonstrate nearly all the classic features of gait ataxia (i.e., reduced stride lengths, increased stride widths, reduced joint excursions, abnormal swing foot trajectories, increased variability in foot placement, and joint-joint decomposition). In contrast, clients with cerebellar damage and significant leg coordination deficits but minimal or no balance deficits typically have very few walking abnormalities (Figure 21-7).101,102 Therefore during typical conditions of level walking, balance deficits contribute much more strongly to cerebellar gait ataxia than do leg coordination deficits.
Oculomotor deficits
Eye movements are often dramatically impaired after cerebellar damage. Saccades are often slowed and dysmetric (can be hypermetric or hypometric).103 Smooth pursuit may be “choppy,” referred to as saccadic pursuit, wherein the smooth tracking of a target is degraded into a series of shorter saccadic movements following behind the target.104 The ability to cancel, or suppress, the VOR may be impaired or absent.105 Finally, abnormal nystagmus may also be present. The nystagmus may occur during central gaze, or there may also be alternating nystagmus or rebound nystagmus. The most common form of nystagmus in cerebellar dysfunction is gaze-evoked nystagmus, indicating nystagmus elicited toward the end ranges of lateral and/or vertical gaze.106,107
Clients with significant oculomotor abnormalities may be referred to vestibular specialists, but these deficits should never be ignored. Impaired eye movements may have a significant negative impact on physical function. For example, impaired saccades can prevent a client from reading, and saccadic pursuit can exacerbate already poor visually guided limb movements.108 Perhaps most devastating, deficits related to impaired oculomotor control and vestibular reflexes often worsen dynamic balance and walking abilities.
Speech impairments
Speech production may also be impaired when the cerebellum is damaged. Classically, the speech deficit associated with cerebellar damage is referred to as scanning speech, though it may be more generally referred to as ataxic dysarthria. Similar to limb control deficits, the primary impairment of speech may be related to the planning and prediction of movements rather than the execution of speech components directly.109 Also like limb movements, most speech impairments appear to be attributable to alterations in timing and coordination.143a The most consistent characteristics of ataxic dysarthria are impaired articulation (the correct pronouncement of speech sounds) and impaired prosody (the pattern of stress and intonation of certain syllables or words). Other common findings include slowed speech and either a lack of or excessive loudness variability.109 Traditionally, speech impairments are treated primarily by speech and language pathologists.
Impaired motor learning
A critical problem associated with cerebellar damage is impaired motor learning. In humans the cerebellum has been linked to learning of a wide variety of motor behaviors, including recovering balance after a perturbation,96,97 learning new walking patterns,64,100,111 adjusting voluntary limb movements,65,112,113 and making eye movements.114,115 The type of learning that appears most reliant on the cerebellum is associative and procedural. Specifically, the cerebellum appears to be essential for learning to adjust a motor behavior through repeated practice of, or exposure to, the behavior and using error information from one trial to improve performance on subsequent trials. It is important to note that cerebellum-dependent motor learning is driven by errors directly occurring during the movement rather than by other types of feedback, such as knowledge of results after the fact (e.g., hit or miss). Studies have suggested that the type of error that drives cerebellum-dependent learning is not the target error (i.e., “How far am I from the desired target?”), but instead what has been referred to as a sensory prediction error (i.e., “How far am I from where I predicted I would be?”).113,116
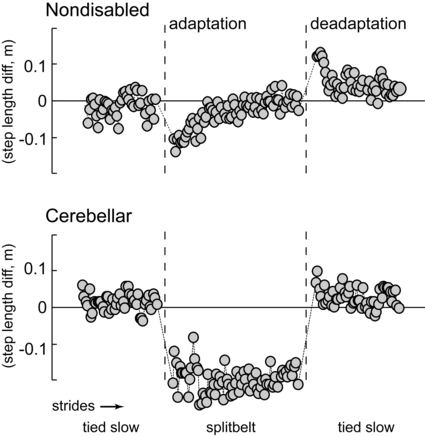
In the laboratory setting, cerebellar learning is most easily tested via motor adaptation, a form of motor learning that requires a modification of an already well learned motor behavior for new environmental or physical demands (in contrast to learning of a completely novel skill). Adaptation is an error-driven learning process that is acquired on a time scale of minutes or hours, as opposed to days or weeks.117,118 It is an active process: movement adaptation takes trial-and-error practice of the task, in which errors during one trial change movement on the subsequent trial. Storage of the adapted movement is shown by the presence of aftereffects when the new demand is removed. Specifically, aftereffects are movement errors in the opposite direction to the original errors during adaptation and provide strong evidence that the central nervous system adjusts the predictive control for body movements with practice.62,119 Thus when the new demand is removed, a process of active “unlearning” or deadaptation must occur to return the movement to its original form. An example of a locomotor adaptation is shown in Figure 21-8. In this case, a walking adaptation is induced by having subjects walk on a split-belt treadmill, where one belt is moving at twice the speed of the other, forcing the two legs to walk at different speeds. Control subjects are able to rapidly restore appropriate step length symmetry after only a few minutes walking on the split-belt treadmill. They also appear to store the newly learned set of (predictive) motor commands, demonstrated by large negative aftereffects (step length asymmetry in the reverse direction compared with early adaptation) when the treadmill belts are initially returned to a regular (nonsplit) pattern. In contrast, individuals with cerebellar damage typically show a slower rate of adaptation, a reduced magnitude of adaptation, or no adaptation at all, and small or no aftereffects (see Figure 21-8). All of these findings indicate a significant deficiency in the capability for motor adaptation in individuals with cerebellar damage. As indicated earlier, adaptation deficits have been demonstrated in this patient population with numerous behavioral tasks.*
Other forms of motor learning may not depend on the cerebellum and thus may be particularly useful for rehabilitation for clients with cerebellar lesions, though this has never been formally tested. One example is use-dependent motor learning, in which a person strengthens a movement pattern with repeated practice of that same pattern.120 It is not clear what mechanisms subserve this form of learning, though a Hebbian-like process in the cerebral cortex seems likely (i.e., repeated use strengthens the synapses in the brain that are engaged). Another form of motor learning is reward or reinforcement learning. This may involve basal ganglia circuits to strengthen movements that are rewarded.121 Whether individuals with cerebellar damage can use either of these other forms of learning has not been experimentally tested. Yet if they can, these learning mechanisms might provide important compensatory strategies for the loss of error-dependent adaptations.
Nonmotor impairments
Within the last 25 years, researchers have exposed a possible role of the cerebellum in a number of nonmotor, cognitive behaviors. Much of the early evidence for cerebellar involvement in nonmotor tasks came from functional imaging studies showing increased activation within the cerebellum during performance of certain tasks with a predominant cognitive component, such as language processing.122,123 Speculation has since arisen that the cerebellum may be involved in not only language, but also working memory,124 learning nonmotor associations between objects,125 and higher-order executive functions.126 Loss of control over emotional behaviors127 and certain neurodevelopmental and neuropsychiatric disorders have also been said to be linked to cerebellar damage.128,129 However, interpretation of some investigations of the relationship between the cerebellum and cognition is limited in that it is sometimes difficult to separate the cognitive and motor components of a task, particularly in imaging studies in which subjects are instructed to perform some motor task to indicate a cognitive choice.130,131 Nevertheless, anatomical studies have shown clearly that the cerebellum has connections to brain areas considered relatively purely cognitive in function, suggesting that a cognitive role for the cerebellum is likely.132
Clinical signs by functional division
Because of the organizational structure of the cerebellum into functional longitudinal divisions, discrete areas are associated with specific signs and symptoms. Thus, depending on the location and volume of cerebellar damage, clients may have just a few or nearly all of the clinical manifestations described earlier. Clients with damage to the flocculonodular lobe or midline zone typically demonstrate some (though usually transient) loss of postural tone, impaired upright posture and balance, gait ataxia, and oculomotor deficits.2,51 On the other hand, damage to the intermediate zone often results in action tremor, dysdiadochokinesia, and dysmetria of the limbs.2,51 Finally, damage to the lateral zone commonly produces dyssynergia, dysmetria, and difficulty planning complex limb movements, especially those that are visually guided (see Table 21-3).2,51
Often, however, multiple longitudinal zones are affected at the same time. For instance, the arteries supplying the cerebellum each span more than one longitudinal zone, such that a stroke involving a single cerebellar artery would be likely to cause signs and symptoms associated with more than one zone (see Table 21-1). Degenerative disorders affecting the cerebellum also are typically pancerebellar, affecting large regions of the cerebellum. Hence these clients will also typically have a wide variety of signs and symptoms. A careful and thorough clinical examination is therefore a requirement before a diagnosis for physical therapy can be made in clients with cerebellar damage.
