Chapter 48 Mosquitoes and Mosquito-Borne Diseases
Mosquitoes are in the biologic phylum Arthropoda, class Insecta, order Diptera, family Culicidae. There are approximately 35 genera, which include Anopheles, Culex, Psorophora, Ochlerotatus, Aedes, Sabethes, Wyeomyia, Culiseta, and Haemagogus. Worldwide there are more than 3000 species and subspecies, and in the United States alone, there are an estimated 200 different species of mosquitoes that vary in habitat and behavior.179 In addition to transmitting the parasitic disease of malaria to 300 to 500 million people per year and thereby killing up to 2.7 million people per year,13 mosquitoes also serve as vectors for arboviruses that cause significant morbidity and mortality to humans worldwide. In addition, mosquitoes are responsible for transmission of the larval nematode that leads to lymphatic filariasis.
Mosquitoes
Mosquito Anatomy
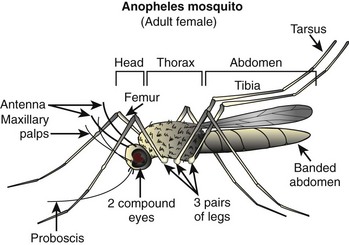
Mosquito anatomy (Figure 48-1) includes a slender body with two wings (rather than the four possessed by flies) and six long, delicate legs. The wings are covered with scales. The body is divided into three parts: head, thorax, and abdomen. The head has two large compound eyes with many lenses angled in different directions, and two antennae. The mouth, or proboscis, looks like a funnel extending downward, and is used to pierce skin and suck blood (for females) or sip nectar (for males). The thin and short neck connects the head to the thorax, which is triangular and holds the wings in place. The thorax also has two pairs of spiracles, which are tubes through which the mosquito can breathe. The shape of the abdomen can be pointed or rounded, depending on the species, and it has eight pairs of spiracles. The legs are divided into the coxa, femur, tibia, and tarsus.
The spiracles form the basis of the mosquito’s tracheal system, which is finely branched so that the cells of the body are directly oxygenated. Mosquitoes have a dorsal blood vessel that extends directly from the eighth abdominal segment into the head. The heart is the portion of the blood vessel located within the thorax and although it is not innervated, it pulses automatically at a rate of 85 beats/min.161
Mosquito Life Cycle
The life cycle of most species of mosquitoes is defined by four stages: egg, larva, pupa, and adult. The time a mosquito spends in each stage depends on the species and temperature. Culex tarsalis, for example, has a 14-day life cycle at 20° C (68° F), and 10-day life cycle at 25° C (77° F). The life cycle varies from 4 days to 1 month, but averages around 2 weeks, which means that several generations can arise within a single year.179 Most mosquitoes that do not have habitats in the tropics lie dormant as eggs, although some, such as the genus Culex, overwinter as larvae or adults.
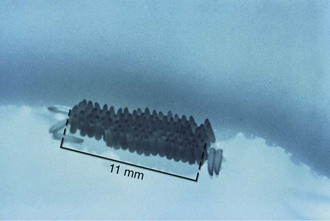
The female mosquito usually mates once in her lifetime and stores sperm in her body. She fertilizes her eggs when she lays them, which is generally on the surface of the water—often in the form of egg rafts, shown in Figure 48-2—or sometimes in groups in empty containers or jars, shown in Figure 48-3. Eggs can survive winter and hatch in the spring. They hatch into larvae (also known as wrigglers), about 1 to 2 cm (0.4 to 0.8 inch) long, that feed on microscopic animals, plant life, and, in some cases, other mosquito larvae. Except for the genus Anopheles, the larvae must come to the surface to employ their air tubes, or siphons, which supplement their gills (Figure 48-4). After molting several times, they develop into pupae (Figure 48-5), floating on the surface of the water and breathing through an air tube, now called a trumpet. While encased in the hard pupal case, they transform into adult mosquitoes. Enough pressure is created to burst the case, and when their wings harden, they can fly and live on land.161
Mechanism of Mosquito Bites
Mosquitoes technically do not sting, because there is no stinger; they simply pierce the skin and suck blood. Only the females of most species have sucking mouth parts that can pierce skin and thus transmit disease (Figure 48-6). The blood protein is required because the normal mosquito diet of nectar and fruit juices does not contain enough protein for egg development. (One genus, Toxorhynchites, does not suck blood, and its larvae prey on other mosquito larvae). Mosquitoes generally identify victims by scent, as well as by the carbon dioxide of exhaled breath and some chemicals found in sweat. Mosquitoes have been shown to have odorant-binding proteins on their antennae that bind human-specific odorants, stimulating their olfactory neurons and allowing them to locate their human prey.154 Other risk factors for getting bitten include male gender, heavier weight, and type O blood.61
When mosquitoes pierce human skin, all six legs are usually positioned on the skin surface, along with the labella (part of the proboscis). The female proboscis is made up of six shafts, four of which cut and pierce skin. One serves as a conduit for blood into the mosquito, and another transports mosquito saliva into the skin. The mosquito lays the labella on the skin, which allows the maxillae of the fascicle (a bundle of feeding stylets, contained within the proboscis) to saw into the skin in an oscillatory cutting fashion. Blood is drawn up into the fascicle, which collects into the labrum held between the mandibles of the mosquito. The female mosquito usually requires about 50 seconds to insert the fascicle into the skin, and approximately 2 minutes to finish feeding. She can withdraw her fascicle in 5 seconds.60,69
Mosquito saliva contains a wide array of antihemostatic molecules that allow feeding upon blood. These molecules include vasodilators, such as amines and prostaglandins; platelet aggregation inhibitors, such as nitric oxide; prostaglandins; apyrase; molecules that sequester adenosine diphosphate (ADP-sequestering molecules), and peptides and proteins specifically targeted to integrin receptors; and anticoagulants, such as thrombin and factor Xa inhibitors. The saliva and its accompanying proteins (specifically, the 33-kilodalton F-1 protein found in mosquito saliva) that remain in human skin after feeding serve as antigens that evoke an immune response.43
Pathophysiology and Clinical Manifestations of Mosquito Bites
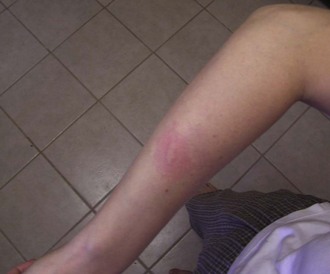
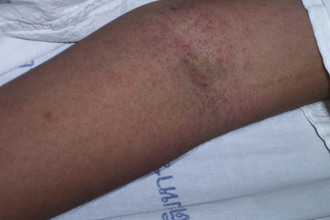
Individuals vary in their responses to mosquito bites. Almost all experience local irritation caused by a type I immunoglobulin E (IgE)-mediated response of a soft, pale, pruritic wheal or plaque (Figure 48-7). The subsequent effects reveal which immune response a person has to the bite. Type I IgE responses can also create immediate hive-like skin lesions (rare), whereas type IV cell-mediated immunity causes delayed pruritic papules or vesicles within 48 hours of the bite. Other type IV reactions caused by mosquito bites include blisters, bullae, and erythema multiforme or purpura, but these are rare.
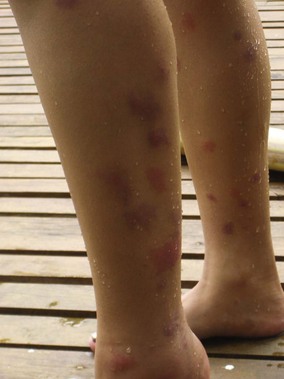
One common type IV hypersensitivity response is papular urticaria, which describes an eruption of pruritic papules measuring 3 to 10 mm (0.1 to 0.4 inch) in diameter that may be surrounded by vesicles grouped in irregular clusters (Figure 48-8). Because repeated exposures may lead to desensitization in adults, these lesions are more common in children. They begin as an erythematous wheal, lasting from 2 to 10 days, and may lead to temporary hyperpigmentation. Children who suffer this hypersensitivity may develop a firm, light-brown papule, whereas most adults have only a transient wheal without formation of a persistent papule. The dermatopathology of the epidermis in papular urticaria shows spongiosis with exocytosis and vesicle formation. The upper dermal layer reveals a localized perivascular infiltrate with lymphocytes, histiocytes, eosinophils, and mast cells, and a spattering of eosinophils and mast cells in the mid-dermis.168
The pathophysiology behind the phenomenon of itching is complex. There are abundant theories on the neurophysiologic basis of itch. The most traditional is that the local reaction induces production of histamine, which stimulates C nerve fibers that travel to the brain and induce the sensation of itching.195 Histamine, however, is only one of many pruritogenic substances that stimulate a subset of specialized skin C fibers. Other mediators of itch include proteases, opioids, lipid peroxidation metabolites (such as leukotrienes and prostaglandins), neuropeptides (e.g., substance P), cytokines, and growth factors (e.g., nerve growth factor). Pruritus is now seen as an interdisciplinary issue that reaches into the fields of neurophysiology, neuroimmunology, neuropharmacology, protease research, internal medicine, and dermatology.
Diseases
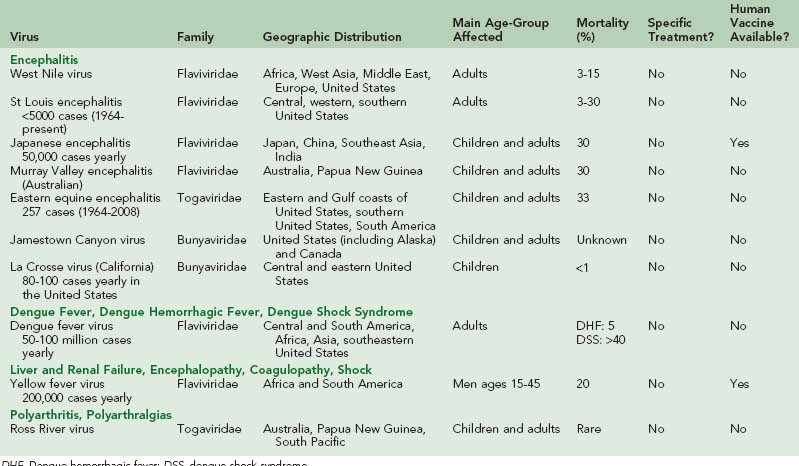
The effects of mosquitoes on human life are far reaching. Every year, they are responsible for transmitting a wide spectrum of diseases, such as malaria, dengue fever, yellow fever, and encephalitis to millions of people. This chapter discusses the main diseases transmitted by mosquitoes, other than malaria (see Chapter 49). Table 48-1 summarizes the viruses discussed in this chapter.
