CHAPTER 12 MOTOR SPEECH AND SWALLOWING DISORDERS
Motor speech relies on a complex interaction of the resonatory, respiratory, articulatory, and laryngeal neuromuscular systems.1 Coordination of the neuromuscular components of the latter three systems is also essential for the execution of swallowing. Different neurological disorders affecting motor speech production may give it particular features that aid in anatomically localizing the disorders; there is, frequently, also an associated abnormality of the swallow mechanism.2
ANATOMY
Corticobulbar Tract
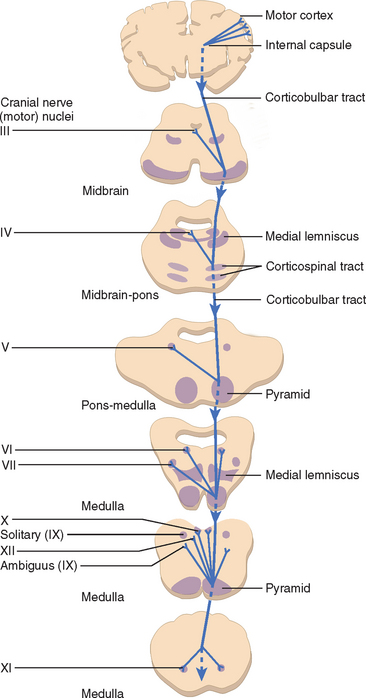
Upper motor neuron (UMN) pathways responsible for motor speech and swallowing originate in the motor cortex in each cerebral hemisphere and descend through the genu and posterior limb of the internal capsule, via the cerebral peduncle, to the pons and medulla (and upper cervical cord for the spinal nucleus of cranial nerve XI). At these levels, they synapse with the various lower motor nuclei responsible for supplying the bulbar muscles: cranial nerves V, VII, IX, and X; the cranial portion of cranial nerve XI (which contributes to the motor component of the vagus nerve [cranial nerve X]); and cranial nerve XII. The UMN pathways are known as the corticobulbar tracts, and are generally bilateral (contralateral and ipsilateral). There are, however, important exceptions, such as cranial nerve XII and the lower facial muscles, which receive their upper motor connection predominantly from contralateral corticobulbar fibers (Fig. 12-1). Emotional involuntary movements and voluntary facial movements may at times be clinically dissociated, which suggests that a separate supranuclear pathway for control of involuntary facial movements probably also exists. These fibers do not pass through the internal capsule, and it appears that the right cerebral hemisphere is dominant for expression of facial emotion.3
Lower Cranial Nerves
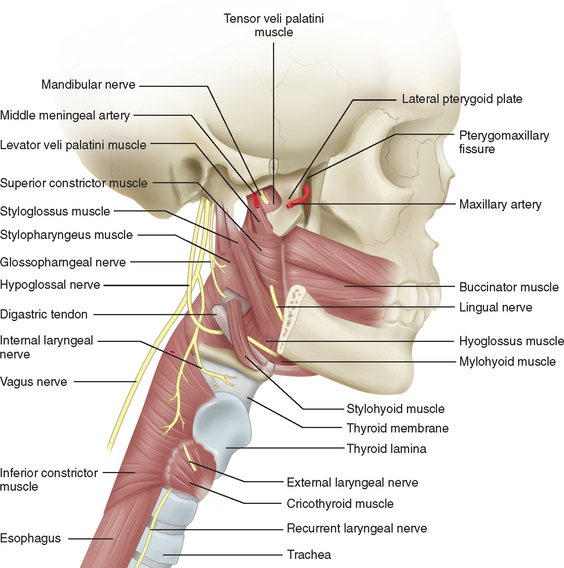
Assessment of the bulbar cranial nerves and their function is extremely important when disorders of motor speech and swallowing are considered. These cranial nerves exit the brainstem at the level of the pons or medulla and leave the cranium through the skull base, traveling either through the retropharynx or across the angle of the mandible to innervate the muscles of the face, mouth, soft palate, pharynx, and larynx (Fig. 12-2). The trigeminal nerve (V) innervates the muscles of mastication and the tensor veli palatini and communicates sensation from the face, mouth, teeth, mucosal lining, and anterior two thirds of the tongue (via the lingual nerve). The facial nerve (VII) supplies the muscles of facial expression and conveys taste from the anterior two thirds of the tongue (via the chorda tympani and lingual nerve). The glossopharyngeal nerve (IX) conveys taste from the posterior one third of the tongue, as well as sensation from this portion of the tongue, the fauces, the pharynx to about the level of the epiglottis, and the eustachian tube. It also provides the motor supply to the stylopharyngeus and, in part, to the superior and middle pharyngeal constrictor muscles through a contribution to the pharyngeal plexus. The vagus nerve (X) conveys sensation from the tympanic membrane, pharynx, larynx, and esophagus. One of its branches, the recurrent laryngeal nerve, innervates all the intrinsic muscles of the larynx other than the cricothyroideus, whereas the superior laryngeal nerve innervates the cricothyroideus and conveys sensation from the larynx and the base of the tongue. The vagus also contributes to the innervation of the pharyngeal constrictors through the pharyngeal plexus. The hypoglossal nerve (XII) innervates the muscles of the tongue, with the exception of the palatoglossus, which is supplied by the vagus nerve.4
Bulbar and Pseudobulbar Palsies
UMN lesions affecting the corticobulbar tracts can be distinguished from disorders of the lower cranial nerves or their nuclei by the distinctive changes to speech that are associated with damage to these tracts, described in the next sections. The features of such corticobulbar tract lesions are collectively known as pseudobulbar palsy, a term used to distinguish them from the true bulbar palsy, which results from pathology affecting the lower cranial nerves or their nuclei. There may, in addition to speech, be other distinguishing features on examination, typical of all UMN disorders, such as increased muscle tone (as evidenced by slow side-to-side movement of the tongue) and exaggerated reflexes (gag or jaw reflex), without signs of muscle wasting, atrophy, or fasciculations. A patient with bulbar palsy, in contrast, has the hallmarks of a lower motor neuron (LMN) disorder: namely, muscle weakness, wasting, and fasciculations.
DISORDERS OF MOTOR SPEECH
Verbal communication involves a sequence of processes culminating in the motor execution of a cortically determined set of instructions to produce speech. Disorders of this complex pathway have been classified as aphasia, apraxia of speech (AOS), and dysarthria, each of which may then be subclassified further, depending on the nature of the dysfunction and its cause. Dysarthria and AOS are termed disorders of motor speech because they exist at the output level of the motor system and disrupt only sound output, sparing semantics and syntax. The patient has a full knowledge of words they are finding difficult to articulate. This serves to distinguish these disorders from aphasia, which is defined as “a disorder of linguistic processing characterized by a disturbance in the comprehension and formulation of language caused by dysfunction in specific brain regions.”5,6 Aphasia is discussed in detail in Chapter 3 and is not considered further here.
