Chapter 25
Motor System II: Corticofugal Systems and the Control of Movement
General Features of Motor Deficits
Motor Cortex and the Control of Movement
Cerebellar and Pallidal Influences
Hierarchical Organization Versus Parallel Distributed Processing in the Motor System
Brushing our teeth seems like a simple voluntary movement. The neural basis for this action is, in fact, richly complex. For example, muscles in the upper limb are used cooperatively with jaw muscles while neck and back muscles provide postural support. Sensory feedback from the teeth and gums is linked to muscle afferents conveying tension and proprioceptive signals from the forearm and hand. This sensory input is an essential feature of efficient motor performance.
Even other less obvious aspects, such as visual system input and memory of prior experience, are involved. The focus of this chapter is on elements of voluntary movement that are regulated by the cerebral cortex.
OVERVIEW
Control of the voluntary movements just described is a complex, multifaceted process that involves many areas of the brain. Several of the principal control sites are located in the cerebral cortex, specifically the primary motor, premotor, and supplementary motor cortices in the frontal lobe along with portions of the parietal lobe. Although the frontal and parietal lobes have direct projections to the spinal cord, they also work cooperatively through the primary motor cortex (upper motor neurons) and the corticospinal and corticonuclear systems to influence the activity of ventral horn and cranial nerve motor neurons (lower motor neurons). The latter cells and their axons represent the final common path that links the central nervous system with skeletal muscles. Lesions that damage the descending cortical systems or lower motor neurons produce signs and symptoms of upper or lower motor neuron disease, respectively. These signs are among the most useful in diagnosis of neurologic deficits related to the control of movement.
GENERAL FEATURES OF MOTOR DEFICITS
Lower Motor Neuron Signs
Lower motor neurons are those cells whose axons synapse directly on skeletal muscle. When these neurons or their axons are damaged, the innervated muscles will show some combination of the following signs: (1) flaccid paralysis followed eventually by atrophy, (2) fibrillations or fasciculations (involuntary contractions of one motor unit or a group of motor units), (3) hypotonia (decreased muscle tone), and (4) weakening or absence of muscle stretch reflexes (hyporeflexia, areflexia).
Upper Motor Neuron Signs
The term upper motor neuron is commonly used in reference to corticospinal or corticonuclear cell bodies and their axons. Other neurons, such as rubrospinal or reticulospinal neurons, can also be included under the strict definition of this term. Corticospinal neurons are also called pyramidal neurons because their axons pass through the medullary pyramid. Therefore, the terms upper motor neuron signs and pyramidal tract signs are often used synonymously. However, as described later in this chapter, these characteristic signs of “pyramidal tract damage” are in fact the result of injury to other descending motor systems in combination with damage to fibers of the pyramidal tract. For example, ischemic lesions of the internal capsule can potentially involve corticostriatal, corticothalamic, and corticoreticular fibers in addition to corticospinal axons.
Damage to upper motor neurons results in muscles that (1) are initially weak and flaccid but (2) eventually become spastic, (3) exhibit increased muscle tone (hypertonia), seen as an increase in resistance to passive movement of an extremity, and (4) show an increase in muscle stretch reflexes (hyperreflexia), as may be seen in clonus. Upper motor neuron lesions usually affect groups of muscles, and certain pathologic reflexes and signs often appear. One of the most common is the inverted plantar reflex, also known as the Babinski sign. This involves dorsiflexion of the great toe in response to firm stroking of the lateral aspect of the sole of the foot with a blunt instrument. The response in the normal adult is plantar flexion of the great toe. In contrast, stroking the sole of the foot in a normal neonate may also result in a Babinski sign. This is related to the incomplete myelination of the corticospinal tract and will disappear as this tract matures.
Spasticity
Muscles that are no longer under the influence of upper motor neurons exhibit spasticity. That is, when tested by the physician, the affected muscles offer an increased resistance to passive movement or manipulation. These effects are most pronounced in the antigravity muscles, which in humans include the proximal flexors in the upper extremity and extensors in the lower extremity. Also, the increased resistance to passive movement is velocity dependent: the more rapidly the examiner moves the affected extremity, the greater the resistance. However, after a relatively brief period of applied force, the increased resistance totally collapses; this response is known as the clasp-knife effect (also called clasp-knife rigidity).
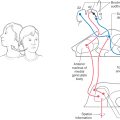
Several hypotheses have been advanced to explain spasticity. One suggests that spasticity, with its associated hypertonia and hyperreflexia, is the result of a release of dynamic gamma motor neurons from descending inhibitory control. This leads to increased gamma motor neuron firing and increased activity transmitted over type Ia muscle spindle afferents, resulting in increased excitatory tonic drive on the associated alpha motor neurons. Another suggestion is that spasticity may represent a generalized failure of the descending cortical activation of spinal cord inhibitory interneurons. For example, supraspinal fibers activate type Ia inhibitory interneurons that contact extensor motor neurons (Fig. 25-1A). If the upper motor neuron input to inhibitory interneurons were to be removed, the extensor motor neurons would be released from inhibitory control, and the result would be hypertonia and spasticity.
Descending cortical fibers also activate a type of inhibitory (glycinergic) interneuron called a Renshaw cell (Fig. 25-1B). The Renshaw cell receives excitatory input from a lower motor neuron via an axon collateral (and in turn it inhibits the same) and potentially from adjacent lower motor neurons. It is also known, for example, that cortical fibers activating ankle flexors also contact Renshaw cells (as well as type Ia inhibitory interneurons) that inhibit the antagonistic ankle extensors (Fig. 25-1B). This circuitry serves to prevent reflex stimulation of the extensors when flexors are active. Therefore, when the cortical fibers are lost (upper motor neuron lesion), the inhibition of antagonists is absent. The result is repetitive, sequential contraction of ankle flexors and extensors. Such a phenomenon is referred to as clonus and is often present in combination with spasticity and hyperreflexia.
CORTICOSPINAL SYSTEM
Before the specifics of corticospinal projections are considered, a general point about laterality should be made. The fibers that form the core of the corticospinal system cross the midline in the pyramidal decussation; this structure is commonly called the motor decussation because it is where corticospinal fibers cross. Corticospinal fibers arising in the left motor cortex therefore influence muscles on the right side of the body, and vice versa. Consequently, lesions of corticospinal fibers rostral to the motor decussation result in contralateral motor deficits, whereas lesions of the corticospinal tract in the spinal cord result in ipsilateral deficits. An understanding of this concept of laterality is essential in the diagnosis of the neurologically impaired patient.
Origin
Neurons that give rise to corticospinal axons are located in deep portions of layer V of the cerebral cortex (Fig. 25-2A). A small number of these pyramidal neurons are especially large, with somata that may reach 100 µm or more in diameter. These neurons are called Betz cells, and at one time it was believed that they were the sole source of corticospinal axons. It is now known that they account for only 1% to 2% of this fiber bundle.
Corticospinal neurons are found primarily in six cortical locations (Fig. 25-2B). The single largest concentration (about 31%) is in Brodmann area 4, which occupies the posterior portion of the precentral gyrus bordering on and extending into the depth of the central sulcus and in the anterior paracentral gyrus. This region is also called MI, the primary motor cortex. The premotor and supplementary motor cortices, which are located in area 6, give rise to about 29% of corticospinal axons. Thus about 60% of all corticospinal axons arise from neurons in the frontal lobe, with more than half of this group coming from the precentral and anterior paracentral gyri (Fig. 25-2B). The remaining approximately 40% arise from the parietal lobe and a few other regions. Included are cells in the postcentral gyrus (areas 3, 1, and 2), the superior parietal lobule (areas 5 and 7), and portions of the cingulate gyrus.
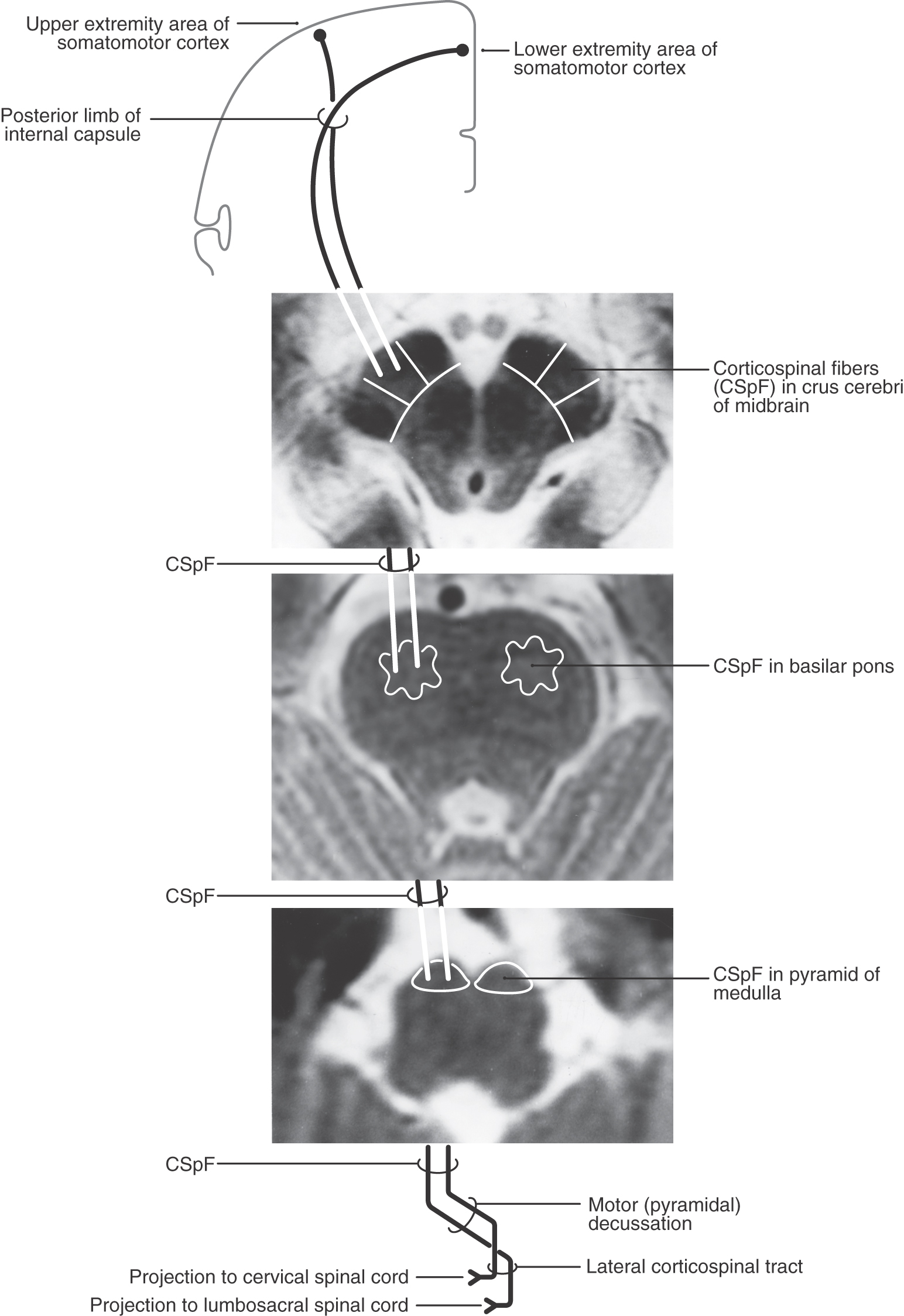
Within MI, corticospinal neurons are somatotopically organized in patterns that reflect their influence over specific muscle groups. The caricature thus created is called the motor homunculus (Fig. 25-3A). Neurons in medial MI, the anterior paracentral gyrus, project to lumbosacral cord levels to influence motor neurons that innervate muscles of the foot, leg, and thigh. Thoracic and cervical cord levels, which contain motor neurons innervating the trunk and upper extremity, receive input from neurons in the medial two thirds of the precentral gyrus. The musculature of the head, face, and oral cavity is influenced by neurons in the lateral third of the precentral gyrus (Fig. 25-3A). These cells contribute to the corticonuclear tract that projects to cranial nerve motor nuclei. The disproportion in body part size in the homunculus reflects the density and distribution of corticospinal neurons devoted to the control of musculature in each particular region of the body (Fig. 25-3A). Complete but less precise body representations are also found in other motor cortical regions. Thus a single muscle or muscle group can be influenced from multiple locations in the cerebral cortex.
The blood supply to MI arises from branches of the anterior and middle cerebral arteries (Fig. 25-3B). The lower extremity area of MI is served by terminal branches of A4 and A5 segments of the anterior cerebral artery. Specifically, these branches arise from the callosomarginal artery. The trunk, upper extremity, and head areas of the motor cortex are supplied by branches of the M4 segment of the middle cerebral artery, mainly its rolandic and prerolandic branches.
Lesions that involve only areas of motor cortex outside the MI usually do not result in paralysis, and the effects may dissipate over time. For example, vascular infarcts of the premotor or supplementary cortex may produce an apraxia. This disorder involves difficulty in using the affected part of the body to perform voluntary actions, such as grasping a pencil, even though there is no obvious spasticity, paralysis, or altered tone in the muscles. For example, a premotor lesion may result in the inability to perform voluntary actions with the contralateral hand, although the strength and tone of the hand muscles are normal. Similarly, unilateral lesions in the supplementary motor cortex affect the ability to coordinate actions on the two sides of the body. The muscles, again, are normal. In contrast, lesions that affect the primary motor cortex in combination with another motor cortical region usually result in spastic paralysis and hyperreflexia, signs characteristic of upper motor neuron lesions.
Course
The largest axons in the corticospinal tract are myelinated, range from 12 to 15 µm in diameter, and have conduction velocities up to 70 m/s, but they make up less than 10% of the total corticospinal population. The remainder are less than 5 µm in diameter, and many are lightly myelinated or unmyelinated with proportionally slower conduction velocities.
Corticospinal fibers pass through the corona radiata and converge to enter the posterior limb of the internal capsule (Fig. 25-4A). Here the fibers are somatotopically organized in about the caudal half of the posterior limb such that the axons that terminate at the highest cord levels are located most rostrally and the axons that terminate at progressively lower levels are located more caudally (Fig. 25-4B).
Unlike with lesions of the cortical gray matter, interruption of axons in the posterior limb of the internal capsule often results in catastrophic motor deficits. A common cause of lesions in this area is hemorrhage from lenticulostriate branches of the M1 segment of the middle cerebral artery (Fig. 25-4A). Motor symptoms of capsular infarcts appear in the contralateral upper and lower extremities and consist of weakness and transient flaccid paralysis of variable duration, which is followed by spastic paralysis (upper motor neuron signs) that typically never resolves. Apparently these symptoms appear because not only corticospinal fibers but also many other types of cortical axons are interrupted. Included are axons projecting to the neostriatum, thalamus, and brainstem as well as thalamocortical axons involved in somatic sensation and vision. Damage to thalamocortical axons explains why hemisensory loss or homonymous hemianopia (in the case of an anterior choroidal artery syndrome) may accompany the motor deficits. Deficits such as spasticity, hypertonia, and hyperreflexia, although commonly associated with pyramidal tract lesions, are in fact due to damage of other descending systems in combination with damage to corticospinal fibers.
