Chapter 24
Motor System I: Peripheral Sensory, Brainstem, and Spinal Influence on Anterior Horn Neurons
Peripheral Sensory Input to the Anterior (Ventral) Horn
Brainstem-Spinal Systems: Anatomy and Function
Functional Role of Brainstem-Spinal Interactions
Posterior (Dorsal) Root Section
Spinal anterior horn motor neurons whose axons innervate skeletal muscles are called lower motor neurons. These cells activate skeletal muscles to produce characteristic movements of a body part. The activity of these motor neurons is influenced by two sources. First, peripheral sensory input arrives via posterior roots and is transmitted to anterior horn motor neurons and interneurons. Second, extensive descending projections from the cerebral cortex and brainstem, called supraspinal systems, terminate at all levels of the spinal cord and are responsible for a mixture of excitatory and inhibitory influence on anterior horn motor neurons. This chapter focuses on the peripheral sensory and brainstem systems that influence anterior horn neurons.
OVERVIEW
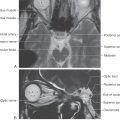
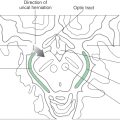
The lower motor neurons of the spinal cord anterior horn form neuromuscular junctions (synapses) with skeletal muscles and are topographically arranged according to the muscle groups they innervate. This is particularly evident in the cervical and lumbosacral enlargements, the levels of the spinal cord that innervate the musculature of the upper and lower extremities, respectively. Motor neurons that supply flexor muscles generally are more posteriorly located in the anterior horn than are extensor motor neurons. In addition, motor neurons that innervate paravertebral and proximal limb muscles are more medial, whereas those that innervate distal musculature are more lateral (Fig. 24-1). The anterior horn motor neurons receive sensory feedback from the muscles they control as well as from synergist and antagonist muscles. The linkage of peripheral sensory input and anterior horn neurons forms the substrate for a number of spinal reflexes (see Fig. 9-9, to Fig. 9-11).
In addition to sensory feedback, the activity of lower motor neurons in the spinal cord is greatly influenced by descending projections from cells in the brainstem and cerebral cortex. These brainstem and cortical neurons are referred to as upper motor neurons, and unlike lower motor neurons, they have no direct synaptic link with muscles. Because of their origin, these descending projections are also called supraspinal systems.
Anterior horn motor neurons represent the only direct link (the final common path) between the nervous system and skeletal muscle. As such, these neurons play a central role in the production of movement. The regulation of motor neuron activity by peripheral sensory input and descending brainstem influences is crucial to the performance of normal, synergistic, and productive movements.
ANTERIOR HORN MOTOR NEURONS
Types and Distribution
There are two varieties of anterior horn motor neurons, alpha and gamma, which are intermingled within the anterior horn. Alpha motor neurons innervate the ordinary, working fibers of skeletal muscles called extrafusal fibers, and gamma motor neurons innervate a special type of skeletal muscle fiber, the intrafusal fibers, which are found only within muscle spindles. Recall that the anterior horn also contains small interneurons whose axons distribute locally within the spinal gray. Interneurons are numerous in the intermediate zone and anterior horn and are functionally essential in the regulation of alpha and gamma motor neurons. Their action on motor neurons may be either excitatory or inhibitory.
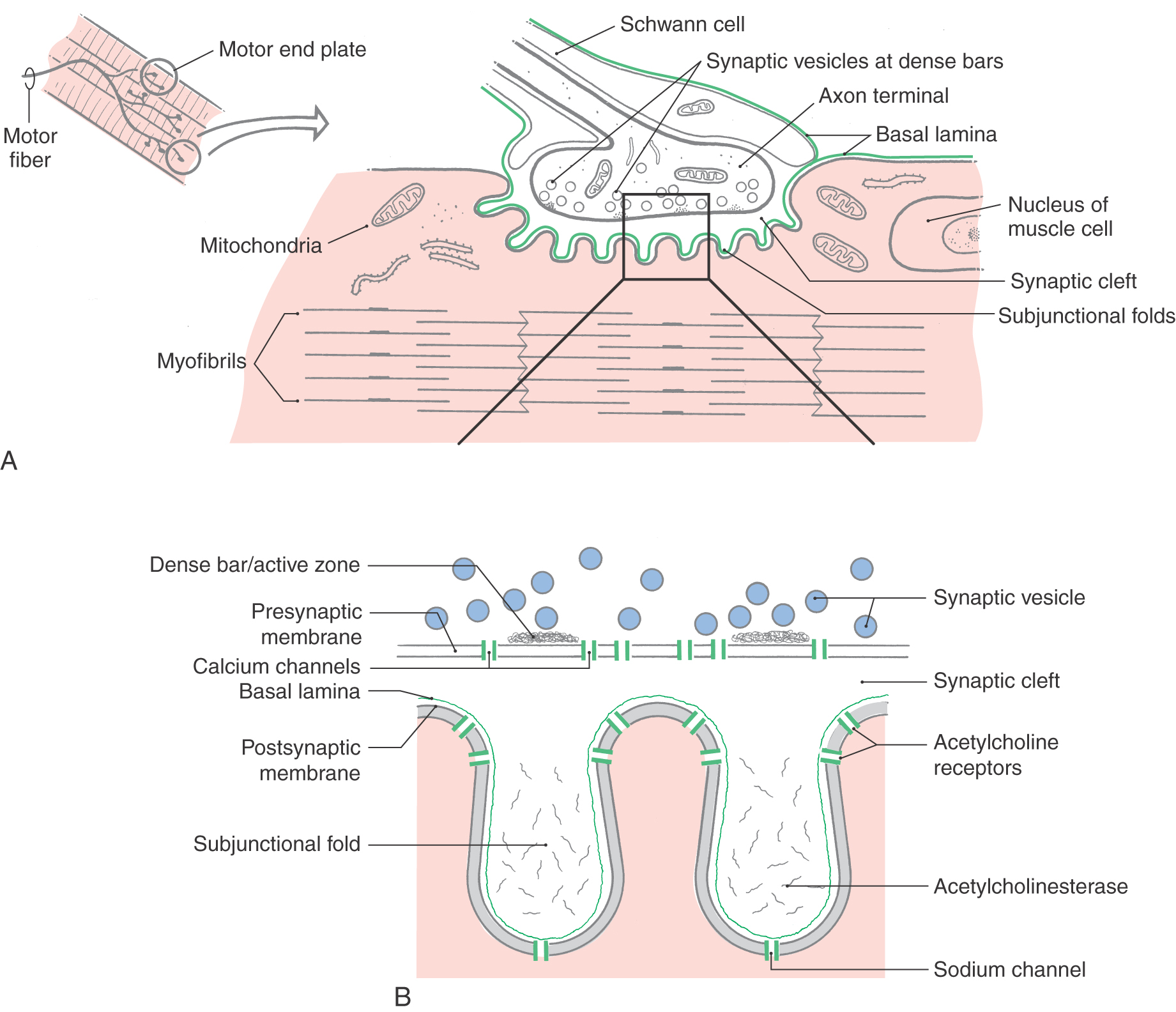
The axons of both types of anterior horn motor neurons exit the spinal cord via the anterior roots and course distally in peripheral nerves. These fibers represent the final common path that links the nervous system and skeletal muscles. As the axon of an alpha motor neuron reaches the muscle it innervates, it loses its myelin sheath and forms a series of flattened boutons that indent the surface of a group of muscle fibers. This specialized type of synapse is called a neuromuscular junction or motor end plate (Fig. 24-2). Damage to or loss of the cell bodies of alpha motor neurons (also called lower motor neurons) or lesions of their axons result in a profound weakness of the skeletal muscles innervated and loss of reflexes (areflexia).
Neuromuscular Junction
Like synapses in the central nervous system, the junction between a motor axon and skeletal muscle fibers consists of presynaptic and postsynaptic components (Fig. 24-2). The presynaptic element, the axon terminal, contains round, clear synaptic vesicles (filled with the neurotransmitter acetylcholine), mitochondria, and small patches of dense material around which the vesicles aggregate at the active site. The presynaptic element is separated from the postsynaptic element by an extracellular space called the synaptic cleft. The postsynaptic membrane, the specialized portion of the muscle cell plasma membrane subjacent to the axon terminal, exhibits a large number of folds that effectively increase the surface area of the muscle cell in contact with the axon terminal (Fig. 24-2). These irregularities, called subjunctional folds, contain nicotinic acetylcholine receptors on their summit facing into the synaptic cleft (Fig. 24-2). These nicotinic acetylcholine receptors are integral membrane proteins with an extracellular domain that actually binds the acetylcholine molecule and a membrane-spanning domain that forms an ion channel (Fig. 24-2B). Such receptors are called ionotropic receptors because binding of the neurotransmitter molecule to the extracellular domain typically opens the ion channel and allows the passage of sodium and potassium ions. Thus the receptor and its associated ion channel mediate the ion flux that underlies the transmission of electrical signals from nerve to muscle. Surrounding the exterior surface of the muscle is a basal lamina that extends into the synaptic cleft, where it becomes continuous with a basal lamina formed by the Schwann cell process that encloses the axon terminal (Fig. 24-2).
When an action potential depolarizes the presynaptic element, there is an influx of calcium through voltage-gated membrane channels. Synaptic vesicles fuse with the presynaptic membrane at the active sites (which are marked by structures called dense bars) and release acetylcholine into the synaptic cleft. The transmitter binds to receptors on the postsynaptic membrane and opens ion channels. Ion flux then occurs, and a depolarizing potential called an end plate potential spreads over the surface of the muscle fiber. This potential triggers the release of calcium (from the sarcoplasmic reticulum), which elicits the movement of actin and myosin filaments, resulting in muscle contraction. Synaptic transmission is terminated by an enzyme called acetylcholinesterase, which is located in the matrix of the basal lamina in the depths of the postjunctional folds. This enzyme inactivates acetylcholine by detaching it from its receptor and hydrolyzing it to acetate and choline.
Myasthenia gravis (MG) is a disease of the neuromuscular junction. It is characterized by antibodies that bind to nicotinic acetylcholine receptors and probable lysis of postsynaptic receptors. Patients with MG may experience weakness that waxes and wanes during minutes, hours, or days; it most frequently affects ocular muscles first (about 65% of patients) and in some cases will remain confined to the extraocular muscles (about 15%, ocular myasthenia). General weakness of limb musculature occurs in about 85% of patients but rarely without concurrent ocular or brainstem (facial, tongue) involvement. If weakness of the respiratory muscles (intercostal, diaphragm) occurs, it is called a myasthenic crisis and may require rapid intervention.