Apraxia of Speech
Abnormalities of speech after neurological insult were subdivided into aphasias and dysarthrias before the contribution of Darley, who with colleagues delivered an unpublished paper on the topic in 1969.7 In this lecture, Darley was the first to use the term apraxia of speech and to attribute a specific disorder of speech—interposed between aphasia and dysarthria—to impaired motor programming,8,9 The term apraxia had long been used in other contexts to describe the inability to carry out a motor command despite normal comprehension and the normal ability to carry out the motor act in another context, such as by imitation or with use of a real object.10
This three-level model of sound-level speech production disorders survived without challenge until the late 1990s. In 1997, van der Merwe10 proposed a four-stage model in which there was an explicit division between “speech motor planning” and “speech motor programming.” Previously, these terms had been used interchangeably. In this model, speech motor planning involves two stages (linguistic-symbolic planning and motor planning) and refers to the planning of the temporal and spatial goals of the articulators. This is followed by a third stage, speech motor programming, which refers to the selection and sequencing of motor programs for the movements of the individual muscles of these articulators (including the vocal cords). The final stage is the execution stage, which refers to the actual realization of speech on an articulatory level. This model makes a clear assignment of AOS to the motor level of impairment as a disorder of speech motor programming.6 Aphasias are disorders of the stages of linguistic-symbolic and motor planning in this system, and dysarthrias are disorders of the execution stage.
AOS is a syndrome in which a sequence of single sounds (phonemes), especially consonant sounds, are disrupted and inconsistently misarticulated, in contrast to the consistently abnormal articulation of dysarthria. A further identifying feature of AOS is that comprehension and automatic or reactive speech are normal, but volitional or purposive speech contains substitutions, additions, prolongations, and reversal of phonemes.9 The sufferer repeats incorrect initial phonemes, words, or phrases, which results in a labored, perseverative speech pattern. This may superficially resemble stuttering, but the effortful blocking on a correct initial phoneme typical of stuttering is not seen. AOS, according to this definition, is commonly encountered during attempted speech production in the aphasias, and the sites of lesions that produce a nonfluent aphasia and AOS may overlap.6 One literature review suggests that cortical-subcortical lesions in the lower part of the left precentral gyrus in most right-handed persons, and a lesion of the corresponding region in the right hemisphere in some left-handed individuals, are the most likely to produce AOS.11 A lesion in Broca’s area may cause a combined syndrome of AOS, orobuccal dyspraxia, and nonfluent aphasia. This symptom complex is frequently referred to as Broca’s aphasia.
Dysarthria
Dysarthria is defined as a group of speech disorders resulting from disturbance in the control of speech mechanisms that, in turn, results from damage to the central or peripheral nervous systems, including muscles and neuromuscular junctions.12 There is consistently abnormal articulation of phonemes during both automatic and volitional speech. It is caused by the impaired functioning of one or several of the components of the motor speech subsystems (respiration, phonation, resonation, and articulation). Dysphonia is a subset of dysarthria, and the term refers specifically to a disruption of phonation, resulting in an abnormal voice sound without disturbance of articulation. The definition encompasses all disorders of voice sound, both organic and psychogenic.
Speech is produced by co-coordinated contraction of the muscles of the larynx, pharynx, and tongue, linked to the expiration phase of respiration. At a cortical level, articulation requires the coordinated bilateral movements of the muscles concerned, which is effected by fibers passing from the inferior region of the left lateral frontal lobe to the corresponding region of the right hemisphere via the corpus callosum.13
The motor speech system relies on the normal function of the various elements of the nervous system involved in the control of motor speech: namely, UMNs and LMNs; the coordinating and regulating influence of extrapyramidal, cerebellar, and sensory pathways; and the final output through neuromuscular junctions and muscles.14 Disorders affecting each part of this extensive control, effector, and feedback network have distinct effects on speech, which can be identified through the clinical examination. The nature of the change in speech therefore has localizing significance, which can be used to classify the motor speech disorder as AOS or a particular type of dysarthria.
Upper Motor Neuron Lesions
In view of the bilateral nature of the majority of the UMN input to the cranial nerves responsible for speech, unilateral UMN lesions produce a relatively mild dysarthria that reflects primarily weakness and some loss of skilled movement. Bilateral UMN lesions have a much more severe effect that reflects both bilateral weakness and loss of skilled movement, as well as an increase in muscle tone (spasticity).15 The dysarthria accompanying such pathology is known as spastic dysarthria and is one of the features of pseudobulbar palsy. The speech changes characteristic of this condition include slow rate of speech, imprecise consonants, distorted vowels, hypernasality, monotone pitch, short phrases, and a strained-strangled quality to the voice. A number of neurological conditions can affect these pathways and cause a spastic dysarthria (Table 12-1).
TABLE 12-1 Examples of Neurological Conditions Causing Dysarthria or Neurogenic Dysphagia
* These are uncommon causes of dysarthria; they more commonly cause dysphagia. dHMN-VII, distal hereditary motor neuropathy type VII; FALS, familial amyotrophic lateral sclerosis; HMSN-IIC, hereditary motor sensory neuropathy type IIC.
Lower Motor Neuron Lesions
Which exact speech disorder accompanies a LMN lesion depends on the nerve or nerves involved. A brainstem stroke, for example, may affect several cranial nerves, whereas a mediastinal mass may affect only the left recurrent laryngeal nerve. Patients are usually able to compensate if damage is unilateral, whereas a bilateral lesion usually results in a severe impairment. The principal causes of flaccid dysarthria are listed in Table 12-1.
Cerebellar Disorders
Disorders involving cerebellar pathways cause loss of the normal coordination and timing of speech output. This can lead to random breaks between words and syllables, vowel distortions, prolongations of sounds, and the use of equal stress on each syllable, thereby creating a “rambling” or “scanning” quality to the speech (ataxic dysarthria) (see Table 12-1). The dysarthria accompanying focal cerebellar lesions tends to occur particularly with lesions of the vermal and (usually) left dorsal intermediate zone of the cerebellum, whereas it is typically not seen with lateral neocerebellar lesions.13
Extrapyramidal Disorders
Extrapyramidal disorders, such as Parkinson’s disease or Huntington’s disease, result in hyperkinetic or hypokinetic dysarthria. The most common dysarthria accompanying Parkinson’s disease is a hypokinetic dysarthria, characterized by rapid speech rate (festination), slurring of words and syllables, and trailing off at the end of sentences. The voice is soft and monotonous, without the usual inflections. The speech disorder accompanying Huntington’s disease is hyperkinetic dysarthria, which results in an uncontrolled loud, harsh voice, poorly coordinated with breathing. Chorea and myoclonus may cause abrupt interruption between or within words by the superimposition of abnormal respiratory, phonatory, or articulatory movements.15
Dysphonia
Dysphonia may also be produced by conditions that cause weakness of the respiratory muscles—such as Guillain-Barré syndrome or a high cervical spinal cord lesion—because insufficient airflow is produced for phonation.15 Abnormalities of phonation become more apparent the longer the subject speaks, and asking the patient to read aloud from a magazine is a good means of bringing out these features.