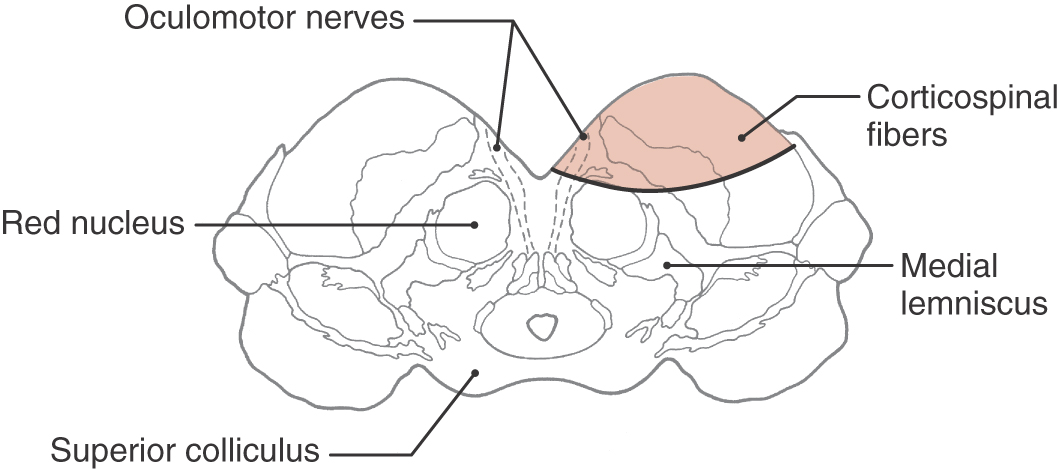
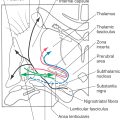
As they pass caudally from the internal capsule, corticospinal fibers traverse the various divisions of the brainstem. In the midbrain they coalesce to form the middle third of the crus cerebri (Figs. 25-5A, 25-6, and 25-7). Within this part of the crus, fibers from forearm-hand (upper extremity) areas of MI are located medially, whereas those from leg-foot (lower extremity) areas are located laterally.
Fibers in the medial two thirds of the crus cerebri (frontopontine, corticonuclear [corticobulbar], and corticospinal) and the exiting rootlets of the oculomotor nerve fibers are served by paramedian branches of P1 and branches from the adjacent posterior communicating artery (Fig. 25-6). Hemorrhage of these vessels will damage these groups of fibers, resulting in (1) contralateral hemiparesis of the arm and leg with spasticity and (2) deviation of the ipsilateral eye down and laterally because of the unopposed action of the superior oblique and lateral rectus muscles. Direct and consensual light reflexes and accommodation may also be lost in the eye on the side of the lesion. This condition is known as superior alternating hemiplegia because cranial nerve signs are seen on one side and corticospinal signs on the “alternate” side; it is also called a crossed deficit. In clinical parlance, this combination of deficits is known as a Weber syndrome (Fig. 25-8; Table 25-1).
Table 25-1 Synopsis of Brainstem Syndromes Involving Corticospinal Fibers and Cranial Nerves*
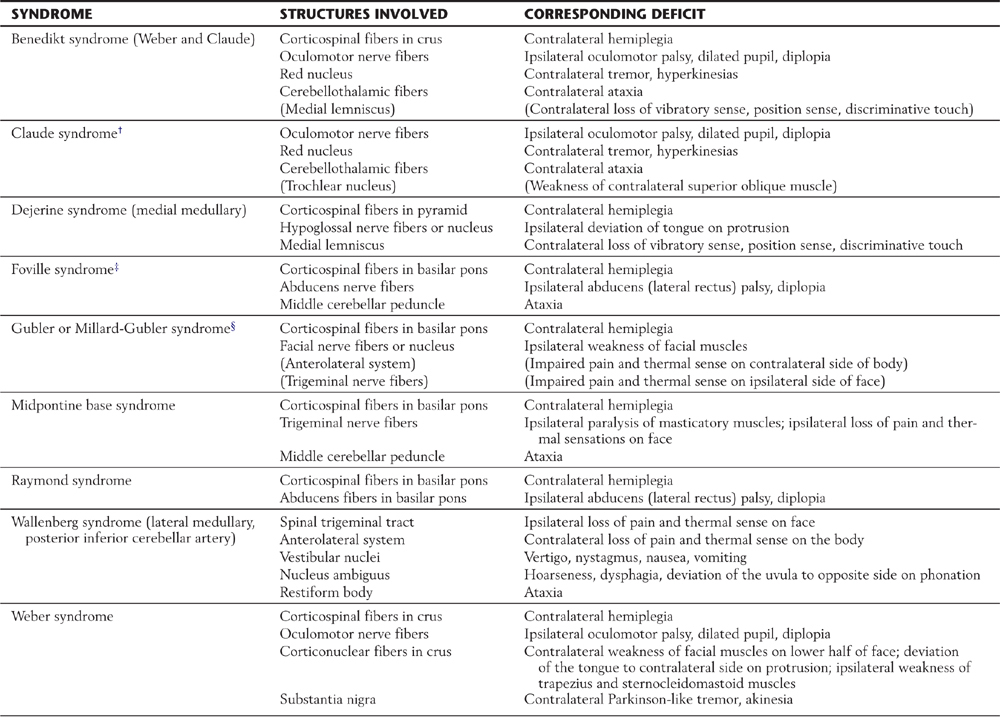
*Syndromes are listed in alphabetical order.
†Although this syndrome does not include corticospinal fibers, it is included here for completeness.
‡This syndrome is described, in some sources, as including the facial nerve or nucleus, anterolateral system fibers, paramedian pontine reticular formation (lateral gaze center), and medial lemniscus, each with their corresponding deficits.
§Some sources also include the fibers of the abducens nerve as being involved in this syndrome.
Note: Those structures or deficits listed in parentheses are inconsistently seen in these respective syndromes. Lesions in the lateral areas of the brainstem interrupt descending hypothalamospinal fibers to the interomediolateral cell column (GVE preganglionic sympathetic cells) of the spinal cord. An ipsilateral Horner syndrome (ptosis, a drooping eyelid; miosis, constricted pupil; anhidrosis, lack of facial sweating) is commonly seen in these patients.
From the midbrain, corticospinal fibers continue into the basilar pons, where they make their way longitudinally between the masses of neurons forming the basilar pontine nuclei (Figs. 25-5B, 25-6, and 25-7). As corticospinal axons pass through the pontine gray, they give rise to collaterals that synapse on these neurons.
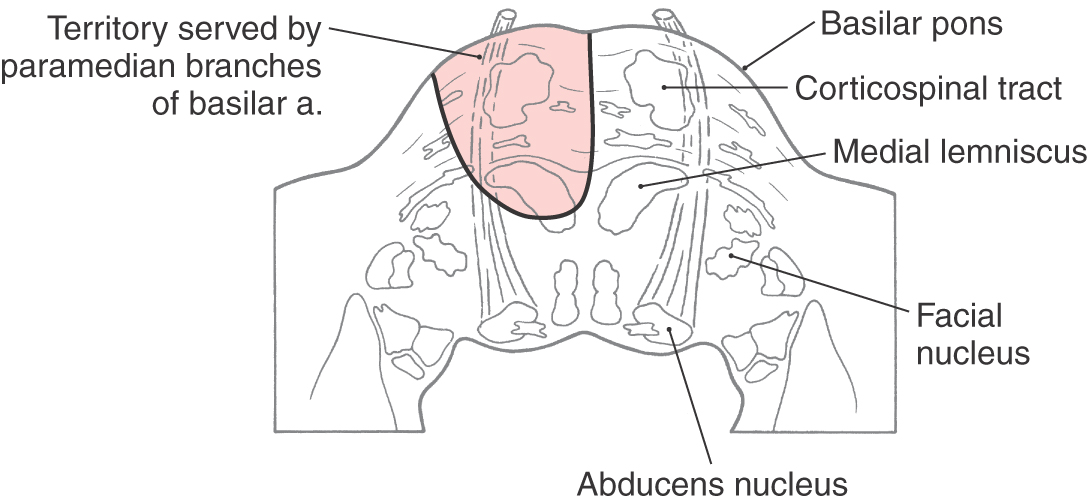
Corticospinal fibers in the basilar pons and the exiting fibers of the abducens nerve in the caudal pons are within the domain of the paramedian branches of the basilar artery. Occlusion or rupture of these vessels results in hemiplegia and upper motor neuron signs in the contralateral extremities. The lesion may also involve intraaxial abducens fibers, resulting in lower motor neuron paralysis of the ipsilateral lateral rectus muscle (Fig. 25-9). This combination of deficits (ipsilateral abducens paralysis and contralateral hemiplegia) is (1) a characteristic of brainstem lesions, that is, a crossed deficit, (2) called a middle alternating hemiplegia, and (3) one of the variations of the Foville syndrome (Table 25-1). The paramedian branches of the basilar artery may penetrate deep into the pons and also serve the medial lemniscus (Fig. 25-9). In such cases, damage to these vessels can produce not only the motor deficits described earlier but also contralateral loss of vibratory sense and two-point tactile discrimination.
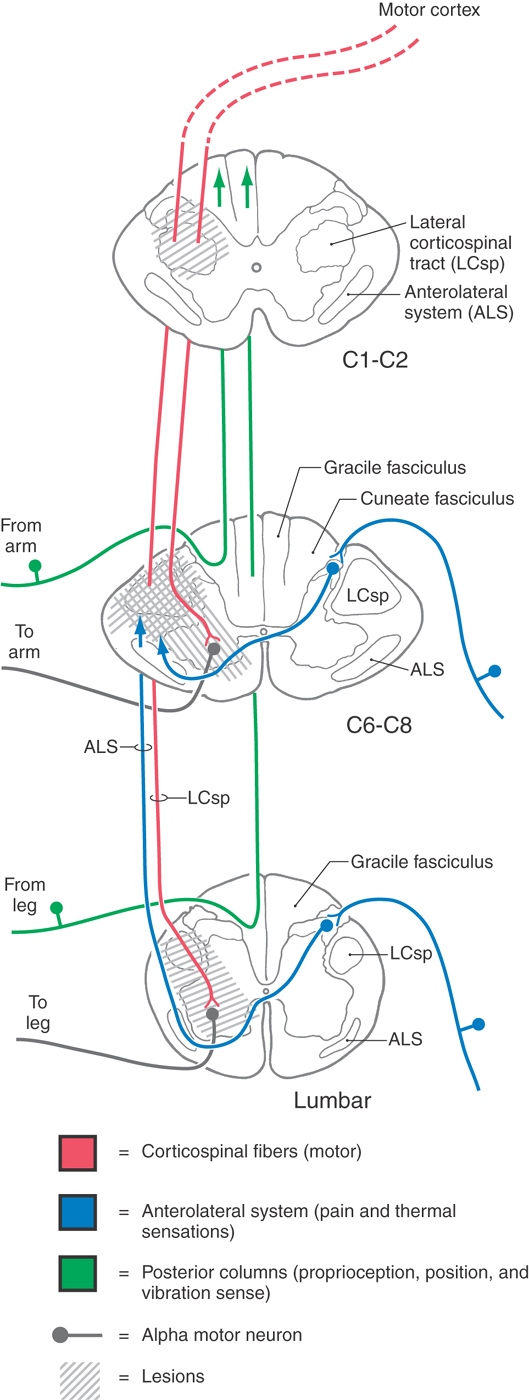
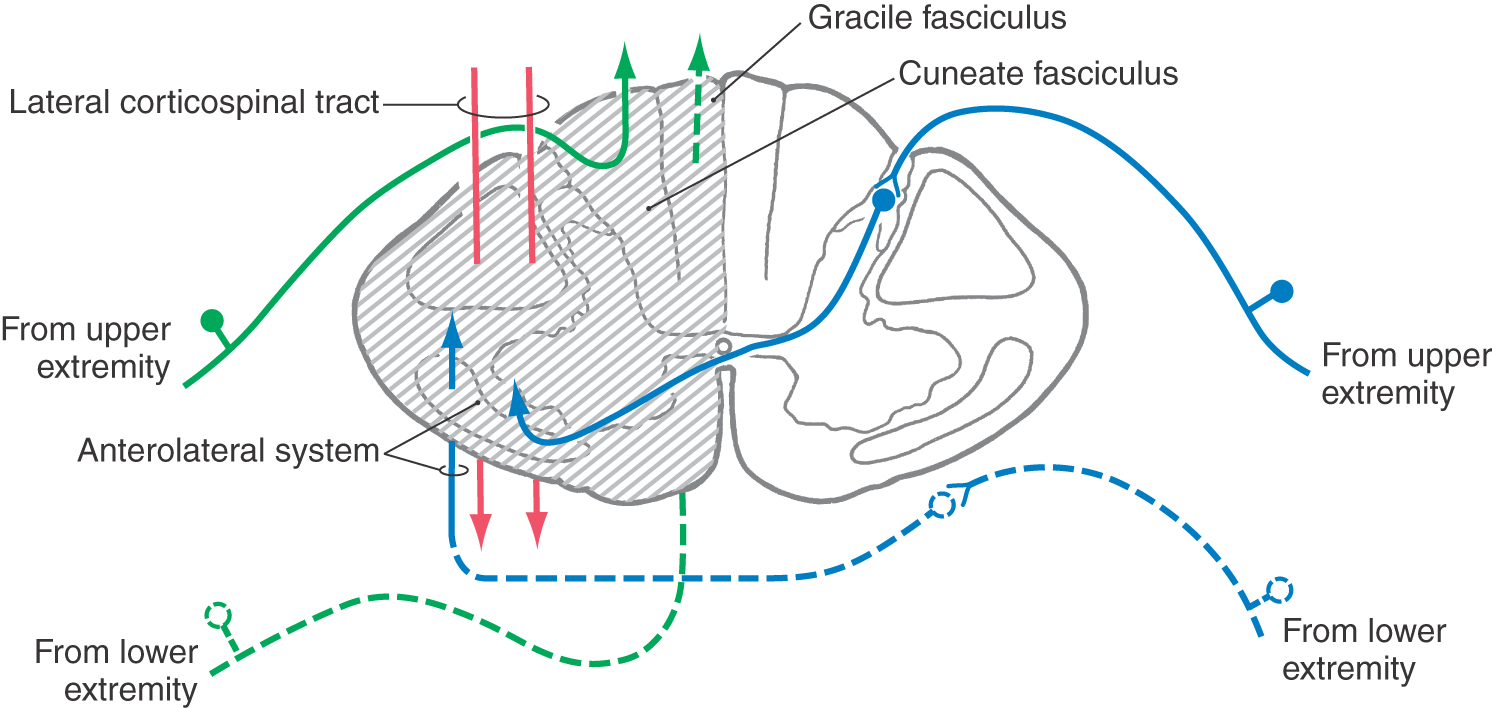
In the medulla, corticospinal fibers aggregate on the anterior surface of the brainstem, where they course within the medullary pyramids (Figs. 25-5C, 25-6, and 25-7). Within the pyramid, fibers that terminate at cervical levels tend to be located medially, whereas those projecting to lumbar and sacral levels are more lateral. Collaterals of these axons innervate the inferior olivary complex, posterior column nuclei, and various medullary reticular nuclei.