Dengue
Dengue was first identified as being caused by a virus in the 1940s, although its existence was probably present much earlier, as reports of dengue fever epidemics occurred as early as 1779. Like West Nile virus (WNV), dengue viruses belong to the family Flaviviridae, genus Flavivirus, and have four distinct but closely related serotypes (DEN-1, DEN-2, DEN-3, and DEN-4), all of which cause infection. Dengue has become a major public health issue over recent decades, as the incidences of cases and dengue hemorrhagic fever (DHF) epidemics have dramatically increased. Currently, it is estimated that 50 to 100 million people in more than 100 countries are infected yearly with dengue viruses.76,189
Epidemiology and Transmission
Several species of Aedes can transmit dengue, but the most important is Aedes aegypti, which is found in temperate zones from latitude 45 degrees north to 35 degrees south. Certain factors make Aedes mosquitoes a particularly effective vector. Specifically, the mosquito itself is quite susceptible to dengue virus; they feed preferentially on human blood; they inhabit highly populated areas and breed inside or close to houses; and their bite is often unnoticed by humans. Moreover, because they feed many times in a breeding cycle, numerous people living in one household can be infected by the same mosquito. Aedes albopictus has a larger geographic distribution than does A. aegypti and can also transmit the virus, but it is less often the culprit responsible for dengue transmission because these mosquitoes breed farther from households and bite humans less frequently.67,76
Dengue virus transmission is classified as epidemic dengue when a single virus strain is introduced into a region with a large number of hosts and mosquitoes, or as hyperendemic when there is uninterrupted circulation of numerous dengue serotypes in one area. Epidemic dengue is the usual transmission pattern for many smaller islands in South America, Africa, and Asia, where several years pass between epidemics and then the disease reemerges. For travelers, this means that the risk for acquiring the infection is high during epidemics, but it is very low between those periods. Generally the frequency of DHF during epidemics seems to be lower than that in areas where the transmission pattern is hyperendemic. In hyperendemic areas, competent mosquito vectors are present throughout the year, and the population of uninfected individuals is sufficiently large to sustain the disease. This latter pattern of transmission accounts for the preponderance of global dengue virus infections.74
As shown in Figure 48-9, dengue virus has been found in Southeast Asia and the western Pacific islands (with hyperendemic transmission of all four serotypes present in Thailand, Vietnam, Indonesia, Malaysia, and the Philippines). Hyperendemic dengue fever (DF) is also found in India, Pakistan, and Sri Lanka. A. aegypti mosquitoes are also distributed across much of sub-Saharan Africa and the Middle East. In the Americas, A. aegypti exists in the southeastern United States, Mexico, Central America, South America, and the Caribbean.76
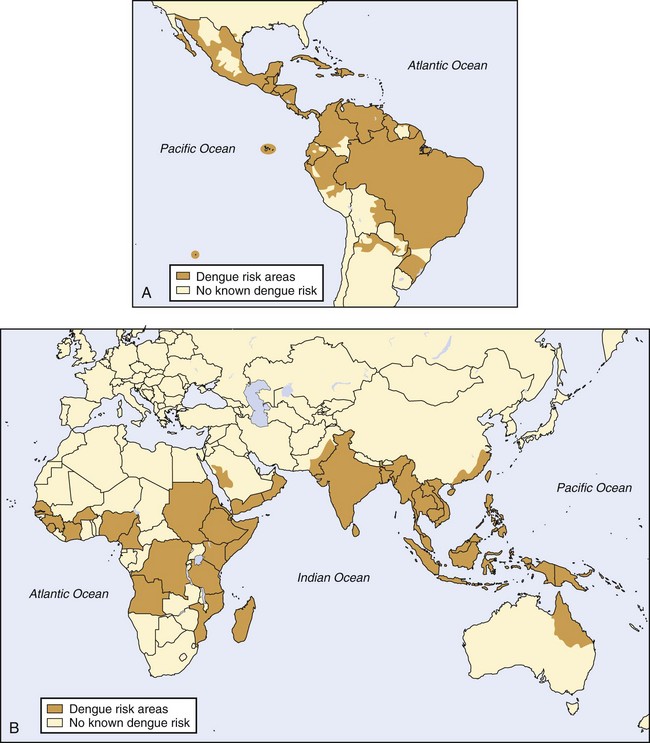
FIGURE 48-9 A, Distribution of dengue, western hemisphere. B, Distribution of dengue, eastern hemisphere.
(From http://wwwnc.cdc.gov/travel/yellowbook/2010/chapter-5/dengue-fever-denguehemorrhagic-fever.aspx.)
Reports of DF and DHF are increasing. In fact, before 1970, only nine countries had suffered DHF epidemics; by 1995, this number had expanded more than fourfold. In the past, DF was considered a relatively benign and nonfatal disease. However, since World War II, during which time Aedes mosquitoes were transported around the world in cargo, there has been expansion of the disease not only in frequency and geographic distribution but also in morbidity and mortality. The 1980s and 1990s saw a rise in DHF (specifically, serotype DEN-3) in southern Asia, and in the 1980s, there was a rise in epidemic DF/DHF (specifically serotype DEN-2) in Taiwan and the People’s Republic of China. Africa has also seen large increases in DF incidence since 1980.72 Dengue has also been emerging much more rapidly in the Americas, with the first major outbreak in Cuba in 1981 and continuing outbreaks in other Central and South American countries since then.145 The World Health Organization (WHO) reports that in 2007 alone, nearly 900,000 cases of dengue were reported in the Americas, with 26,000 cases of DHF. Countries such as Venezuela reported devastating epidemics in the same year, with more than 80,000 cases of DF and more than 6000 cases of DHF.189
Demographic changes, such as population growth and increased human density, contribute to transmission and also to emergence of DHF. Moreover, unplanned urbanization resulting in substandard housing situations and poor water and sanitation systems leads to the same outcome. The situation seems to be potentiated by deterioration of mosquito control programs and of health systems in general in dengue-endemic countries, where the disease cannot be well controlled.110 Climate change alters the interaction between infective mosquitoes and humans. Increased rainfall during certain seasons in tropical areas causes an increased density of mosquitoes. Not only do warmer temperatures decrease the incubation period of the virus inside the mosquito so that the virus can be transmitted earlier, but they may also allow greater mosquito population growth and raise the number of susceptible humans, who perpetuate the disease.79
Clinical Presentation
The clinical presentation of dengue virus can range from asymptomatic infection to self-limited mild febrile illness to life-threatening shock syndrome. Risk factors influence disease severity and development of DHF or dengue shock syndrome (DSS), as opposed to simply dengue fever. These factors include serotype, prior exposure, age, malnutrition, and genetic factors such as human leukocyte antigen (HLA) type. All four serotypes can cause DHF, but DEN-2 appears to be most virulent. The four serotypes do not provide cross-protective immunity, so it is possible for one individual to sustain four separate dengue infections over a lifetime. Although previous infection provides homologous immunity to one serotype, individuals who experience secondary infection by another serotype are at higher risk for more severe forms of disease. This theory supports the observation that infants less than 1 year of age with passively acquired maternal antibodies have a higher rate of DHF/DSS. This phenomenon, known as immune enhancement or antibody-dependent enhancement, is thought to occur because the Fc receptors of monocytes by dengue viruses complexed to IgG antibodies are more accessible to infectious immune complexes and result in a higher number of cells being infected with this different serotype.75,114,123 In terms of age distribution, DHF in the past was seen as a disease of children (especially in Asia, where the disease was previously more predominant). This distribution, however, is slightly different in the Americas, and epidemics have shown increasing numbers of adult patients. Moreover, some epidemiologic studies have demonstrated that most children less than 15 years infected with dengue virus tend to be asymptomatic or have a mild fever. Malnourished children, compared with well-nourished children, seem less inclined to develop DHF/DSS than other diseases, perhaps secondary to immunosuppression. Whites have a higher frequency of developing severe disease than do blacks.114
The incubation period for the virus in the mosquito is 8 to 12 days before it is transmissible, and in humans there is a 4- to 6-day incubation period from bite to clinical infection. Pathogenesis of the virus is hypothesized to be a result of endothelial cell dysfunction, activation of cytokines and complement, and suppression of the reticuloendothelial system.76 At autopsy, the virus is commonly found in the liver, spleen, thymus, lymph nodes, and lung cells.187
WHO currently classifies symptomatic dengue infections into three categories for surveillance purposes. These include undifferentiated fever, classic dengue fever, and DHF. Although this classification system remains controversial,51 the clinical characteristics of DF, DHF, and DSS are discussed here.
The majority of children infected with dengue viruses are asymptomatic or present with mild self-limited fever. Typically, older children and adults with DF present with high fever (greater than 39° C [102.2° F]), myalgias, headache, arthralgias, and rash.187 Patients may also describe retro-orbital pain, and suffer gastrointestinal (nausea, vomiting, and diarrhea) and respiratory (cough, sore throat, and nasal congestion) symptoms.49 A particular characteristic of dengue infection is central nervous system (CNS) involvement in both DF and DHF. Patients present with symptoms that range from irritability and depression to encephalitis with seizures and death, with the more severe symptoms noted in DHF. Despite these complications, the prognosis for patients with DF is generally good, with an acute phase of 1 week and up to 2 weeks of convalescence with general malaise and anorexia. Case fatality rates for dengue fever are less than 1%.33
The main characteristic that differentiates DHF from DF is development of increased vascular permeability resulting in plasma leakage. After 2 to 7 days of high fever, patients with DHF have bleeding (ranging from petechiae, ecchymoses, epistaxis, and mucosal bleeding, to more severe gastrointestinal bleeding and hematuria), thrombocytopenia (<100,000/mm3), and, paradoxically, hemoconcentration and hepatomegaly in some settings.114 Symptoms include severe abdominal pain, nausea and vomiting, and restlessness or lethargy. Plasma leakage may cause pleural effusion, ascites, and hypoproteinemia. This may lead to circulatory failure, resulting in DSS with hypotension, cool extremities, and altered mental status. Encephalopathy is possible in DHF and can result from cerebral anoxia, edema, or intracranial hemorrhage.165 It is still currently unknown whether dengue viruses infiltrate the CNS directly or if neurologic manifestations are nonspecific complications. Case fatality rates for DHF are approximately 5%38; in DSS they can be greater than 40%.33
Diagnosis
Dengue is typically diagnosed clinically, because laboratory confirmation is typically only available in specialized reference laboratories in the developed world. Clinical characteristics are as discussed above; however, it may remain difficult to differentiate dengue from other febrile illnesses. One recent systematic review evaluating 15 studies conducted between 1990 and 2007 attempted to identify clinical criteria to help differentiate such disease processes. In this review, thrombocytopenia, leukopenia, neutropenia, transaminitis, and petechiae were all associated with dengue across multiple studies.147 Another study of 1200 patients from Vietnam and Singapore with acute febrile illness demonstrated a decision algorithm using clinical and hematologic parameters (platelet count, white blood cell count, neutrophil and lymphocyte counts, hematocrit, and body temperature), which accurately identified 84.7% of the dengue cases in their study population. This algorithm had a 71.2% sensitivity and 90.1% specificity for predicting the diagnosis of dengue but has not been externally validated.171
Such studies use both clinical and laboratory findings to identify patients with DF. The tourniquet test (Figure 48-10) for capillary fragility, which describes the appearance of 20 or more petechiae over a square-inch patch on the forearm after deflation of the blood pressure cuff (held for 5 minutes between systolic and diastolic pressures), may also be positive. Although WHO typically uses a positive tourniquet test to help distinguish DHF from DF, the test has been shown to be positive in both disease states.184 Patients may have neutropenia with subsequent lymphocytosis marked by atypical lymphocytes, hyponatremia, hypoproteinemia, and mild elevation of liver enzymes. Other findings include albuminuria and occult blood in the stool. In patients with DHF, either thrombocytopenia (defined by WHO as less than 100,000/mm3) or hemoconcentration (elevation of hematocrit 20% or greater than the average for age, gender, and population) is used by WHO to reach a provisional diagnosis of DHF if certain clinical observations (e.g., acute onset of high fever, hemorrhagic manifestations, hepatomegaly, shock) are made. In cases with extensive liver involvement, vitamin K–dependent prothrombin factors, such as II, VII, IX, and X, may be reduced, and assays of coagulation factors may reveal reduced fibrinogen, prothrombin, factors VIII and XII, or antithrombin III. About one-third to one-half of patients with DHF have increased partial thromboplastin and prothrombin times. Chest radiographs may show a unilateral (usually right-sided) pleural effusion in more severe disease.187
Definitive diagnosis of dengue can be made by isolating the virus or detecting specific antibodies. Immunofluorescence assay of serum or cerebrospinal fluid (CSF) with serotype-specific monoclonal antibodies (IgM enzyme-linked immunosorbent assay [ELISA]) is the test of choice to confirm diagnosis. Although the IgM immunoassay has a sensitivity of 90% to 97%,76 samples collected within the first 4 to 5 days of illness may lead to false negative results.184 Serologic diagnosis of DF then commonly depends on demonstration of a fourfold or greater increase in IgM or IgG antibody titer between acute-phase and convalescent-phase serum samples by hemagglutination inhibition or enzyme immunoassay (and less commonly complement fixation and neutralization test). Other methods for laboratory detection include detection of DF genomic sequences from autopsy tissue, serum, or CSF with polymerase chain reaction (PCR).187 Several protocols have been written for PCR detection of DF, because this technology can aid in detection of the virus within 1 to 2 days.46,47,52,95 However, this method is not widely available, and thus its use is limited to detecting dengue viruses in pathologic tissue, cell cultures, and mosquito specimens.76 Unfortunately, attempts to develop rapid bedside tests using immunochromatographic or immunoblot technologies remain relatively unsuccessful.11 Virus identification can also be confirmed by inoculation of serum into mosquitoes or certain mosquito cell lines (most commonly A. albopictus [C6-36] and Aedes pseudoscutellaris [AP61], but with better sensitivity when mosquitoes in the genus Toxorhynchites are used), but these methods are not commonly available.