Spasmodic Dysphonia
Spasmodic dysphonia appears to result from a dystonia restricted to the phonatory apparatus, but it can occasionally co-occur with other dystonias, such as writer’s cramp and blepharospasm. More sufferers are women, and speaking gradually becomes more of an effort. Usually, attempts to speak result in co-contraction (adduction) of the vocal folds, causing a strained, strangled-sounding voice. In rare cases, the problem may be a breathy, soft voice; in these patients, the dystonia causes abduction of the vocal cords. In either instance, other activities involving use of the same muscles, such as swallowing and singing, are usually unimpeded.15
Nonorganic Disorders of Voice
Voice disorders may have a psychological basis, rather than being the result of pathology affecting neural pathways or muscular control. The most common psychogenic speech disorders affect voice, fluency, or prosody. Prosody is the term used to describe all the variations in time, pitch, and loudness that accomplish emphasis, lend interest to speech, and characterize individual and dialectical modes of expression.16 Psychogenic speech disorders are not unusual and can account for up to 5% of acquired communication disorders.15 The most common is aphonia (hoarseness), but psychogenic spasmodic dysphonia, particularly adductor spasm, is also encountered.
Psychogenic Spasmodic Dysphonia
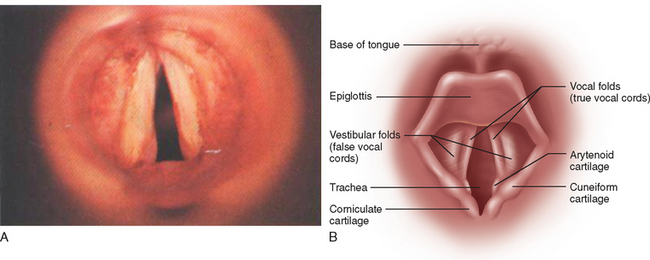
Establishing the diagnosis of this condition is very difficult in some cases, and distinction from an organic disorder such as a focal dystonia (see previous discussion) can be challenging. Symptom reversibility in some patients may be the only way to confirm the diagnosis. The adductor form is the most common and is characterized by a continuous or intermittent strained, jerky, grunting, squeezed, groaning, and effortful quality to the voice.15 The nature of the voice disorder is very similar to that in neurogenic spasmodic (adductor) dysphonia, although underlying voice tremor or evidence of laryngeal dystonia (e.g., adduction of the vocal cords during inspiration seen during laryngoscopy) (Fig. 12-3) is not ordinarily encountered in psychogenic etiologies unless a combination of causes is present.
CLINICAL ASSESSMENT OF SPEECH
Clinical examination of speech requires the assessment of three aspects of speech production:15
An ataxic dysarthria is usually accompanied by other signs of cerebellar dysfunction (see Chapter 7), particularly a wide-based gait and, often, incoordination of the limbs and abnormalities in eye movement. Patients with Parkinson’s disease and a hypokinetic dysarthria ordinarily have other features of the disease, such as masklike, expressionless facies, bradykinesia, rigidity, and tremor, whereas a patient with a hyperkinetic dysarthria may exhibit other features of a movement disorder, such as choreiform movements or myoclonic jerks.
MANAGEMENT OF MOTOR SPEECH DISORDERS
Treatment options for the dysarthric patient depend on the etiology of the speech defect. Recovery of function may be a realistic goal for a patient with a mild dysarthria as a result of a stroke, but is not a possibility for a patient with a progressive neurological disease such as motor neuron disease.15 In this case, compensation of function is more appropriate. Compensation may involve speech strategies such as overarticulation, alternative communication devices, management of the environment, or prosthetic devices.
Management approaches can be separated into three broad areas: medical, prosthetic, and behavioral.15 Because of the heterogeneous nature of the dysarthric population, a single approach is very rarely adequate; often, all three approaches are used in conjunction.
Medical Management
Pharmacological
The first consideration in the management of any motor speech disorder is to ensure that any underlying neurological problem receives appropriate pharmacological treatment. This can have a dramatic positive effect on speech: For example, acetylcholinesterase antagonists for the treatment of myasthenia gravis can improve or restore speech,17 and botulinum toxin has been shown to reduce vocal fold spasm in cases of spasmodic dysphonia.18 There are inconsistent reports of the effects of dopaminergic agents on speech intelligibility in Parkinson’s disease: Some investigators have reported a trend for improvement of speech,19 whereas others have reported no difference.20,21 Some drugs—for example, benzodiazepines and anticonvulsants—may have a negative effect on speech, and this may result in a worsening of the dysarthria.17
Surgical
Surgery may be the treatment of choice for patients who have severe hypernasality as the result of velopharyngeal incompetence.22 Pharyngoplasties and construction of pharyngeal flaps can be performed by otolaryngologists to rectify such speech defects. Patients with unilateral vocal fold paralysis may be referred for medialization procedures such as thyroplasty23,24 or vocal fold augmentation techniques.25,26
Prosthetic Management
Numerous available appliances can improve the communication of dysarthric speakers. Palatal lifts may be used for hypernasal patients who have a hyporeflexive gag.27 Voice amplifiers allow patients with reduced modal loudness to increase their speech volume. Other devices, such as pacing boards and delayed auditory feedback, are used during behavioral therapy to slow speech production rate.19 Patients who are severely dysarthric or anarthric may need to use augmentative or alternative communication devices.28 These range from simple devices such as picture or alphabet boards to more advanced devices such as computers with voice synthesizers. Assessment and decision making about the appropriateness of prosthetic devices is the realm of the speech pathologist.