Motor Units
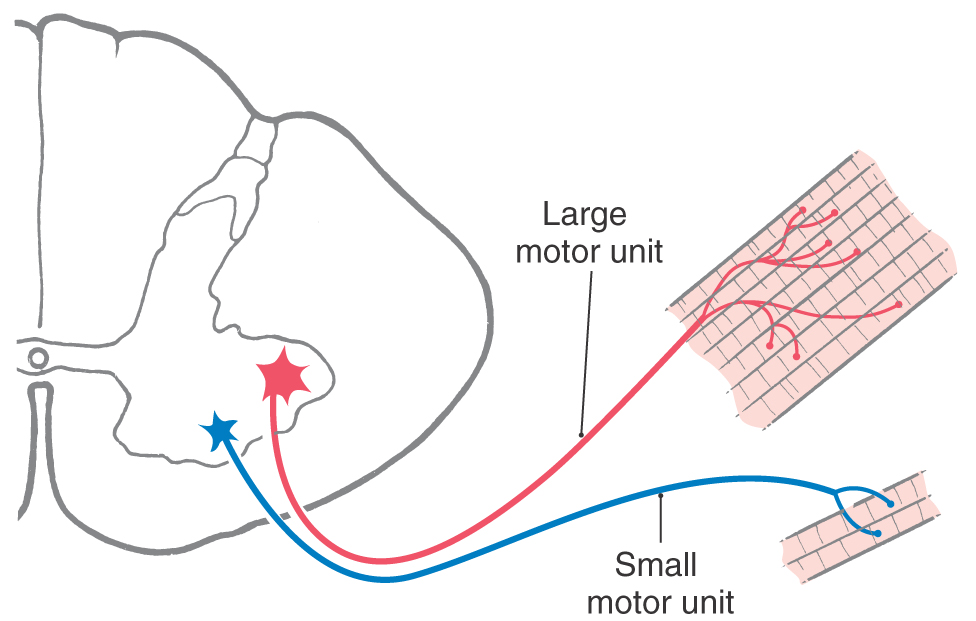
Each muscle fiber receives only one motor end plate, but the number of muscle fibers innervated by a single alpha motor neuron axon varies from a few to many. The aggregate of a motor neuron axon and all the muscle fibers it innervates is called a motor unit (Fig. 24-3). In general, as the need for fine control of a muscle increases, the size or innervation ratio of its motor unit decreases. That is, the number of muscle fibers innervated by a single axon decreases. The size of a motor unit is also related to the mass of the muscle and its speed of contraction. Small muscles that generate low levels of force typically have small motor units (10 to 100 muscle fibers per motor neuron axon), whereas large, powerful muscles that generate high levels of force are usually innervated by large motor units (600 to 1000 muscle fibers per motor neuron axon). An example of small motor units are the extraocular muscles and their innervation by oculomotor, trochlear, and abducens motor neurons. On the other hand, the quadriceps muscles (rectus femoris and vastus medialis, lateralis, and intermedius) and their motor neurons in the lumbosacral spinal cord are examples of large motor units. Large muscles also contain some small motor units; the small units are initially recruited for precision, and the larger units are recruited later in the movement for increased strength.
 Figure 24-3. Large and small motor units.
Figure 24-3. Large and small motor units.
Motor units can be divided into two categories (slow twitch and fast twitch) on the basis of the metabolic and physiologic properties of the muscle fibers and their innervation. Type I units are composed of “red” (dark) muscle fibers referred to as slow-twitch (S) fibers. These muscles are rich in mitochondria and contain a (red) heme protein that helps bind and store oxygen. Because of their ability to use glucose and oxygen from the bloodstream, these fibers can generate abundant adenosine triphosphate (aerobic metabolism) and fuel the contractile apparatus for long periods of contraction time, making these motor units resistant to fatigue. The trade-off, however, is that these muscle fibers can generate only relatively small levels of force or tension. The postural muscles (deep back muscles) are composed predominantly of this fiber type; these muscles may contract at a low level of tension but for exceedingly long times.
In contrast, the type II or fast-twitch units (white or pale muscles) generate much higher levels of force but for comparatively brief periods. Muscles used during strenuous exercise are examples of type II fibers: they contract with greater force than postural muscles but for shorter periods. The fast-twitch fibers actually come in two varieties. The fast-fatigable type (type IIb or FF) contains large stores of glycogen that provide the energy necessary to phosphorylate adenosine diphosphate (glycogen converted to lactic acid) and produce relatively greater amounts of force compared with slow-twitch fibers. However, the rapid depletion of glycogen coupled with the accumulation of lactic acid (anaerobic catabolism) contributes to the relatively brief contraction time. A second fast-twitch unit (type IIa) is actually intermediate between the type I slow-twitch and type II fast-twitch units because it exhibits sufficient aerobic capacity to resist fatigue yet is able to generate nearly as much force as the type IIb units. These units are referred to as fast fatigue-resistant fibers (FFR).
Muscles generally contain a mixture of motor units, and the proportions vary according to the demands placed on the muscle. For example, the soleus muscle is a slow-twitch postural muscle containing mainly S-type units. The relatively slow conduction time of the small-diameter alpha axons serving these motor units is adequate for the demands of this muscle. By contrast, the gastrocnemius muscle is a dynamic, powerful muscle used in running and jumping. It is considered to be a fast-twitch muscle and contains mainly FF motor units innervated by large-diameter, rapidly conducting axons.
Size Principle
The nervous system uses the size and functional properties of motor units as a means of grading the force of muscle contraction. When an excitatory input reaches a group of motor neurons in the anterior horn, that input will produce a larger change in the membrane potential of the smaller motor neurons than in the larger motor neurons. This is because the firing threshold of a neuron is determined by its total electrical resistance, which is inversely proportional to its surface area. Therefore, a given synaptic input to a pool of motor neurons will first recruit the smaller neurons (linked to small motor units) followed in sequence by progressively larger cells (and larger motor units). This is known as the size principle of motor neuron recruitment. Thus in a fine movement that requires sustained output with little force, the smaller cells and the small motor units (slow twitch) are activated first. As the need for a more forceful rapid movement increases, progressively larger cells and larger motor units (fast twitch) are activated, and the movement transitions smoothly from low force to high force with strong bursts of contraction. The force of contraction is also influenced by the firing rate of the participating motor neurons. As the requirement for greater force and speed of contraction increases, the synaptic input increases and recruits more of the larger neurons. The firing rate of the activated larger motor neurons also increases and enhances the speed and force of the movement.
PERIPHERAL SENSORY INPUT TO THE ANTERIOR (VENTRAL) HORN
Muscle Spindles
Signals that transmit information from skeletal muscles into the nervous system enter the spinal cord via the posterior roots. For the most part, these signals are generated in specialized structures in muscles called neuromuscular spindles (commonly called muscle spindles; also known as Kühne spindles). The output of the muscle spindle signals a change in muscle length and the rate of change in muscle length.
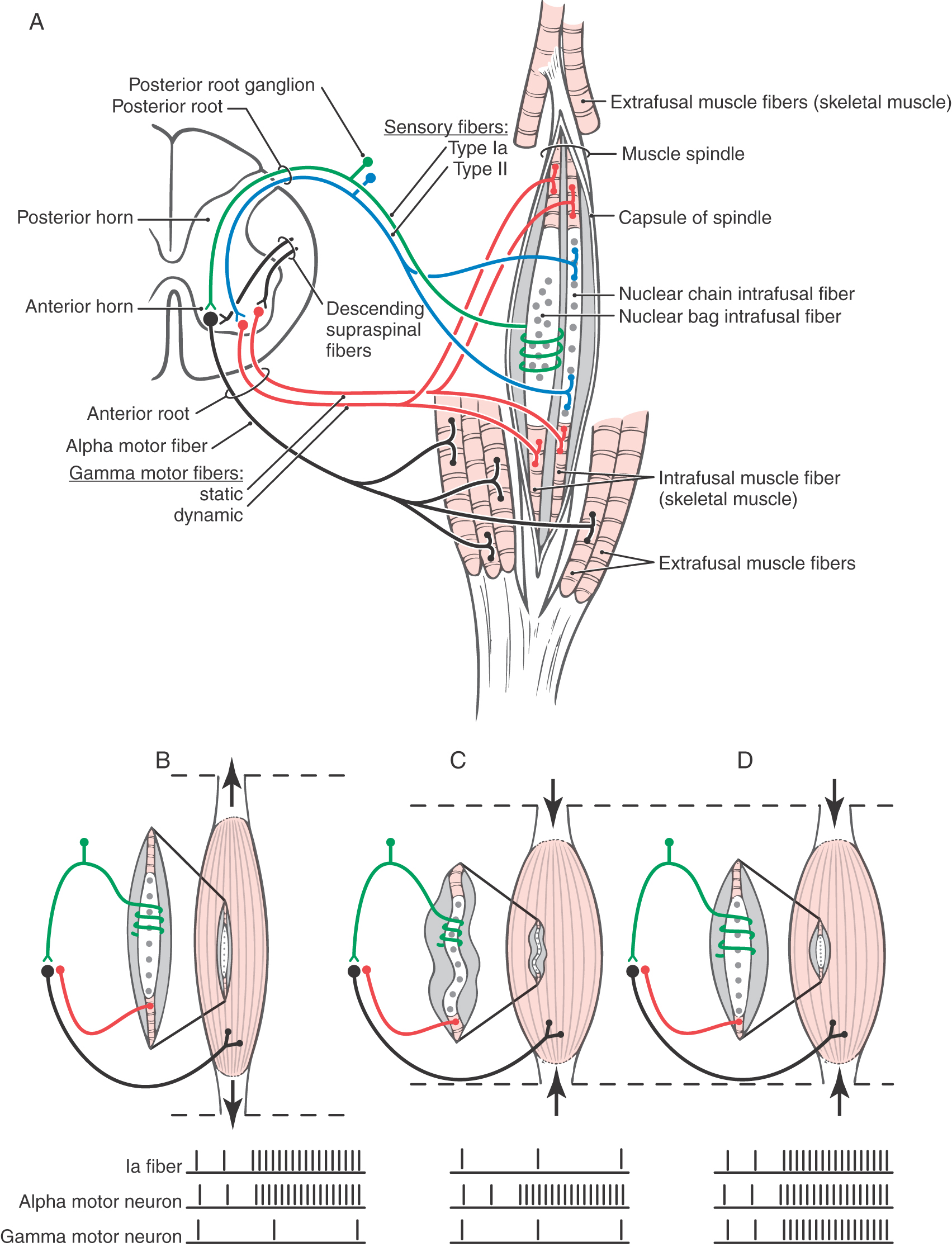
A muscle spindle (Fig. 24-4) is a long, thin, encapsulated structure that typically contains about seven intrafusal muscle fibers, which are striated. Spindles range in length from 4 to 10 mm. The capsule of the spindle (along with its intrafusal muscle fibers) is attached to and oriented in parallel with the extrafusal fibers that constitute the bulk of the muscle.
There are two basic types of intrafusal fibers: nuclear bag fibers and nuclear chain fibers (Fig. 24-4; Table 24-1). Like other skeletal muscle cells, intrafusal fibers are multinucleated, and the arrangement of the nuclei is the most obvious structural feature distinguishing the two types. In both types, the nuclei occupy the central (equatorial) region of the cell. In nuclear bag fibers, the nuclei are clustered centrally and give the equatorial region a swollen appearance. In nuclear chain fibers, the nuclei are arranged in a linear row, and the equatorial region is not obviously expanded. The contractile elements of both types of cells are located entirely in the two distal (polar) regions of the cell.