The recommendation of the Centers for Disease Control and Prevention (CDC) is that, for testing and reporting, both acute-phase and convalescent-phase (6 days after the onset of symptoms) serum samples be sent to the state health department, which will forward them to the CDC for testing. The acute-phase specimen should be stored on dry ice (or unfrozen at 4° C [39.2° F] if sent within a week), and the convalescent-phase specimen should be shipped on ice (or in a rigid container without ice if next-day delivery is possible).33
Despite all this, however, dengue infections are often diagnosed clinically because laboratory-based diagnosis is often unavailable and has limitations as well.184 That said, dengue should be considered only after other common tropical infections such as malaria are ruled out.184 History is of utmost importance; symptoms in a traveler or immigrant that begin 2 weeks after departure from an endemic area cannot be attributed to dengue, because the incubation period is less than 2 weeks.158
Treatment and Prevention
Treatment includes supportive therapy, including aggressive fluid and electrolyte resuscitation. Early recognition is important, because the acute increase in vascular permeability may cause extreme amounts of plasma to be lost from vascular compartments for 24 to 48 hours. Fever should be treated with antipyretics (especially in children to avoid febrile seizures), and WHO recommends treatment with acetaminophen (paracetamol) if the temperature is greater than 39° C (102.2° F), with dosing as follows: for infants less than 1 year of age, 60 mg per dose; 1 to 3 years of age, 60 to 120 mg per dose; 3 to 6 years of age, 120 mg per dose; and 6 to 12 years of age, 240 mg per dose. WHO recommends no more than six doses in a 24-hour time period, and advises against salicylates to prevent bleeding complications and Reye-like syndrome in children.187
Volume expanders can include normal saline, Ringer’s lactate, or 5% glucose diluted in half-normal or normal saline administered in 10 to 20 mL/kg boluses initially, and then continued with maintenance therapy as needed. One recent double-blind randomized study suggests that Ringer’s lactate at 25 mg/kg over 2 hours should be given for initial resuscitation in children with moderately severe DSS, and that 6% hydroxyethyl starch (also in the amount of 25 mg/kg) may be preferable in children with severe shock.185 Corticosteroids have also been attempted in DSS for their antiinflammatory properties. However, one recent Cochrane meta-analysis of 284 patients demonstrated no reductions in mortality, length of hospital stay, DSS-related complications, or need for blood transfusions when comparing patients who received corticosteroids with those those who received placebo.138 Because of plasma loss, the hematocrit may initially increase, but blood transfusion should be given if there is a clinical indication of internal bleeding. WHO recommends fresh-frozen plasma and concentrated platelets only if there is massive risk for bleeding from coagulopathy. At the same time, because of the volume resuscitation, a drop in hematocrit does not automatically indicate internal hemorrhage, because reabsorption of extravasated plasma may occur when shock ceases. Continued overzealous administration of fluids at this point can lead to pulmonary edema or heart failure. All these measures must be based on clinical judgment, in turn based on indicators such as vital signs, urine output, and hematocrit.187
Despite ongoing vaccine initiatives, there is not yet a licensed vaccine against dengue viruses. As discussed above, a primary infection with one serotype induces long-term protective immunity to reinfection with the homologous serotype but not the heterotypic serotypes. In addition, infection of any serotype can lead to DHF, and sequential exposure to DF may predispose a person to DHF. Thus a successful vaccine must induce long-lasting neutralizing antibodies to all four dengue serotypes. Tetravalent vaccines that enable immunity to all four serotypes are in development and have shown promising results in preliminary clinical testing.58,129 However, vaccine development remains difficult, because administration of four live viruses can result in competition between serotypes and incomplete stimulation of neutralizing antibodies. At this point, vector control through environmental, biologic, and chemical means remains a mainstay of prevention (see information regarding vector control in Global Programs, later).
Yellow Fever
The first epidemic caused by the yellow fever virus is thought to have been in the Yucatan Peninsula in 1648. This single-stranded RNA flavivirus causes infection in up to 200,000 persons per year, with an estimated 30,000 deaths annually, mainly in Africa (90% of cases) and South America (10% of cases).8,182
Epidemiology and Transmission
Unfortunately, factors such as increased urbanization and waning yellow fever immunization programs have contributed to resurgence of the disease since the 1980s.8,150 Accurate data regarding the burden of disease are difficult to obtain because of underreporting (especially in remote areas), lack of diagnostic facilities, and delayed recognition of outbreaks. Nevertheless, of particular concern are the increasing incidence of yellow fever in West Africa (which includes multiple countries suffering humanitarian crises that hinder effective immunization campaigns) and the high (>50%) case fatality rate in South America. Given such concerns, the Yellow Fever Initiative was launched in 2006 as a collaborative effort toward improved yellow fever surveillance and prevention through mass vaccination campaigns.193,194 Figures 48-11 and 48-12 show the most recent endemic areas in Africa and the Americas.
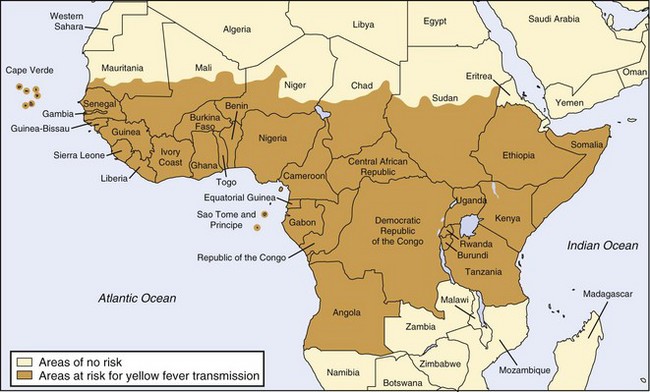
FIGURE 48-11 Yellow fever–endemic zones in Africa, 2009.
(From http://wwwnc.cdc.gov/travel/yellowbook/2010/chapter-2/yellow-fever.aspx#668.)
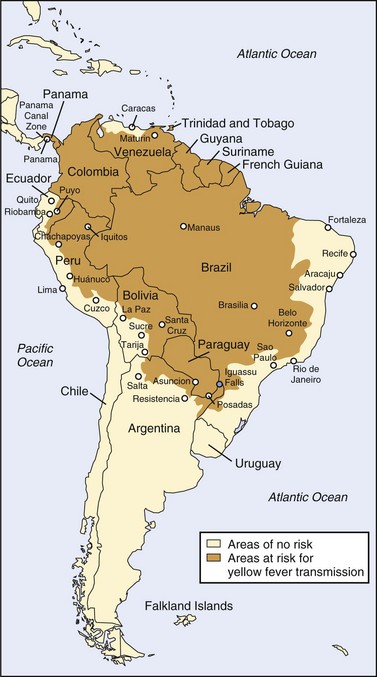
FIGURE 48-12 Yellow fever–endemic zones in the Americas, 2009.
(From http://wwwnc.cdc.gov/travel/yellowbook/2010/chapter-2/yellow-fever.aspx#668.)
Yellow fever in travelers to South America and Africa has been very rare, likely due to routine vaccination. Between 1970 and 2002, only 10 cases of yellow fever were reported, 9 of which occurred in unvaccinated individuals.38
The mosquito species A. aegypti was hypothesized as early as 1881 to be the vector, and it is indeed one of the main mosquitoes that transmit yellow fever in urban settings. There are three patterns of yellow fever transmission: urban, intermediate, and sylvatic (jungle). In urban yellow fever, no monkeys are involved in transmission, and domestic mosquitoes, such as A. aegypti, carry the virus from person to person. In intermediate yellow fever, mosquitoes of the genus Haemagogus, or other forest-canopy species, are known to transmit this disease to both monkeys and humans; this is the most common type of transmission in recent outbreaks in Africa. Finally, in sylvatic yellow fever, nonhuman primates, such as monkeys, are the zoonotic focus, and wild mosquitoes can become infected by the monkeys and pass the disease to humans.38,190
Interestingly, there is also vertical transmission of the virus within mosquitoes; a female mosquito can pass the virus to her offspring. Thus mosquitoes serve as additional reservoirs and vectors of the virus, which ensures endemicity of the disease.182 Peak transmission in Central and South America is during the rainy season between January and March, whereas in Africa it is highest through the end of the rainy season into the early dry season (July through October).38
Clinical Presentation
The main risk factor for disease is exposure, as evidenced by the fact that a large portion of cases are in men of ages 15 to 45 years who have occupational exposure in mosquito-infested areas. Unvaccinated individuals (such as persons too young to have been immunized) in epidemics are also at higher risk, as are individuals in areas with poor health care.182
There are three phases in the classic description of yellow fever: infection, remission, and intoxication. After an incubation period of 3 to 6 days, the infection stage consists of sudden onset of fever, headache, photophobia, malaise, back and epigastric pain, anorexia, and vomiting. Helpful clinical signs include Faget sign (bradycardia occurring at the height of the fever), conjunctival injection, and a coated tongue with pink edges. The infection stage may resolve rapidly within 3 to 4 days and be followed by up to 48 hours of remission.8,128,182
The intoxication period occurs in an estimated 15% or more of individuals infected with this virus, manifesting with liver and renal failure (jaundice, azotemia, and oliguria), myocardial involvement, encephalopathy, and shock. Viral-induced thrombocytopenia and acquired coagulopathy can cause severe hemorrhage, ranging from epistaxis, metrorrhagia, and gastrointestinal bleeding (the reason Spanish-speaking peoples called the disease vomito negro, or black vomit, before the disease was officially named).16 Severe illness has a case fatality rate of about 20%, which may approach 50% in some outbreaks.117
Diagnosis
Preliminary diagnosis is based on clinical presentation and travel history. However, given the clinical overlap with several other disease processes (e.g., leptospirosis, louse-borne relapsing fever, DHF, viral hepatitis), virus detection or serologic examination is required for definitive diagnosis. Laboratory findings may also be supportive in making the diagnosis. They may include leukopenia with relative neutropenia; thrombocytopenia with reduction of all liver-produced clotting factors; elevation of liver enzymes (but usually a normal alkaline phosphatase); elevated blood urea nitrogen (BUN) and creatinine levels; and albuminuria.128
Definitive diagnosis is typically obtained by IgM ELISA and is best done with paired acute and convalescent sera. The acute sample should be drawn within several days of symptom onset, and the convalescent sample should be drawn about 14 days later. A neutralization test, which is done by showing loss of infectivity through reaction of the virus with a specific antibody, can also be done to confirm the diagnosis of yellow fever. Given cross-reactivity between antibodies from other flaviviruses, such confirmatory tests are especially important in Africa, where multiple flaviviruses coexist. The virus can be isolated from serum specimens or liver tissue after death, via inoculation of mice, mosquitoes, or cell cultures, but these techniques are most effective in the preicteric state. Once the intoxication period begins, the viral RNA is usually undetectable and thus such methods are generally not used for diagnosis. Other methods, including PCR for viral genome identification and liver biopsy for demonstration of characteristic midzonal necrosis, have been discussed but are not used for diagnosis.7,38,128,182
Treatment and Prevention
Although there is no treatment other than supportive care, there is a vaccine against yellow fever (17D-204 strain YF-VAX); 0.5 mL of the reconstituted vaccine is administered subcutaneously.42 This vaccine is a live, attenuated vaccine that is generally considered very effective and safe. Seroconversion rates exceed 95%, and side effects are usually limited to injection site pain, mild headaches, myalgias, or low-grade fevers for several days.9,65,128,131 As more travelers enter endemic areas without receiving the vaccine, the number of yellow fever cases imported into the United States and Europe has increased.117 The CDC roughly estimates the risk for acquiring yellow fever for an unvaccinated traveler going to endemic areas of West Africa and South America to be 50 per 100,000 and 5 per 100,000, respectively.38 The CDC and the Advisory Committee on Immunization Practices recommend that travelers (older than 9 months) to certain areas in Africa and South America be vaccinated. Some countries (not the United States) require vaccination before allowing travelers to enter from endemic areas. Vaccination guidelines (and authorized centers for vaccination) can be found on the CDC Yellow Book website at http://wwwnc.cdc.gov/travel/yellowbook/2010/chapter-2/yellow-fever.aspx.
International travelers must receive yellow fever vaccines approved by WHO and administered at an approved yellow fever vaccination center and then obtain an international certificate of vaccination. Although the International Health Regulations require boosters every 10 years, many studies suggest that vaccine immunity endures for over 30 years and possibly for life.190
Immediate hypersensitivity reactions in people with allergies to eggs or other substances are thought to occur in 1 per 131,000 vaccinations, whereas anaphylaxis is reported to occur in 1.8 per 100,000 doses administered. Additional significant adverse reactions to the yellow fever vaccine typically occur in first-time vaccinees and are categorized as yellow fever vaccine–associated neurotropic disease (YEL-AND) and yellow fever vaccine–associated viscerotropic disease (YEL-AVD). YEL-AND is typically due to neuroinvasion by the 17D virus and represents multiple different clinical syndromes, including Guillain-Barré syndrome and meningoencephalitis. Historically the vaccine was associated with encephalitis in children, but standardization of the virus vaccine has decreased the incidence in the United States to 0.8 case per 100,000 doses administered. Although the incidence is thought to be higher in the older adult population, the case fatality rate remains low.9,38 YEL-AVD is a severe illness resembling wild-type disease, in which vaccine virus replication often leads to multisystem organ failure and death. The incidence in the United States is 0.4 cases per 100,000 doses administered, but host factors such as increased age, history of thymus disease, and male gender are thought to increase the risk. The case fatality rate remains a staggering 53%.9,38 Caution is advised in infants less than 9 months old; in individuals with a history of hypersensitivity to vaccine products, thymus disease, or human immunodeficiency virus (HIV); and in individuals who are pregnant, nursing, immunocompromised, older adults, or receiving other vaccines simultaneously.9,38,42 The CDC encourages health care providers to report febrile illness thought to be caused by the yellow fever vaccination to the CDC/FDA Vaccine Adverse Events Reporting System (VAERS) at http://vaers.hhs.gov/esub/index.
