Chapter 21 Motor Cortex Stimulation for Relief of Chronic Pain
 MCS has been available for many years but has recently drawn interest as a viable therapy for the intractable pain patient.
MCS has been available for many years but has recently drawn interest as a viable therapy for the intractable pain patient. The Food and Drug Administration (FDA) has not approved MCS as a labeled therapy for the use of humans in the United States. Many neurosurgeons use this therapy in an off-label fashion.
The Food and Drug Administration (FDA) has not approved MCS as a labeled therapy for the use of humans in the United States. Many neurosurgeons use this therapy in an off-label fashion. Patients should be treated with spinal cord or peripheral nerve stimulation before MCS if appropriate.
Patients should be treated with spinal cord or peripheral nerve stimulation before MCS if appropriate.Introduction
With the advent of modern therapies, a need to determine a proper treatment algorithm is essential to proper use of new advances. Spinal cord stimulation (SCS), peripheral nerve stimulation (PNS) and intrathecal drug delivery (IDD) are options for the patient suffering from severe pain who does not respond to new treatment options.1,2 Motor cortex stimulation (MCS) is viewed as a new therapy that may play a role in those who do not respond to the treatments noted previously. In fact, MCS is not a new option. The initial report of MCS appeared in the literature 20 years ago and that of deep brain stimulation in the early 1970s, but there is no Food and Drug Administration (FDA) approval for the use of stimulation devices over the cortex of the brain or in the brain to treat pain problems in the United States, making access to these motor cortex and deep brain stimulators for pain difficult.3,4 This chapter examines the current knowledge base regarding MCS and helps the reader understand critical issues for considering this option for patients.
Establishing Diagnosis
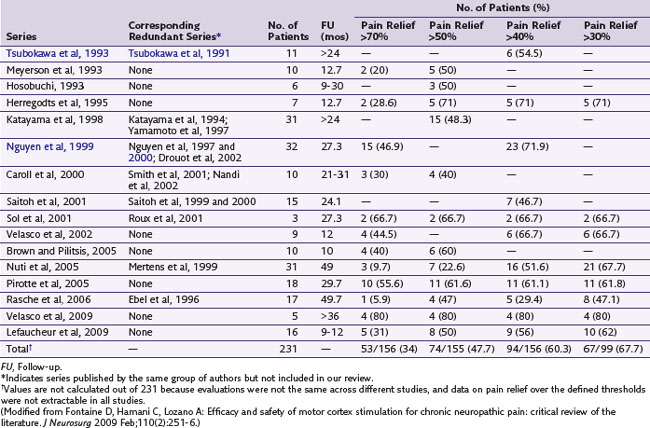
Although the original report by Tsubokawa and associates4 of MCS for chronic pain considered a group of seven patients who suffered from thalamic pain, this procedure has been used in patients presenting with varied facial pain syndromes in particular, without apparent regard to physiological presentation.5 In a review of the literature, Nguyen and associates6 did find results suggestive of modest-to-good improvement of pain in some patients with neuropathic pain. Fontaine, Hamani, and Lozano7 did an additional review and noted evidence for improvement of patients who experienced neuropathic pain on a variety of causal bases (e.g., phantom limb, central pain and plexus avulsion). Given absence of sensory paresthesias with MCS, two recent studies examined its efficacy using double-blinded stimulation parameters over a 30-day period (Velasco et al, 2009; Lefaucheur et al, 2009). Patients in both studies were then followed long term with active open-label stimulation. One study examined 16 patients with limb or facial neuropathic pain who underwent MCS; 13 were crossed over in a double-blind fashion between active and no stimulation. There were no significant differences between both groups based on visual analog scale (VAS) scores, Brief Pain Inventory (BPI) scores, McGill Pain Questionnaire–Pain Rating Index, Sickness Impact Profile (SIP), or the medication quantification scale. However, in the 12 patients who completed the open-label study, the VAS and SIP scores were significantly reduced compared to baseline. On the other hand, a 2009 study by Velasco and associates trialed five patients with refractory complex regional pain syndrome (CRPS) and implanted four. Clinical signs, VAS, and McGill Pain Scale were monitored while the implanted MCS in each patient was either turned off or on for 30 days between days 30 and 60 and days 60 and 90 following implant in a double-blind fashion. Compared to the off mode, MCS resulted in significant improvement in VAS and McGill Pain Scale and clinical improvements in allodynia, hyperalgesia and sympathetic signs. Table 21-1 examines studies using MCS for the treatment of pain.