The pyramid, the laterally adjacent exiting fibers of the hypoglossal nerve, and the medial lemniscus receive their blood supply through penetrating branches of the anterior spinal artery (Fig. 25-6). Occlusion of these branches results in a contralateral hemiparesis of the extremities (with spasticity) and an ipsilateral flaccid paralysis of the tongue. When it is protruded, the tongue deviates toward the side of the lesion (the weak or flaccid side). This combination of symptoms is called inferior alternating hemiplegia. Because branches of the anterior spinal artery also serve the medial lemniscus, an inferior alternating hemiplegia is typically accompanied by a contralateral loss of two-point discrimination and vibration sense. Lesions of the medial medulla characterized by crossed (or alternating) deficits, as described earlier for other brainstem levels, are also known as the Dejerine syndrome (Table 25-1). Additional brainstem syndromes that may involve cranial nerves and corticospinal fibers in various combinations are summarized in Table 25-1.
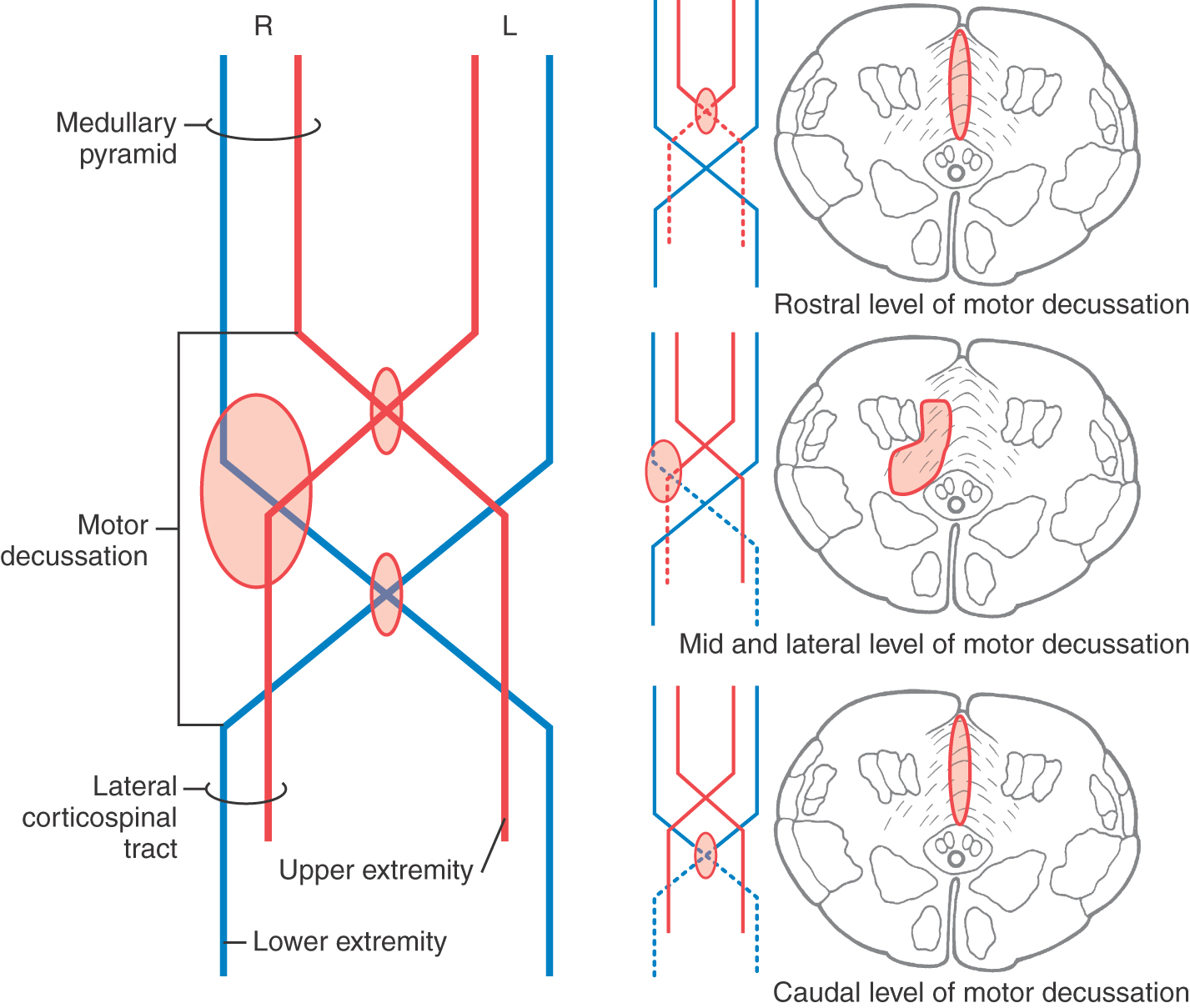
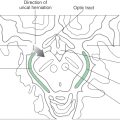
At the medullospinal junction, 85% to 90% of the corticospinal fibers cross the midline as the motor decussation (sometimes called decussation of the pyramids) (Fig. 25-6). The corticospinal fibers within the motor decussation are somatotopically arranged (Fig. 25-10). Fibers that originate in the upper extremity portion of the MI cortex cross in rostral portions of the decussation and preferentially terminate in cervical cord levels. In similar manner, fibers that arise in the lower extremity portion of MI cross in the caudal parts of the decussation and terminate primarily in lumbosacral cord levels. This arrangement explains why small vascular lesions in the motor decussation (which is also served by branches of the anterior spinal artery) may result in selective bilateral weakness or paralysis of only the upper extremities or only the lower extremities (Fig. 25-10). The pattern of crossing fibers in the motor decussation also explains the somewhat unusual picture of weakness of the upper extremity on one side and of the lower extremity on the opposite side. Lesions in the rostral half of the decussation on one side, and not on the midline, damage upper extremity fibers that have already crossed (ipsilateral upper extremity weakness) and lower extremity fibers that have not crossed (contralateral lower extremity weakness) (Fig. 25-10). If a lesion producing this alternating upper extremity–lower extremity pattern of deficits extends laterally, it may damage the accessory nucleus and the anterolateral system with corresponding deficits. The decussating fibers extend into the lateral funiculus to form the lateral corticospinal tract. The corticospinal axons that do not cross in the decussation continue into the ipsilateral anterior funiculus of the spinal cord as the anterior corticospinal tract (Figs. 25-6 and 25-7). Damage to this tract is of little clinical significance. This is because most of the fibers in this tract cross in the spinal cord before termination.
The crossing of corticospinal fibers at the motor decussation is the anatomic basis for the contralateral deficits seen in a patient with a lesion in which these fibers are rostral to (above) this decussation. For example, a patient with a capsular lesion on the right side will have hemiparesis of the upper and lower extremities on the left (Fig. 25-11). This patient may also exhibit additional deficits related to damage of corticonuclear fibers in the genu of the internal capsule, such as drooping of the face and weakness of the sternocleidomastoid muscle; these deficits are discussed later in the section on the corticonuclear system.
Termination
Fibers of the lateral corticospinal tract are topographically organized (Figs. 25-6 and 25-7). Axons terminating in cervical cord levels are most medial in this tract, whereas those distributing to lumbosacral levels are most lateral. This pattern means that as the medially located fibers enter and terminate in the spinal gray, the adjacent, more lateral fascicles shift medially.
Corticospinal fibers that arise from the frontal lobe terminate primarily in the intermediate zone and anterior horn (laminae VII to IX), whereas fibers arising from the parietal lobe terminate in the base of the posterior horn (laminae IV to VI). As might be expected, most fibers terminate in the spinal cord enlargements that serve the extremities; about 55% terminate in the cervical enlargement, and about 25% terminate in the lumbosacral enlargement. The remaining fibers terminate at thoracic levels. As mentioned earlier, some corticospinal fibers terminate, via their collateral branches, at multiple levels. However, the influence exerted by any single axon or its collaterals depends on the number of synapses it forms and the locus of the synaptic contacts on the postsynaptic neuron. Thus a given corticospinal axon may have a powerful action on some spinal cord neurons and only a weak influence on others.
At their level of termination, particularly in the cord enlargements, corticospinal fibers synapse primarily on interneurons in laminae V to VII. In animals capable of dexterous finger movements, such as humans, monkeys, and raccoons, some corticospinal fibers terminate among clusters of lamina IX alpha motor neurons, which innervate distal flexor muscles. However, most corticospinal fibers, at least in nonhuman primates, synapse with excitatory and inhibitory interneurons, which in turn influence flexor and extensor motor neurons, respectively.
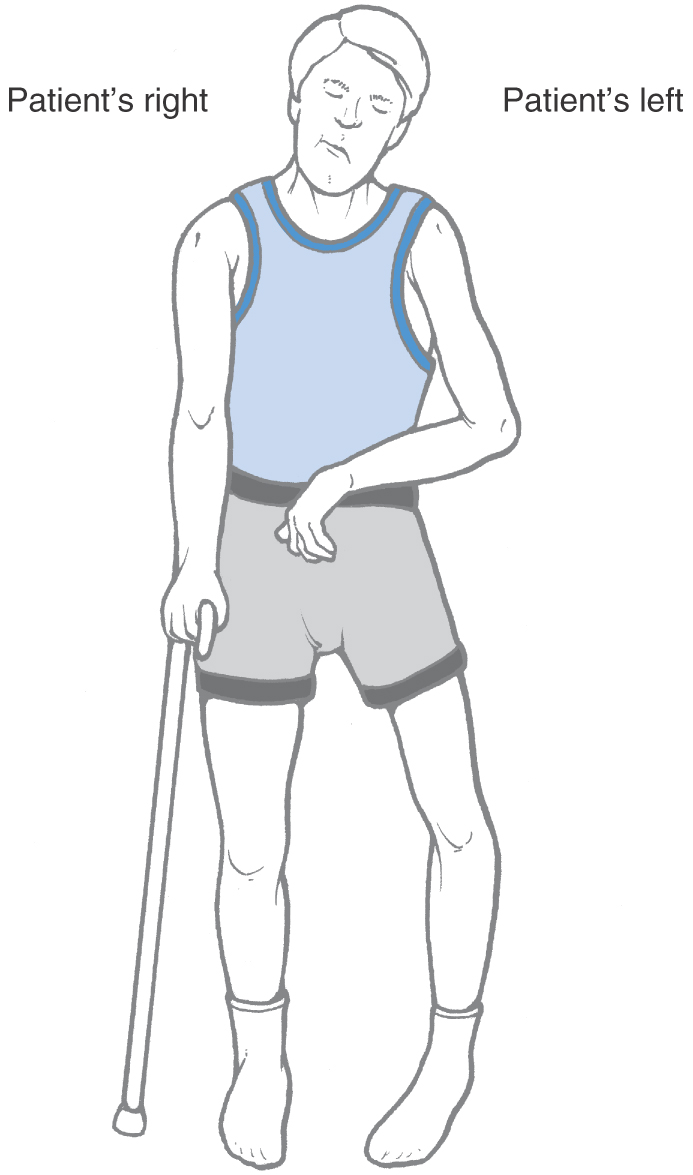
Interruption of lateral corticospinal axons in the upper cervical cord (C1, C2) results in spastic hemiplegia involving the ipsilateral upper and lower extremities (Fig. 25-12). Common upper motor neuron signs, such as hypertonia, hyperreflexia, and the Babinski sign, will be present ipsilateral to the lesion. If the lesion is sufficiently large, the innervation of the diaphragm (from C3 to C5 via the phrenic nerve) may be disrupted, necessitating the use of a respirator.
A lesion of the cervical enlargement results in a different pattern of motor deficits (Fig. 25-12). If the damage involves only the lateral funiculus, the ipsilateral upper and lower extremities will exhibit typical upper motor neuron signs. If, however, the C6 to C8 anterior horn gray matter and the lateral funiculus white matter are both included in the lesion, lower motor neuron signs will appear in the upper extremity ipsilaterally, whereas upper motor neuron signs will be seen in the ipsilateral lower extremity. When anterior horn motor neurons or their axons are damaged, the affected muscles exhibit lower motor neuron signs despite the fact that supraspinal axons providing input to these cells may also have been interrupted.
At lumbosacral levels, injury to the spinal cord frequently affects motor neurons in the anterior horn as well as descending supraspinal fibers (Fig. 25-12). Characteristically, affected patients exhibit lower motor neuron signs in the ipsilateral lower extremity if both the corticospinal fibers and anterior horn motor neurons are damaged.
The blood supply to the lateral corticospinal tract is derived from penetrating branches of the arterial vasocorona and sulcal branches of the anterior spinal artery (Fig. 25-6). The former serve fibers in lateral parts of the tract, and the latter serve the medially located fibers. Hyperextension of the neck may result in injury to the cord or in occlusion of the sulcal arteries (central cord syndrome); either can result in bilateral hemiparesis of the upper extremities secondary to vascular infarcts involving medial regions of both lateral corticospinal tracts. In addition, affected patients may also exhibit both urinary retention and a bilateral, patchy loss of pain and temperature sensations below the lesion.
A functional hemisection of the spinal cord, such as may be caused by a tumor or by trauma, results in a characteristic set of deficits known as the Brown-Séquard syndrome (Fig. 25-13; also see Fig. 18-10). These deficits begin about two levels below the lesion and consist of (1) ipsilateral loss of two-point discrimination and vibration (from damage to the dorsal columns), (2) contralateral loss of pain and thermal sensation (from damage to the anterolateral system), and (3) ipsilateral paresis or paralysis (from damage to the corticospinal tract). The paralysis involves the upper and lower extremities or only the lower extremity, depending on the level of the injury. Also, if the lesion is large enough that it involves several spinal cord levels, damage to a sufficient number of primary afferent fibers entering the cord may result in a narrow band of complete anesthesia on the side ipsilateral to the lesion in dermatomes corresponding to the damaged cord segments.
Figure 25-13. Hemisection of the spinal cord (Brown-Séquard syndrome).
CORTICONUCLEAR SYSTEM
Origin
Organized in parallel with the corticospinal system is the corticonuclear (corticobulbar) system (Fig. 25-14). As defined here, the corticonuclear system consists of the cortical neurons that influence the movements of striated muscles innervated by the motor nuclei of cranial nerves V, VII, and XII; by the nucleus ambiguus (cranial nerves IX and X); and by the accessory nucleus.
The term “corticobulbar” was historically used to describe all cortical projections to cranial nerve nuclei of the brainstem. The suffix -bulbar comes from bulb, an obsolete term for the medulla oblongata. The international committee (see Preface) charged with establishing anatomic terminology studied the inherent problems with this term; for example, how can there be “corticobulbar” projections to cranial nerve nuclei that are not in the “bulb”? After detailed consideration by the committee and with the publication of the new terminology in 1998, the term corticobulbar was replaced with terms that accurately describe these connections. These terms are as follows: fibrae corticonucleares bulbi for cortical projections to cranial nerve nuclei in the bulb/medulla (medullary corticonuclear fibers), fibrae corticonucleares pontis for cortical projections to cranial nerve nuclei of the pons (pontine corticonuclear fibers), and fibrae corticonucleares mesencephali for similar projections to the midbrain (mesencephalic corticonuclear fibers). Here these terms are shortened to simply corticonuclear, and this new and preferred term is used synonymously with the replaced term corticobulbar.