Eradication of A. aegypti via well-executed control campaigns in the 1970s has been successful in many Central and South American countries, with much credit owed to the Pan American Health Organization. However, vigilance must be maintained, because A. aegypti has reestablished itself in many of these areas. Much work must be done in Africa, where the mosquito has never been eradicated.117
Japanese Encephalitis
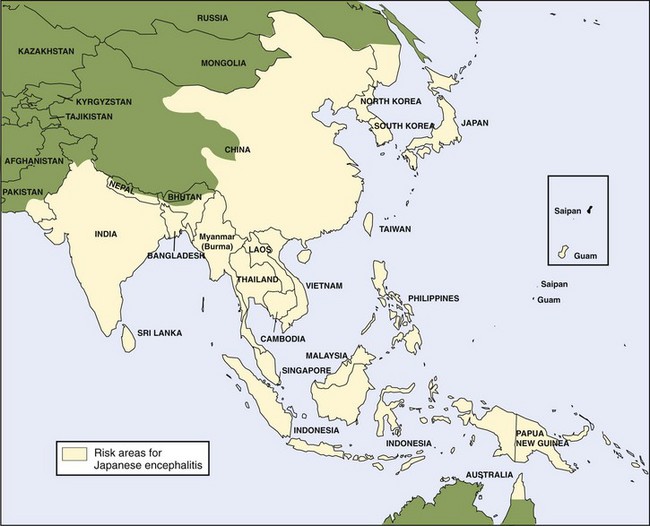
The Japanese encephalitis (JE) virus, a member of the Flavivirus genus, is the major cause of viral encephalitis in Asia, with as many as 50,000 cases reported every year. Although JE virus is a leading cause of viral encephalitis in Asia, there is less than one case per year in U.S. civilians and military personnel living in or traveling to Asia, with rare outbreaks in U.S. territories in the western Pacific. There has been more recent expansion of JE into northern Australia (Figure 48-13).39,40,81,135
Epidemiology and Transmission
Culex mosquitoes (usually Culex tritaeniorhynchus, C. vishnui, and C. pseudovishnui) are the vectors for this virus, and vertebrate hosts such as pigs and birds sustain the virus. Because humans are not necessary for transmission, the virus can still be maintained in an enzootic cycle even where JE immunity is high (either in endemic regions or through vaccinations) and human cases do not occur. The mosquitoes breed in flooded fields associated with rice production. Because these settings contain factors that enhance the JE transmission cycle (e.g., large numbers of pigs and birds to serve as reservoirs for infection), JE infection rates may be as high as 3% in these areas.117
JE has two main transmission patterns in Asia. Seasonal transmission occurs in the more temperate northern regions (e.g., Japan, People’s Republic of China, Taiwan, northern India) and can cause large epidemics. The second pattern of year-round transmission presents in tropical southern regions (e.g., southern Thailand, southern Vietnam, southern India, Indonesia, Malaysia, Philippines, Sri Lanka) and is usually more endemic or sporadic.54,117,159
Clinical Presentation
Persons at increased risk include individuals (residents, active duty military, or expatriates) living in endemic areas. Travelers have a low risk for acquiring the disease, estimated to be less than one in a million American travelers going to endemic areas.19 Most residents of endemic areas are infected during childhood or early adulthood,86 with about 10% of the susceptible population infected yearly. The disease appears to affect mainly children less than 15 years of age, although there also seems to be a secondary increase of disease in older adults, posited to be caused by waning immunity.19
The incubation period is from 5 to 15 days. Most infections with JE are subclinical. Early symptoms include sudden onset of fever, headache, nausea and vomiting, and abdominal pain. JE may also manifest with coryza, diarrhea, and rigors. In some individuals, abnormal behavior is the only clinical manifestation, so JE has been misidentified as mental illness.163 In the Korean conflict, for example, American military personnel with JE were sometimes diagnosed with “war neurosis.”113 After several days, JE may develop into aseptic meningitis with or without encephalopathy, or into encephalitis (less than 1% of infected individuals) presenting with delirium and abnormal motor movement. Children in particular seem to have more convulsions than do adults infected with the virus.56,107,126,164 In fact, 98.7% of children hospitalized for JE during a recent epidemic in India presented with seizures.109 Seizures are more often generalized tonic–clonic than focal.126 Abrupt onset of flaccid paralysis in one or more limbs has also been reported.166
The case fatality rate of JE is approximately 30%, and almost 50% of survivors have severe neurologic sequelae, including motor deficits, upper and lower motor neuron weakness, and cerebellar or extrapyramidal signs.40,163,164
Diagnosis
Laboratory analysis demonstrates that patients can have peripheral leukocytosis. Patients may also have hyponatremia secondary to the syndrome of inappropriate antidiuretic hormone secretion (SIADH). Fifty percent of patients have high CSF opening pressure on lumbar puncture, and analysis of CSF may show pleocytosis (predominantly lymphocytic) of 10 to 100 cells/mm3, with normal glucose and slightly elevated protein. With early disease, however, there may be no CSF pleocytosis, and leukocytes are predominantly neutrophilic.109,174
Definitive diagnosis is achieved by demonstration of IgM antibody by enzyme immunoassay antibody captured in CSF or serum; by a fourfold or greater rise in serum antibody titers against JE virus; or by isolation of virus from or demonstration of viral antigen or genomic sequence in tissue, blood, CSF, or other body fluid.174 Of note, it can be difficult to isolate JE virus from blood because of the low viral burden. The most common method of diagnosing JE in the past was with a serologic hemagglutination inhibition test. Since the 1980s, however, IgM-capture ELISA has been used, and confirmation is recommended with another assay for IgG antibody. One recent study looked at anti-JE virus IgM levels in both serum and CSF of 155 confirmed JE cases in Thailand between 2002 and 2004. Specific IgM was detected in nearly all serum samples (23/26) collected at least 9 days after symptom onset, and in all but one CSF sample collected at least 7 days after symptom onset. In fact, in most JE cases, anti-JE virus IgM was detectable in CSF as early as day 1 after symptom onset.45
Both noncontrast and contrast computed tomography (CT) and magnetic resonance imaging (MRI) may show nonenhancing low-density areas in the thalamus, basal ganglia, midbrain, pons, or medulla, and an electroencephalogram may show theta and delta coma, burst suppression, and epileptiform activity.97,125,127,160
Treatment and Prevention
There is no specific treatment for JE beyond supportive care. Other modalities, such as reducing intracerebral pressure with mannitol and providing seizure prophylaxis, have been suggested by some experts, but there have been no studies on agents to use and whether these interventions are beneficial in JE.117,174 Therapies such as corticosteroids, interferon-α, and ribavarin have all been studied in randomized, double-blind, placebo-controlled trials. JE patients do not seem to benefit from any of these treatment measures.87,108
In March 2009, a new inactivated Vero cell culture–derived vaccine (Ixiaro) was approved for use in the United States for individuals 17 years and older.34,172 This vaccine has not been studied in children. It produces a 97% seroconversion rate after two doses administered 28 days apart. In a multicenter, multinational randomized clinical phase III trial comparing Ixiaro with JE-Vax, the vaccines were shown to have equal clinical efficacy and safety profiles.172
Historically the most widely available JE vaccination has been an inactivated mouse brain–derived vaccine (JE-Vax) typically prepared using the Nakayama or Beijing-1 strain of JE virus. This was the only vaccine available to international travelers (adults and children older than 1 year of age) from nonendemic countries. For adult travelers, the recommendation for primary immunization is a series of three subcutaneous vaccinations of 1.0 mL each (0.5 mL for children 1 to 3 years of age), on days 0, 7, and 30 (an abbreviated schedule of days 0, 7, and 14 is used if the longer schedule is not feasible). Although the literature regarding this vaccine’s dose-dependent efficacy is somewhat conflicting, one recent retrospective study evaluated over 30 years of JE-Vax use in Taiwan. They estimated that the vaccine’s long-term efficacy was 87% for even a single dose, whereas it was 98% effective for the three-dose series.192 The last dose should be given at least 10 days before the actual date of travel to allow a sufficient immune response. There have been no studies on administering this vaccine to infants younger than 1 year of age.18
JE-Vax has a low rate of side effects.18 It is associated with a moderate frequency of local adverse effects, as well as mild systemic effects (e.g., fever, headache, malaise, rash), in 10% to 20% of vaccinated individuals. More serious events, including angioedema, can appear up to 2 weeks after administration and sometimes after the second or third dose when the first was received without complications. There have also been concerns about vaccine-related neurologic events, including encephalitis, seizures, and even death.146,162,92 WHO estimates that the rates of such severe adverse reactions are in the range of 18 to 64 per 10,000 vaccinated individuals.92 However, since the 1990s, the reported rate for all adverse events for this inactivated vaccine is 2.8 per 100,000 in Japan and 15 per 100,000 in the United States.117 The usefulness of this vaccine is further limited due to high production costs and the need for serial doses in addition to booster doses. At this time, JE-Vax is no longer being produced and given a shortage of supplies, the CDC recommends that this vaccine now be used only in children 1 to 16 years old.40
A live, attenuated vaccine based on the strain SA14-14-2 is also available. This vaccine has been used in China for over 20 years and has recently been licensed in Nepal, Sri Lanka, South Korea, and India. Numerous large field studies and case-control studies have shown efficacy of over 95% following two doses given 1 year apart. WHO estimates that this vaccine was helpful in reducing the burden of JE in China from 2.5 per 100,000 in 1990 to less than 0.5 per 100,000 in 2004.140,153,162,92
It is difficult to assess which travelers should receive the vaccine. The low rate of human disease must be balanced against the severe effects of JE infection. Furthermore, the JE infection rate in travelers is low, and there are legitimate concerns regarding the side effects of vaccination. The vaccine should not be given to all travelers to Asia; according to the CDC, it should be offered to those persons older than 9 months staying a prolonged amount of time (>1 month) in endemic areas during transmission season, and if travel includes rural areas. Persons staying less than 1 month but with specific risk factors for exposure (e.g., extensive amount of time spent outdoors during seasons of peak transmission) may also consider vaccination.18
West Nile Virus
First identified in 1937 from the blood of a febrile woman in the West Nile province of Uganda, WNV is a flavivirus that before 1999 was found only in Africa, West Asia, and the Middle East. WNV has received significant press in North America because of its intrusion into the United States, with the first report in the summer of 1999 in New York.5,137 The most accurate epidemiologic trend data include neuroinvasive disease (meningitis/encephalitis), because most patients with West Nile fever (WNF) are asymptomatic and do not seek care. Through 2007, more than 11,000 cases of WNV neuroinvasive disease have been reported, and it is estimated that 1.5 to 3 million individuals have been infected in the United States alone. Since 1999, cases of human disease have been found in all 50 of the United States except Hawaii, Alaska, and Maine.37,142
Epidemiology and Transmission
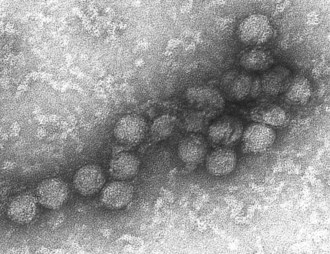
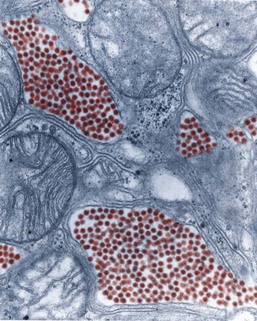
WNV is a single-stranded RNA virus that is part of the family Flaviviridae, genus Flavivirus. All flaviviruses have a size of approximately 40 to 60 nm (Figure 48-14), are enveloped with icosahedral nucleocapsids, and have single-stranded RNA of about 10,000 to 11,000 bases.37
Mosquitoes are the vectors for this virus, and birds are the most common reservoir. The most commonly named vector is the Culex mosquito species, but WNV has recently been identified in more than 64 different mosquito species in North America, which contributes to its permanent establishment and wide distribution in the United States (Figure 48-15). The different species of mosquitoes have varying host preferences and behavior, and frequent transmission to migrating birds extends the reach of the virus.70 Newly infected birds sustain viremia for about 1 to 4 days (after which they develop lifelong immunity), and during that time, mosquitoes can contract the virus. Humans and other mammals such as horses are considered incidental or “dead-end” hosts, because they often do not sustain viremia long enough to serve as reservoirs for the virus. The effects on nonhuman life can be significant; the 2002 WNV epidemic and epizootic in the United States, for example, reported 16,471 dead birds and 14,751 equine cases. In horses, as in humans, WNV crosses the blood–brain barrier and interferes with CNS functioning, ultimately leading to possible death.26 Another recent study conservatively estimated the American crow population to have declined 45% since WNV arrival, and only two of the seven avian species documented to have been impacted returned to pre-WNV levels by 2005.111
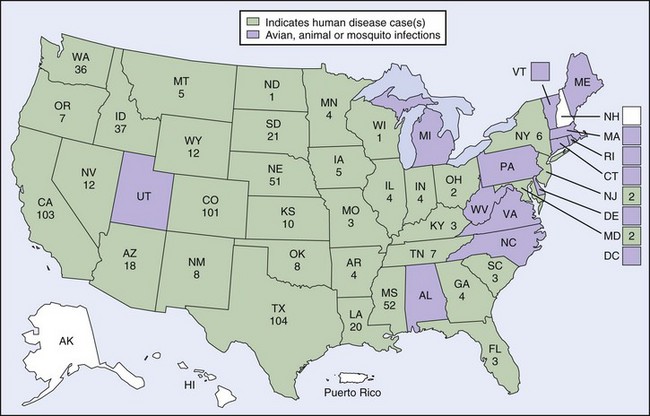
FIGURE 48-15 2009 West Nile virus activity in the United States.