Table 24-1 The Muscle Spindle
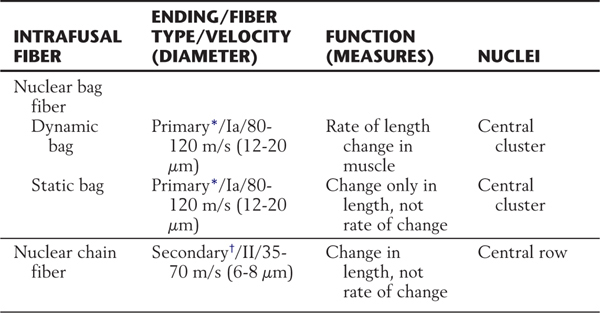
*Annulospiral ending.
†Flower-spray ending.
The two ends of the spindle are anchored to extrafusal muscle fibers (Fig. 24-4A–D). This arrangement allows two conditions under which the equatorial region of the spindle may be stretched and the afferent fibers of the spindle activated. First, passive stretch of extrafusal muscle fibers will elongate the spindle, stretch its equatorial region, and activate its afferent fibers. Second, the intrafusal muscle fibers, when activated by a gamma motor neuron, will contract, stretch the equatorial region of the spindle, and also result in activation of its afferent fiber (Fig. 24-4A-D).
The two types of intrafusal fibers perform different sensory functions. The nuclear bag fibers are actually subdivided into two different categories that have different elastic properties and, correspondingly, different functions (Table 24-1). One type, the dynamic nuclear bag fiber, is sensitive mainly to the rate of change in muscle length. The other, the static nuclear bag fiber, signals only a change in muscle length but not the rate of that change. Nuclear chain fibers, like static bag fibers, are mainly sensitive to changes in muscle length (Table 24-1).
Intrafusal muscle fibers are associated with two types of sensory fibers, the terminals or receptive ends of which are concentrated at the equatorial (noncontractile) region of intrafusal fibers. The type Ia fiber is heavily myelinated, has a conduction velocity of 80 to 120 m/s, and is typically associated with dynamic and static nuclear bag fibers (Fig. 24-4A; Table 24-1). The distal end of this sensory fiber is wrapped around the central (noncontractile) region of the intrafusal muscle fibers. Because of this relationship, the type Ia afferent terminations are called annulospiral endings. These endings are, in effect, mechanoreceptors. Stretching of the central region of the intrafusal fiber will also stretch the sensory fiber and mechanically open ion channels that will enable sodium and potassium ion flux through the membrane. If the induced ion flux raises the membrane potential above threshold, an action potential is initiated in the sensory fiber. The firing frequency is directly proportional to the degree to which the spindle is stretched.
The other type of muscle spindle sensory fiber, the type II fiber, is principally associated with nuclear chain fibers (Fig. 24-4A; Table 24-1). Its connection with the equatorial region of the target intrafusal fiber has the form of a cluster of thin, radiating branches and is called a secondary ending or flower-spray ending. This sensory fiber is also activated by mechanical stretch, but it codes only the change in muscle length, not the rate of the stretch.
Each type of intrafusal fiber is also innervated by a gamma motor neuron. Dynamic nuclear bag fibers are associated with dynamic gamma motor neurons, whereas static nuclear bag fibers and nuclear chain fibers are innervated by static gamma motor neurons. When the gamma motor neuron is active, contractile elements at both poles of the intrafusal muscle fiber are activated, resulting in increased stretch on its central region. This increases the frequency of action potentials generated in the Ia sensory fibers. As explained further on, dynamic and static gamma motor neurons function to maintain spindle sensitivity and length, respectively.
Gamma Loop
Muscle spindles play an essential role in movement and in the maintenance of muscle tone. Consider two situations: one in which a muscle—for example, the biceps brachii—is passively stretched and another in which it contracts and shortens actively against a load.
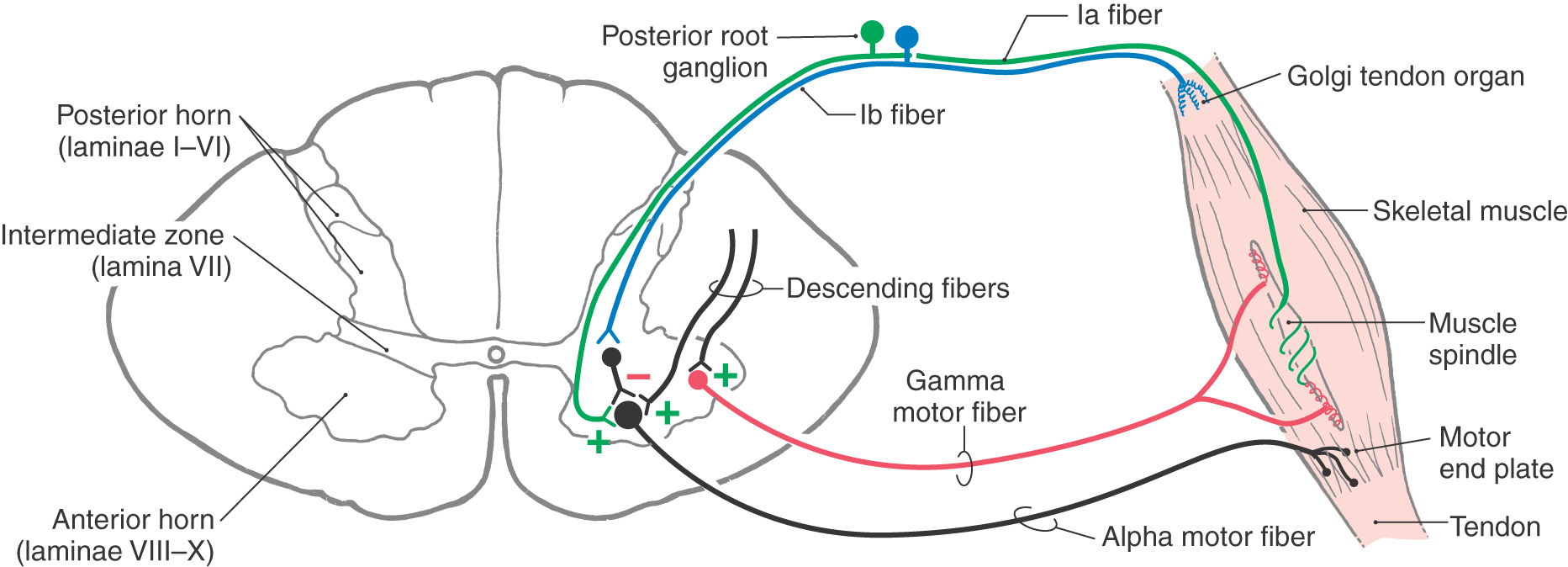
A passive stretching of the biceps muscle, produced, for example, by tapping on its tendon, will elongate the muscle spindles. The stretching of the equatorial region of the nuclear bag fibers results in an increase in the firing rate of the Ia fibers (Figs. 24-4B and 24-5). These sensory fibers enter the cervical spinal cord and form monosynaptic excitatory synapses with alpha motor neurons that innervate the biceps brachii (Figs. 24-4A, B and 24-5). In this situation, the extrafusal muscles that contract are those in which the activated spindle is located. This is the circuit that forms the basis of the muscle stretch reflex explained in Chapter 9 (see Fig. 9-9).
 Figure 24-5. Circuits related to input and output mediated by Golgi tendon organs and to alpha-gamma coactivation.
Figure 24-5. Circuits related to input and output mediated by Golgi tendon organs and to alpha-gamma coactivation.
The connection between the Ia sensory fibers and the alpha motor neurons of a muscle also functions in a more complex mechanism called the gamma loop, which is absolutely crucial to the maintenance of stretch reflexes and muscle tone. In this mechanism, extrafusal muscle contraction is indirectly produced by supraspinal activation of gamma motor neurons (Figs. 24-4D and 24-5; Table 24-2). Like alpha neurons, gamma motor neurons receive supraspinal input from the cerebral cortex and brainstem. In the gamma loop, this supraspinal input activates the gamma motor neurons so that the intrafusal muscle fibers contract. Because the contraction of an intrafusal fiber has the effect of stretching the equatorial region between its two polar regions, it results in increased Ia fiber activity. In the spinal cord, this increase in Ia fiber discharge activates alpha motor neurons, which then activate extrafusal muscle fibers, resulting in muscle contraction (Figs. 24-4D and 24-5). This circuit involving gamma motor neurons, intrafusal muscle fibers, Ia primary afferent fibers, alpha motor neurons, and extrafusal muscle fibers is called the gamma loop (Table 24-2).
Table 24-2 Gamma Loop

Now consider the situation in which a muscle is contracting actively against a load. Because a muscle spindle is attached parallel to the adjacent extrafusal fibers, one might infer that overall spindle length is determined by the length of the surrounding extrafusal muscle fibers; when the muscle contracts, the spindle shortens. This is not so; if the intrafusal fibers remained passive during extrafusal muscle fiber contraction, the shortening of the spindle would relax the equatorial region of the intrafusal fibers, and the Ia fibers would cease firing (Fig. 24-4C). For example, if only the alpha motor neuron is activated, the extrafusal fibers contract, the intrafusal fibers do not, and the spindle is not responsive. This slack, inactive spindle would be useless for reporting of muscle length. In reality, the spindle retains its sensitivity (Fig. 24-4D) and the Ia sensory fibers continue to fire during voluntary muscle contraction because when the brain signals the alpha motor neurons to initiate muscle contraction, it sends parallel impulses to the gamma neurons to cause the intrafusal fibers to contract. Therefore, when the extrafusal muscle fibers shorten, the intrafusal fibers also shorten because their gamma motor neurons are activated at the same time. As a result, the equatorial regions of the intrafusal fibers remain under nearly constant tension, and the spindle retains its ability to signal changes in muscle length as movement (muscle contraction) occurs. This mechanism is called alpha-gamma coactivation (Fig. 24-5; Table 24-3).
Table 24-3 Alpha-Gamma Coactivation

Golgi Tendon Organ
Sensory feedback to the spinal anterior horn is also derived from the Golgi tendon organs. These structures are located in tendons near their junctions with muscle fibers and consist of networks of thin nerve fibers intertwined with the collagen fibers of the tendon (Fig. 24-5; Table 24-4). These nerve fibers, like the sensory fibers of muscle spindles, are mechanoreceptors. However, unlike muscle spindles, the sensory fibers of tendon organs are connected in series between the tendon and the extrafusal muscle fibers. When force is applied to the tendon, the sensory fibers are stretched, which opens ion channels in the nerve fiber membrane. The fibers that lead from the tendon organs to the spinal cord are type Ib fibers. These fibers are large in diameter and heavily myelinated, with a conduction velocity of 70 to 110 m/s (Table 24-4). After entering the spinal cord, the type Ib fibers traverse the intermediate zone to reach the anterior horn, where they form excitatory synapses with interneurons. These interneurons in turn inhibit alpha motor neurons that innervate the muscle associated with the activated Golgi tendon organ. This action of the Golgi tendon organ is exactly opposite that of the muscle spindle; activation of the muscle spindle leads to excitation of the muscle associated with the activated spindle, whereas activation of the tendon organ leads to inhibition of neurons innervating the muscle from which the afferent input originated (Table 24-4).