Some corticonuclear axons project directly to cranial motor neurons, but most terminate on reticular formation interneurons immediately adjacent to the cranial nerve nuclei. The corticonuclear system originates for the most part from the face and head area of the precentral gyrus (Fig. 25-14). Because most of the musculature innervated by cranial nerves is located in the facial region, this area of MI is typically called face motor cortex.
The oculomotor, trochlear, and abducens nuclei do not receive direct input from the face motor cortex. Instead, voluntary control of eye movement is mediated via cortical projections from frontal and parietal motor eye fields to eye movement (gaze) control centers in the midbrain and pons. These centers in the midbrain reticular formation and the paramedian pontine reticular formation in turn relay input from the cortical eye fields to somatic motor neurons in the nuclei of cranial nerves III, IV, and VI (see Chapter 28 for details). Although the cortex of each hemisphere influences these nuclei bilaterally, the resulting eye movements are conjugate and are toward the side contralateral to the cortex from which the input originated. Because these cortical axons are often several synapses removed from the actual cranial nerve motor neurons, they are not considered part of the corticonuclear system as defined in this text.
Course
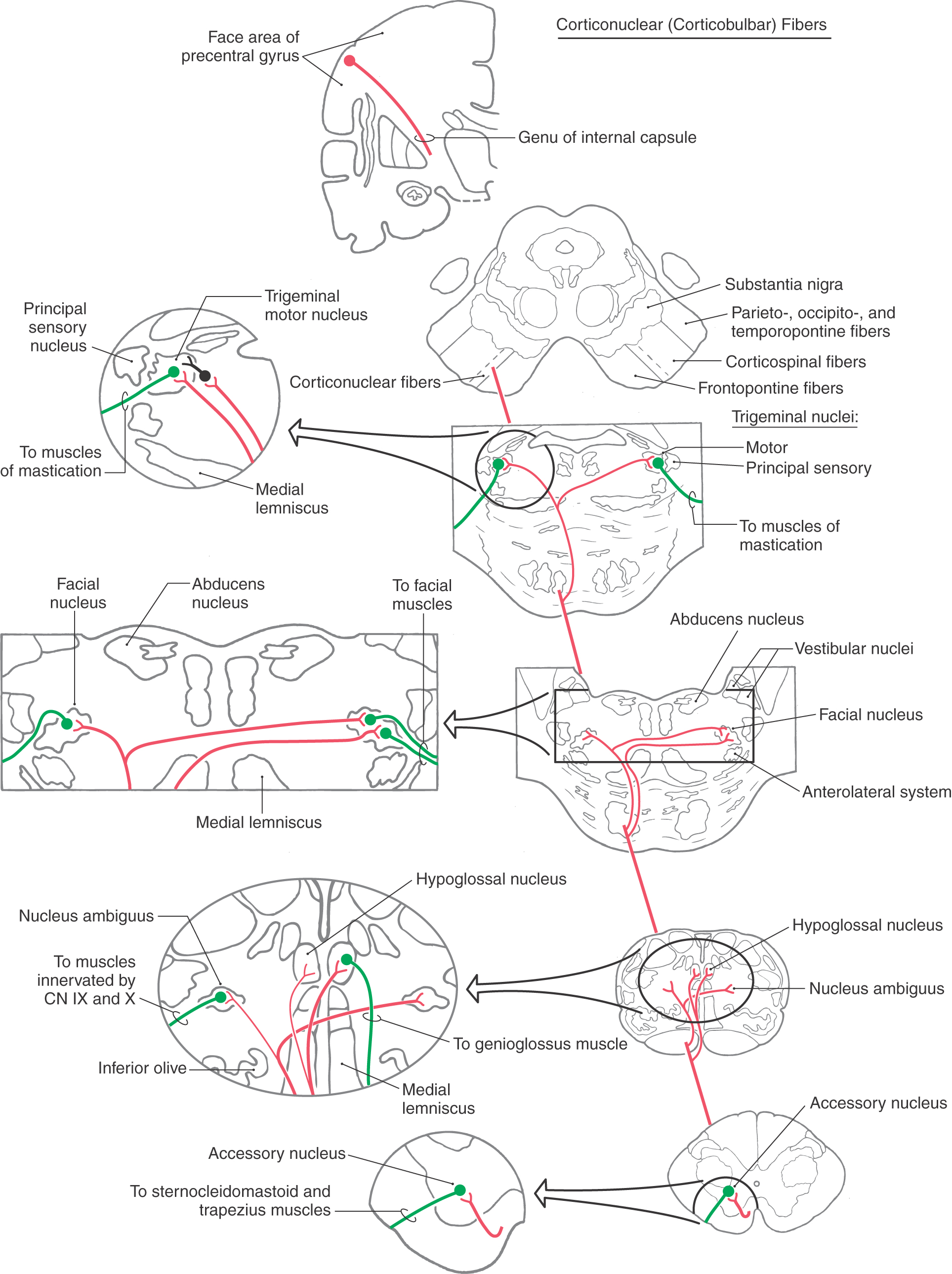
Corticonuclear axons that originate from cells in layer V of the face motor cortex funnel into the genu of the internal capsule (Fig. 25-4). Corticonuclear fibers continue into the crus cerebri, where they are located medial to corticospinal fibers traveling to cervical cord levels (Figs. 25-6 and 25-14). Within about the middle third of the crus, the body is somatotopically arranged, lower extremities most lateral, head-face most medial.
From here, these axons descend into the pons and medulla in association with corticospinal fibers. Corticonuclear fibers in the genu and in the crus cerebri receive their blood supply from lenticulostriate arteries and from the paramedian branches of the basilar bifurcation, respectively.
Termination
As corticonuclear fibers pass through the basilar pons, branches arch superiorly into the pontine tegmentum to terminate bilaterally in the areas of the trigeminal and facial motor nuclei (Fig. 25-14). The fibers to the trigeminal motor nuclei terminate on interneurons adjacent to the nuclei. The corticonuclear system sends nearly equal numbers of fibers to the left and right trigeminal motor nuclei. Consequently, unilateral damage to corticonuclear fibers does not result in any discernible weakness of masticatory muscles on either side.
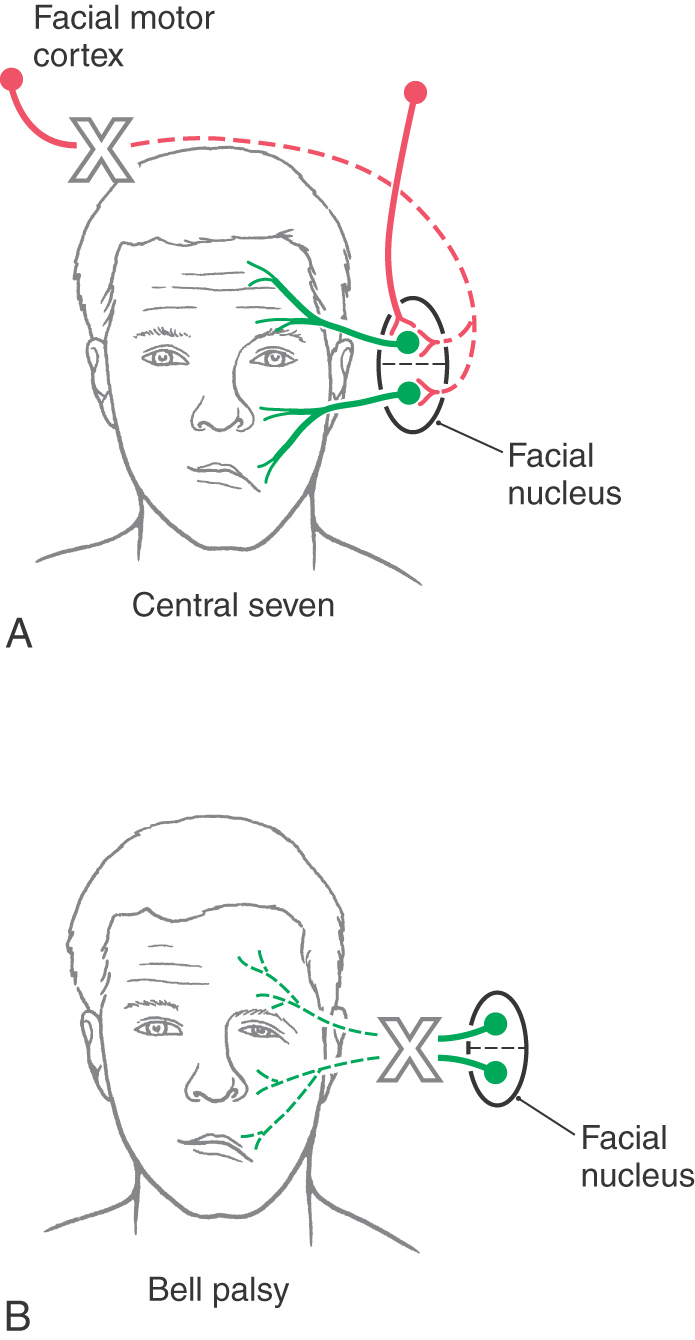
Likewise, nearly equal numbers of fibers are sent to the left and right facial motor nuclei. Whereas the muscles of facial expression in the upper half of the face are controlled about equally from both hemispheres, muscles in the lower half of the face are influenced primarily from the contralateral hemisphere. Consequently, a lesion of corticonuclear fibers rostral to the facial motor nucleus results in drooping of muscles at the corner of the mouth and on the lower portion of the face on the side opposite the lesion (Figs. 25-11 and 25-15A). This deficit is called a central facial paralysis (central seven). In contrast, a lesion of the root of the facial nerve will result in a flaccid paralysis of facial muscles of upper and lower portions of the face on the ipsilateral side (Fig. 25-15B); this deficit is called a Bell (facial) palsy.
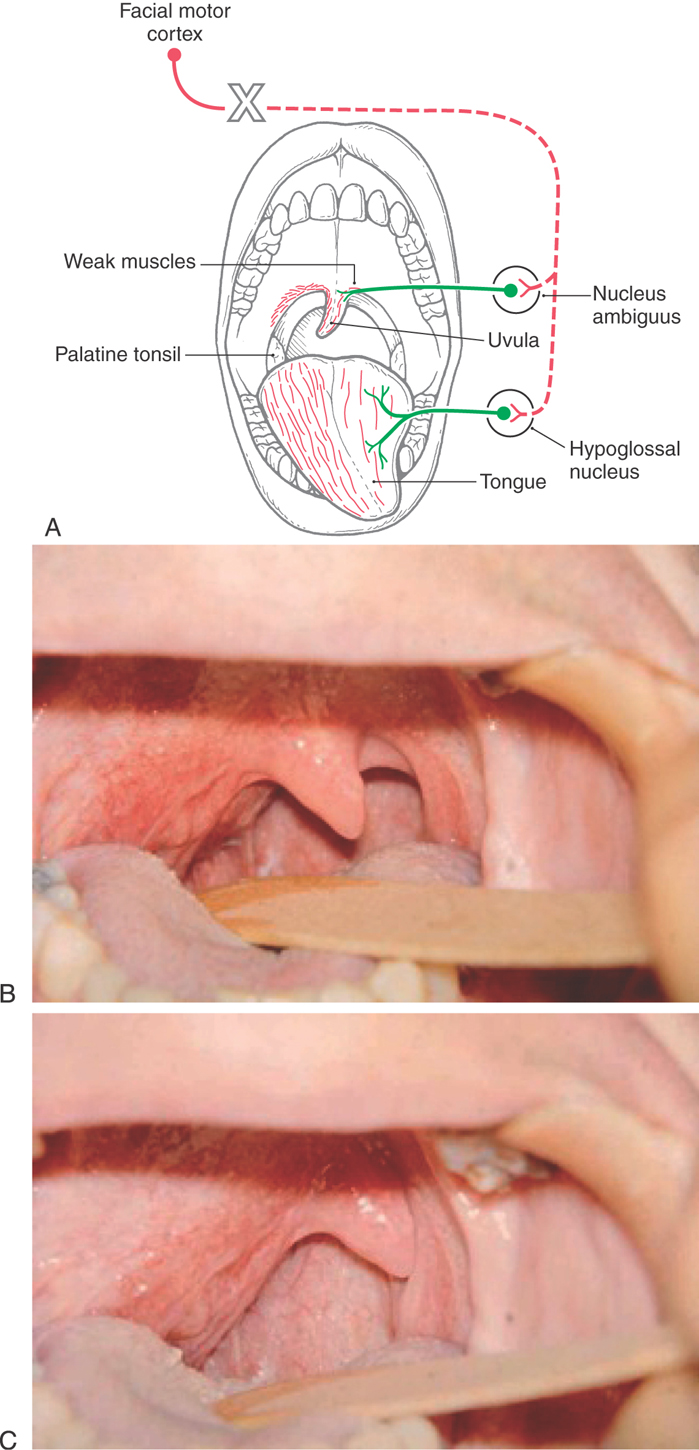
At midmedullary levels, corticonuclear fibers pass superiorly to reach the ambiguus and hypoglossal nuclei (Fig. 25-14). Projections to nucleus ambiguus motor neurons are generally bilateral. However, the motor neurons that innervate muscular parts of the soft palate and uvula receive mainly a contralateral input. Consequently, a lesion of corticonuclear fibers on the right (Fig. 25-16A) projecting to the left nucleus ambiguus may produce weakness of the palatal arch muscles on the left (the weak side), a slight drooping of the palatal arch and failure to elevate on the weak side, and deviation of the uvula to the right (the strong side) on attempted phonation (the patient tries to say “ah”). In contrast, lesions that damage the root of the vagus nerve, as in several of the jugular foramen syndromes, result in weakness and slight drooping of the arch on the same side of the lesion, a noticeable deviation of the uvula to the strong side (opposite the lesion side) at rest, and acute deviation on phonation (Fig. 25-16B, C).
Figure 25-16. Deviation of the uvula and tongue after a lesion of corticonuclear fibers (on the right) that project to the left nucleus ambiguus and hypoglossal nucleus (A) and deviation of just the uvula consequent to a lesion of the right vagal root (B and C). The large X indicates the side of the lesion in A. Note in A that the uvula deviates toward the side of the corticonuclear lesion and away from the weak side and the tongue deviates away from the side of the corticonuclear lesion but toward the weak side. Compare the tongue deviation with Figure 25-17. In B and C, the lesion is on the patient’s right, the right side of the palate is affected, and the uvula deviates to the left at rest (B) and on phonation (C). (B and C Courtesy of Dr. James Corbett.)