(From http://www.cdc.gov/ncidod/dvbid/westnile/Mapsactivity/surv&control09Maps.htm.)
Other, rarer methods of transmission include organ transplantation,28,89 blood transfusion,24,25,141 transplacental infection,22 and possibly human breast milk.23 Because transmission from blood transfusions reached 2.7 cases per 10,000 units during the peak of the 1999 outbreak in New York, and because there were confirmed cases of WNV transmission to organ transplant recipients, routine screening of blood donors for WNV RNA began in the summer of 2003. In 2003 and 2004, 519 donors were identified by this method, resulting in removal of more than 1000 potentially infectious units of blood products from the Red Cross supply. All donated blood continues to be screened for WNV with nucleic acid amplification tests, either on individual samples or on “minipools” of up to 16 donations.169 A recent study of targeted nucleic acid amplification testing of individual donations in high-prevalence regions suggests that this approach may be cost-effective, because units with low levels of viremia may not be detected in minipools of blood donations.17 Despite reduction in transfusion-related transmission with universal screening of blood, this limitation has prevented eradication and cases are still reported.31
Clinical Presentation
The virus has an incubation period of 3 to 14 days.143 Disease usually occurs in temperate zones either in late summer or early fall, or year-round in milder climates. Clinical syndromes caused by WNV include asymptomatic infection, WNF, and West Nile neuroinvasive disease (WNND). Most WNV infections in humans are asymptomatic. Of the 20% of symptomatic cases, the majority of patients develop WNF, which usually involves a mild influenza-like illness with malaise, headache, nausea, vomiting, anorexia, lymphadenopathy, and rash. In a case-series review of 98 community-dwelling patients with laboratory evidence of WNV infection but no evidence of neuroinvasive disease, fatigue was present in 96% of patients, fever in 81%, headache in 71%, and difficulty concentrating in 53%.181 Even in cases of WNF, the impact on quality of life can be severe. Of the 98 patients, 39% reported ongoing symptoms at 5 month follow-up, 82% reported limitations in household activities, and there was a median of 10 days of missed school or work.181 In another follow-up study done in a population-based cohort of 656 nonfatal cases, 91% of patients reported limitations in routine daily activities secondary to WNV infection. Moreover, in cases of WNF alone, there was a median number of 16 school or work days missed, and 53% of this subset of patients reported symptoms of at least 30 days’ duration. The impact was even more significant in patients with WNND.139
West Nile neuroinvasive disease occurs in less than 1% of individuals infected with WNV and includes encephalitis, meningitis, meningoencephalitis, and acute flaccid paralysis/poliomyelitis.50 Older adult and immunocompromised patients are at increased risk for severe neurologic disease from WNV. In a retrospective medical chart review of 221 hospitalized WNV patients, 16% of those with meningitis and 32% of those with encephalitis had autoimmune disease, were immunosuppressed, or were organ transplant recipients. Age over 50 years, alcohol abuse, and diabetes were also found to be associated with WNND.12
Clinical criteria for WNND are listed in Table 48-2. Although the clinical presentation of meningitis and encephalitis in WNV is nonspecific, one particular feature of WNV encephalitis is that it may manifest with parkinsonian features or other movement disorders, such as myoclonus or intention tremor. In a small prospective case-series, 94% of WNV-seropositive patients experienced dyskinesias compared with 13% in the control group.156 Another clinical syndrome associated with severe WNV disease is acute flaccid paralysis, which can rapidly progress to paralysis of one or up to all four limbs. It may also manifest as severe, asymmetric weakness, usually with sensation preserved. Affected limbs show decreased or completely absent deep tendon reflexes, most likely from anterior horn cell involvement similar to that seen in poliomyelitis.50,70,155,156
TABLE 48-2 Criteria for Clinical and Definite Diagnoses of West Nile Neuroinvasive Disease
| Possible Clinical Diagnosis of West Nile Neuroinvasive Disease (before serology results available) |
| Requirements: 1, 2, 3 (A, B, or C), 5 with 4 supportive |
| New onset of: |
|
3 Acute neurological signs and symptoms (A, B, or C required)
|
From Davis LE, DeBiasi R, Goade DE, et al: West Nile virus neuroinvasive disease, Annals of Neurology 60:294, 2006.
Other clinical findings may include ocular complications (e.g., optic neuritis, chorioretinitis, retinal hemorrhage, uveitis, conjunctivitis),44,102,191 bladder dysfunction,70 myocarditis, pancreatitis, and fulminant hepatitis.143
Diagnosis
For public health and surveillance purposes, the CDC uses specific criteria for arboviral infections and includes disease caused by California serogroup viruses, eastern and western equine encephalitis viruses, Powassan virus, St Louis encephalitis virus, and WNV. This was most recently revised in 2004 (Box 48-1).35
BOX 48-1
2004 CDC Case Definition for Neuroinvasive and Non-Neuroinvasive Domestic Arboviral Diseases
Clinical Criteria for Diagnosis
Laboratory Criteria for Diagnosis
Confirmed Case
From Centers for Disease Control and Prevention. http://www.cdc.gov/ncphi/disss/nndss/casedef/arboviral_current.htm.
Typical findings for severe disease usually include normal or slightly elevated leukocyte counts in peripheral blood, possible hyponatremia in individuals with WNV encephalitis, pleocytosis and increased protein in the CSF, and no findings on CT scans of the brain.37,143 Although MRI may yield abnormal results in 25% to 35% of cases, findings may be nonspecific.37,196 For definitive diagnosis, serum is ideally collected within 8 to 14 days of symptom onset, and CSF is collected within 8 days to give the highest diagnostic sensitivity. Serum or CSF should show IgM antibodies to WNV by IgM-capture ELISA (MAC-ELISA). There is, however, a chance that persistent IgM antibodies can be found in people years after living in places where WNV is epidemic. The diagnosis can be further confounded by the fact that these IgM ELISA tests have a cross-reactivity of up to 44% with other flaviviruses during acute infection.37,70
The most specific test for arthropod-borne flaviviruses is the plaque-reduction neutralization test (PRNT). It can be used both to negate the false-positive results sometimes seen in MAC-ELISA or other assays and to facilitate discrimination among the flaviviruses.37
Efforts have been made to develop more specific and rapid serologic assays. Promising candidates include a microsphere immunoassay that discriminates among different flavivirus infections, as well as an immunofluorescence assay that has less cross-reactivity among WNV IgM antibodies and closely related flaviviruses. PCR (as used for screening blood products in the United States) or viral culture can also isolate the virus, but sensitivity is not high because of the transient and low levels of viremia, because the virus quickly disappears from the periphery into the CNS in severe disease.37,143 However, one recent study showed that the combination of serologic assay and nucleic acid testing allowed for more accurate identification of WNV cases compared with either approach alone.173 Such studies have recommended that these measures be used as an adjunct to serologic testing.
Treatment and Prevention
Currently there is no specific treatment for WNV, and supportive care remains the mainstay of therapy.66 Certain antivirals, antibodies, and drugs altering the inflammation process (e.g., interferon) are being tested. Nucleoside analogs have been studied in vitro,2,134 but the most advanced testing in vivo has been with ribavirin, with no reduction in disease88 or viremia116 shown. In a retrospective, uncontrolled, nonblinded study of 233 human WNV cases, ribavirin was associated with poorer outcome in bivariate analysis. Although this effect was not seen in multivariate analysis, ribavirin was ineffective.48 Similarly, there have been promising in vitro studies and human case reports evaluating the usefulness of interferon-α2,96,133 and intravenous immunoglobulin,1,10 but these treatment options remain controversial. Currently there are two randomized, double-blind, placebo-controlled studies being conducted to evaluate the potential therapeutic effect of interferon and a monoclonal antibody. Information regarding these trials can be found at http://www.nyhq.org/posting/rahal.html and http://clinicaltrials.gov/ct2/show/NCT00927953, respectively.
For prevention of disease, general control measures such as blood donor screening and precautions against mosquitoes can be taken, but so far there is no specific way to prevent WNV. Although vaccines have proved efficacious in multiple animal species, unfortunately no human vaccine exists. Theoretically there is potential for an inactivated virus to induce long-term immunity, because such a vaccine exists for JE and other flavivirus diseases. Multiple candidate vaccines against WNV are being evaluated, a couple of which have shown promise in small human studies. These include a chimeric virus (ChimeriVax-WN) based on the live, attenuated yellow fever 17D vaccine virus containing the premembrane and envelope proteins of WNV, and a single-plasmid recombinant DNA vaccine. ChimeriVax-WN was evaluated in a randomized, double-blind placebo-controlled study of 45 healthy adults. The vaccine was well tolerated, and within 21 days of receiving a single inoculation dose, all study subjects developed high titers of WN-specific neutralizing antibodies. In addition, 43 of 45 participants developed a WNV-specific T-cell response.130 The DNA vaccine was recently evaluated in an open-label phase 1 trial of 15 healthy adults. The vaccine was well tolerated, and in the 12 participants that completed the three-dose vaccination schedule, all developed WNV-specific neutralizing antibodies.120 Other WNV vaccines under development include a candidate live-attenuated chimeric WN vaccine that uses an infectious dengue virus type 4 cDNA clone as a backbone and a recombinant subunit WN vaccine.188
Surveillance and Reporting
Reporting requirements for suspected WNV infections vary across local and state health departments, but WNV encephalitis is on the list of designated nationally notifiable conditions. The CDC may use results from commercial laboratories but may also request samples for diagnosis in its own laboratory. Information on how to send specimens can be found at http://www.cdc.gov/ncidod/dvbid/misc/arboviral_shipping.htm.