Behavioral Management
When providing behavioral treatment, the speech pathologist analyzes the severity of the patient’s dysarthria and identifies the cluster of deviant speech symptoms. In the case of a mild-to-moderate dysarthria, patients usually receive direct therapy aimed at recovering as much speech function as possible; the goal is to improve intelligibility while maintaining speech naturalness.15,29 Direct therapy approaches that are supported by the literature include use of various biofeedback devices aimed at improving respiratory support30 and improving respiratory/phonatory coordination and control.31,32 Effortful closure techniques to increase adduction of vocal folds in cases of hypoadduction33,34 and tension-reducing strategies to reduce hyperadduction are also supported.35 A large body of research has demonstrated the efficacy of an intensive treatment program, which focuses on increased vocal loudness, in mild to moderate cases of Parkinson’s disease.27 Various studies have shown benefits of rate-control techniques, such as pacing and delayed auditory feedback, to slow speech rate.36–39 In cases of severe dysarthria, indirect therapy may be used; this is aimed at optimizing the communication environment, and training caretakers to repair communication breakdowns.40
Behavioral management is the foundation of treatment for AOS. Unlike treatment for dysarthria, in which the aim is to improve physiological support for speech that is appropriately planned and programmed, the treatment of AOS focuses on reorganizing the disturbed programs for speech movements, which are then able to be implemented by an intact neuromuscular speech system. Most treatment goals for AOS are aimed at improving articulation and prosody through the use of imitation and cuing in a progressive hierarchy of intensive drills.15
NEUROGENIC DYSPHAGIA
Neuromuscular Control of Swallowing
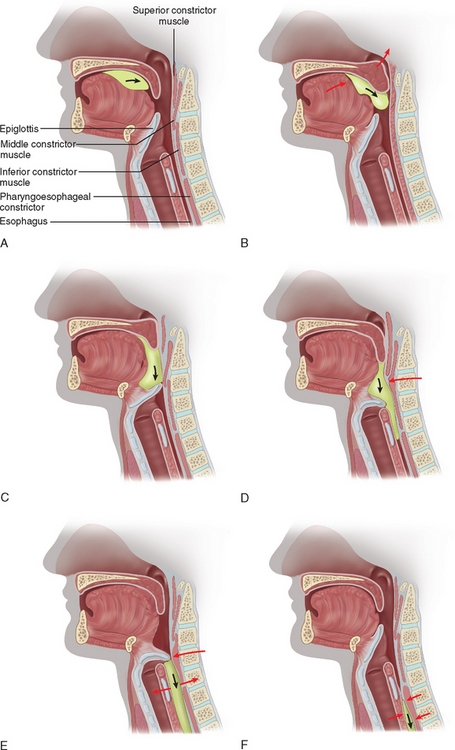
Normal swallowing is a complex sensorimotor behavior involving the coordinated contraction and inhibition of the muscles around the mouth and the tongue, larynx, pharynx, and esophagus bilaterally (Fig. 12-4).41 The act of swallowing has been subdivided into three phases: oral, pharyngeal, and esophageal, after Magendie’s classic description (Fig. 12-5).42 The initial (oral) phase is voluntary, whereas the latter two phases are semiautonomous reflex responses. The motor events of swallowing, however, are best described as occurring in two stages: the first (“oropharyngeal”) stage, which incorporates the first two phases of swallowing, and the subsequent (“esophageal”) stage or phase.43,44
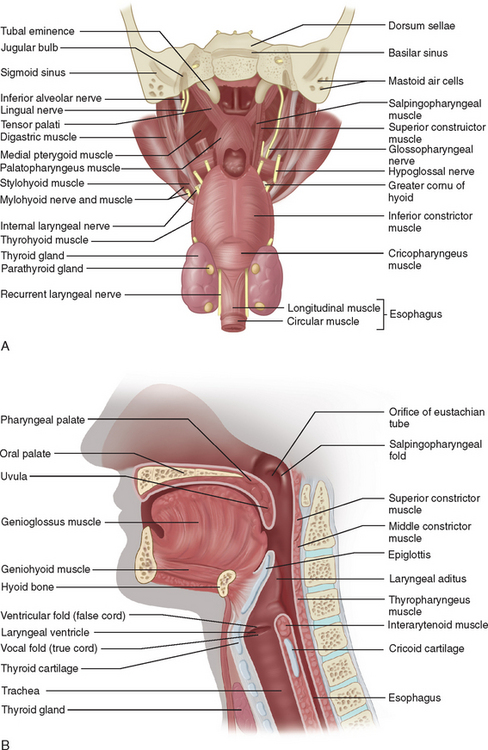
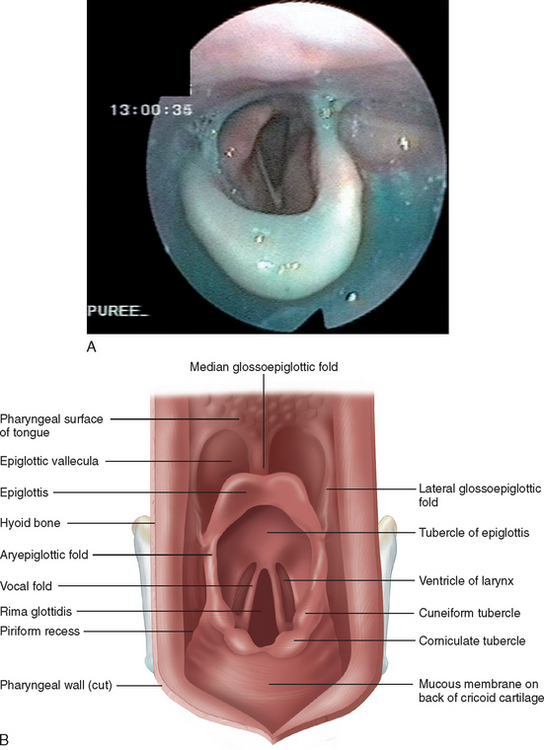
Figure 12-4 The nasopharynx opened to view the soft palate from behind (A) and in midline section (B).
(A from Last RJ: Anatomy: Regional and Applied, 7th ed. Edinburgh: Churchill Livingstone, 1984. B from Groher ME: Dysphagia: Diagnosis and Management, 2nd ed. Boston: Butterworth-Heinemann, 1992.)
Oral Phase of Swallowing
The act of swallowing commences before food or fluid is actually placed in the mouth. The anticipation, smell, or presence of food stimulates saliva production in the mouth, which is necessary to commence the digestive processes and lubricate the bolus of food for the swallow. Once the bolus enters the mouth, the duration of the oral phase is highly variable, depending on the taste, texture, and consistency of the food and on the hunger, motivation, and consciousness of the subject.43 The motor events accompanying this phase involve lip closure, tension within the buccinator muscles, downward movement of the soft palate (allowing breathing during mastication), and actual chewing of the food. The final step generally takes place with the positioning of the adequately chewed and mixed bolus of manageable size, usually on the mid-dorsum of the tongue. The tongue subsequently sweeps the bolus posteriorly, forming a rolling wave of contact stripping against the hard palate, pushing the bolus toward the posterior tongue surface and into the oropharynx.1 The oral phase may be disrupted by disorders of the motor nerves responsible for muscle function in this area (cranial nerves V, VII, IX, X, and XII) or by disorders of their central control, impairing coordinated mastication and posterior bolus movement (e.g., Huntington’s disease.)