Medical management of cerebellar damage
The greatest limitation in caring for clients with cerebellar dysfunction is the lack of any curative treatment for most forms of cerebellar damage. Pharmacological agents have been used, but with limited success. Often, clients with ataxia and degenerative forms of cerebellar damage may be prescribed vitamin E, coenzyme Q10, and/or its synthetic analogs, but the effectiveness of these drugs in minimizing symptoms or otherwise slowing or halting the disease is largely unsubstantiated.133,134 Other drugs that may be used include acetazolamide, amantadine, 4-aminopyridine, and buspirone chlorhydrate,6,135–137 but these also have questionable effectiveness. Studies of the effectiveness of medications for cerebellar ataxia have been significantly limited by inadequate sample sizes, inappropriate outcome measures, and/or limited follow-up. Given the unsatisfactory results from pharmacological interventions thus far, most clients with cerebellar ataxias must rely primarily or solely on physical rehabilitation approaches to restore or reduce the symptoms of their disease.
Physical and occupational therapy management of cerebellar movement dysfunction
As is the case with all brain lesions, there is nearly always some level of natural, or spontaneous, recovery after damage to the cerebellum. The extent of recovery depends on complex interactions among numerous factors including the source of damage; the severity, location, and volume of damage; the presence or absence of damage to other brain regions; the presence or absence of other coexisting medical conditions; age; and other factors. Several studies have now indicated that motor recovery from a first-ever ischemic cerebellar stroke is generally excellent, with minimal to no residual deficits in up to 83% of patients.138–140 On the other hand, individuals with degenerative cerebellar disorders tend to have progressively worsening clinical signs and symptoms.141 One study has shown that people with damage to the deep nuclei do not recover as well as those with damage to only the cerebellar cortex and white matter.2 Another consideration is the degree to which other brain regions are lesioned. Individuals with cerebellar stroke or tumor may also have damage of the brain stem. Clients with multiple sclerosis or head injury often also have cerebral and spinal cord lesions. Moreover, the majority of the spinocerebellar ataxias (SCAs) affect other neural structures well beyond the cerebellum; these are often the corticospinal tract, cerebral cortex, basal ganglia, and sometimes the peripheral nervous system. Generally speaking, the presence of multiple lesion locations is associated with a poorer outcome, possibly because circuits that could otherwise serve a compensatory role are also damaged.142
Physical/occupational therapy evaluation
A majority of the components of the physical therapy examination for clients with cerebellar dysfunction are the same as would be performed with any client with a health condition that is primarily neurological in origin. Therefore we will discuss only the features that are unique or of critical importance for the client with cerebellar pathology. In the following sections we highlight just a few of these specific tests. Box 21-1 contains a more complete list. One important note is that many of the tests for cerebellar movement dysfunction are sensitive but not specific to cerebellar pathologies. That is, although they often will reveal abnormalities in a client with cerebellar damage, their results are frequently also observed to be abnormal in clients with other, noncerebellar disorders of a neuromuscular origin. Therefore they should not be used in isolation to rule in or rule out cerebellar pathologies.