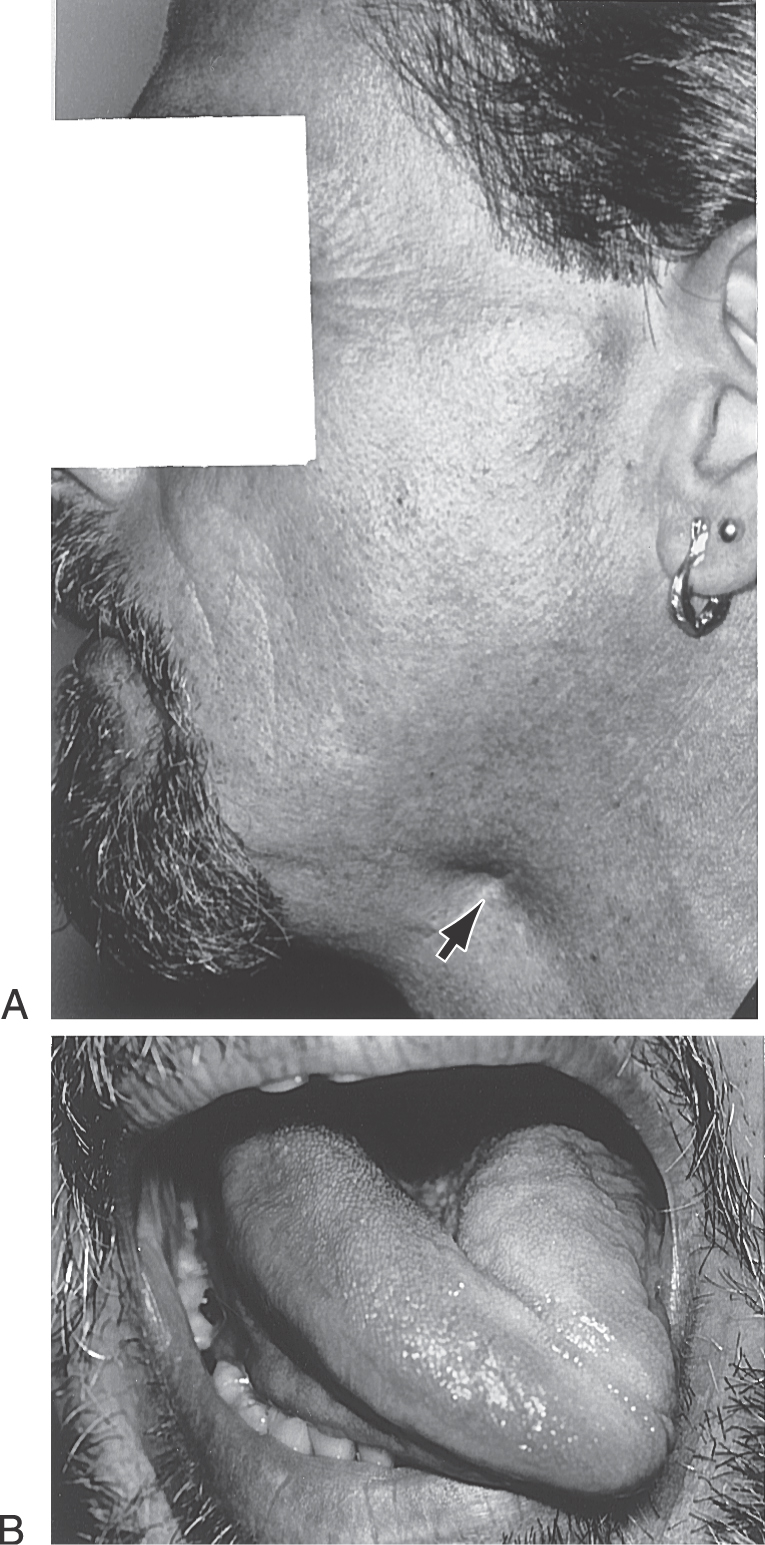
In the case of the hypoglossal nuclei, although corticonuclear fibers in general distribute bilaterally, those motor neurons that innervate the genioglossus muscles receive primarily contralateral corticonuclear input. Each genioglossus muscle pulls its half of the tongue anteriorly and slightly medially. When the two muscles function together and symmetrically, the tongue protrudes straight out of the mouth. However, a lesion of corticonuclear fibers to the hypoglossal nucleus will cause the tongue to deviate toward the weak (contralateral to the lesion) side when it is protruded because of the unopposed pull of the intact muscle (Fig. 25-16A). In this example of a lesion of corticonuclear fibers (upper motor neurons), the tongue will deviate (on protrusion) toward the side opposite the lesion (Fig. 25-16A), and the patient may have other symptoms characteristic of a lesion of the genu of the internal capsule, such as a central seven or a deviation of the uvula. In contrast, an injury to the hypoglossal nerve (lower motor neurons) will result in deviation of the tongue toward the side of the lesion (weak side) on protrusion (Fig. 25-17) and ultimately lead to the appearance of lower motor neuron signs in the tongue, such as muscle atrophy and a flaccid paralysis. If, however, the lesion is in the medial medulla and involves the root of the hypoglossal nerve, pyramid, and medial lemniscus, the patient may experience an ipsilateral deviation of the tongue along with a contralateral hemiparesis (corticospinal fiber involvement) and a contralateral loss of posterior column modalities (medial lemniscus involvement). This combination of deficits is an inferior alternating hemiplegia (medial medullary or Dejerine syndrome; Table 25-1). Hypoglossal root fibers, corticospinal fibers, and the medial lemniscus have a common blood supply, in the medulla, from the anterior spinal artery.
The final contingent of corticonuclear (corticobulbar) fibers innervates the spinal portion of cranial nerve XI (accessory nucleus) (Fig. 25-14). These fibers continue into the upper cervical spinal cord along with corticospinal fibers. Clinical observations in patients with cortical or internal capsule lesions reveal that the sternocleidomastoid and trapezius muscles (targets of accessory motor neurons) are affected mainly on the side ipsilateral to the lesion. The patient is unable to shrug or to elevate that shoulder (especially against resistance) or to turn the head away from the side of the lesion (Fig. 25-11). This finding suggests that corticonuclear fibers distribute primarily to the ipsilateral accessory nucleus.
Because the corticospinal and corticonuclear systems course adjacent to each other, it is common for lesions of the internal capsule or midbrain to affect both fiber bundles. For example, lenticulostriate arteries serve portions of the genu and most of the posterior limb of the internal capsule (Fig. 25-4A). Consequently, hemorrhage of these vessels on the right side results in (1) a left spastic hemiparesis of the extremities (corticospinal damage), (2) a central facial paralysis on the left, (3) a deviation of the uvula to the right on phonation, and (4) a deviation of the tongue to the left when it is protruded. The last three deficits are the result of damage to corticonuclear fibers. Effects on the trapezius and sternocleidomastoid muscles are variable but if present will usually involve the muscles ipsilateral to the lesion of corticonuclear fibers (Figs. 25-11, 25-15, and 25-17).
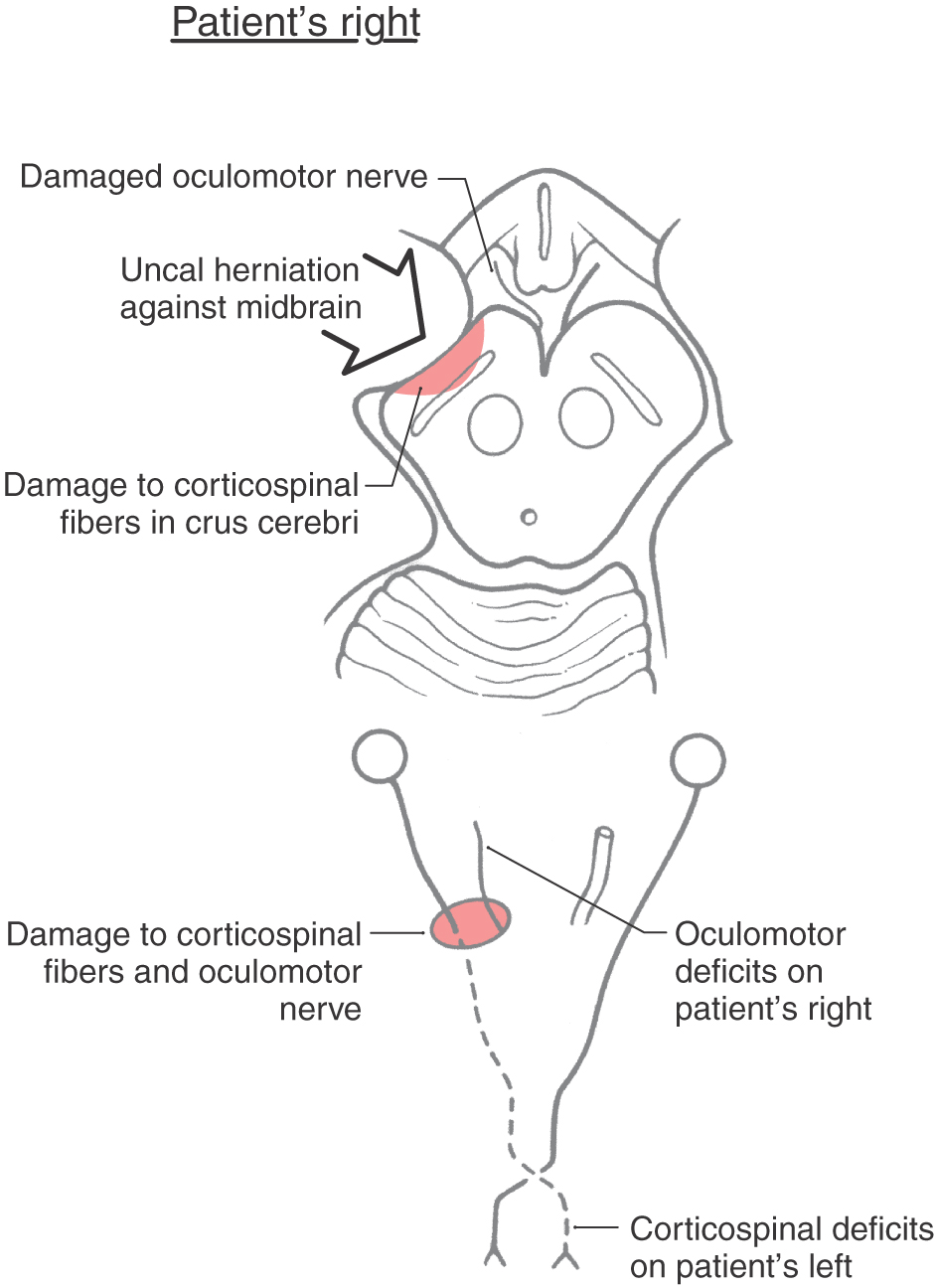
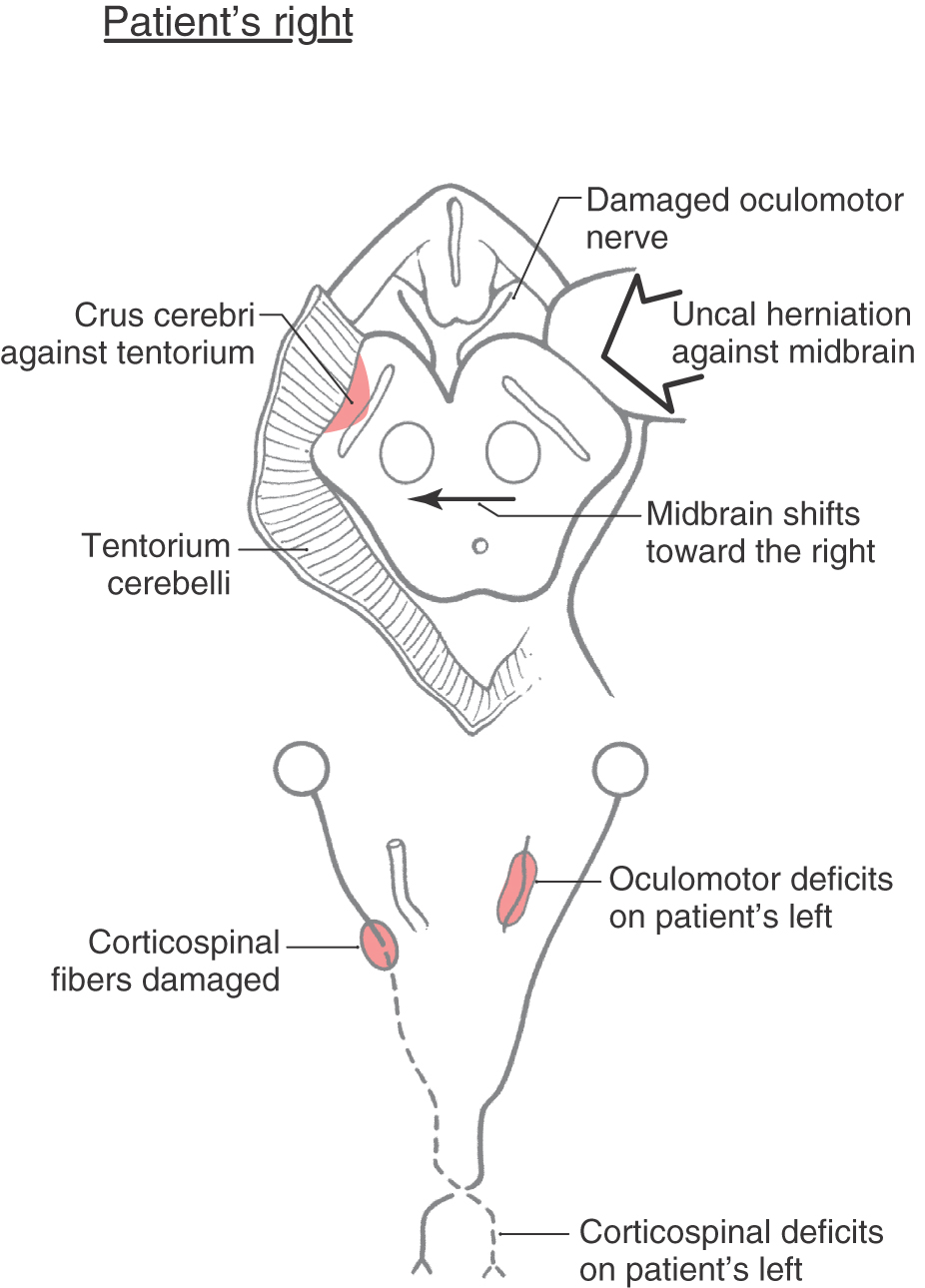
Lesions that impinge on or are located within any level of the brainstem may produce corticospinal and corticonuclear signs in various combinations, depending on the level of the brainstem involved and what cranial nerve root is damaged along with the corticospinal fibers. One example is the case of herniation of the uncus through the tentorial notch (Fig. 25-18). Increased intracranial pressure in a supratentorial compartment forces the uncus over the edge of the tentorium and into the midbrain, damaging the oculomotor nerve and the crus cerebri on that side. The deficits experienced by the patient are an ipsilateral paralysis of most eye movement, diplopia, and a dilated pupil (oculomotor damage) and a contralateral hemiplegia (damage to corticospinal fibers in the crus) (Fig. 25-18). This is an example of one lesion that results in a Weber syndrome (Table 25-1). In patients with corticospinal signs accompanied by cranial nerve signs on the opposite side of the body, two important facts come to mind. First, these alternating or crossed deficits are a hallmark of brainstem lesions. Second, the cranial nerve deficit is the best localizing sign because it provides, in combination with a long tract deficit (corticospinal in this example), the most precise location and level of the lesion.