Anatomy
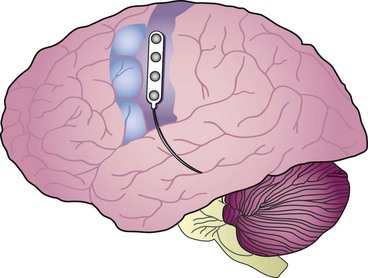
MCS brings with it the anatomy of the cortex itself as a potential challenge, producing possible limitations to the therapy. The face and hand area are over the convexity of the brain and thus are accessible to stimulation without any significant dissection. However, the leg area is in the interhemispheric fissure, which produces technical challenges, both from the point of view of achieving lead placement and retaining the lead in proper position once implanted (Fig. 21-1).
Basic Science
Tsubokawa’s initial report5 noted as a rational basis for his series burst suppression in cats that were subjected to MCS. In addition, it was suggested that relevant mechanisms for benefit in these patients included an increase in regional cerebral blood flow and glucose metabolism in both the cortex and the thalamus contralateral to the side of pain. In a later report, Tsubokawa and associates8 report that patients obtaining benefit from MCS were also found to have pain that was responsive to thiamylal and ketamine infusions but resistant to morphine infusion, again suggesting that patients experiencing relief had neuropathic pain.
Equipment
Patients have been implanted most commonly with quadripolar plate electrodes and conventional power supplies (Fig. 21-2). Other arrays are usable, and today consideration would be given to more robust electrode arrays to have a better opportunity to improve the patient’s pain. New devices are being developed that may have different contours and shapes to better solve the issue of how to best deliver current to this challenging anatomy.
Technique
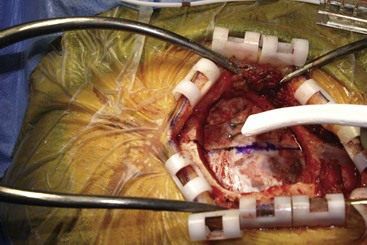
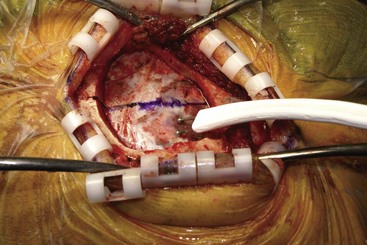
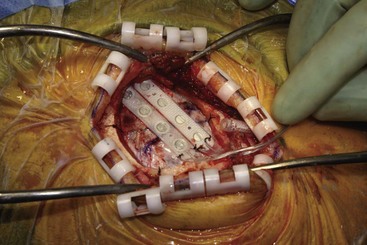
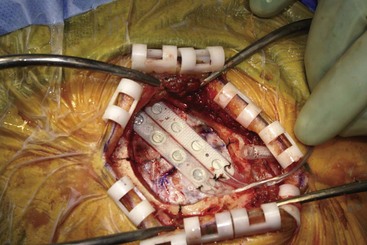
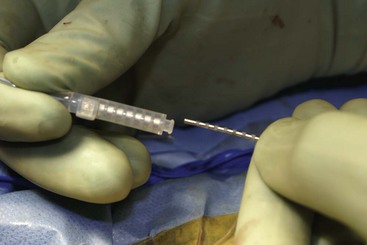
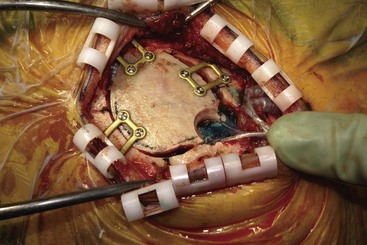
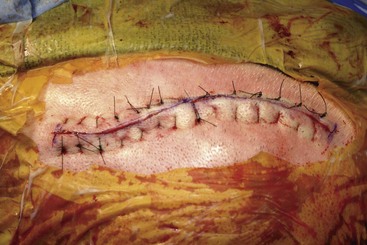
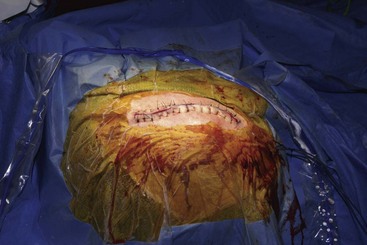
Before moving forward with MCS the patient should undergo a careful preoperative evaluation. This includes a work-up of the primary pain etiology and a stabilization of concomitant diseases. Issues to consider are infection risks, blood sugar control, clotting function, and nutrition status. Once the patient is considered stable for surgery, intravenous antibiotics are administered, the hair is removed from the surgical site, and the patient is draped and positioned (Figs. 21-3 to 21-8). A small craniotomy is performed to gain access to the target area of the motor cortex (Figs. 21-9 to 21-16). Although operative technique varies somewhat in this procedure, the essential common ground is insertion of a plate electrode over the motor cortex in the area that is somatotopically relevant to the patient’s pain (Figs. 21-17 to 21-27). This most often involves preoperative imaging with magnetic resonance imaging and intraoperative physiological corroboration using somatosensory data to confirm lead placement over the motor cortex. Leads are most commonly placed epidurally; however, some have used subdural placements for leads, as would be expected in attempts to treat patients with leg pain.