Tests of impairments of body functions or structures
A major component of almost all physical therapy examinations of clients with cerebellar dysfunction is the posture and balance examination. Posture and balance should always be observed in both static and dynamic conditions and in both sitting and standing positions, as the client’s capabilities allow. The examination is performed in the same manner as for clients with other neurological disorders and so is not described in detail here (see Box 21-1 for suggested components to emphasize with clients with cerebellar dysfunction). Important considerations specific for clients with cerebellar dysfunction include careful monitoring for symptoms of nausea or vertigo (common in acute cerebellar stroke, may resolve quickly), observation for postural tremor or titubation, and observation of the recovery from perturbations and the presence of lack of check. Although physical therapists most often deal with posture and balance, occupational therapists also need to identify and examine these components in order to analyze movement activity problems that are based upon a functional trunk such as dressing, feeding, and other daily living activities.
It is also important to examine the client’s initial level of endurance (fatigability), both in the cardiovascular and muscular systems. For the cardiovascular system, this can be approximated by recording the response to sustained aerobic exercise on one or more measurement scales (e.g., perceived exertion, heart rate, blood pressure, respiratory rate). See chapter 30 for additional information. For the muscular system, this can be approximated by recording the maximal number of repetitions of a specific set of muscle contractions or limb movements that can be tolerated before force output or range of motion is reduced. These types of measures can provide a gross gauge of the overall level of cardiovascular and musculoskeletal fitness of the client. Because cerebellar dysfunction often leads to movements being much more effortful and often exaggerated, it is extremely important that the client with cerebellar dysfunction obtain adequate endurance for safe participation in daily activities.
Tests of activity limitations
Tests of activity limitations should typically proceed similarly to the standard neurological examination. The observation of gait should be given particular attention, as gait ataxia is considered one of the most sensitive signs of cerebellar damage and the inability to walk safely is a major participation limitation for most clients with motor disorders.143 Box 21-1 provides suggested components of the gait analysis for clients with cerebellar dysfunction. In addition, it may be necessary to evaluate the activity limitations related to speech, visual, and/or oculomotor deficits common to cerebellar dysfunction. With careful interviewing, the client may relay information about limitations in conversation and communication, reading, and so on that should be addressed in rehabilitation. The physical or occupational therapist may be the first health care professional to note these types of activity limitations and should refer the client to the proper professional for additional services.
Standardized clinical scales
There are two common standardized rating scales that quantify the severity of cerebellar ataxia. The more well known is the International Cooperative Ataxia Rating Scale (ICARS).143a This scale was first published in 1997 by the Ataxia Neuropharmacology Committee of the World Federation of Neurology in response to an established need for a universal scale to quantify ataxia in randomized clinical trials of pharmacological interventions for treating ataxia. The ICARS measures a client’s ability to perform 19 specific activities or movements using an ordinal scale. The activities are grouped into categories based on whether they relate to cerebellar dysfunction affecting (1) posture and gait, (2) limb movements, (3) speech, or (4) oculomotor performance. A subscore is tallied for each category and a total score is obtained, ranging from 0 (no ataxia) to 100 (most severe ataxia). The ICARS has been found to be reliable in clients with cerebellar dysfunction and has established criterion-related and external validity.144–147 More recently, it was shown that the ICARS is sensitive to increases in ataxia severity over one year in persons with chronic cerebellar degeneration.141 The Scale for the Assessment and Rating of Ataxia (SARA) is a newer tool that was devised, at least in part, out of concern over the construct validity of the ICARS subscale structure.148 The SARA is similar to the ICARS in that it quantifies performance of specific movements or activities on an ordinal scale (many of the test activities are the same or similar to those in the ICARS), but it does not categorize individual test items by body part. The SARA has fewer test items (only eight) and can therefore be administered faster in the clinical setting. The SARA has been shown to be reliable and valid for clients with SCAs.148 Either the ICARS or SARA scale may be useful for longitudinal tracking of disease progression in cerebellar degeneration or for quantifying improvement of ataxia in clinical trials. The scales are detailed in Appendixes 21-A and 21-B.