St Louis Encephalitis
Epidemiology and Transmission
The Culex mosquito is the main species that transmits StLE virus, with birds as a reservoir and humans as another host. The viremia, however, does not cause illness in birds or mosquitoes. Around the Gulf Coast, Ohio and Mississippi River Valleys, the culprit mosquitoes are Culex pipiens and C. quinquefasciatus; in Florida, C. nigripalpus; and in the western states, C. tarsalis.30
StLE virus is found throughout much of the Americas. In temperate parts of the United States, disease usually occurs in late summer or early fall, but it can occur year-round in the southern United States. Outbreaks can occur almost anywhere in the United States, though epidemics have occurred mainly in the Midwest and Southeast. Between 1964 and 2008, less than 5000 human cases of StLE were reported in the United States.32,30 In some areas, it appears that WNV is displacing previously endemic StLE because of cross-protective avian herd immunity and differences in transmission efficiency.149 Historically few cases were reported from Central and South America, although there have been recent large outbreaks in both Argentina and Brazil.55,132,167
Clinical Presentation
StLE has an incubation period of approximately 5 to 15 days, with a 300 : 1 ratio of asymptomatic to symptomatic cases. Risk factors for infection include low socioeconomic status, outdoor occupations, and older age. Patients with symptomatic StLE present with high fever and headache, aseptic meningitis and encephalitis. Infants in particular may have occasional convulsions and spastic paralysis. Of recognized cases, 95% are hospitalized for CNS involvement. The case fatality rate is 3% overall and up to 30% in older adults.30
Diagnosis
As with other arboviral diseases, clinical and epidemiologic features must be taken into account to make a presumptive diagnosis. However, StLE may be difficult to differentiate on clinical features alone. Thus definitive diagnosis is typically obtained by testing serum or CSF for serotype-specific monoclonal antibodies (IgM ELISA). Further confirmatory tests, such as demonstrating at least a fourfold increase in antivirus antibody titer between acute and convalescent period, may be done.30 Antigenic cross-reactivity with other flaviviruses is a known problem, encouraging some facilities to use PRNT or develop discriminatory algorithms to differentiate infections.119 A potential additional option for diagnosis includes a newly developed rapid microsphere-based IgM assay that would shorten the test processing time to less than 5 hours.93,94
Treatment and Prevention
As is the case for many viral illnesses, treatment is supportive care, because there are neither specific antiviral therapies nor vaccines. One interventional pilot study of 15 patients with meningoencephalitis due to StLE showed that treatment with interferon-α2b reduced severity and length of complications. Given the small number of participants and the nonrandomized nature of the study, further investigation must be conducted.148
Eastern Equine Encephalitis
First discovered in the brain of a horse with encephalitis in 1933 in New Jersey, eastern equine encephalitis (EEE) is a serious mosquito-borne disease that appears in the eastern half of the United States with a high case fatality rate. The virus is a member of the family Togaviridae, genus Alphavirus, and it is related to western and Venezuelan equine encephalitis viruses.118
Epidemiology and Transmission
The transmission cycle involves birds and several species of mosquitoes, predominantly Culiseta melanura (Figure 48-16). Other possible mosquito vectors include species in the genera Aedes and Coquillettidia.118 Humans and horses are considered hosts with incidental infections that can progress to severe disease.
Between 1964 and 2008, there were approximately 257 confirmed cases in the United States, with an average of 5 cases per year. Most of the cases have been in Florida, Georgia, Massachusetts, and New Jersey, near coastal areas and freshwater swamps. The real incidence is probably higher because of underreporting and underrecognition. In fact, in 2005, 21 cases were reported to the CDC, compared with an average of 8.2 cases per year from 2000 to 2004. This included an outbreak of 7 cases in New Hampshire, a state which was previously not known to have locally acquired EEE.32,29,41
Clinical Presentation
Clinical presentation can be within the spectrum from mild influenza-like illness to encephalitis, coma, and death. One study collecting the 36 cases from 1988 to 1994 in the United States showed that clinical signs included fever (83%), headache (75%), nausea and vomiting (61%), confusion (4%), myalgias and arthralgias (36%), chills (25%), seizures (25%), focal weakness (23%), abdominal pain (22%), respiratory symptoms (11%), and cranial nerve palsies (8%).53 The case fatality rate for EEE is 33%, making it the most severe of the arboviral encephalitides. Moreover, 50% of individuals who survive are left with mild to severe neurologic deficits.41,53
Diagnosis
Peripheral blood may show leukocytosis, and analysis of CSF may reveal elevated protein with red and white cells in the range of 600 to 2000/mm3 (white cells being predominantly lymphocytes). Preliminary diagnosis may be completed by demonstration of IgM antibodies by capture ELISA. Definitive diagnosis lies in serologic evidence based on paired sera (acute and convalescent samples) in hemagglutinin-inhibition assays or in neutralization assays. Acute samples should be obtained within 10 days of symptom onset, and convalescent samples about 2 or 3 weeks after the symptom onset. It is possible to isolate the virus directly from the serum in acute infection, but this method is used less commonly.20,35,118
Other helpful diagnostic modalities include MRI, which may show focal changes, particularly in the basal ganglia, thalamus, and brainstem.53,68
Treatment and Prevention
No specific treatment is available. There has been one case report of a child being treated with corticosteroids and intravenous immunoglobulin,68 but this has not been further studied. No human vaccine is available, but there are equine vaccines that should be employed in endemic areas.
Australian Encephalitis (Murray Valley Encephalitis)
Clinical Presentation
Most infections are asymptomatic, and only 1 in 1000 to 2000 infections actually develops into clinical illness.115 The clinical course is similar to that of JE, with headache, fever, myalgias, nausea and vomiting, and anorexia. The neurologic involvement can involve lethargy, irritability, confusion, or meningismus, as well as seizures, spastic paresis, and coma. Individuals who succumb to progressive MVE infection have a poor prognosis; more than 30% die and 30% to 50% suffer neurologic sequelae, with a bimodal risk for children and older adults.57
Diagnosis
As is true for many other viral illnesses, definitive diagnosis of MVE is by identifying IgM antibody via immunoassay of the CSF or a fourfold rise in serum antibody titers. Direct viral isolation or PCR can also prove the infection in tissue, blood, or CSF. However, these tests can be obscured by cross-reactive antibodies to Kunjin, JE, Edge Hill, Alfuy, Sepik, dengue, and other flaviviruses.177
Two-thirds of patients have normal CT scans. If the scans are abnormal, they may show nonspecific signs of encephalitis, including mild hydrocephalus and cerebral edema.103
California (La Crosse) Encephalitis
La Crosse (LAC) virus is a California serogroup bunyavirus that can cause encephalitis (usually in children) in the central and eastern United States. La Crosse encephalitis constitutes 8% to 30% of all cases of encephalitis in the United States, and is one of the major causes of arboviral illness.152 In fact, its incidence in endemic areas (20 to 30 cases per 100,000 population per year) exceeds that of bacterial meningitis.124
Epidemiology and Transmission
Aedes triseriatus, the eastern tree hole mosquito, is the main vector, although A. albopictus, or Asian tiger mosquito, may be emerging as another significant species. Vertebrate hosts include chipmunks, tree squirrels, and foxes. The annual incidence in the United States is about 80 to 100 cases.27 Most infections are asymptomatic and occur from late summer to early fall in the central and eastern United States.21,27,59
Clinical Presentation
The majority of cases are children less than 15 years of age.27,78,122 For 80% to 90% of infected persons, the clinical course is mild, with only headache, fever, and vomiting after an incubation period of 3 to 7 days. Approximately 10% to 20% of infected individuals develop a severe form of the disease within the first 8 to 24 hours of symptom onset.123 In one pediatric study of 127 hospitalized children, it was noted that 70% of patients presented with headache, fever, and vomiting. Of children, 46% had seizures (generally focal seizures, with some progression to status epilepticus), and 20% had focal neurologic findings. Encephalitis does not always occur, because aseptic meningitis was also found. Although all patients survived, over 10% had neurologic deficits at time of hospital discharge, and preliminary analysis suggested patients may suffer long-term cognitive and behavioral deficits as well.122 Only one case report has mentioned California encephalitis causing infarction of the basal ganglia, leaving a child with acute hemiparesis.112 The CDC recently described a case of possible congenital infection after identification of IgM antibodies in umbilical cord serum. The mother declined collection of infant serum to document seroconversion, but fortunately the infant remained asymptomatic and development was normal at 6-month follow-up. The CDC reports the case fatality rate to be less than 1%, although recent studies have found this to be an underestimate.36,77,78
Diagnosis
Laboratory findings may reveal leukocytosis with polymorphonuclear leukocyte predominance and pleocytosis in the CSF (either neutrophilic or lymphocytic predominance), although studies of laboratory values for LAC and non-LAC cases show no statistically significant differences.82 More than one-half of patients have abnormal electroencephalographic findings. A CT scan may show nonspecific cerebral edema, if there are any findings at all, and MRI may reveal gadolinium enhancement in cortical areas.122
Definitive diagnosis is made with IgM antibody by capture immunoassay of CSF, a fourfold rise in serum antibody titers against LAC virus, or isolation of virus from or demonstration of viral antigen or genomic sequences in tissue, blood, or CSF.20 As in almost all other viral infections discussed in this chapter, IgM antibody capture with ELISA is the most common method, with the recommendation to confirm IgG antibody with another serologic assay such as a neutralization test.
Epidemic Polyarthritis (Ross River Virus)
A mosquito-borne alphavirus in the family Togaviridae, Ross River virus (RRV) is a single-stranded, enveloped RNA virus in the same family as chikungunya, Sindbis, and eastern and western equine encephalitis. The virus is endemic and enzootic in Australia and Papua New Guinea and causes epidemic polyarthritis.84 It is the most common human arboviral disease in Australia, causing approximately 5000 reported cases a year.175 In the late 1970s, RRV spread to the South Pacific islands, but it disappeared after the end of an epidemic in which 500,000 people were infected; recent reports, however, show that there are new cases reappearing in Fiji.104
Epidemiology and Transmission
The main vectors are Culex and Aedes (particularly Aedes vigilax) mosquitoes, and marsupials such as kangaroos and wallabies are important as vertebrate-amplifying hosts.151 Vertical transmission is possible from mosquito to offspring, and human-mosquito-human transmission is also thought to occur. Tropical coastal regions with salt marsh habitats are the primary habitats for the mosquito vector species, and infections are most common in the spring, after summer rains, or after inundation of salt marshes by rain or tides.104
Clinical Presentation
After inoculation, the virus is thought to replicate within macrophages. It causes local cell-mediated immune responses that appear to be the cause of arthritis. After an incubation of 3 to 9 days (or as long as 21 days), patients can present either with acute febrile illness with arthritis and rash; with acute fever, rash, or arthritis alone; with polyarthralgia; or with arthritis.63 The joints involved are usually symmetric and include the wrists, knees, ankles, and metacarpophalangeal and interphalangeal joints of the hands, with true arthritis present in 40% of infected individuals.170 About 50% have fever and a sparse maculopapular rash on the limbs and trunk that may occur before or after the arthritis. Whereas fever may resolve within several days, rash may persist for several months. Joint symptoms and fatigue, on the other hand, have been reported to last years, even after fever and rash have subsided.118 However, recent literature suggests that patients with chronic symptoms may have additional underlying comorbidities, such as rheumatic disease or depression.85,136,170
Diagnosis
There are no laboratory findings specific for RRV infection. Patients often have normal leukocyte counts (although mild neutrophilia or atypical lymphocytes can be found) and possibly elevation in the nonspecific erythrocyte sedimentation rate. Joint aspiration may reveal viscous fluid with predominantly mononuclear cells and points to viral arthritis.63
Serum can be screened for IgM antibody against Ross River virus IgM, which can then be confirmed by PRNT. These tests are available at only a few reference laboratories, including the CDC.104 Viral culture and PCR are not commonly used for diagnosis of RRV infection.
Treatment and Prevention
There is no specific treatment. Management of arthralgias and myalgias is with standard analgesic and nonsteroidal antiinflammatory drugs. There does not seem to be any direct association of mortality with RRV. As with other arboviral diseases, prevention depends on adequate mosquito control and avoidance of mosquito bites. One recent prospective case-control study showed that protective measures such as mosquito coils, repellants, and citronella candles decreased this risk for RRV infection twofold with a dose response for the number of protective measures used. In addition, light-colored clothing decreased risk threefold, whereas camping in the 3 weeks before symptom onset increased the risk eightfold.83 There has been additional research on understanding the environmental, anthropogenic, and social factors responsible for disease outbreaks. Some believe factors such as climate variability can affect virus replication, vector demographics, and even human behavior, each of which can be modeled to predict onset and severity of RRV epidemics. Early warning systems could potentially enable improved disease control measures.*
Jamestown Canyon Virus
Jamestown Canyon virus is a California serogroup bunyavirus widely distributed throughout the United States and Canada. White-tailed deer, Odocoileus virginianus, are the principal vertebrate host and boreal Aedes and Ochlerotatus mosquitoes are the primary vectors.3,4,64 Documented human infections are scant, but the virus usually causes a mild febrile illness, and rarely meningitis and encephalitis. There is a case report of Jamestown Canyon virus–induced encephalitis in an 8-year-old girl in rural southwestern Michigan, who presented with symptoms of headache and fever that quickly progressed to seizures and coma.71 The infection is difficult to distinguish from other California serogroup bunyaviruses, such as California encephalitis, snowshoe hare, Keystone, and trivittatus viruses, because of cross-reactivity in the more common serologic tests and difficulty in isolating the actual virus.178 The virus has been posited as a possible emerging infectious disease,71,121 but there have been few identified cases and the literature on this subject is scant.