Having made this distinction concerning the localizing sign, it is important to realize that there are examples of what are called false localizing signs. These are cases in which the signs are counter to what one would expect. One example of this is the Kernohan syndrome (also called the Kernohan notch phenomenon). This is a variation on uncal herniation against the midbrain (Fig. 25-19). In a Kernohan lesion, the herniating uncus displaces the midbrain (and crus cerebri) against the edge of the tentorium cerebelli on the side contralateral to the herniation (Fig. 25-19). This may result in an oculomotor nerve palsy and a hemiplegia of the extremities, both on the side of the herniation (Fig. 25-19). The oculomotor nerve palsy indicates damage to the oculomotor nerve root (by avulsion of the root or by compression of its blood vessels with resultant necrosis of the root) on the side of the herniation, and the hemiplegia results from the crus cerebri being forced against the edge of the tentorium on the contralateral side with subsequent damage to the corticospinal fibers on that side (Fig. 25-19). In this example (ipsilateral oculomotor paralysis plus ipsilateral hemiplegia), the hemiplegia is the false localizing sign. The combination of oculomotor and corticospinal deficits suggests that uncal herniation may be the underlying cause of these symptoms.
The Kernohan syndrome illustrates an important general concept concerning posterior fossa lesions that may affect the brainstem. Lesions at any brainstem level (midbrain, pons, medulla) may displace the stem to one side, damage ipsilateral cranial nerve roots at that level (for example, oculomotor, trigeminal, facial, hypoglossal), and damage long tracts on the contralateral side by compression of the stem against bony or meningeal structures. The result is a patient with cranial nerve and long tract signs on the same side of the body.
OTHER CORTICOFUGAL SYSTEMS
Corticorubral System
Cortical projections to the red nucleus arise primarily from areas 4 and 6 and to a lesser extent from areas 5 and 7. Both large and small neurons in the red nucleus receive ipsilateral corticorubral input. Although pyramidal tract neurons provide some collaterals to the red nucleus, many corticorubral axons are not collaterals of pyramidal tract fibers. In general, the corticorubral-rubrospinal projection is topographically organized. For example, the upper extremity region of the MI cortex projects to cells of the red nucleus that in turn send their axons to contralateral cervical levels of the spinal cord (see Fig. 24-8, to Fig. 24-10). Because the rubrospinal system primarily influences flexor musculature, this pathway may supplement the function of the corticospinal tract. It is known from experimental studies that section of corticospinal fibers in the medullary pyramid leaves the animal still able to walk, to climb, and to pick up food but unable to perform fine, dexterous movements with its digits. This finding suggests that the corticorubrospinal system may partially compensate for the loss of the corticospinal tract. In addition, in a patient with decorticate rigidity, the flexion of the upper extremities reflects an intact red nucleus and rubrospinal tract. If the supratentorial lesion producing decortication extends through the tentorial incisure (tentorial notch) and damages the midbrain, the flexed upper extremities convert to extended upper extremities; this signals destruction of the red nucleus and rubrospinal fibers and the onset of decerebrate posturing.
The red nucleus also receives input from the contralateral interposed and lateral nuclei of the cerebellum (see Chapter 27). Consequently, this relatively small population of brainstem upper motor neurons is capable of integrating signals from motor-related areas of the cerebral cortex and from the cerebellum. Input from the interposed nuclei is excitatory, and this projection may be part of a circuit specialized for rapid control or adjustment of movements based on sensory processing by the cerebellum.
Corticoreticular System
The pontine and medullary nuclei that give rise to the reticulospinal tracts receive cortical input from the premotor cortex and to a lesser extent from the supplementary motor cortex. Because reticulospinal systems primarily influence extensor muscles, including the paravertebral extensors as well as those of the limbs, the corticoreticulospinal system may provide the cortex with the means to influence extensor musculature in parallel with its regulation of flexors (see Fig. 24-8, to Fig. 24-10). The cerebellar nuclei project to the motor-related areas of the reticular formation, thus providing for a cerebellar influence on extensor musculature.
Corticopontine System
Axons from nearly all regions of the cerebral cortex contribute to the corticopontine projection, and this pathway is particularly well developed in the human brain. Although most of these fibers originate from motor-related areas and the somatosensory cortex, nonmotor regions in the frontal lobe and in parietal, temporal, and occipital association cortices also contribute fibers. Corticopontine axons descend through the internal capsule and continue into medial and lateral parts of the crus cerebri. Frontopontine fibers are located medially, and parietopontine, occipitopontine, and temporopontine fibers are laterally placed. These corticopontine projections synapse in the ipsilateral basilar pontine nuclei. Although most neurons in the pontine nuclei send their axons into the contralateral cerebellum via the middle cerebellar peduncle, there is a notable ipsilateral pontocerebellar projection.
Although the function of this vast corticopontocerebellar system is not fully understood, it certainly must be involved in aspects of motor control. However, studies in humans have shown that the cerebellum is also active during mental problem solving and internal (nonvocal) language functions. This finding, in conjunction with animal experiments showing the presence of basilar pontine local circuit interneurons, implies that the corticopontine system is an important route of communication between the cerebral cortex and the cerebellum, structures that have no direct connections in the mature brain.
MOTOR CORTEX AND THE CONTROL OF MOVEMENT
The classic view of voluntary movement control is that the various motor-related areas of the cerebral cortex function hierarchically. At one time, the primary motor cortex was thought to form the apex of this hierarchy, the output of the other cortical areas being funneled through it. Recent findings suggest that the motor-related cortical areas outside MI and their respective descending projections carry out the tasks involved in planning and executing a movement in parallel with MI and its descending projections.
Primary Motor Cortex
Recall that the primary motor cortex (MI) is organized into a detailed somatotopic map of the body (Fig. 25-2) and that many corticospinal fibers originate from the pyramidal neurons in its layer V. What is this area’s contribution to the control of movement?
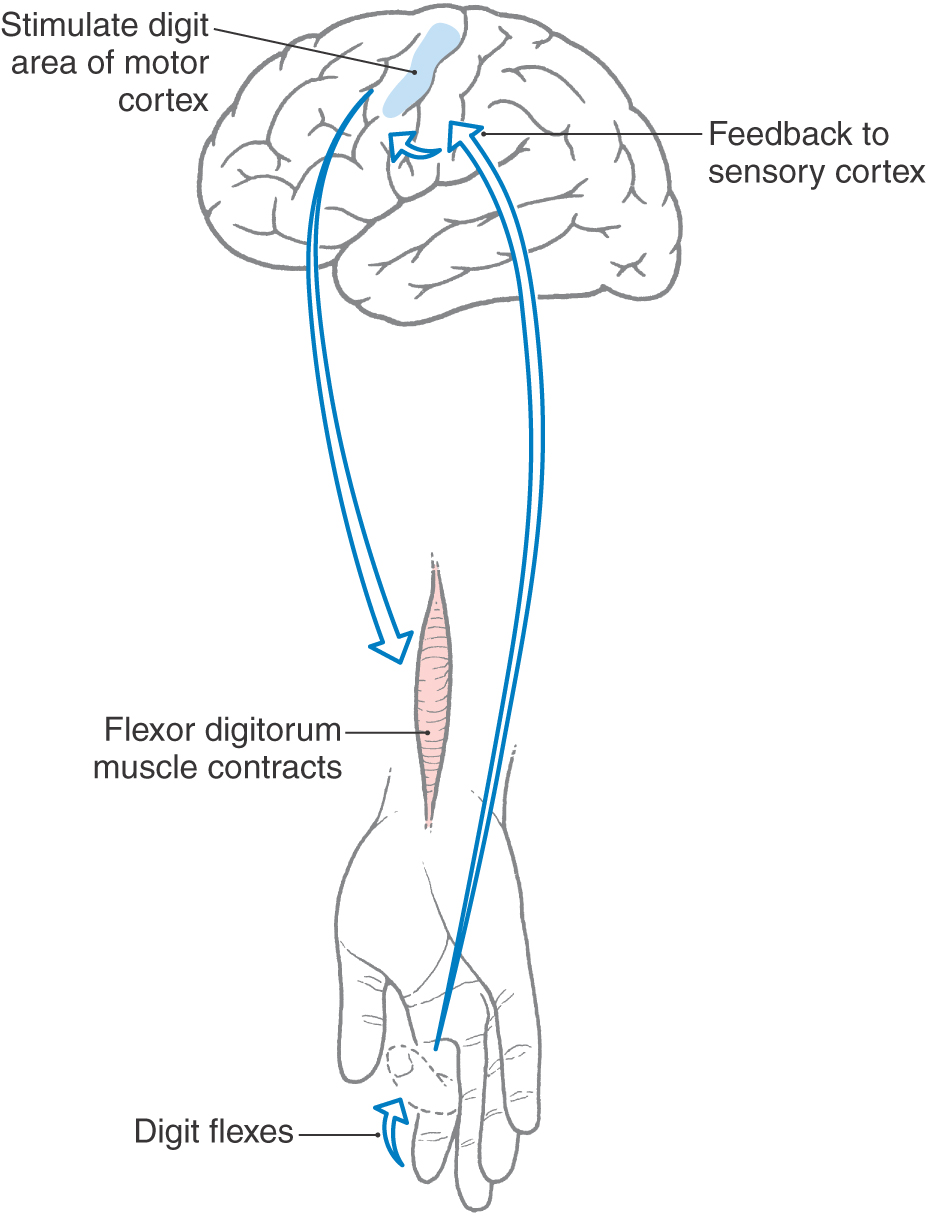
Like other cortical areas, such as the striate and primary somatosensory cortices, MI is organized into a series of modules or vertical columns. Microstimulation in MI can result in discrete movements of individual muscles. For example, stimulation in a vertical column in the hand area of MI may evoke flexion of a digit (Fig. 25-20). Neurons in the same columnar array receive somatosensory feedback from the patch of skin on the volar (glabrous) side of the digit, which is the area that would come in contact with a surface when the digit is flexed, as to grasp an object. These connections are part of long-latency reflex circuits. The sensory information reaches MI from the ascending somatosensory systems indirectly via synapses in the thalamus and the primary somatosensory cortex (SI). The inference here is that motor cortical neurons are informed of the result of their output.
Figure 25-20. Schematic representation of a long-latency reflex.
However, studies have shown that many muscles, particularly distal muscles of the upper extremity, are regulated from more than one cortical location. Conversely, microstimulation at one cortical locus can often activate more than one muscle. Thus, the classic view that a discrete, somatotopically organized projection emanating from area 4 is primarily responsible for the control of individual muscles may be an oversimplification.
The activity of corticospinal neurons in MI can also be modified during a movement. In experiments involving nonhuman primates, microelectrodes placed in layer V identified single corticospinal neurons by their response to antidromic stimulation of a medullary pyramid. These monkeys were trained to make wrist flexion or extension movements in a situation in which the movement was either assisted or impeded by a weight attached to the wrist by a pulley system. Under these conditions, corticospinal neurons were found to be active slightly in advance of the movement, and they did not simply code for flexion or extension but rather for the amount of force required to make the movement. For example, when the weight was arranged to oppose the movement, increases in the amount of weight were matched by increases in the activity of corticospinal neurons, and vice versa; if the weight assisted the movement, the cortical neurons decreased their firing rates.
Other populations of MI cortical neurons encode movement direction. When a monkey is trained to move a handle toward one of several targets arranged concentrically around a central starting location, the activity of individual cortical neurons varies with the direction of the required movement. That is, some neurons fire rapidly for a movement in one direction but are silent for a movement in the opposite direction. This directional tuning is rather broad, however, with most neurons firing with movement in a preferred direction but exhibiting less vigorous activity in relation to movements in other directions.
Supplementary Motor Cortex
The supplementary motor cortex occupies the portion of Brodmann area 6 that lies rostral to MI near the convexity of the hemisphere and extends onto the medial wall of the hemisphere rostral to the paracentral gyri (Fig. 25-2). It contains a map of the body musculature that is complete although less precisely organized than that of MI. It receives input from the parietal lobe and projects to MI and directly to the reticular formation and spinal cord.
Stimulation of the supplementary cortex can evoke movements. In contrast to the single-muscle movements evoked by MI stimulation, however, these movements involve sequences or groups of muscles and orient the body or limbs in space. In addition, stimuli of higher intensities are required to activate supplemental motor cortex. Under these conditions bilateral movements of the hands or upper extremities may be produced.
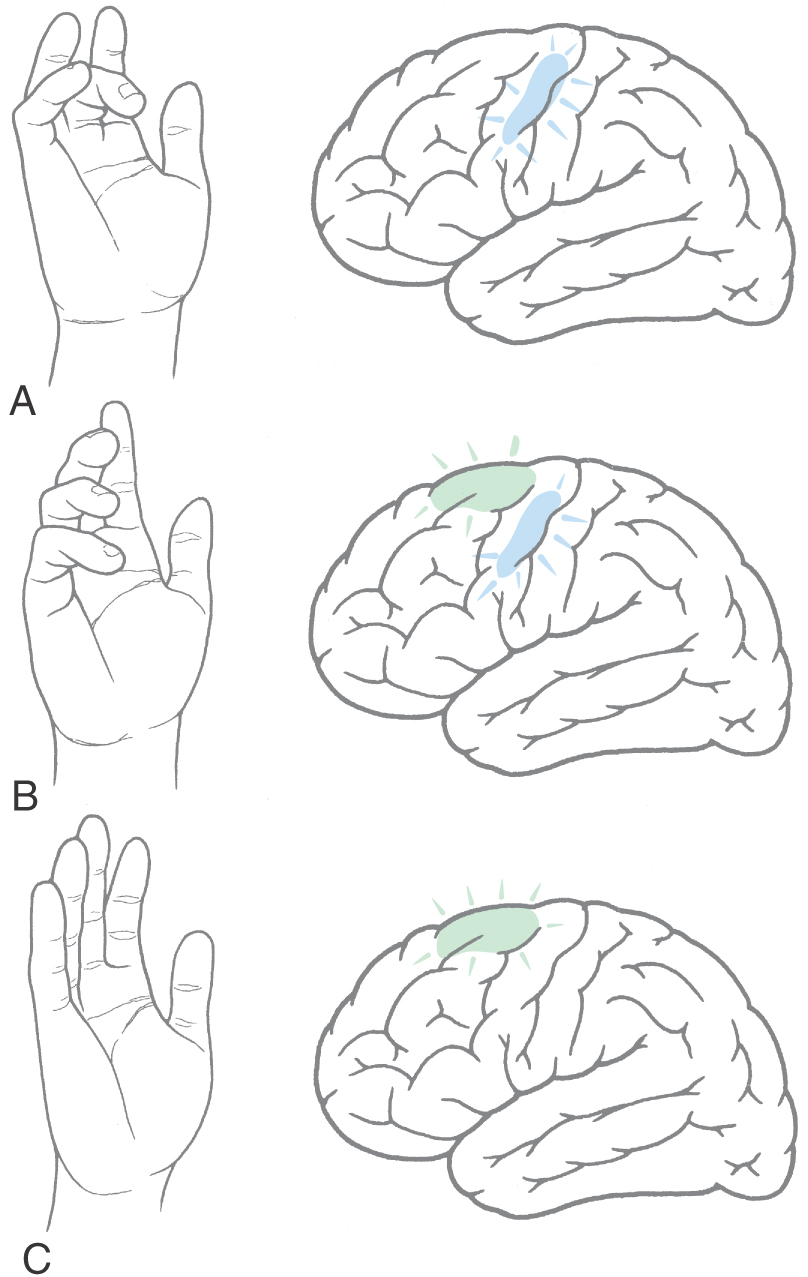
New clinical and experimental imaging techniques have provided important new insights concerning the organization of the cerebral cortex. This is especially the case for functional magnetic resonance imaging (fMRI), which measures and visualizes increases in blood flow in specific brain areas. When brain activity increases, as during the execution of a specific task, the blood flow within the activated region increases. At the same time, there is a corresponding decrease in regional deoxyhemoglobin. Because deoxyhemoglobin functions as an endogenous paramagnetic substance, the cortical area in which activity (and blood flow) is increased has a decreased level of deoxyhemoglobin; this is a signal that is read by the MRI as a region of increased activity. When a volunteer made a series of random finger movements, an increase in neural activity was observed only over the hand region of MI (Fig. 25-21A). The subject was then asked to make movements with several fingers of the same hand but in a specific sequence. Activity was increased both in the supplementary motor cortex and in the hand region of MI (Fig. 25-21B). Finally, when the subject was asked to mentally rehearse a specific sequence of finger movements without actually moving the fingers, increased activity was restricted to the supplementary cortex, and the hand region of MI was silent (Fig. 25-21C). These findings indicate that the supplementary motor cortex is involved in organizing or planning the sequence of muscle activation required to make a movement, whereas the primary motor cortex functions mainly to execute the movement.