Table 24-4 Golgi Tendon Organ

*Whereas some tendon organs may discharge at high rates under conditions of high force (and may serve a protective function), it is well known that the discharge of many tendon organs forms a continuum from low (rate/force) to high (rate/force).
Reflex Circuits
Afferent fibers from muscle spindles and Golgi tendon organs take part in a variety of reflex circuits that directly or indirectly influence the activity of anterior horn motor neurons. Several of the more prominent of these circuits are described in Chapter 9 (see Fig. 9-9, to Fig. 9-11); they are summarized only briefly here. As mentioned earlier, many type Ia spindle afferents form monosynaptic excitatory connections with alpha motor neurons that innervate the muscle from which the afferents originated. This circuitry is the basis for the muscle stretch reflex (see Fig. 9-9). At the same time, these Ia fibers activate Ia interneurons that inhibit motor neurons innervating antagonist muscles; this is called reciprocal inhibition (see Fig. 9-9). Incoming muscle afferents can also activate interneurons that project to the contralateral side of the spinal cord as well as propriospinal neurons that link the spinal segment at which the spindle afferents entered to more rostral or caudal spinal cord levels. Circuits of the first type, which convey cutaneous somatic inputs, form the basis for the crossed extensor reflex (see Fig. 9-11).
In general, the various local spinal reflex pathways primarily target alpha motor neurons or spinal interneurons. For the most part, the activity of these basic spinal reflexes occurs in the background and is not under direct volitional control. However, certain so-called long loop reflexes transmit muscle sensory information through ascending pathways that reach the cerebral cortex by way of a thalamic relay. The cortex can then increase or decrease the gain of spinal reflexes via descending supraspinal pathways.
BRAINSTEM-SPINAL SYSTEMS: ANATOMY AND FUNCTION
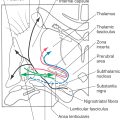
Of the several pathways that project to the spinal cord from the brainstem or cerebral cortex, four are particularly relevant to voluntary movement. Three of the four originate from cell groups in the brainstem. Two of them, the vestibulospinal and reticulospinal systems, travel in the ventral funiculus of the spinal cord. The other two, the rubrospinal and lateral corticospinal tracts, travel in the lateral funiculus. The following sections focus on the three systems that originate in the brainstem: the vestibulospinal, reticulospinal, and rubrospinal tracts.
Vestibulospinal Tracts
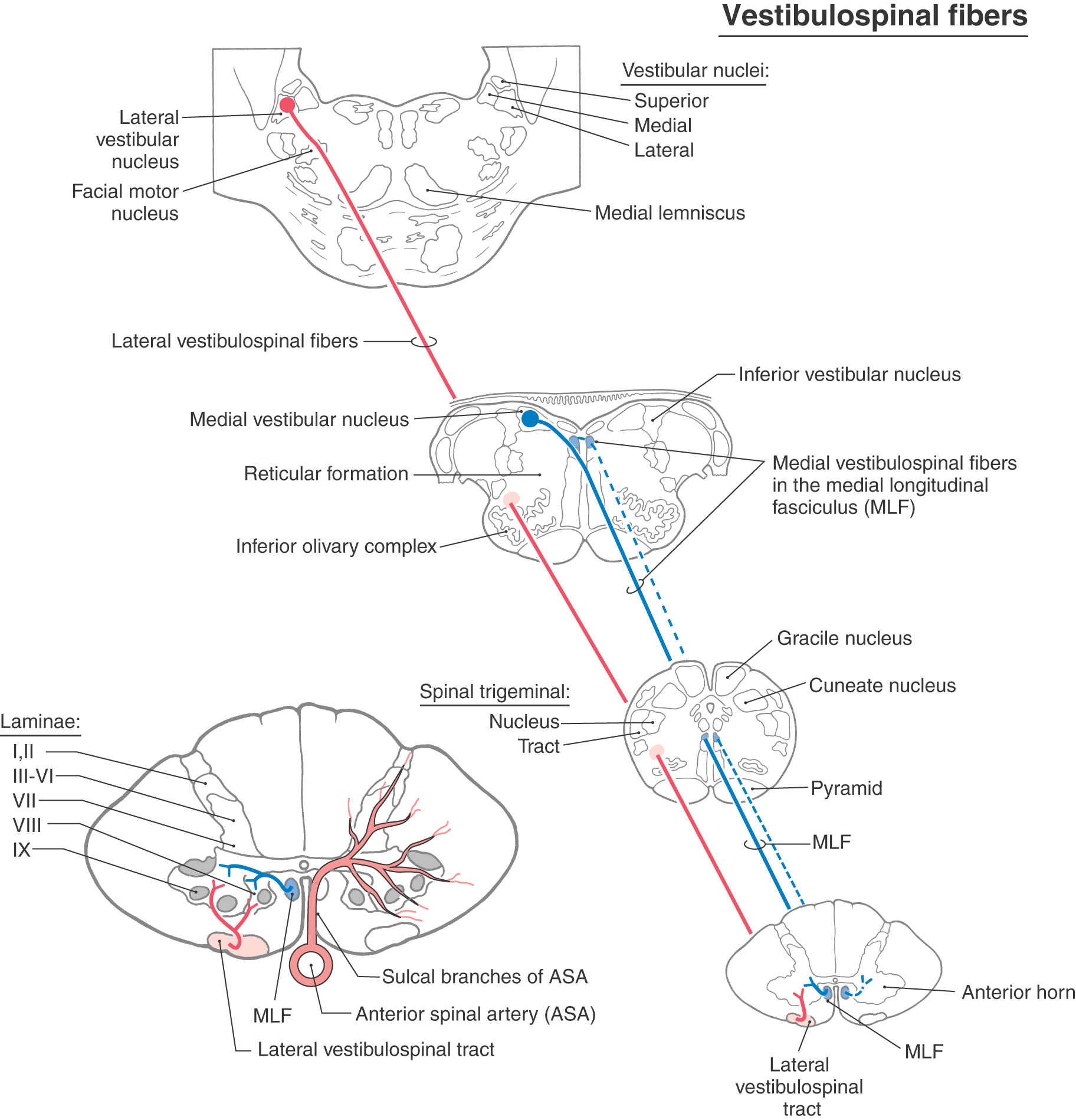
The vestibulospinal system comprises medial and lateral vestibulospinal tracts (Figs. 24-1 and 24-6). The medial vestibulospinal tract is made up of axons that originate in the medial and inferior vestibular nuclei and descend bilaterally into the spinal cord as part of the medial longitudinal fasciculus. The lateral vestibulospinal tract is formed by axons that originate in cells of the lateral vestibular nucleus and descend ipsilaterally through the anterior portion of the brainstem to course in the anterior funiculus of the spinal cord.
 Figure 24-6. Medial and lateral vestibulospinal tracts.
Figure 24-6. Medial and lateral vestibulospinal tracts.
The medial vestibulospinal tract projects only as far as cervical or upper thoracic spinal cord levels and influences motor neurons controlling neck musculature. The lateral vestibulospinal tract, in contrast, extends throughout the length of the cord. Cells in rostral portions of the lateral vestibular nucleus project to the cervical cord, cells in the middle portion project to the thoracic cord, and cells in the caudal part terminate in lumbosacral levels. The fibers of this tract terminate in the medial portions of laminae VII and VIII and excite motor neurons that innervate paravertebral extensors and proximal limb extensors (Fig. 24-6). These muscles function to counteract the force of gravity and therefore are commonly called antigravity muscles. Through their effects on these extensor muscles, lateral vestibulospinal fibers function in the control of posture and balance and, therefore, are also sometimes specified as postural muscles. Evidence from experimental studies suggests that some vestibulospinal axons synapse directly on alpha motor neurons but that most exert their influence through spinal interneurons.
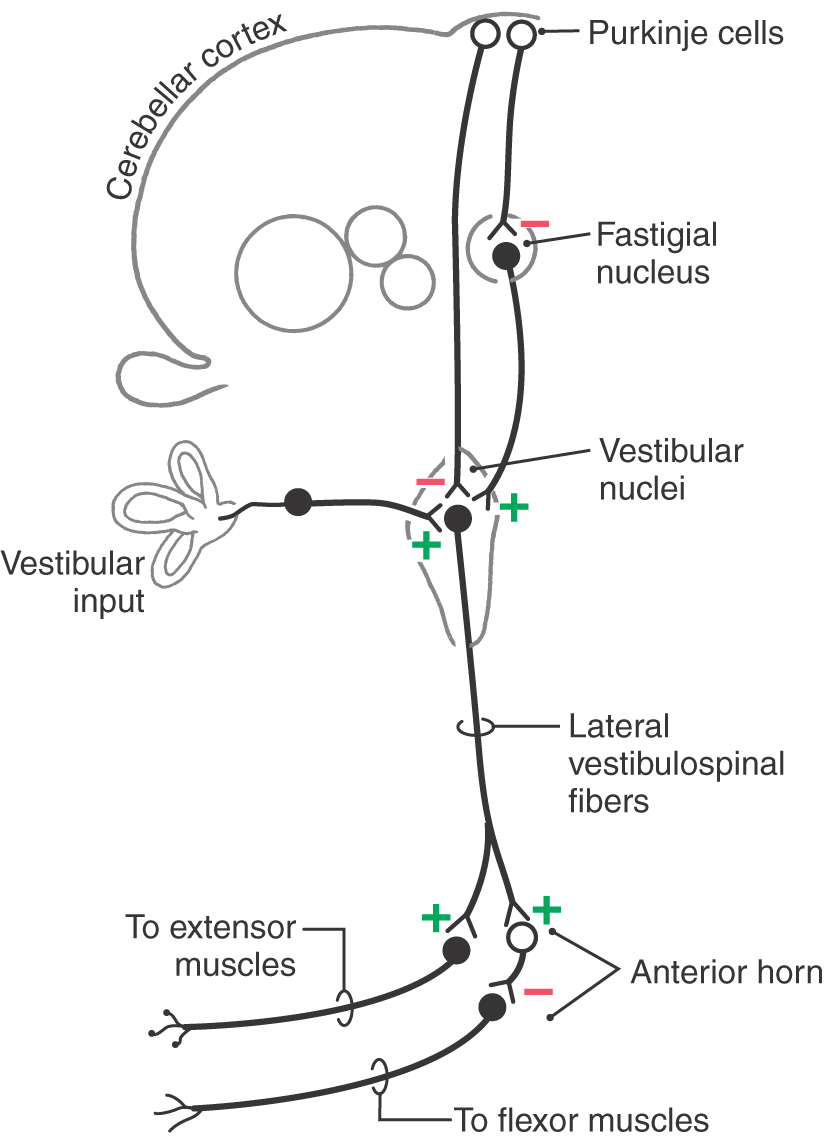
Activity in the lateral vestibulospinal tract is driven primarily by three ipsilateral inputs—two excitatory and one inhibitory (Fig. 24-7). The two sources of excitatory input are the vestibular sensory apparatus and the cerebellar nuclei, mainly the fastigial nucleus. The inhibitory input consists of Purkinje cell axons from the cerebellar cortex.