Outcomes Evidence
As noted previously, Nguyen and associates6 and Fontaine and associates7 have reviewed the literature and found a variance of positive outcomes from this procedure in the treatment of neuropathic disorders. Notably published reports on this therapy are typically small, poorly controlled, usually retrospective, and have short follow-up. Nonetheless benefit is noted on average in over 50% of patients. These patients report greater than 40% or 50% pain relief, depending on the study measurement. Taking into consideration that these patients have no other reasonable available therapy, this is remarkable.7
Risk and Complication Avoidance
Common complications of this procedure are infection, which represents ≈5% to 6% incidence; seizures, ≈12%; and hardware dysfunction, ≈5%. Additional, less common complications of extraaxial hemorrhages, motor deficit, and death have been reported. Immediate postoperative deaths from pulmonary embolism and extraaxial hemorrhage have been reported. From the perspective of hardware malfunction and infections, these rates are average to low.7 Hardware malfunction/breakage is the most common complication in SCS, expected to occur between 25% and 30% of the time in contemporary practice. The infection rate is similar to that expected with SCS. The mortalities noted occurred from reasons consistent with other intracranial procedures.7,9
1 Krames E, et al. rethinking algorithms of pain care: the use of the SAFE principles. Pain Med. 2009;10(Issue 1):1-5.
2 Taylor RS, et al. The cost effectiveness of spinal cord stimulation in the treatment of pain: a systematic review of the literature. J Pain Symptom Manage. 2004;27(Issue 4):370-378.
3 Levy R, Deer TR, Henderson J. Intracranial neurostimulation for pain control: a review. Pain Physician. 2010;13(2):157-165.
4 Tsubokawa T, et al. Treatment of thalamic pain by chronic motor cortex stimulation. Pacing Clinical Electrophysiol. 1991;14(Issue 1):131-134.
5 Nguyen JP, et al. Motor cortex stimulation in the treatment of central and neuropathic pain. Arch Med Res. 2000;31(Issue 3):263-265.
6 Nguyen JP, et al. Chronic motor cortex stimulation in the treatment of central and neuropathic pain: correlations between clinical, electrophysiological and anatomical data. Pain. 1999;82(Issue 3):245-251.
7 Fontaine D, Hamani C, Lozano A. Efficacy and safety of motor cortex stimulation for chronic neuropathic pain: critical review of the literature. J Neurosurg. 2009 Feb;110(2):251-256.
8 Tsubokawa T, Katayama Y, Yamamoto T, et al. Chronic motor cortex stimulation in patients with thalamic pain. J Neurosurg. 1993 Mar;78(3):393-401.
9 Turner J, et al. Spinal cord stimulation for patients with failed back surgery syndrome or complex regional pain syndrome: a systematic review of effectiveness and complications. Pain. 2004;108(Issue 1):137-147.