Physical/occupational therapy interventions for clients with cerebellar dysfunction
The literature on the effectiveness of rehabilitation interventions for individuals with primary cerebellar damage is extremely limited: there have been no randomized controlled trials published. Of the few studies on the effects of rehabilitation interventions in this patient population, all have been nonrandomized, noncontrolled small group101,149,150 or case study151–155 designs. The fact that there are so few studies available, each featuring different client populations (e.g., poststroke versus post–surgical tumor resection versus cerebellar degeneration) and different outcome measures, makes determining the most effective interventions difficult. Therefore we provide here only a description of the major themes that have arisen from these studies to date.
Gait and balance interventions
Many of the intervention studies for cerebellar ataxia emphasize stability and balance, especially during gait.101,149,153,155 This likely is a reflection of the tight link between gait and balance102 and the fact that gait ataxia is one of the most common and debilitating signs of cerebellar damage.100 Common interventions include combinations of exercises targeting gaze, static stance, dynamic stance, gait, and complex gait activities.101,153 Some examples of exercises in each of these categories are detailed in Box 21-2. Dynamic balance activities performed while sitting, kneeling, and quadruped have also been advocated.101 Other interventions specific to the client’s individual impairments of body structure or function should be implemented as necessary, for example, stretching of short or tight ankle plantarflexors and exercises for the VOR.101,153 Locomotor training over ground and on treadmills and with and without body weight support has also been used with some success in single case examples.151,156 It is not clear how imbalance is corrected in the body weight support environment, however. With all gait and balance activities, it is critical that the exercise be sufficiently and increasingly challenging, so as to facilitate plasticity in the nervous system.157,158 In a recent study in mice, it was suggested that trial-and-error practice is a requirement for regaining full motor recovery during cerebellar remyelination.159
Aerobic exercise and resistance training
Integration of aerobic exercise and resistance training into the treatment plan is recommended for a majority of clients with cerebellar dysfunction, particularly if it is expected the client will not regain premorbid status. If full recovery is not attained, nearly all types of movements will be generally more effortful, requiring increased energy expenditure and demanding greater concentration. It is well known that repetitive fatiguing activity worsens postural control160,161 and therefore may contribute to trips, falls, or other injuries.162 Because imbalance is such a common outcome for clients with cerebellar dysfunction, incorporating both aerobic exercise to improve cardiovascular endurance and submaximal resistive exercise to improve muscle fatigue resistance appears appropriate. Aerobic exercise activities might include walking, dance, recumbent or stationary cycling, rowing, arm ergometry, swimming and aquatic exercise, as well as many other possibilities.
Consider more intensive, longer duration interventions
Because the cerebellum is thought to be a primary site of motor learning and individuals with cerebellar damage often have motor learning deficits, it is reasonable to consider whether these clients are capable of benefiting from any intervention that relies on trial-and-error motor practice.101 It has been suggested that rehabilitation for clients with significant cerebellar dysfunction may take longer (more trials, more sessions) and might not ever be complete. The question remains unanswered thus far. Of course, cerebellum-dependent motor adaptation is only one of many motor learning mechanisms, so it is possible that other processes can be engaged during rehabilitation. In support of this idea, it now appears that at least partial “re-learning” of more normal movement patterns is possible with selected cerebellar patient populations.101,150 Notably, in the literature, gains were reported under conditions of very frequent (10 hours/week) or very long (6 months) training schedules. This could be a necessity for clients with health conditions in which motor learning is impaired. Figure 21-9 shows improvements in SARA scores and self-selected walking speeds in a group of individuals with significant progressive ataxia, in response to an intensive rehabilitation program targeting balance and dynamic intersegmental control. Much more research is needed to determine the full range of improvements possible, the minimal dosage of intervention required, and whether the benefits can be retained over the long term in this difficult patient population.