Premotor Cortex
The premotor cortex occupies the portion of area 6 lying just rostral to the anterolateral part of MI (Fig. 25-2). Like the supplementary motor cortex, this region contains a somatotopic representation of the body musculature that is complete although less precisely organized than that of MI. The premotor cortex receives considerable input from sensory areas of the parietal cortex and projects to MI, the spinal cord, and the reticular formation. The reticular formation gives rise to reticulospinal fibers, which in turn influence spinal motor neurons that innervate paravertebral and proximal limb musculature.
On the basis of these connections, the premotor cortex, like the supplementary cortex, is involved in the preparation to move. That is, it organizes those postural adjustments that are required to make a movement. To test this concept, monkeys were trained to move one hand to a specific target location that differed from trial to trial. The monkey was first given a cue signaling which target to reach for and then a “go” signal to actually make the movement. Recordings of cell activity revealed that premotor neurons were active only during the interval between presentation of the cue and the go signal. The premotor cortex is most active in directing the control of proximal limb muscles that are used to position the arm for movement tasks or, more generally, to orient the body for movement.
Posterior Parietal Cortex
The motor regions of the posterior parietal cortex comprise Brodmann areas 5 and 7, which largely occupy the superior parietal lobule (Fig. 25-2). These areas carry out some of the “background computations” necessary to make movements in space. To organize such a movement, it is necessary to collate input from a variety of sensory systems to create a map of space and to compute a trajectory by which a body part can reach its target. Area 5 receives extensive projections from the somatosensory cortex and input from the vestibular system, whereas area 7 processes visual information related to the location of objects in space. Both areas project primarily to supplementary and premotor cortices and have few spinal or brainstem targets.
Experiments in monkeys provide the best insight into the function of areas 5 and 7. In area 5, arm projection neurons are active only when the monkey reaches for a specific object of interest. They are not active when the same arm movement is made but the object is not present; hand manipulation neurons fire only when the hand manually explores an object of interest. In area 7, many different types of neurons are present. One type, the eye-hand coordination neurons, is vigorously active only when the eyes fixate a target and the hand reaches for that target.
Cingulate Motor Cortex
Two aggregates of corticospinal neurons are associated with the cingulate gyrus (Fig. 25-2). One occupies the inferior bank of the cingulate sulcus, and the other, located slightly more caudally, occupies both the superior and inferior banks of the cingulate sulcus. Each is topographically organized with respect to its spinal cord projections, and each also projects to the primary motor cortex. Little is known about the functional role of these areas, other than that stimulation in either area produces motor effects. Because of their proximity to the limbic cortex, these motor neurons may be involved in movements that have an intense motivational or emotional component.
Cerebellar and Pallidal Influences
The basal nuclei and cerebellum play essential roles in the control of movement through their interaction with motor-related areas of the cerebral cortex. Although these pathways are detailed in Chapters 26 and 27, their general relationships are summarized here. The cerebellar nuclei and the globus pallidus each project primarily to their own spatially segregated regions in the ventral anterior, ventral lateral, and oral parts of the ventral posterolateral nuclei of the dorsal thalamus. These so-called motor areas of the thalamus give rise to thalamocortical projections to MI and the supplementary motor area. The thalamic areas that receive input from the globus pallidus project mainly to the supplementary motor cortex, and the thalamic areas that receive input from the cerebellum project to MI. The degree to which these two lines of communication are separate is not clear. Therefore, signals transmitted through the corticospinal and corticonuclear systems can be modified by outputs that reach the cortex from the cerebellum, basal nuclei, and thalamus.
HIERARCHICAL ORGANIZATION VERSUS PARALLEL DISTRIBUTED PROCESSING IN THE MOTOR SYSTEM
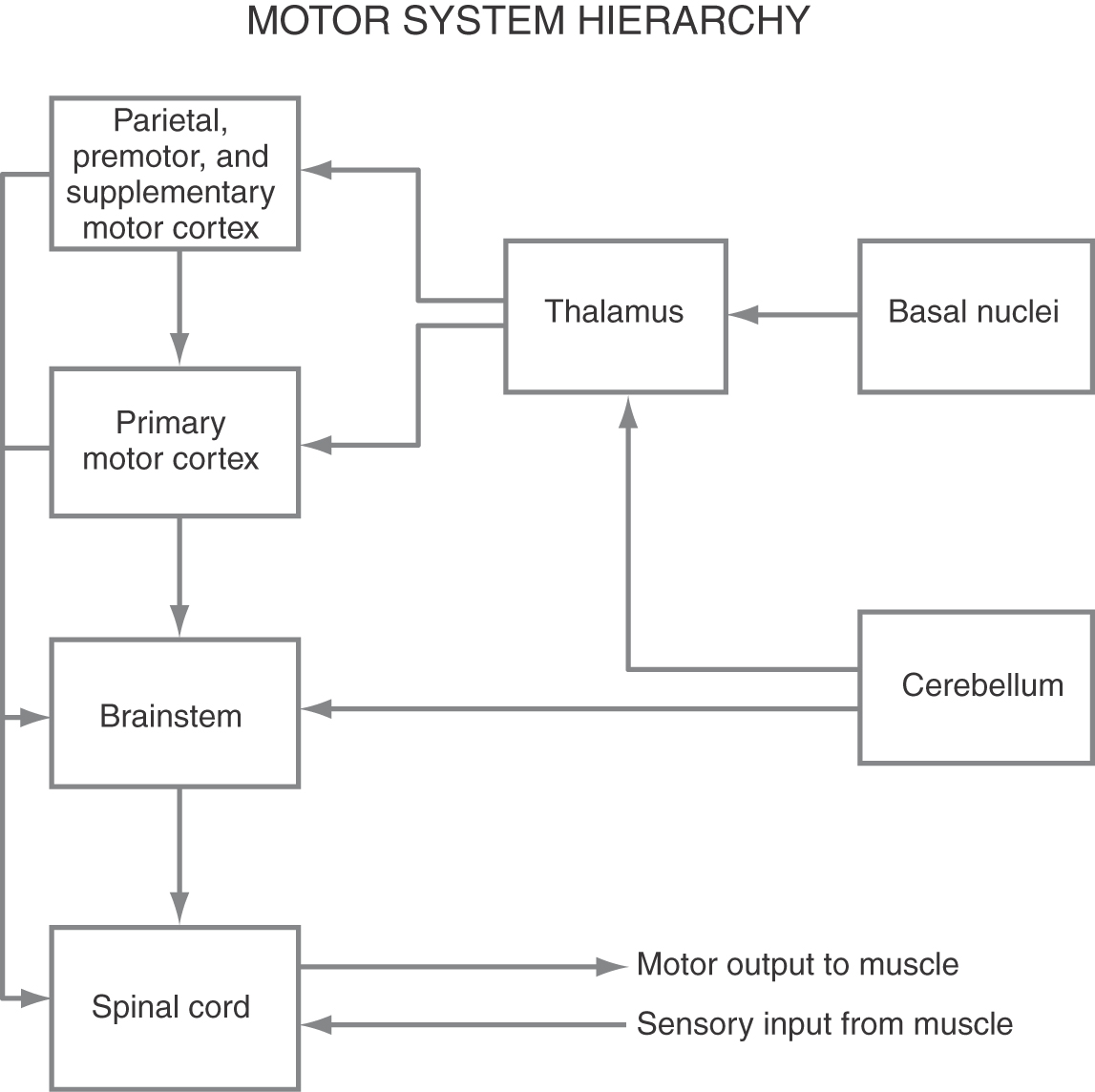
Until recently, it was generally thought that the control of voluntary movement could be satisfactorily described by the hierarchical scheme shown in Figure 25-22. In this plan, lower motor neurons and their associated interneurons are influenced by (1) segmental peripheral sensory feedback circuits, (2) descending brainstem-spinal systems modulated by the cerebral cortex, and (3) the corticospinal system.
Furthermore, the motor areas in the cortex are hierarchically organized such that the output of the corticospinal system is regulated and modulated by higher order motor cortices. When a voluntary movement is desired, a plan for the movement is organized by the combined efforts of the higher order motor areas and then transmitted to MI. The primary motor cortex then executes the plan by communicating with the spinal motor apparatus either directly or indirectly via brainstem-spinal systems (Fig. 25-22). However, a reexamination of the origins of the corticospinal tract indicates that more of these fibers than had been previously thought originate from cells located outside MI. In addition, circuits linking the basal nuclei, thalamus, and motor cortical regions are segregated to some extent from pathways linking the cerebellum, thalamus, and motor cortical areas.
These observations have led to an evolving hypothesis that motor system control is achieved by a series of parallel systems formed by somatotopically organized, descending cortical projections that link the various motor-related areas of cortex more directly with spinal motor circuits. Included here are spinal projections originating from the so-called higher order motor regions, such as the premotor and supplementary motor areas. The concept is that each descending cortical pathway contributes its own element or series of elements to movement control. This idea is supported by the fact that stimulation in different cortical locations can elicit movements that involve the same muscles or muscle groups, but the characteristics of the movement are different and depend on the site of stimulation.
Sources and Additional Reading
Asanuma H. The Motor Cortex. New York: Raven Press; 1988.
Georgopoulos AP. Higher order motor control. Annu Rev Neurosci. 1991;14:361–377.

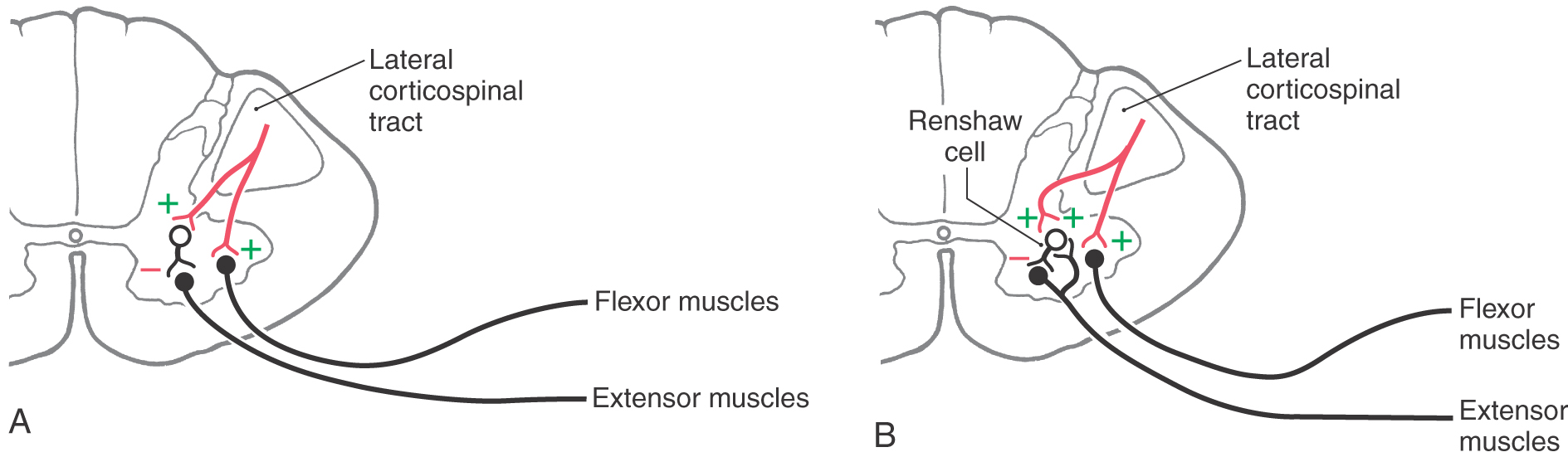
 Figure 25-1.