Pharyngeal Phase of Swallowing
The oral and pharyngeal phases are highly interrelated, and the distinction between them is often unclear. All of the events, from the initiation of the swallowing reflex until the esophageal phase, are probably under the control of a central pattern generator in the brainstem.44,45
As the bolus enters the pharynx, it triggers the swallow reflex. The nature of the triggering is not known.43 It is assumed that the afferent pathway is conveyed through the trigeminal, glossopharyngeal, and vagus nerves from sensory fibers innervating the pharynx. These fibers converge in the brainstem in the tractus solitarius and synapse in the nucleus tractus solitarius. Cortical descending inputs reach similar areas of the nucleus tractus solitarius. There is also sensory input to cortical regions involved in initiating swallowing.46,47 The initiation or triggering of swallowing is probably more complex than a simple brainstem reflex and may depend on structures above the brainstem. The swallow reflex consists of several movements (see Fig. 12-5):48
Esophageal Phase of Swallowing
The esophageal phase starts from the time the bolus enters the esophagus at the upper esophageal sphincter until it passes through the lower esophageal sphincter and enters the stomach. This transit time ranges between 8 and 20 seconds. A peristaltic wave begins at the top of the esophagus and pushes the bolus ahead of it as it travels toward the stomach. The upper esophageal sphincter is formed by the cricopharyngeus muscle and the cricoid cartilage and, as such, can be affected by disorders of voluntary skeletal muscle, whereas the lower esophageal sphincter is formed by smooth muscle. Opening of the upper esophageal sphincter is partially under voluntary control, and it is actively closed in the resting state. It is opened by a complex series of actions, including laryngeal elevation.39 There is no voluntary control of the lower esophageal sphincter.
Assessment of Patients with Dysphagia
Clinicians should be alert to the clinical signs of swallowing disorders (dysphagia), which may suggest that oral feeding is not safe. Eating is a demanding cognitive process requiring planning and judgment, intact bulbar musculature, and neural control systems.49 Patients presenting with confusion after any cause of neurological impairment may not be mentally able to eat safely. All patients presenting with dysarthria should also be suspected of having dysphagia, because of shared neuroanatomical pathways. However, dysphagia may also occur independently of dysarthria. Dysphagia is potentially life-threatening and must be evaluated promptly. During history taking, patients may complain of difficulties during the act of swallowing, such as food sticking in the back of the throat, regurgitation, or dribbling. Others may not report dysphagia at all and present with complications such as recurrent chest infections caused by “silent” aspiration. Other manifestations that may warrant a dysphagia examination include wet-sounding voice, coughing on saliva or food, pain on swallowing (odynophagia), slowed eating rate, unexplained weight loss, and difficulty chewing. Neurogenic dysphagia may result from UMN or LMN disorders, including those affecting neuromuscular junctions or muscle (see Table 12-1).
Oral Peripheral Examination
Impaired dentition and any dryness or inflammation of the oral mucosa should be particularly noted. The cranial nerve examination should include the assessment of taste. Sweet, sour, salty, bitter, and, possibly, umami (the taste of the glutamate moiety in monosodium l-glutamate, a compound that occurs naturally in protein-rich and other foods50) constitute the basic taste qualities; all others are flavors, their appreciation depending on an intact sense of smell. Taste is tested on the anterior two thirds of the tongue (enervated by the facial nerve) with sugar, salt, vinegar, quinine, and, if required, monosodium l- glutamate, in that order. The patient sticks out the tongue to one side, keeps it out through the test, and does not talk. The four or five possible tastes are written on a card. The tip of the tongue is held gently with a piece of gauze, and the side of the tongue is moistened about one inch from the tip with a little of the test substance. The patient indicates the taste by pointing to the appropriate line on the card. Between tests, the patient rinses out the mouth with water. Alternatively, an electrical device (Rion Electrogustometer, Sensonics, Inc.) can be used, but this is expensive and a little cumbersome for easy bedside use. Testing taste on the posterior one third of the tongue (glossopharyngeal nerve) is so difficult by conventional means that it is hardly worth attempting. An electrogustometer is needed to acquire the information. Olfaction should also be tested by a smell identification test, such as those produced by Sensonics, Inc. (see Chapter 13). Hyposmia or anosmia may impede swallowing by impairing anticipatory salivation.
Oral Trials
Patients with oral and pharyngeal dysphagia as a result of bulbar impairment are generally referred for speech pathology assessment. Speech pathologists provide oral trials, usually starting with the consistency that would be easiest for the patient to manage. In a neurologically impaired patient, the safest consistency is generally a thickened fluid rather than a thin fluid.51 This is because weak or uncoordinated swallowing mechanisms render it difficult to initiate reflexes rapidly enough to protect the airway and do not have the precise, well-timed movements necessary to move boluses of thin fluids, which tend to fragment, safely through the hypopharynx. The examiner palpates the patient’s thyroid notch between the hyoid bone and larynx and feels for the anterosuperior excursion of the larynx during swallowing trials (Fig. 12-6).51 In cases of severe brainstem stroke, the swallow reflex may be absent. This palpation position also gives an indication of tongue movement during the oral phase and may reveal repetitive tongue pumping, as is frequently observed in patients with Parkinson’s disease. Examiners should make note of any delay between tongue movement and laryngeal excursion, because this may be correlated with delayed initiation of the swallow reflex. Patients should be asked to phonate after oral trials, as an indication of laryngeal protection. If the voice sounds wet after swallow, it may indicate either laryngeal penetration or aspiration. Laryngeal penetration occurs when foreign material enters the laryngeal vestibule to the level of the vocal folds. Aspiration occurs when foreign material enters the larynx below the level of the vocal folds (see Fig. 12-3). When material is aspirated in the absence of the protective cough reflex, as may be indicated by a wet-sounding voice, it is referred to as silent aspiration. This is a common phenomenon, occurring in up to two thirds of patients in the acute poststroke phase.52
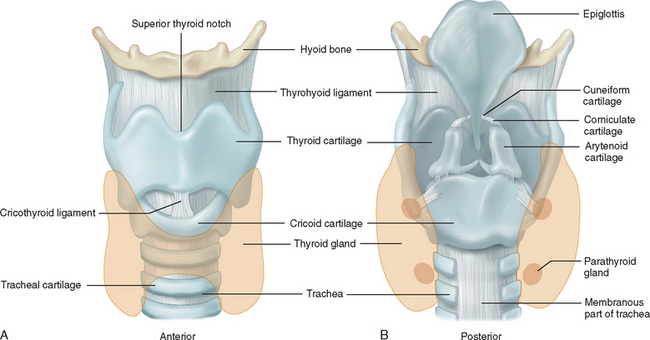
Figure 12-6 Anatomy of the larynx. A, Anterior view. B, Posterior view.
(From Seeley RR, Stephens TD, Tate P: Anatomy and Physiology. St. Louis: Mosby–Year Book, 1992.)