The lateral vestibulospinal tract is the path by which input from the vestibular sensory apparatus is used to coordinate orientation of the head and body in space. Maintenance of body and limb posture is also influenced by extensive cerebellovestibular projections, which can be either excitatory or inhibitory. The cerebral cortex essentially has no direct projections to the vestibular nuclei; consequently, the vestibulospinal tract is not directly influenced by cortical mechanisms.
Reticulospinal Tracts
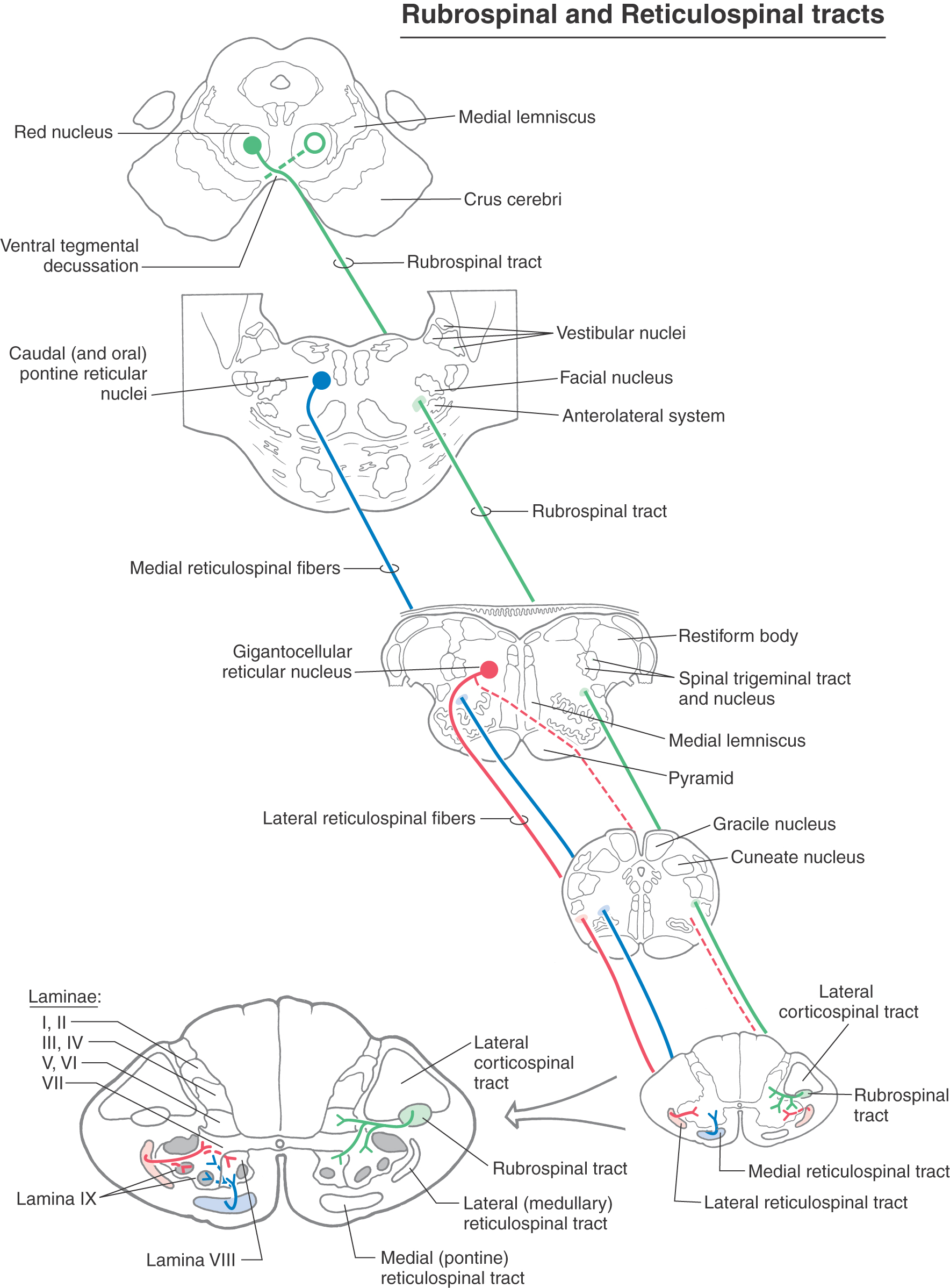
Cells at many levels of the reticular formation contribute to the reticulospinal system, and these fibers can be found in the lateral and anterior funiculi throughout the spinal cord (Fig. 24-1). Reticulospinal fibers participate in a wide variety of functions ranging from pain modulation to visceromotor activity. Most of the fibers involved in somatomotor function originate either from the oral and caudal pontine nuclei or from the gigantocellular reticular nucleus (Fig. 24-8). The fibers from the oral and caudal pontine reticular nuclei descend bilaterally, but with an ipsilateral predominance, in the anterior funiculus. They constitute the medial reticulospinal (or pontine reticulospinal) tract, which runs the full length of the spinal cord. The fibers from the gigantocellular reticular nucleus originate at medullary levels. Most of these medullary reticulospinal fibers remain ipsilateral and descend in the anterior funiculus, although a few decussate (Fig. 24-8). Most take up a new position somewhat lateral and anterior to the anterior horn, where they are called the lateral reticulospinal tract.
 Figure 24-8. Rubrospinal and reticulospinal tracts.
Figure 24-8. Rubrospinal and reticulospinal tracts.
Like the vestibulospinal fibers, reticulospinal fibers terminate in the anteromedial portion of laminae VII and VIII, where they influence motor neurons supplying paravertebral and limb extensor musculature. However, in contrast to the vestibulospinal tract, individual reticulospinal fibers commonly terminate at multiple spinal levels by means of collateral branches, and there is little evidence for monosynaptic contact with alpha motor neurons.
The reticulospinal system is activated by ipsilateral descending cortical projections (corticoreticular fibers) as well as by ascending somatosensory systems (spinoreticular fibers), mainly those conveying nociceptive signals traveling in the anterolateral system. Through its influence on gamma motor neurons, the reticulospinal system is involved in the maintenance of posture and in the modulation of muscle tone. Pontine reticulospinal fibers tend to mediate excitatory effects, and medullary reticulospinal fibers usually produce inhibitory effects.
Rubrospinal Tract
In the midbrain, neurons in the red nucleus give rise to axons that cross the midline in the anterior (ventral) tegmental decussation (Fig. 24-8). These fibers descend through the brainstem contralateral to their origin and enter the spinal cord anteriorly adjacent to the lateral corticospinal tract. The red nucleus consists of magnocellular and parvocellular subdivisions. In mammals that have been investigated and probably also in humans, the magnocellular part gives rise to most rubrospinal fibers, and the parvocellular part gives rise to rubroolivary fibers. In general, the rubrospinal fibers descend to the contralateral spinal cord and the rubroolivary fibers to the ipsilateral inferior olivary nuclei.
Each rubrospinal fiber terminates in a restricted area of the spinal cord; they do not innervate multiple cord levels by means of collaterals, as do reticulospinal fibers. In the spinal gray, rubrospinal fibers terminate in laminae V, VI, and VII. For the most part, they provide excitatory influence to motor neurons innervating proximal limb flexors (Fig. 24-8).
The magnocellular portion of the red nucleus is relatively smaller in humans than in other mammals, and the rubrospinal tract is correspondingly small. In addition, relatively few rubrospinal axons appear to extend caudal to the cervical enlargement in humans, suggesting that this system is primarily involved with the upper extremity. Clinical findings in patients are consistent with this conclusion, indicating that the rubrospinal system exerts its control mainly over the upper extremity and has little, or no, influence over the lower extremity.
The rubrospinal system is influenced by the cerebral cortex and the cerebellar nuclei via corticorubral (uncrossed projection) and cerebellorubral (crossed projection) fibers, respectively. Precentral and premotor cortices project to the ipsilateral red nucleus, and the supplementary motor area contributes contralateral input. The latter pathways provide a route through which the cortex might influence flexor motor neurons and thus serve as a supplement to the corticospinal system. Connections that link the cerebellar nuclei, inferior olive, red nucleus, and rubrospinal tract may represent circuitry important for modification of motor performance or acquiring of new motor skills.
FUNCTIONAL ROLE OF BRAINSTEM-SPINAL INTERACTIONS
Insight into the functional role of the brainstem and spinal systems has come from animal studies in which lesions have been created in specific locations above or within the brainstem. The resulting deficits mimic those of humans known to have or suspected of having damage in the same structures.