Compensatory strategies
Compensation is a common component of the plan of care for clients with cerebellar dysfunction. Many clients begin using compensations subconsciously, whereas others need to be taught these strategies and when to use them. If the client is not expected to recover premorbid movement patterns, compensation can enable the individual to regain a certain prior level of activity or societal participation despite an abnormal movement pattern. Instruction in compensation can also be valuable in situations when full recovery is expected but the client would benefit from the use of compensatory strategies for the short term, such as for safety purposes. One compensatory strategy that works well for clients with cerebellar dysfunction is the instruction to simply slow down movements. Recall that slower movements are less dyssynergic and less hypermetric.49 Voluntarily reducing the number of segments moving at the same time (i.e., decomposition) also helps reduce dyssynergia and dysmetria.49,83 In some cases, reminding clients to use visual cues can also be helpful, for instance, to use vertical markings of a doorway to maintain upright stability, although for certain types of cerebellar damage this is not effective.93,94 For gait, deliberately widening stance can be helpful, both for maintaining balance and for preventing tripping over a foot. The use of assistive devices for gait should be considered on a case-by-case basis. For some clients, facilitating upper-extremity support and increasing the base of support improve balance during gait. However, for others, the level of skill required to coordinate controlling the device with the movements of arms and legs is too difficult, and using the device actually worsens the instability. Generally speaking, clients with significant imbalance and limb ataxia but who are ambulatory will often not be safe with any type of cane and instead require a wheeled walker and/or the physical assistance of a caretaker to walk. A final strategy is to remove or minimize distractions during effortful activities. Distraction, for example, dividing attention between a motor and a cognitive task, can worsen ataxia.163
The controversy of weighting
Rehabilitation textbooks have traditionally supported the use of weights for adults and particularly children with cerebellar dysfunction and ataxia. Either the trunk may be weighted by having the client wear a vest or pack that is weighted, increasing the load on the body axially,164 or the limbs may be weighted individually165,166 with simple wrist or ankle weights. Presumably this trend was based on the common finding, first noted by Holmes,74 that clients with cerebellar dysfunction appear improved (move more smoothly and with reduced tremor) if they are asked to hold a heavy weight in the hand while moving the arm. Today it is known that cerebellar tremor is at least in part mechanical in nature, such that anything that adds inertia to the moving segment would reduce the amplitude of the tremor.86 This most likely explains the improvement that has been reported. However, this apparent benefit would certainly not continue once the weight is removed, and a reduction in tremor does not indicate a normalization of any other parameters of movement. In fact, weighting of the extremities would seem a questionable choice, given the known difficulty of clients with cerebellar dysfunction to predict and account for limb dynamics or to account for interaction torques.49,50 Addition of weights, particularly to the distal segments, would seem only to make this task more difficult. Evidence for this has been demonstrated in a study of patients with cerebellar ataxia in which it was shown that weighting the extremities worsens the amount of hypermetria during a simple single-joint wrist movement task.87
One unintended consequence of weighting is that it tends to slow movements down. As described earlier, slower movements are less hypermetric and less dyssynergic than preferred-speed or fast movements. In early studies of weighting, speed was not monitored or controlled for, so a simple reduction in movement speed could account for the immediate apparent improvement described in some reports.165,166 Therefore weighting may indeed provide a benefit to some clients with ataxia but it is most likely an indirect one, related more to decreased movement speed. Accordingly, the significant potential for negative consequences of weighting (i.e., worsened hypermetria, increased interaction torques, and the annoyance and physical awkwardness of weights) makes this intervention option seem inferior to the simpler strategy of instructing the client in voluntarily moving more slowly.167