Extreme caution is needed when oral trials are initiated in patients with illnesses often associated with neurogenic dysphagia, because there is the potential to cause serious negative health consequences, including aspiration pneumonia, malnutrition, and death.53–55 Ice chips are frequently used when cautious testing is required, because very small amounts are able to be presented, and the cold provides heightened sensory input that may help to stimulate a swallow in some patients.56,57 Some clinicians use cervical auscultation to listen to laryngeal and pharyngeal noises during swallowing, to determine whether pooling or aspiration has occurred,57,58 although the reliability of this technique is not yet established. Pulse oximetry may be used to observe drops in oxygen saturation levels. It has been suggested that a decline of more than 4% during swallowing may be indicative of episodes of penetration or aspiration.59,60 Other clinical signs of aspiration include gurgling breath sounds, wet-sounding voice, and dyspnea. Fatigue is a factor that affects patients with neurogenic dysphagia; several boluses should therefore be tried before a decision regarding oral feeding is made.
The cough is the most important protective mechanism preventing aspiration, and patients who are unable to produce a volitional cough at the clinical examination may be unable to expectorate material from their upper airway, should the need arise. The bedside clinical examination has been shown to miss up to 40% of cases of silent aspiration,51 and patients without adequate airway protection, as provided by a volitional cough, may benefit from more objective assessments such as videofluoroscopic swallowing studies (VFSS) or fiberoptic endoscopic evaluation of swallowing (FEES). These procedures, described later, enable clinicians to perform conservative swallowing examinations of the dysphagic patient and more objectively determine the risks associated with oral feeding.
The presence of a tracheostomy tube, as may be encountered in patients with brainstem strokes or patients with motor neuron disease, necessitates a specific dysphagia assessment approach.61 It has been shown that tracheostomy tubes anchor the larynx and that cuffed tubes splint open the airway, thereby impeding the protective laryngeal closure mechanism. In addition, the upper airway sensory characteristics are altered, and the pressure gradients within the larynx, which help prevent penetration by foreign material, are reduced. It is generally accepted that tracheostomy cuffs should be deflated during oral trials. It has also been suggested that a one-way speaking valve may help normalize the upper airway characteristics and therefore possibly improve swallowing safety and function.61,62
Instrumental Assessment
Videofluoroscopic Swallowing Study
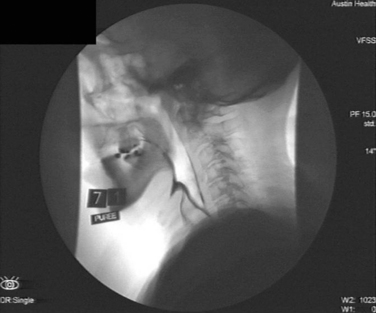
VFSS is a dynamic radiographic study used to evaluate the oral and pharyngeal phases of the swallow. The patient is provided with foods and fluids of differing consistencies that are mixed with a radiopaque substance such as barium. Moving images provide an indication of the interrelationships between the swallowing structures during transit of the bolus. These images allow observation of any pooled material in the pharynx and help delineate the timing and quantity of aspiration in real time. VFSS may aid in clarifying the physiological basis of the patient’s dysphagia. If possible, it is beneficial to include a full esophageal view in each VFSS to provide further information about possible retrograde aspiration and reflux, as well as esophageal causes of dysphagia. Although the procedure is usually performed in the lateral plane, an anteroposterior view may be useful for highlighting asymmetrical dysphagia (e.g., with unilateral stroke) and assist in implementing appropriate swallowing strategies (Fig. 12-7).
Fiberoptic Endoscopic Evaluation of Swallowing
FEES, first described in 1988,63 involves insertion of a fiberoptic nasoendoscope into the nasopharynx to obtain a view of the hypopharynx and larynx. The image is displayed on a video monitor, allowing the patient and health professionals to view the patient’s swallow clearly as foods and fluids of different consistencies are provided. Direct images of the mucosa, secretion management, and the biomechanical relationships of the structures crucial in swallowing are obtained. Because of the lack of radioactive exposure in this procedure, in comparison with VFSS, FEES is a useful biofeedback and therapy tool (Fig. 12-8).
Manometry
Manometric studies are used to assess peristalsis of the pharynx and esophagus. Pharyngeal manometry requires pressure sensors that are sensitive enough to detect the rapid changes in pressure that occur during the swallow reflex. These studies usually need to be combined with VFSS in order to make accurate judgments regarding the causes of pressure changes49 and are not commonly used in clinical practice.
Management of Patients with Neurogenic Dysphagia
Oral Feeding
Patients with neurological swallowing disorders who are able to tolerate oral intake may require modifications to the consistency of their diet. Patients with oral-stage problems affecting tongue and lip function often benefit from pureed food.48 However, some of these patients require increased sensory input to stimulate saliva flow, mastication, and triggering of the swallowing reflex and may respond better to semisolid textures or foods of different temperatures and strong flavors.64 Patients with delay of the swallow reflex may need fluids to be thickened so that they pass slowly as a cohesive bolus in a more controlled manner through the pharynx. In patients with weak pharyngeal musculature, in which pharyngeal dysmotility and pooling are a problem, fluids may need to be of a slightly thickened nectar-like consistency, making pharyngeal pooling less likely. Flaky textures and foods consisting of dual consistencies (e.g., cereal flakes with milk) are notoriously difficult for patients with neurogenic dysphagia to manage, because of the level of swallowing coordination necessary to maintain these textures in a cohesive bolus.
Nonoral Feeding
When patients are unable to eat an oral diet sufficient to sustain their nutritional needs, they require enteral feeding. Enteral feeding may accompany oral intake or, in cases of severe dysphagia, may be the only means of nutrition. If recovery of swallowing function to full quantities of oral diet is expected in the short term, a nasogastric tube is appropriate. For long-term nutrition, an endoscopic or radiographic percutaneous gastrostomy may be performed; in patients with gastroesophageal reflux, a jejunostomy may be a more appropriate option.64,65
Swallowing Therapy
Swallowing Strategies/Maneuvers
Altered swallowing postures may increase safety during oral intake. Postures include chin tuck, in which the chin is placed on the chest to provide more airway protection66; supraglottic swallow, which closes the airway at the level of the vocal folds, thereby affording more control in protecting the airway during the swallow67; and head turn to the side of pharyngeal weakness, to move the bolus through the stronger side.68 There is some preliminary evidence that the following techniques may also strengthen weakened pharyngeal musculature: effortful swallow to improve pharyngeal clearance69 and the Mendelsohn maneuver, a prolonged laryngeal elevation that helps in opening the upper esophageal sphincter.70
Biofeedback
Positive results have been obtained with different types of biofeedback to instruct patients on expected targets.71,72 FEES may enable a patient to visualize and perform vocal fold closure techniques, thereby increasing airway protection during swallowing. Surface electromyography has been used to demonstrate effort behind swallowing.71 Investigators have reported the use of electrical stimulation techniques as a therapeutic tool to improve swallowing.73,74 The use of such devices is not well supported; many questions regarding the type and degree of stimulation, the placement of electrodes, and patients who may gain the most benefit from the technique remain unanswered.