Decerebration
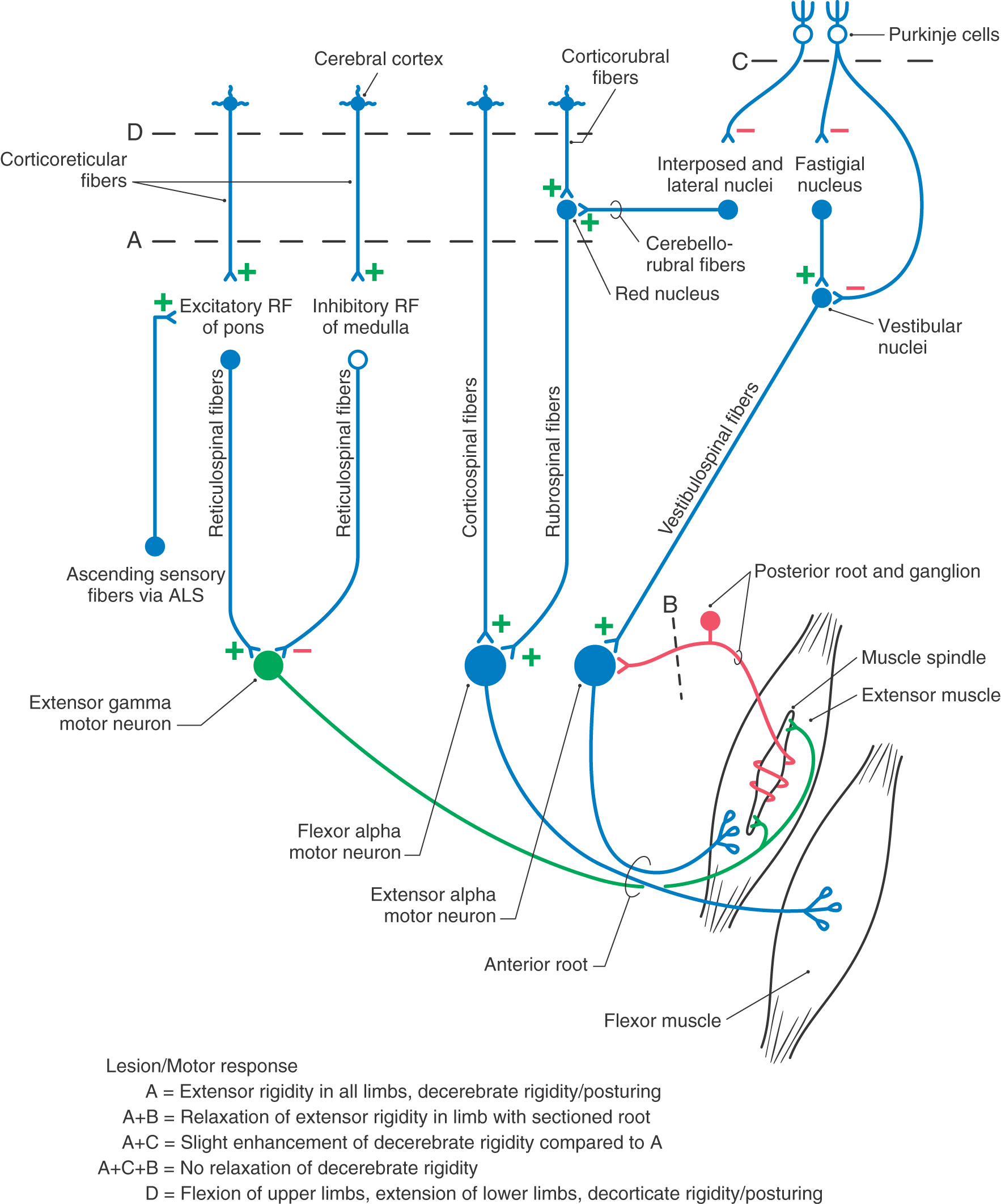
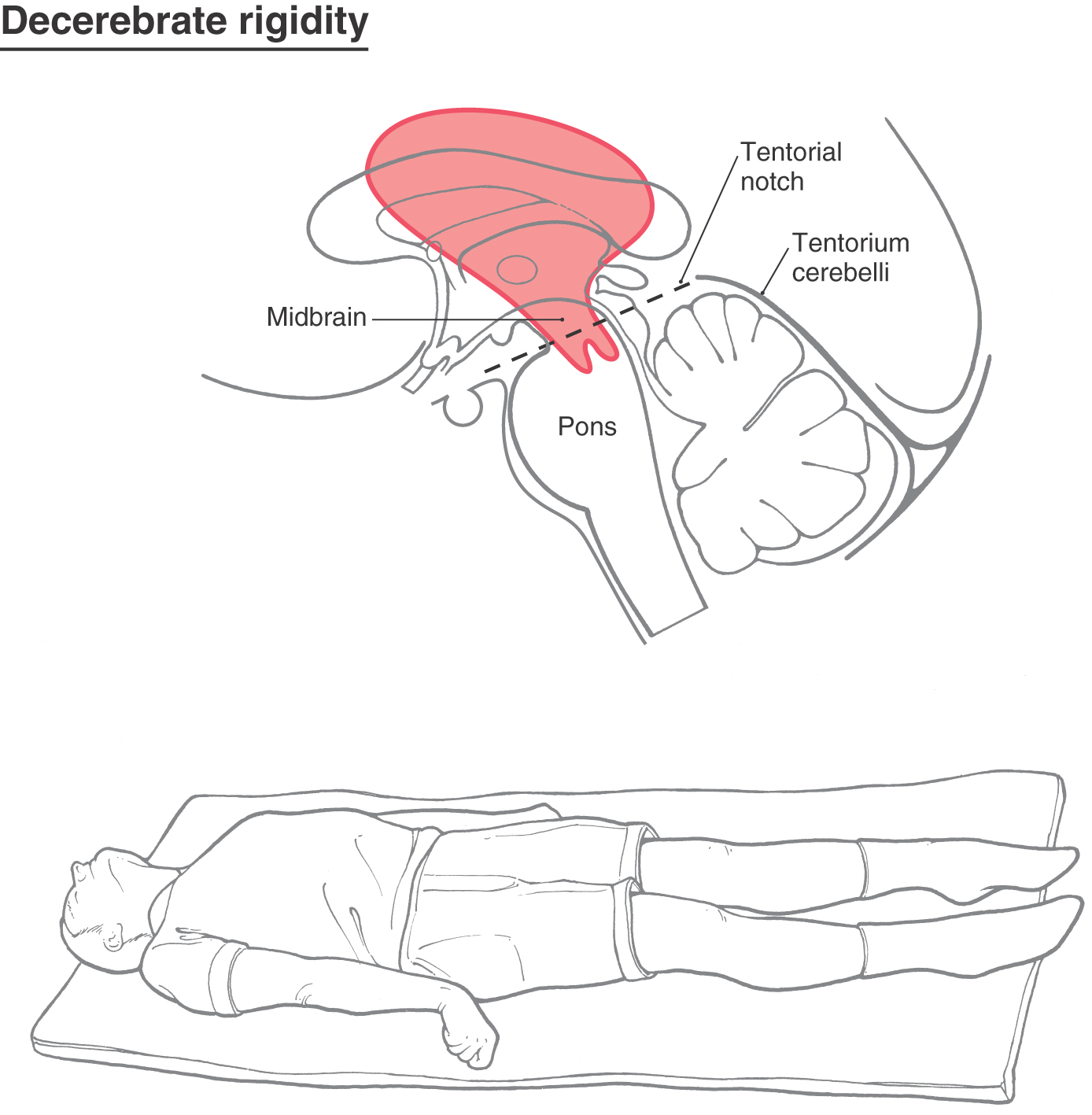
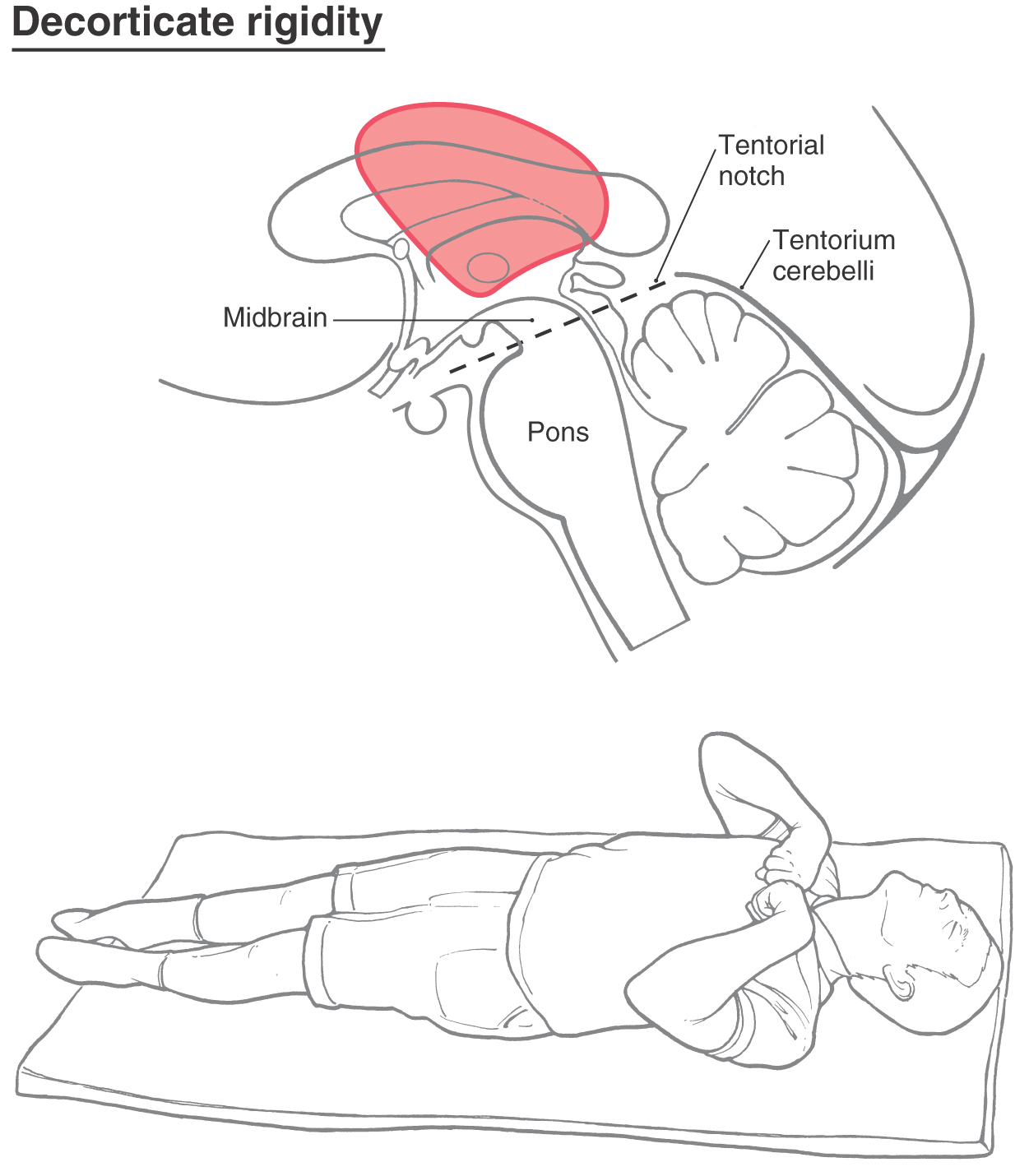
Removal of the influence of the cortex and other higher centers on brainstem-spinal systems was the premise in this experiment, with the idea that whatever functions remained were controlled predominantly by the brainstem-spinal systems. In the basic experiment, under deep anesthesia, the brainstem was completely transected bilaterally between the superior and the inferior colliculi (Figs. 24-9A and 24-10A). This procedure results in a constellation of deficits that closely resemble those seen in humans with supratentorial lesions that cause herniation of the midbrain downward through the tentorial notch; this is central herniation, also called transtentorial herniation (Fig. 24-11; see p. 335). The experimental lesion in animal models, like the comparable lesion in humans, results in unopposed hyperactivity in extensor musculature in all four extremities, a condition called decerebrate rigidity (Fig. 24-12).