Mosquito Control
General Guidelines for Individual Protection
For personal protection and prevention, individuals can take precautions such as avoiding mosquitoes at their most active time (from dusk to dawn) and wearing loose-fitting cotton clothing covering their arms and legs (see Chapter 47). Individuals may also apply repellents to exposed areas of skin. The most effective preparations contain N,N-diethyl-3-methylbenzamide (DEET),144 and generally concentrations need not exceed 20% to 35%.62 DEET is safe in children older than 2 months of age and pregnant women.37,106 However, it can rarely cause neurologic toxicity. Thus it should be kept away from mucous membranes and used sparingly to avoid systemic absorption. Studies have shown that a single application of a product containing 24% DEET confers an average of 5 hours (and up to 12) of protection against A. aegypti mosquitoes. Compared with DEET, newer formulations containing the compound IR3535 fared poorly.62 Picaridin (KBR 3023), a shorter-acting plant-derived repellent, is thought to be as effective as DEET and better tolerated.6,98 Agents such as p-menthane-3,8-diol (PMD) and BioUD have not been evaluated as well but are thought to be less effective compared with DEET. Permethrin-containing compounds and other residual insecticides that kill rather than simply repel mosquitoes may be applied to clothing and netting. Other strategies include using mosquito coils and sprays containing pyrethroids in sleeping areas. Citronella has been also shown to be mildly effective in reducing the number of bites, but it requires frequent applications if used topically (as opposed to the candle form).61 Current research in insect repellents involves sophisticated appreciation of the olfactory senses of mosquitoes and creating proteins to inactivate human-specific odorants.98,154
Global Programs
In general, global eradication programs17 for all infectious diseases, including mosquito vector surveillance and control, have declined dramatically in the past few decades. Because of the success of control programs in the 1970s and the resultant decreased public health threat, less attention has been paid and fewer resources have been allocated to maintaining good control. One result is fewer trained personnel, because training programs are less well funded. The effect has been detrimental, with a reversal of previous gains. In Central and South America, for example, dengue fever was largely eliminated with eradication in the 1950s and 1960s of the A. aegypti mosquito, the main vector for the disease. However, because these programs were largely dismantled in the 1970s, the mosquito species has reinfested most of these tropical regions.73
Vector control has three arms: environmental management, biologic control, and chemical control.105 Environmental management consists of monitoring and intervening to reduce the vector population, as well as transmission factors that sustain the human-vector-pathogen contact. For example, simply removing old tires, covering water storage containers with tight lids, keeping floor drains cleaned and covered, and chlorinating ornamental pools and fountains (or populating them with larvivorous fish) can help prevent the breeding of mosquitoes.
Chemical control is the most well-known vector control. It consists of using products such as pyrethrins (e.g., deltamethrin, resmethrin, permethrin) or organophosphate insecticides (e.g., fenthion, malathion, fenitrothion, temephos). These can be applied in several forms: larvicidal application, perifocal treatment, or space spraying. Larvicidal application is focal treatment to domestic water supplies that cannot be eliminated (e.g., for A. aegypti, either 1% temephos or methoprene can be used to regulate larvicidal growth, or BTI can be used to safely treat drinking water). Insecticides such as fenthion, malathion, and fenitrothion can be used as perifocal treatment for nonpotable sources of water to eliminate larvae and adult mosquitoes. Finally, space spraying can be used against adult mosquitoes, often in emergency situations. The chemicals mentioned here can be used as aerosols or mists applied by portable machines, vehicle-mounted generators, or aircraft. Parameters for use (e.g., grams of active ingredient per hectare, wind conditions that may limit effectiveness) depend on the type of chemical, equipment, and target vector. Table 48-3 shows selected insecticides and dosages for cold-spray control of A. aegypti. Precautions must be taken seriously when using these products, such as following instructions on labels, using physical protection such as gloves and masks, and correctly disposing of chemicals.187
TABLE 48-3 Insecticides and Cold-Spray Control of Aedes aegypti
| Insecticide | Dosage (Grams of Active Ingredient per Hectare) |
|---|---|
| Organophosphates | |
| Malathion | 112-693 |
| Fenitrothion | 250-300 |
| Naled | 56-280 |
| Pirimiphos-methyl | 230-330 |
| Pyrethroids | |
| Deltamethrin | 0.5-1 |
| Resmethrin | 2-4 |
| Bioresmethrin | 5 |
| Permethrin | 5 |
| Cypermethrin | 1-3 |
| Lambda-cyhalothrin | 1 |
From World Health Organization: Dengue haemorrhagic fever: Diagnosis, treatment, prevention and control, ed 2, Geneva, 1997, World Health Organization.
Integrated vector control programs that incorporate all three methods, along with public education and campaigns, are the most effective ways to control disease.183 One recent study demonstrated success of a low-technology strategy for vector control involving community education and participation, as well as biologic control of mosquito larvae using Mesocyclops copepods. Although A. aegypti and dengue transmission were eliminated in 32 rural Vietnamese communities, it remains unclear and unlikely that such a strategy would be as effective in more urban or westernized areas.80,99 Nevertheless, such integrated initiatives should also encourage vaccination, not only of humans, but of the animal reservoirs for which vaccines are available. This includes, for example, equine vaccinations against West Nile virus.
Recently, leading health organizations have been trying to reinstate vector and disease control, as can be seen by a short history of their efforts. In the 1950s, WHO embarked on a mission to eradicate malaria. Despite some early success, by the mid-1970s, it was increasingly difficult to achieve the eradication goal for a number of reasons, including resistance to DDT and other insecticides. However, in 1998, WHO, the United Nations Development Programme, the World Bank, and UNICEF established the Roll Back Malaria initiative with a special focus on Africa. One arm of the campaign is to encourage widespread use of antimalarial drugs in clinics and health centers with a simple diagnosis. The other objectives are to plan and implement sustainable vector control. Experience has shown that pyrethroid-impregnated bed nets may be a better global strategy for eradicating disease, because this approach is less expensive than repeated spraying of household walls, reduces infections (and thus the human reservoir for infection in malaria), and is more effective in terms of horizontal implementation than top-down, or vertical, programming.14 Although source reduction through environmental management has been shown to be effective in some places,180 it is generally very difficult and may not be feasible everywhere.157
Resistance to insecticides is a very real problem. However, it is often a local phenomenon, so certain chemicals and insecticides should not be completely abandoned once reports of resistance are raised. For example, sampling sites only a few kilometers apart in Guatemala showed a large difference in resistance for Anopheles albimanus mosquitoes. Similarly, in the United States, resistance of Culex species to organophosphates is high in areas where vector control is well implemented, but is lower in rural areas.73
WHO has developed bioassays to determine resistance and keeps a database of resistance. This database, however, can be misleading because it is based on a single dataset from a single point in time that may be several years, or even decades, old and no longer relevant.73 There are newer diagnostic methods to test resistance, including genetic linkage and physical maps, that may elucidate factors in vector competence.15
A further step is to detect and contain epidemics through epidemiologic surveillance and then to train personnel and build local capacity to sustain these efforts. Vector surveillance is of primary importance, not only to learn the geographic distribution and density of mosquito vectors and to evaluate control programs but also to predict and intervene to stop the advance of preventable diseases. For mosquitoes, indices have been created to study immature and adult populations (e.g., the “house index” [percentage of houses infected with larvae or pupae], and an index of adult mosquitoes’ landing or biting rates per person-hour). Surveillance also includes verification of control measures, which includes periodically testing the vector’s susceptibility to certain insecticides. Ongoing inspection of areas free of disease and taking measures to prevent reinfestation by vectors (e.g., removing standing water sources and environmental habitats, such as tires and cemetery vases) should be instituted.189 A list of references the WHO currently uses toward the goal of integrated vector management can be found at http://www.who.int/malaria/publications/vector-management/en/index.html.
1 Agrawal AG, Petersen LR. Human immunoglobulin as a treatment for West Nile virus infection. J Infect Dis. 2003;188:1.
2 Anderson JF, Rahal JJ. Efficacy of interferon alpha-2b and ribavirin against West Nile virus in vitro. Emerg Infect Dis. 2002;8:107.
3 Andreadis TG, Anderson JF, Armstrong PM, et al. Isolations of Jamestown Canyon virus (Bunyaviridae: Orthobunyavirus) from field-collected mosquitoes (Diptera: Culicidae) in Connecticut, USA: A ten-year analysis, 1997-2006. Vector Borne Zoonotic Dis. 2008;8:175.
4 Armstrong PM, Andreadis TG. Genetic relationships of Jamestown Canyon virus strains infecting mosquitoes collected in Connecticut. Am J Trop Med Hyg. 2007;77:1157.
5 Asnis DS, Conetta R, Teixeira AA, et al. The West Nile Virus outbreak of 1999 in New York: The Flushing Hospital experience. Clin Infect Dis. 2000;30:413.
6 Badolo A, Ilboudo-Sanogo E, Ouedraogo AP, et al. Evaluation of the sensitivity of Aedes aegypti and Anopheles gambiae complex mosquitoes to two insect repellents: DEET and KBR 3023. Trop Med Int Health. 2004;9:330.
7 Bae HG, Drosten C, Emmerich P, et al. Analysis of two imported cases of yellow fever infection from Ivory Coast and The Gambia to Germany and Belgium. J Clin Virol. 2005;33:274.
8 Barnett ED. Yellow fever: Epidemiology and prevention. Clin Infect Dis. 2007;44:850.
9 Barrett AD, Teuwen DE. Yellow fever vaccine: How does it work and why do rare cases of serious adverse events take place? Curr Opin Immunol. 2009;21:308.
10 Ben-Nathan D, Lustig S, Tam G, et al. Prophylactic and therapeutic efficacy of human intravenous immunoglobulin in treating West Nile virus infection in mice. J Infect Dis. 2003;188:5.
11 Blacksell SD, Newton PN, Bell D, et al. The comparative accuracy of 8 commercial rapid immunochromatographic assays for the diagnosis of acute dengue virus infection. Clin Infect Dis. 2006;42:1127.
12 Bode AV, Sejvar JJ, Pape WJ, et al. West Nile virus disease: A descriptive study of 228 patients hospitalized in a 4-county region of Colorado in 2003. Clin Infect Dis. 2006;42:1234.
13 Breman JG. The ears of the hippopotamus: Manifestations, determinants, and estimates of the malaria burden. Am J Trop Med Hyg. 2001;64:1.
14 Brogdon WG, McAllister JC. Insecticide resistance and vector control. Emerg Infect Dis. 1998;4:605.
15 Brown SE, Severson DW, Smith LA, et al. Integration of the Aedes aegypti mosquito genetic linkage and physical maps. Genetics. 2001;157:1299.
16 Bryan CS, Moss SW, Kahn RJ. Yellow fever in the Americas. Infect Dis Clin North Am. 2004;18:275.
17 Busch MP, Caglioti S, Robertson EF, et al. Screening the blood supply for West Nile virus RNA by nucleic acid amplification testing. N Engl J Med. 2005;353:460.
18 Centers for Disease Control and Prevention. Inactivated Japanese encephalitis virus vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 1993;42:1.
19 Centers for Disease Control and Prevention. Recommendations of the Advisory Committee on Immunization Practices (ACIP): Use of vaccines and immune globulins for persons with altered immunocompetence. MMWR Recomm Rep. 1993;42:1.
20 Centers for Disease Control and Prevention. Case definitions for infectious conditions under public health surveillance. MMWR Recomm Rep. 1997;46:1.
21 Centers for Disease Control and Prevention. Arboviral infections of the central nervous system: United States, 1996-1997. MMWR Morb Mortal Wkly Rep. 1998;47:517.
22 Centers for Disease Control and Prevention. Intrauterine West Nile virus infection: New York, 2002. MMWR Morb Mortal Wkly Rep. 2002;51:1135.
23 Centers for Disease Control and Prevention. Possible West Nile virus transmission to an infant through breast-feeding: Michigan, 2002. MMWR Morb Mortal Wkly Rep. 2002;51:877.
24 Centers for Disease Control and Prevention. Update: Investigations of West Nile virus infections in recipients of organ transplantation and blood transfusion: Michigan, 2002. MMWR Morb Mortal Wkly Rep. 2002;51:879.
25 Centers for Disease Control and Prevention. West Nile virus activity: United States, October 10-16, 2002, and update on West Nile virus infections in recipients of blood transfusions. MMWR Morb Mortal Wkly Rep. 2002;51:929.