Duffy JR. Motor Speech Disorders. Substrates, Differential Diagnosis and Management. St. Louis: Mosby, 1995.
Freed DB. Motor Speech Disorders: Diagnosis and Treatment. San Diego, CA: Singular Publishing Group, 2000.
Groher ME, editor. Dysphagia: Diagnosis and Management. 3rd ed. Boston: Butterworth-Heinemann; 1997:223-243.
Logemann JA. Evaluation and treatment of swallowing disorders, 2nd ed. Austin, TX: Pro-Ed, 1998.
1 Rosenfield DB, Barroso AO. Difficulties with speech and swallowing. In Bradley WG, Daroff RB, Fenichel JM, et al, editors: Neurology in Clinical Practice, 3rd ed., New York: Butterworth-Heinemann, 2000.
2 Brazis PW, Masdeu JC, Biller J. Localization in Clinical Neurology, 4th ed. Philadelphia: Lippincott Williams & Wilkins, 2001.
3 Borod JC, Koff E, Lorch MP, et al. Emotional and nonemotional facial behaviour in patients with unilateral brain damage. J Neurol Neurosurg Psychiatry. 1988;51:826-832.
4 Seikel JA, King DW, Drumright DG. Anatomy and physiology for speech and language. San Diego, CA: Singular Publishing Group, 1997.
5 Damasio AR. Aphasia. N Engl J Med. 1992;326:531-539.
6 Maassen B, Kent RD, Peters HFM, et al, editors. Speech Motor Control in Normal and Disordered Speech. Oxford, UK: Oxford University Press, 2004.
7 Darley FL, Aronson AE, Brown JR. Audio Seminars in Speech Pathology—Motor Speech Disorders. Philadelphia: WB Saunders, 1975.
8 Darley FL, Aronson AE, Brown JR. Motor Speech Disorders. Philadelphia: WB Saunders, 1975;250-269.
9 Kirshner HS. Aphasia. In: Bradley WG, Daroff RB, Fenichel JM, et al, editors. Neurology in Clinical Practice. 3rd ed. New York: Butterworth-Heinemann; 2000:141-169.
10 van der Merwe A. A theoretical framework for the characterization of pathological speech sensorimotor control. In: McNeil MR, editor. Clinical Management of Sensorimotor Speech Disorders. New York: Thieme; 1997:1-25.
11 Sugishita M, Konno K, Kabe S, et al. Electropalatographic analysis of apraxia of speech in a left hander and in a right hander. Brain. 1987;110:1393-1417.
12 Pryse Phillips W. Companion to Clinical Neurology, 2nd ed. Oxford, UK: Oxford University Press, 2003.
13 Brain R. Speech Disorders—Aphasia, Apraxia and Agnosia. London: Butterworths, 1965.
14 Guenther FH. Neural control of speech movements. In: Meyer A, Schiller N, editors. Phonetics and Phonology in Language Comprehension and Production: Differences and Similarities. Berlin: Mouton de Gruyter, 2002.
15 Duffy JR. Motor Speech Disorders. Substrates, Differential Diagnosis and Management. St. Louis: Mosby, 1995.
16 Monrad-Krohn GH. Dysprosody or altered “melody of language.”. Brain. 1947;70:405-415.
17 Vogel D, Carter JE. The Effects of Drugs on Communication Disorders. San Diego, CA: Singular Publishing Group, 1995.
18 Duffy JR, Yorkston KM, Buekelman DR, et al. Medical interventions for spasmodic dysphonia and some related conditions [Technical Report 2]. Minneapolis, MN: Academy of Neurological Communication Disorders and Sciences, 2001.
19 Rigrodsky S, Morrison EB. Speech changes in parkinsonism during L-dopa therapy: preliminary findings. J Am Geriatr Soc. 1970;18:142-151.
20 Larson KK, Ramig LO, Scherer RC. Acoustic and glottographic voice analysis during drug-related fluctuations in Parkinson’s disease. J Med Speech Pathol. 1994;2:227-239.
21 Poluha PC, Teulings HL, Brookshire RH. Handwriting and speech changes across the levodopa cycle in Parkinson’s disease. Acta Psychol (Amsterdam). 1998;100:71-84.
22 Johns DF. Surgical and prosthetic management of neurogenic velopharyngeal incompetency in dysarthria. In: Johns DF, editor. Clinical Management of Neurogenic Communicative Disorders. 2nd ed. Boston: Little, Brown; 1995:168-173.
23 Isshiki N, Okamura H, Ishikawa T. Thyroplasty type 1 (lateral compression) for dysphonia due to vocal cord paralysis or atrophy. Acta Otolaryngol. 1975;80:465.
24 Sasaki CT, Leder SB, Petcu L. Longitudinal voice quality changes following Isshiki thyroplasty type I: the Yale experience. Laryngoscope. 1990;100:849-852.
25 Ford CN, Bless DM. Clinical experience with injectable collagen for vocal fold augmentation. Laryngoscope. 1986;96:863-869.
26 Mikaelian DO, Lowry LD, Sataloff RT. Lipoinjection for unilateral vocal cord paralysis. Laryngoscope. 1991;101:465-468.
27 Bedwinek AP, O’Brian RL. A patient selection profile for the use of speech prosthesis in adult disorders. J Commun Disord. 1985;18:169-182.
28 Beukelman DR, Mirenda P. Augmentative and Alternative Communication, 2nd ed. Baltimore: Paul H. Brooks, 1998.
29 Brookshire RH. An Introduction to Neurogenic Communication Disorders, 4th ed. St. Louis: Mosby-Year Book, 1992.
30 Yorkston KM, Spencer MS, Duffy J. Behavioral management of respiratory/phonatory dysfunction from dysarthria: a systematic review of the evidence. J Med Speech Lang Pathol. 2003;11(2):13-38.
31 Murdoch BE, Pitt G, Theodoros DG, et al. Real-time continuous visual biofeedback in the treatment of speech breathing disorders following childhood traumatic brain injury: report of one case. Pediatr Rehabil. 1999;3(1):5-20.
32 Thompson-Ward EC, Murdoch BE, Stokes PD. Biofeedback rehabilitation of speech breathing for an individual with dysarthria. J Med Speech Lang Pathol. 1997;5:277-290.
33 Dworkin J, Meleca R. Vocal pathologies: diagnosis, treatment and case studies. San Diego, CA: Singular Publishing Group, 1997.
34 Yamaguchi H, Yotsukura T, Sata H, et al. Pushing exercise program to correct glottal incompetence. J Voice. 1993;7:250-256.