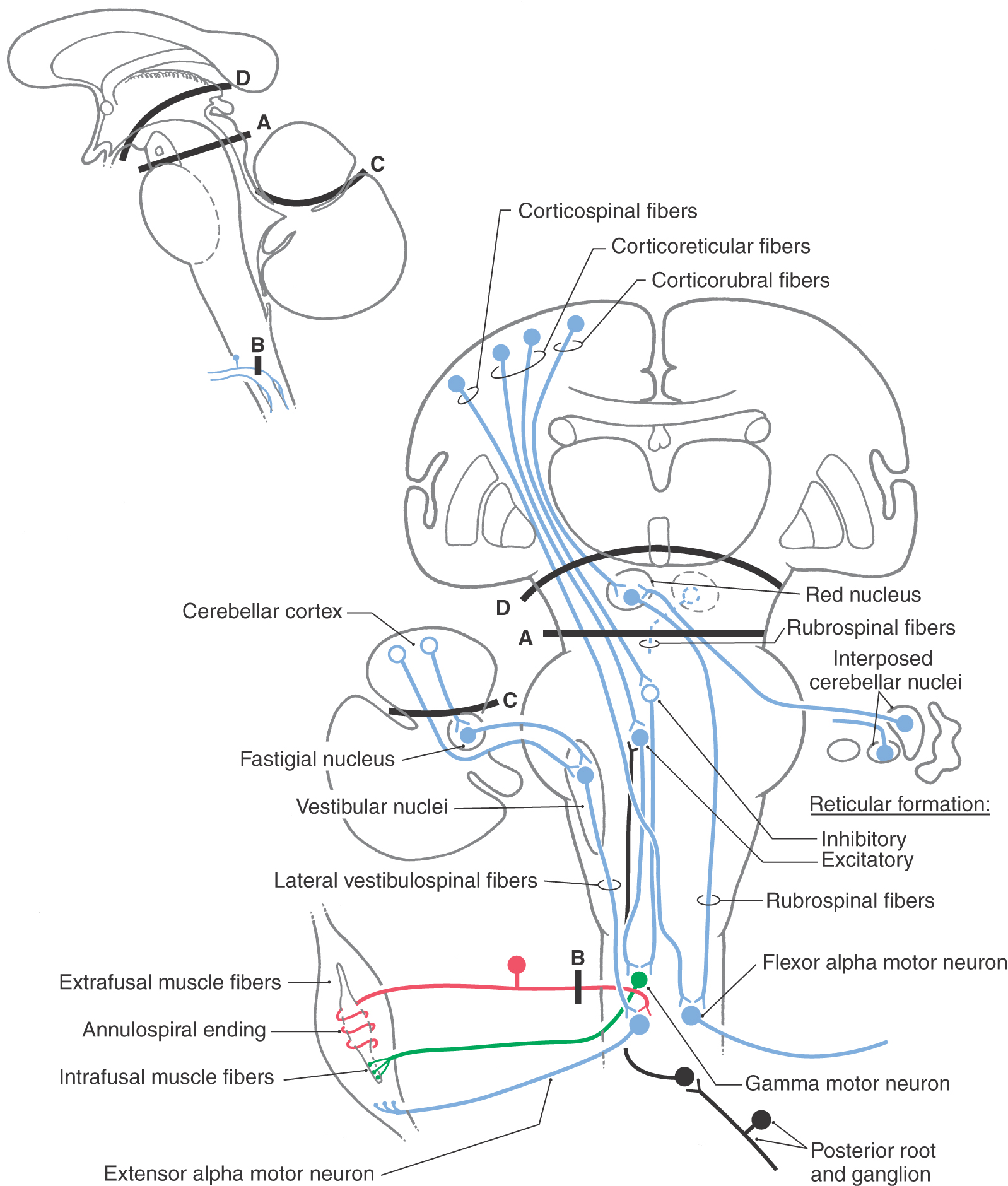
Figure 24-10. A circuit drawing representing the lesions produced in experimental animals to replicate the decerebrate and decorticate lesions or deficits seen in humans. Bilateral transection lesions are indicated by dashed lines A, B, C, and D. The decerebration lesion is at a midcollicular level (A), the decortication lesion is rostral to the superior colliculus (D), the posterior roots are sectioned for one extremity (B), and the anterior lobe of the cerebellum is removed (C). The objective was to identify the anatomic substrate responsible for the decerebrate or decorticate rigidity or posturing seen in humans with lesions that either isolate the forebrain from the brainstem or separate the rostral brainstem from the caudal brainstem and spinal cord. Compare with Figure 24-9. ALS, anterolateral system; RF, reticular formation.
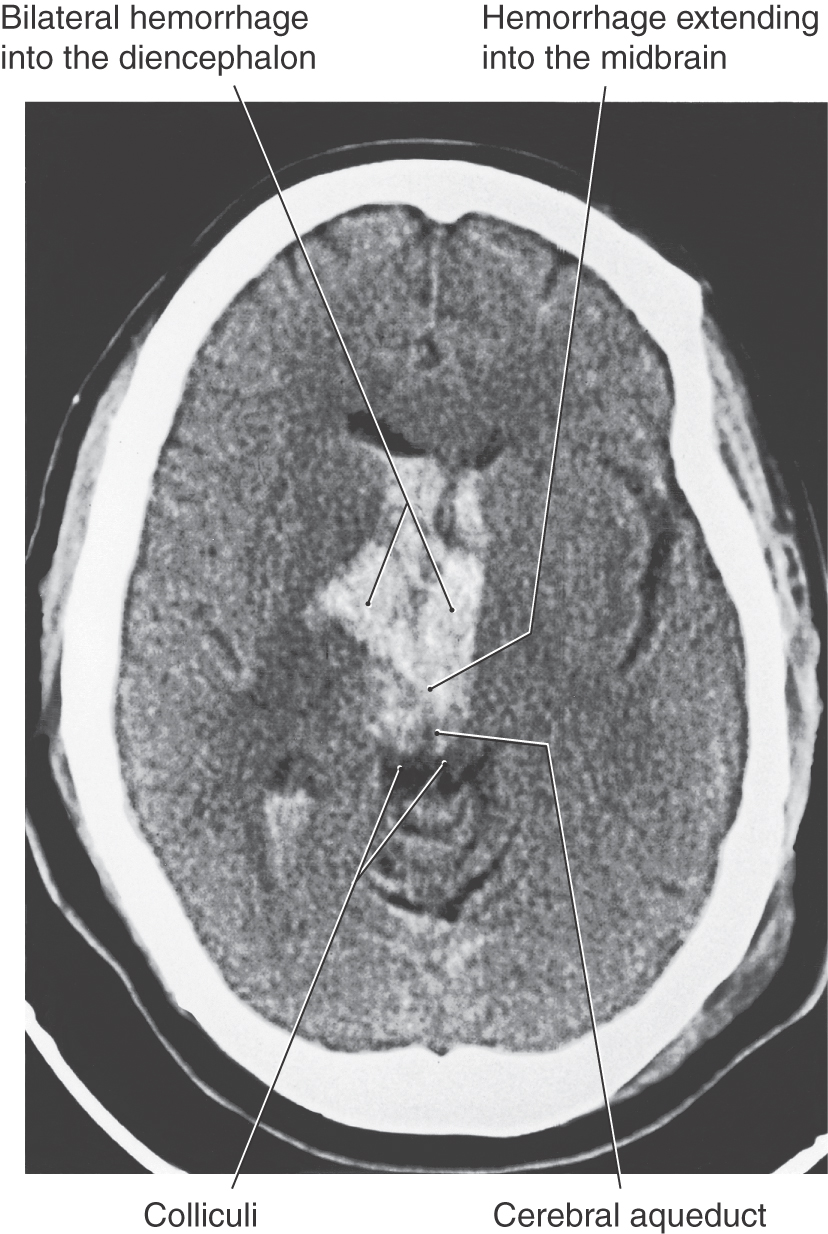
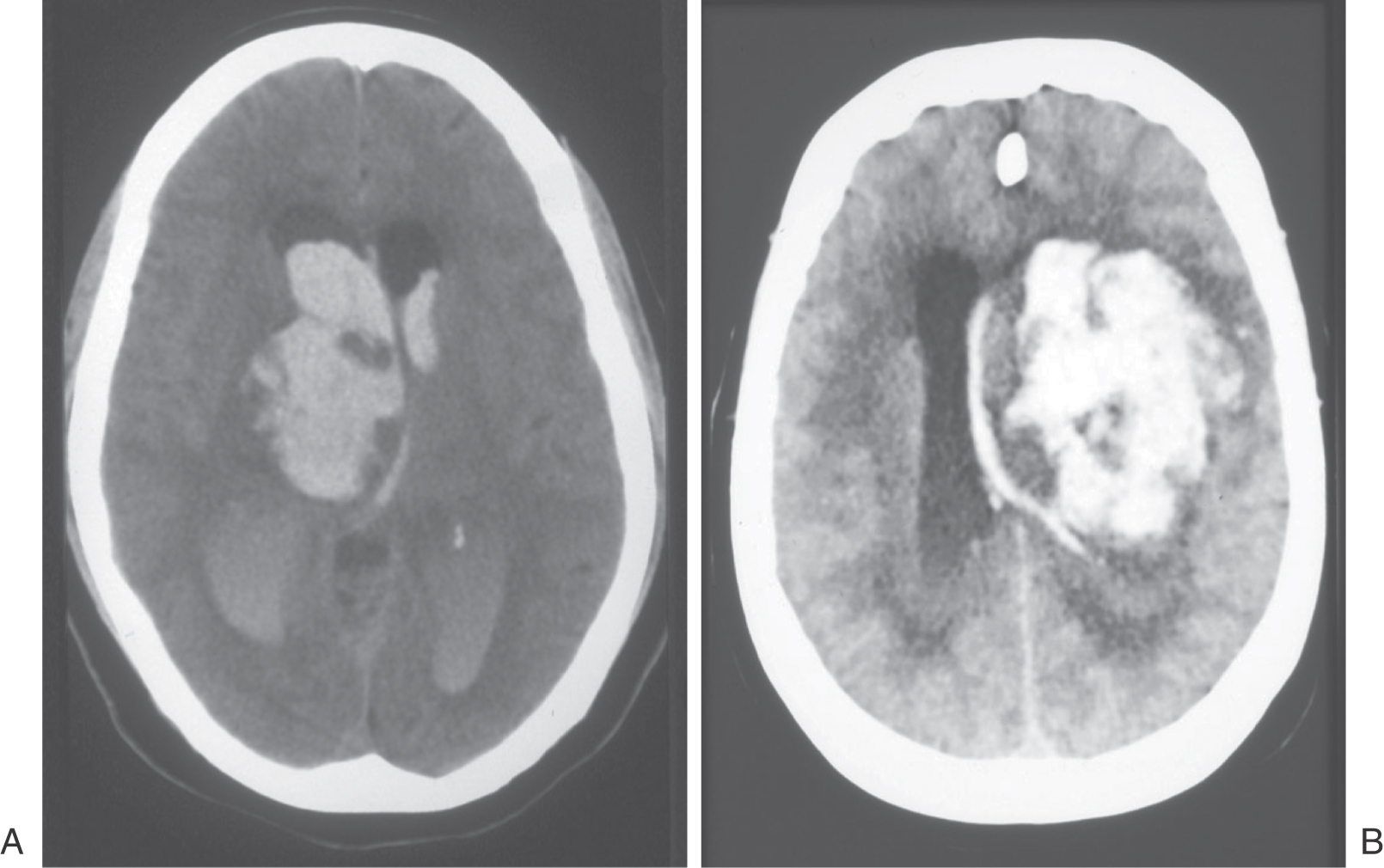
Figure 24-11. Axial computed tomography scan of a patient with a lesion in the forebrain that has herniated through the tentorial notch, into the midbrain, and resulted in decerebrate rigidity (compare with Figure 24-12).
Figure 24-12. Decerebrate rigidity. A supratentorial lesion has extended through the tentorial notch. The patient’s lower extremities are extended, with the toes pointed inward; the upper extremity is extended, with the fingers flexed and the forearms pronated; and the neck and head are extended. The rigidity may be so extreme that the patient’s back is arched up off the bed. A patient may become decerebrate after a period of decorticate posturing (see Fig. 24-11).