Figure 25-1. 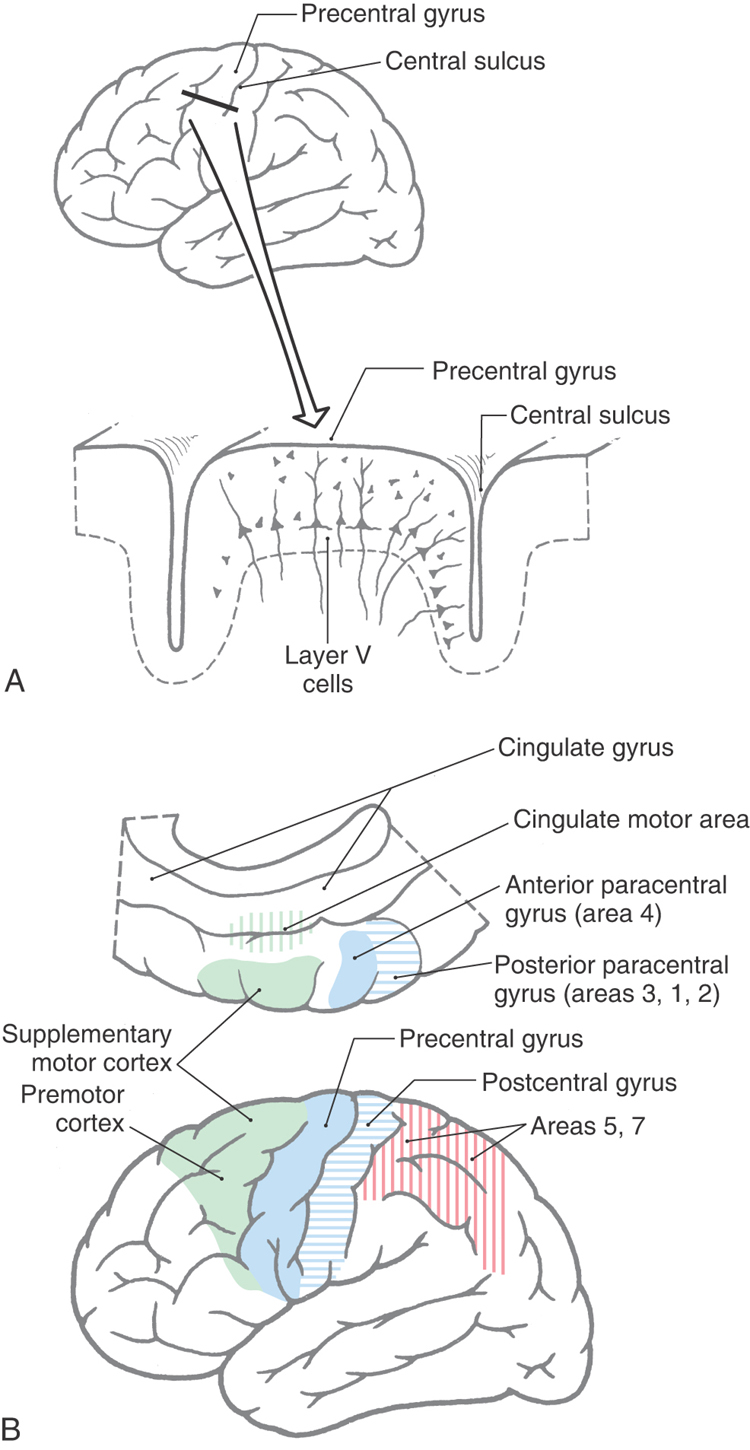
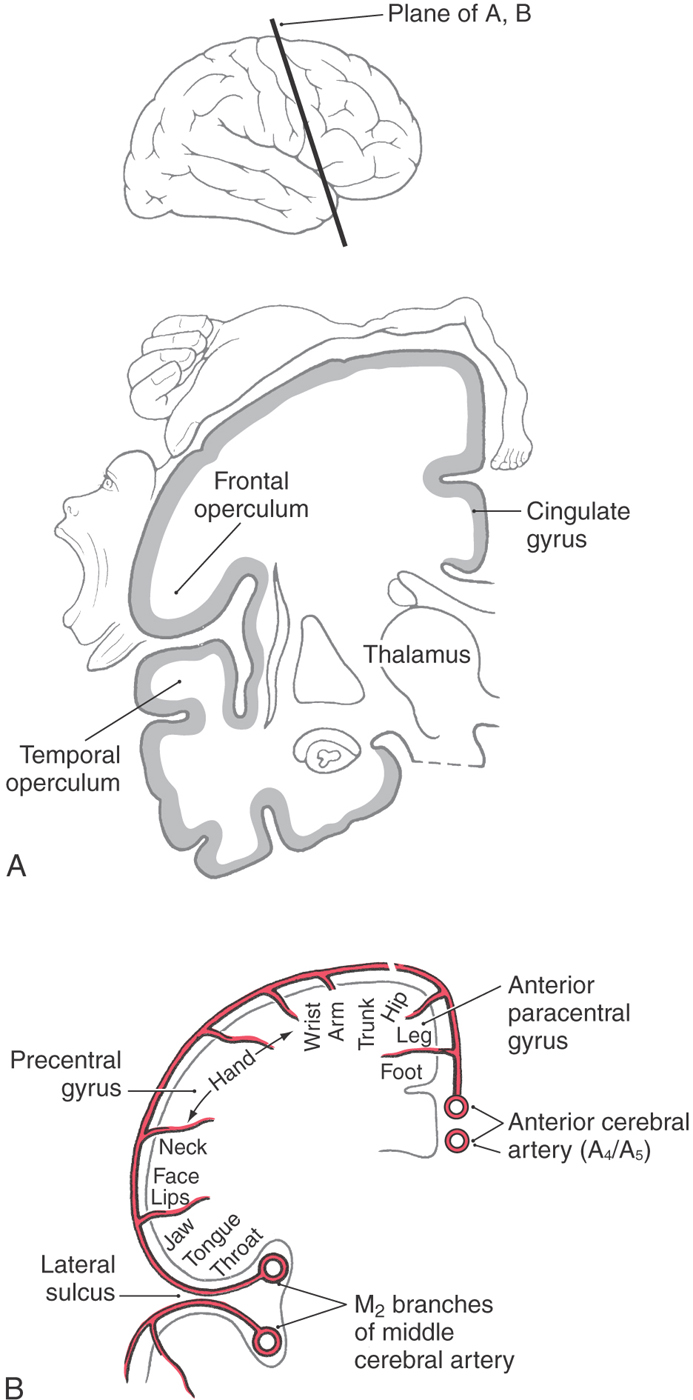
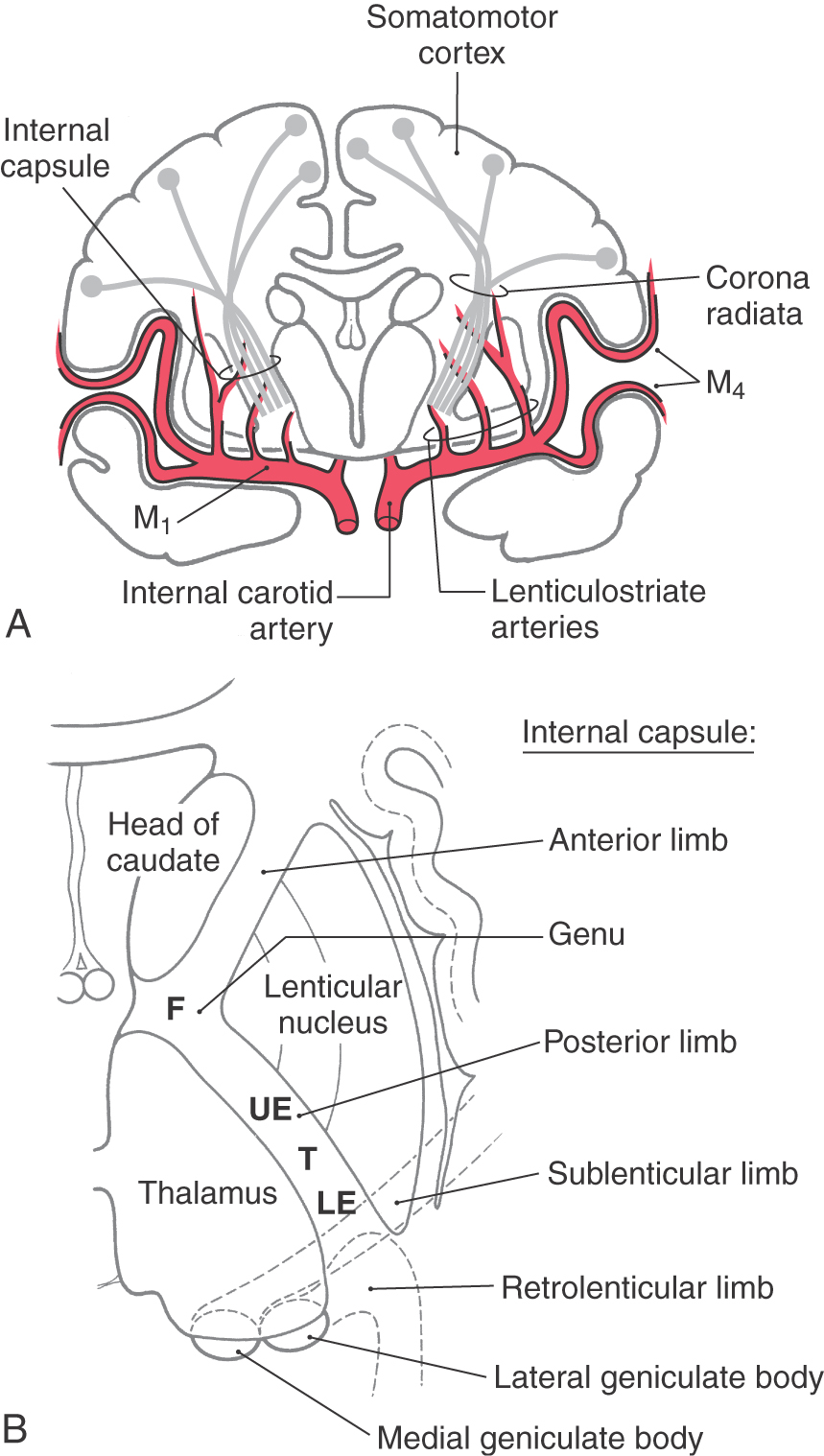
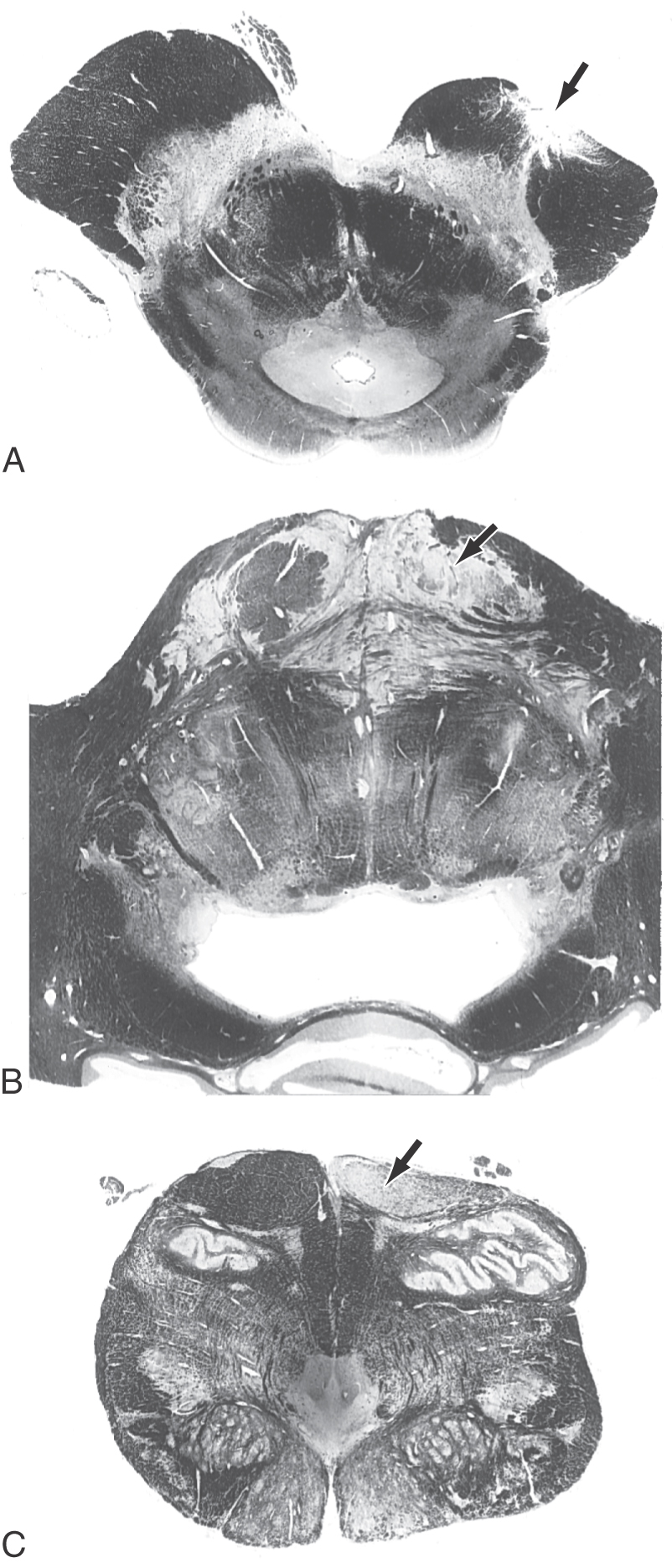
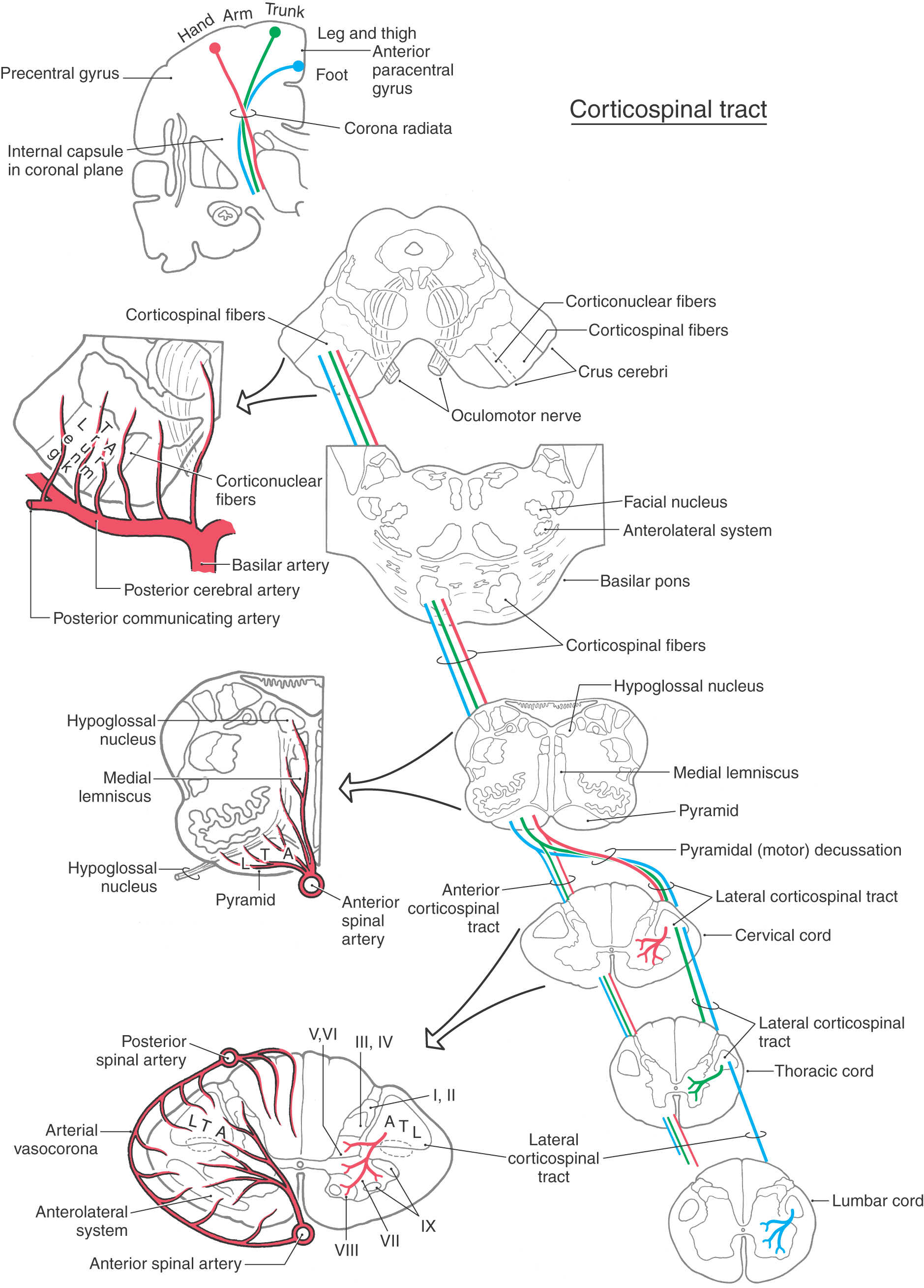
 Figure 25-6.
Figure 25-6.