35 Murry T, Woodson G. Combined-modality treatment of adductor spasmodic dysphonia with Botulinum toxin and voice therapy. J Voice. 1995;9:460-465.
36 Ramig LO, Pawlas AA, Countryman S. The Lee Silverman Voice Treatment. Iowa City, IA: National Center for Voice and Speech, 1995.
37 Yorkston KM, Hammen VL, Beukelman DR, et al. The effect of rate control on the intelligibility and naturalness of dysarthric speech. J Speech Hear Disord. 1990;55:550-560.
38 Thomas-Stonell N, Leeper HA, Young P. Evaluation of a computer-based program for training speech rate with children and adolescents with dysarthria. J Med Speech Lang Pathol. 2001;9:17-29.
39 Downie AW, Low JM, Lindsay DD. Speech disorder in parkinsonism: usefulness of delayed auditory feedback in selected cases. Br J Disord Commun. 1981;16:135-139.
40 Berry WR, Sanders SB. Environmental education: the universal management approach for adults with dysarthria. In: Berry WR, editor. Clinical Dysarthria. Boston: College-Hill, 1983.
41 Ropper A, Victor M. Disorders of speech and language. In: Victor M, Ropper A, editors. Adam’s and Victor’s Principles of Neurology. New York: McGraw-Hill, 2001.
42 Magendie F. Précis Elémentaire de Physiologie. vol 2. Paris: Mequignon-Marvis; 1836:628.
43 Ertekin C, Aydogdu I. Neurophysiology of swallowing. Clin Neurophysiol. 2003;114:2226-2244.
44 Miller AJ. Deglutition. Physiol Rev. 1982;62:129-184.
45 Jean A, Amri M, Calas A. Connections between the medullary swallowing area and the trigeminal motor nucleus of the sheep studied by tracing methods. J Auton Nerv Syst. 1983;7:87-96.
46 Miller AJ. The Neuroscientific Principles of Swallowing and Dysphagia. San Diego, CA: Singular Publication Group, 1999.
47 Jean A. Brainstem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev. 2001;81:929-969.
48 Logemann JA. Evaluation and Treatment of Swallowing Disorders, 2nd ed. Austin, TX: Pro-Ed, 1998.
49 Miller RM. Clinical examination for dysphagia. In: Groher ME, editor. Dysphagia: Diagnosis and Management. 3rd ed. Boston: Butterworth-Heinemann; 1997:223-243.
50 Chaudhari N, Landin MA, Roper SD. A metabotropic glutamate receptor variant functions as a taste receptor. Nature Neurosci. 2000;3:113-119.
51 Linden P, Siebens AA. Dysphagia: predicting laryngeal penetration. Arch Phys Med Rehabil. 1983;64:28.
52 Ramsey DJ, Smithard DG, Kalra L. Early assessments of dysphagia and aspiration risk in acute stroke patients. Stroke. 2003;34:1252-1257.
53 Langmore SE, Terpenning MS, Schork A, et al. Predictors of aspiration pneumonia; how important is dysphagia? Dysphagia. 1998;13:69-81.
54 Odderson R, Keaton JC, McKenna BS. Swallow management in patients on an acute stroke pathway: quality is cost effective. Arch Phys Med Rehabil. 1995;76:1130-1133.
55 Smithard DG, O’Neill PA, Park C, et al. Complications and outcome after acute stroke: Does dysphagia matter? Stroke. 1996;27:1200-1204.
56 Lazzara G, Lazarus C, Logemann JA. Impact of thermal stimulation on the triggering of the swallowing reflex. Dysphagia. 1986;1:73-77.
57 Hamlet SL, Penney DG, Formolo J. Stethoscope acoustics cervical auscultation of swallowing. Dysphagia. 1994;9:63-68.
58 Takahashi K, Groher ME, Mihi K. Methodology for detecting swallowing sounds. Dysphagia. 1994;9:54-62.
59 Sellars C, Dunnet C, Carter R. A preliminary comparison of videofluoroscopy of swallow and pulse oximetry in the identification of aspiration in dysphagic patients. Dysphagia. 1998;13:82-86.
60 Sherman B, Nisenboum JM, Jesberger BL, et al. Assessment of dysphagia with the use of pulse oximetry. Dysphagia. 1999;14:152-156.
61 Dikeman KJ, Kazandjian MS. Communication and Swallowing Management of Tracheostomized and Ventilator-Dependent Adults, 2nd ed. San Diego, CA: Singular Publishing Group, 2003.
62 Elpern EH, Scott MG, Petro L, et al. Pulmonary aspiration in mechanically ventilated patients with tracheostomies. Chest. 1994;105:563-566.
63 Langmore SE, Schatz K, Olsen N. Fiberoptic endoscopic examination of swallowing safety: a new procedure. Dysphagia. 1988;2:216-219.
64 Miller RM, Groher ME. General treatment of neurologic swallowing disorders. In: Groher ME, editor. Dysphagia: Diagnosis and Management. 3rd ed. Boston: Butterworth-Heinemann; 1997:223-243.
65 Workman JR, Pillsbury HCIII, Hulka G. Surgical intervention in dysphagia. In Groher ME, editor: Dysphagia: Diagnosis and Management, 3rd ed., Boston: Butterworth-Heinemann, 1997.
66 Welch MV, Logemann JA, Rademaker AW, et al. Changes in pharyngeal dimensions effected by chin tuck. Arch Phys Med Rehabil. 1993;74:178-181.
67 Martin BJW, Logemann JA, Shaker R, et al. Normal laryngeal valving patterns during three breath-hold maneuvers: a pilot investigation. Dysphagia. 1993;8:11-20.
68 Logemann J, Kahrilas P, Kobara M, et al. The benefit of head rotation on pharyngesophageal dysphagia. Arch Phys Med Rehabil. 1989;70:767-771.
69 Crary MA. A direct intervention program for chronic neurogenic dysphagia secondary to brainstem stroke. Dysphagia. 1995;10:6-18.
70 Jacob P, Kahrilas PJ, Logemann JA, et al. Upper esophageal sphincter opening and modulation during swallowing. Gastroenterology. 1989;97:1469-1478.
71 Huckabee ML, Cannito M. Outcomes of swallowing rehabilitation in chronic brainstem dysphagia: a retrospective evaluation. Dysphagia. 1999;14:93-109.
72 Pouderoux P, Kahrilas PJ. Eglutitive tongue force modulation by volition, volume, and viscosity in humans. Gastroenterology. 1995;108:1418-1426.
73 Freed ML, Freed L, Chatburn RL, et al. Electrical stimulation for swallowing disorders caused by stroke. Respir Care. 2001;46:466-474.
74 Leelamanit V, Limsakul C, Geater A. Synchronized electrical stimulation in treating pharyngeal dysphagia. Laryngoscope. 2002;112:2204-2210.