In this situation, all descending cortical systems are interrupted. This includes the corticospinal tract as well as the corticorubral and corticoreticular projections. In addition, the red nucleus and rubrospinal tract are damaged, but the excitatory and inhibitory components of the reticular formation lie caudal to the level of the lesion and thus the reticulospinal projections remain intact. More important, the ascending somatosensory input (anterolateral system) that is preferentially directed to the excitatory elements of the reticular formation remains intact, and thus the corresponding excitatory component of the reticulospinal system remains functional. Consequently, a noxious stimulus delivered to a decerebrate patient may exacerbate decerebrate rigidity that is already present or evoke decerebrate posturing when it is otherwise not apparent.
Central (or transtentorial) herniation in humans may be seen in patients with large tumors in the hemisphere or after a large hemorrhage in the hemisphere (Figs. 24-11 and 24-12). In the diencephalic stage (before herniation through the tentorial notch), the patient may have a decreased level of consciousness, lethargy, small but poorly reactive pupils, and eye movement disorders. In addition, the withdrawal reflex to noxious stimuli is intact, reflexes are hyperactive, and there is a bilateral Babinski response. The extremities may be weak, and the patient may become decorticate (see Fig. 24-15), first on the ipsilateral side, then contralaterally. Once the herniation occurs, there is a rapid decline. The patient is decerebrate (Fig. 24-12) and comatose, pupils are dilated and fixed (do not react to light), and eye movement is absent. As the damage extends downward through the midbrain, respiration is compromised (Cheyne-Stokes, tachypnea, followed by shallow rapid rates), and survival is highly unlikely.
Posterior (Dorsal) Root Section
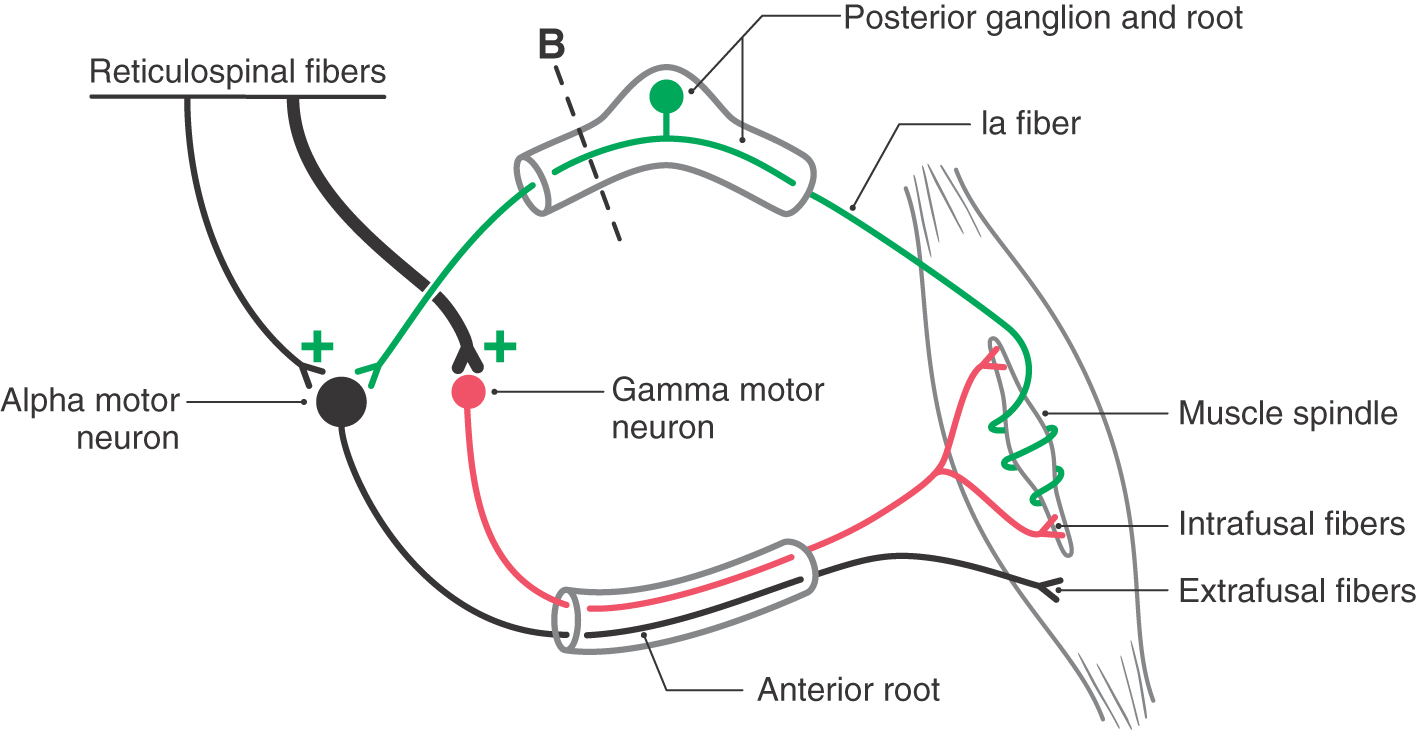
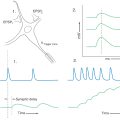
An important question that arose in relation to the decerebration experiment was whether the extensor hypertonus was due to excessive activation of alpha or of gamma extensor motor neurons. To answer this question, the posterior root input from one extremity was interrupted in a decerebrate animal (Figs. 24-9B, 24-10B, and 24-13). Immediately, the extensor hypertonus in that limb collapsed. What does this result indicate? Remember that supraspinal input can produce muscle contraction by two routes: by direct activation of alpha motor neurons and indirectly via the gamma loop. In the latter, the supraspinal input activates gamma motor neurons, leading to contraction of intrafusal fibers, and the resulting increase in Ia sensory input activates alpha motor neurons, which activates extrafusal fibers and leads to muscle contraction.
In the decerebrate condition, flexor muscles are inactive because of the loss of descending corticospinal and corticorubrospinal input to flexor motor neurons. Conversely, extensor motor neurons are unaffected by the loss of descending cortical fibers because they are activated by descending reticulospinal and vestibulospinal inputs that are not involved by the decerebration lesion; consequently, these tracts remain intact. Whereas the vestibulospinal system receives no significant descending input from the cortex, the reticular formation is clearly influenced by descending cortical fibers, and this influence would be eliminated by the decerebrate lesion. However, the ascending somatosensory input to the reticular formation remains intact, and this input primarily reaches the excitatory components of the reticular formation (Figs. 24-9 and 24-10). Because the extensor hypertonus collapses when the posterior roots are sectioned, it can be suggested that the descending reticulospinal influence on extensor motor neurons is focused primarily on gamma rather than on alpha extensor motor neurons (Fig. 24-13). The posterior root lesion interrupts the gamma loop and eliminates the circuit that would be used by the gamma motor neuron to produce indirect activation of extensor alpha motor neurons (via activation of Ia sensory fibers) and subsequently the stimulation of extensor extrafusal muscle fibers (Fig. 24-13). Therefore, decerebrate rigidity has come to be known also as gamma rigidity. To support the conclusion that the basic decerebrate paradigm involves a disruption in the balance of excitatory and inhibitory control of extensor gamma motor neurons, other studies were initiated to determine if the influence of the vestibulospinal system might be focused on extensor alpha motor neurons.
Cerebellar Anterior Lobe Section
Assuming that extensor gamma motor neurons receive preferential input from the reticulospinal system, it is reasonable to ask if extensor alpha motor neurons are preferentially driven by vestibulospinal fibers. To investigate this point, the cerebellar anterior lobe was removed in a decerebrate animal (by midcollicular transection) (Figs. 24-9C and 24-10C). Under these conditions, the extensor hypertonus actually proved to be enhanced compared with that seen with decerebration alone, and the condition was called decerebellate rigidity. Subsequently, section of the posterior roots from one extremity in such an animal produced only a slight decline in extensor rigidity of the limb. Removal of the cerebellar anterior lobe cortex has two effects (Figs. 24-9C and 24-10C). First, it eliminates direct Purkinje cell inhibition of the vestibular nuclei, resulting in enhanced output from the vestibular nuclei over the vestibulospinal tract. Second, Purkinje cell inhibition of fastigial neurons is eliminated. This increases the fastigial excitatory output to the vestibular nuclei and further augments the activity in the vestibulospinal tract. Therefore, the overall effect of cerebellar cortex removal is to substantially increase activity in the vestibulospinal system. When cerebellar anterior lobe removal was followed by posterior root section in one limb, the extensor hypertonus persisted in that limb. This suggests that the hypertonus in that limb is not due to enhanced excitatory input to gamma motor neurons from the gamma loop, but instead there is enhanced direct input to extensor alpha motor neurons resulting from increased excitatory activity in the vestibulospinal system. Consequently, decerebellate extensor rigidity is referred to as alpha rigidity.
Decortication
An extension of these observations explains the neural substrate for the phenomenon called decorticate posturing or decorticate rigidity observed in humans (Figs. 24-14 and 24-15). The clinical events that lead to this type of posturing are frequently large lesions of the hemisphere. These may be vascular or rapidly growing tumors that are located in one hemisphere, damage major tracts on that side, efface the midline, and compromise tracts on the opposite side by mass effect and compression (Fig. 24-14). The net result is a removal of cortical influence on brainstem motor nuclei.
In the clinic, the patient presents with flexion of the upper extremities (intact rubrospinal fibers) at the elbow combined with extensor hypertonus in the lower extremities (intact reticulospinal fibers), an altered state of consciousness and respiration, oculomotor deficits, and a range of motor responses from weakness to motionless.
In experimental animals, this posture can be mimicked by transection of the brainstem at a level just rostral to the superior colliculus (Figs. 24-9D and 24-10D). This lesion leaves the rubrospinal tract intact while eliminating the cortical input to the red nucleus. The rubrospinal system can still be activated because excitatory projections to the red nucleus from the cerebellar nuclei are unaffected by the lesion. The rubrospinal tract influences primarily flexor muscles, and most of this activity, in humans, is limited to the upper extremity. The upper extremities do not exhibit extensor hypertonus but instead show an increase in flexor tone due to the intact rubrospinal system. In contrast, the lower extremities exhibit extensor hypertonus for the same reasons as in decerebration. This characteristic type of posturing is called decorticate rigidity (Fig. 24-15).
These two conditions, decerebration and decortication, are frequently seen (Figs. 24-12 and 24-15), and knowledge of these symptoms and the underlying brain pathologic process is important in the diagnosis and clinical management of these patients. In some cases, the patient may be comatose and initially exhibit decorticate posturing that subsequently converts to decerebrate posturing. This is an ominous sign as it suggests that the lesion has continued to progress and now involves more caudal portions of the brainstem. The patient’s cardiovascular and respiratory control centers in the medulla may soon be compromised, necessitating prompt intervention.
Sources and Additional Reading
Boyd IA. The isolated mammalian muscle spindle. Trends Neurosci. 1980;3:258–265.
Shepherd GM. Neurobiology. New York: Oxford University Press; 1994.
Taylor A, Prochazka A, eds. Muscle Receptors and Movement. London: Macmillan; 1981.


 Figure 24-1.
Figure 24-1.