26 Centers for Disease Control and Prevention. Epidemic/epizootic West Nile virus in the United States: Guidelines for surveillance, prevention, and control, ed 3. Fort Collins, Colo: U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Center for Infectious Diseases, Division of Vector-Borne Infectious Diseases; 2003.
27 Centers for Disease Control and Prevention. Fact sheet: La Crosse encephalitis. November 7 http://www.cdc.gov/ncidod/dvbid/arbor/lacfact.htm, 2005.
28 Centers for Disease Control and Prevention. West Nile virus infections in organ transplant recipients: New York and Pennsylvania, August-September, 2005. MMWR Morb Mortal Wkly Rep. 2005;54:1021.
29 Centers for Disease Control and Prevention. Eastern equine encephalitis: New Hampshire and Massachusetts, August-September 2005. MMWR Morb Mortal Wkly Rep. 2006;55:697.
30 Centers for Disease Control and Prevention. St Louis encephalitis fact sheet. June 11 http://www.cdc.gov/ncidod/dvbid/sle/Sle_FactSheet.html, 2007.
31 Centers for Disease Control and Prevention. West Nile virus transmission through blood transfusion: South Dakota, 2006. MMWR Morb Mortal Wkly Rep. 2007;56:76.
32 Centers for Disease Control and Prevention. Arboviral encephalitis cases reported in humans, by type, United States, 1964-2008. June 8 http://www.cdc.gov/ncidod/dvbid/arbor/arbocase.htm, 2008.
33 Centers for Disease Control and Prevention. Dengue and dengue hemorrhagic fever: Information for health care practitioners. August 27 http://www.cdc.gov/dengue/, 2009.
34 Centers for Disease Control and Prevention. Japanese encephalitis among three U.S. travelers returning from Asia, 2003-2008. MMWR Morb Mortal Wkly Rep. 2009;58:737.
35 Centers for Disease Control and Prevention. Neuroinvasive and non-neuroinvasive domestic arboviral diseases: 2004 Case definition. December 9 http://www.cdc.gov/ncphi/disss/nndss/casedef/arboviral_current.htm, 2009.
36 Centers for Disease Control and Prevention. Possible congenital infection with La Crosse encephalitis virus: West Virginia, 2006-2007. MMWR Morb Mortal Wkly Rep. 2009;58:4.
37 Centers for Disease Control and Prevention. West Nile virus. December 8 http://www.cdc.gov/ncidod/dvbid/westnile/index.htm, 2009.
38 Centers for Disease Control and Prevention. The yellow book: Health information for international travel. http://www.nc.cdc.gov/travel/content/yellowbook/home-2010.aspx, 2010.
39 Centers for Disease Control and Prevention. Global distribution of arboviral encephalitides. http://www.cdc.gov/ncidod/dvbid/arbor/worldist.htm.
40 Centers for Disease Control and Prevention. Japanese encephalitis fact sheet. http://www.cdc.gov/ncidod/dvbid/jencephalitis/qa.htm.
41 Centers for Disease Control and Prevention. Technical fact sheet: Eastern equine encephalitis. http://www.cdc.gov/EasternEquineEncephalitis/FactSheet.html.
42 Cetron MS, Marfin AA, Julian KG, et al. Yellow fever vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2002. MMWR Recomm Rep. 2002;51:1.
43 Champagne DE. Antihemostatic strategies of blood-feeding arthropods. Curr Drug Targets. 2004;4:375.
44 Chan CK, Limstrom SA, Tarasewicz DG, et al. Ocular features of West Nile virus infection in North America: A study of 14 eyes. Ophthalmology. 2006;113:1539.
45 Chanama S, Sukprasert W, Sa-ngasang A, et al. Detection of Japanese encephalitis (JE) virus-specific IgM in cerebrospinal fluid and serum samples from JE patients. Jpn J Infect Dis. 2005;58:294.
46 Chao DY, Davis BS, Chang GJ. Development of multiplex real-time reverse transcriptase PCR assays for detecting eight medically important flaviviruses in mosquitoes. J Clin Microbiol. 2007;45:584.
47 Chien LJ, Liao TL, Shu PY, et al. Development of real-time reverse transcriptase PCR assays to detect and serotype dengue viruses. J Clin Microbiol. 2006;44:1295.
48 Chowers MY, Lang R, Nassar F, et al. Clinical characteristics of the West Nile fever outbreak, Israel, 2000. Emerg Infect Dis. 2001;7:675.
49 Cobra C, Rigau-Perez JG, Kuno G, et al. Symptoms of dengue fever in relation to host immunologic response and virus serotype, Puerto Rico, 1990-1991. Am J Epidemiol. 1995;142:1204.
50 Davis LE, DeBiasi R, Goade DE, et al. West Nile virus neuroinvasive disease. Ann Neurol. 2006;60:286.
51 Deen JL, Harris E, Wills B, et al. The WHO dengue classification and case definitions: Time for a reassessment. Lancet. 2006;368:170.
52 de Oliveira Poersch C, Pavoni DP, Queiroz MH, et al. Dengue virus infections: Comparison of methods for diagnosing the acute disease. J Clin Virol. 2005;32:272.
53 Deresiewicz RL, Thaler SJ, Hsu L, et al. Clinical and neuroradiographic manifestations of eastern equine encephalitis. N Engl J Med. 1997;336:1867.
54 Diagana M, Preux PM, Dumas M. Japanese encephalitis revisited. J Neurol Sci. 2007;262:165.
55 Diaz LA, Re V, Almiron WR, et al. Genotype III Saint Louis encephalitis virus outbreak, Argentina, 2005. Emerg Infect Dis. 2006;12:1752.
56 Dickerson RB, Newton JR, Hansen JE. Diagnosis and immediate prognosis of Japanese B encephalitis: Observations based on more than 200 patients with detailed analysis of 65 serologically confirmed cases. Am J Med. 1952;12:277.
57 Douglas MW, Stephens DP, Burrow JN, et al. Murray Valley encephalitis in an adult traveller complicated by long-term flaccid paralysis: Case report and review of the literature. Trans R Soc Trop Med Hyg. 2007;101:284.
58 Edelman R. Dengue vaccines approach the finish line. Clin Infect Dis. 2007;45:S56.
59 Erwin PC, Jones TF, Gerhardt RR, et al. La Crosse encephalitis in Eastern Tennessee: Clinical, environmental, and entomological characteristics from a blinded cohort study. Am J Epidemiol. 2002;155:1060.
60 Foster WA. Mosquito sugar feeding and reproductive energetics. Annu Rev Entomol. 1995;40:443.
61 Fradin MS. Mosquitoes and mosquito repellents: A clinician’s guide. Ann Intern Med. 1998;128:931.
62 Fradin MS, Day JF. Comparative efficacy of insect repellents against mosquito bites. N Engl J Med. 2002;347:13.
63 Fraser JR. Epidemic polyarthritis and Ross River virus disease. Clin Rheum Dis. 1986;12:369.
64 Fulhorst CF, Hardy JL, Eldridge BF, et al. Ecology of Jamestown Canyon virus (Bunyaviridae: California serogroup) in coastal California. Am J Trop Med Hyg. 1996;55:185.
65 Galler R, Pugachev KV, Santos CL, et al. Phenotypic and molecular analyses of yellow fever 17DD vaccine viruses associated with serious adverse events in Brazil. Virology. 2001;290:309.
66 Gea-Banacloche J, Johnson RT, Bagic A, et al. West Nile virus: Pathogenesis and therapeutic options. Ann Intern Med. 2004;140:545.
67 Gibbons RV, Vaughn DW. Dengue: An escalating problem. BMJ. 2002;324:1563.
68 Golomb MR, Durand ML, Schaefer PW, et al. A case of immunotherapy-responsive eastern equine encephalitis with diffusion-weighted imaging. Neurology. 2001;56:420.
69 Gordon RM, Lumsden WH. A study of the behavior of the mouth-parts of mosquitoes when taking up blood from living tissue together with some observations on the ingestion of microfilariae. Ann Trop Med Parasitol. 1939;33:259.
70 Granwehr BP, Lillibridge KM, Higgs S, et al. West Nile virus: Where are we now? Lancet Infect Dis. 2004;4:547.
71 Grimstad PR, Shabino CL, Calisher CH, et al. A case of encephalitis in a human associated with a serologic rise to Jamestown Canyon virus. Am J Trop Med Hyg. 1982;31:1238.
72 Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11:480.
73 Gubler DJ. Resurgent vector-borne diseases as a global health problem. Emerg Infect Dis. 1998;4:442.
74 Gubler DJ. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol. 2002;10:100.
75 Guzman MG, Alvarez M, Rodriguez R, et al. Fatal dengue hemorrhagic fever in Cuba, 1997. Int J Infect Dis. 1999;3:130.
76 Guzman MG, Kouri G. Dengue: An update. Lancet Infect Dis. 2002;2:33.
77 Haddow AD, Jones CJ, Odoi A. Assessing risk in focal arboviral infections: Are we missing the big or little picture? PloS One. 2009;4:e6954.
78 Haddow AD, Odoi A. The incidence risk, clustering, and clinical presentation of La Crosse virus infections in the eastern United States, 2003-2007. PloS One. 2009;4:e6145.
79 Hales S, de Wet N, Maindonald J, et al. Potential effect of population and climate changes on global distribution of dengue fever: An empirical model. Lancet. 2002;360:830.
80 Hales S, van Panhuis W. A new strategy for dengue control. Lancet. 2005;365:551.
81 Hanna JN, Ritchie SA, Phillips DA, et al. Japanese encephalitis in north Queensland, Australia, 1998. Med J Aust. 1999;170:533.
82 Hardin SG, Erwin PC, Patterson L, et al. Clinical comparisons of La Crosse encephalitis and enteroviral central nervous system infections in a pediatric population: 2001 surveillance in East Tennessee. Am J Infect Control. 2003;31:508.
83 Harley D, Ritchie S, Bain C, et al. Risks for Ross River virus disease in tropical Australia. Int J Epidemiol. 2005;34:548.
84 Harley D, Sleigh A, Ritchie S. Ross River virus transmission, infection, and disease: A cross-disciplinary review. Clin Microbiol Rev. 2001;14:909.
85 Hickie I, Davenport T, Wakefield D, et al. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: Prospective cohort study. BMJ. 2006;333:575.
86 Hoke CH, Nisalak A, Sangawhipa N, et al. Protection against Japanese encephalitis by inactivated vaccines. N Engl J Med. 1988;319:608.
87 Hoke CHJr, Vaughn DW, Nisalak A, et al. Effect of high-dose dexamethasone on the outcome of acute encephalitis due to Japanese encephalitis virus. J Infect Dis. 1992;165:631.
88 Huggins JW. Prospects for treatment of viral hemorrhagic fevers with ribavirin, a broad-spectrum antiviral drug. Rev Infect Dis. 1989;11:S750.
89 Iwamoto M, Jernigan DB, Guasch A, et al. Transmission of West Nile virus from an organ donor to four transplant recipients. N Engl J Med. 2003;348:21963.
90 Jacups SP, Whelan PI, Currie BJ. Ross River virus and Barmah Forest virus infections: A review of history, ecology, and predictive models, with implications for tropical northern Australia. Vector Borne Zoonotic Dis. 2008;8:283.
91 Jacups SP, Whelan PI, Markey PG, et al. Predictive indicators for Ross River virus infection in the Darwin area of tropical northern Australia, using long-term mosquito trapping data. Trop Med Int Health. 2008;13:943.
92 Japanese encephalitis vaccines. Wkly Epidemiol Rec. 2006;81:331.
93 Johnson AJ, Cheshier RC, Cosentino G, et al. Validation of a microsphere-based immunoassay for detection of anti-West Nile virus and anti-St Louis encephalitis virus immunoglobulin M antibodies. Clin Vaccine Immunol. 2007;14:1084.
94 Johnson AJ, Noga AJ, Kosoy O, et al. Duplex microsphere-based immunoassay for detection of anti-West Nile virus and anti-St Louis encephalitis virus immunoglobulin M antibodies. Clin Diagn Lab Immunol. 2005;12:566.
95 Johnson BW, Russell BJ, Lanciotti RS. Serotype-specific detection of dengue viruses in a fourplex real-time reverse transcriptase PCR assay. J Clin Microbiol. 2005;43:4977.
96 Kalil AC, Devetten MP, Singh S, et al. Use of interferon-alpha in patients with West Nile encephalitis: Report of 2 cases. Clin Infect Dis. 2005;40:764.
97 Kalita J, Misra UK. Neurophysiological changes in Japanese encephalitis. Neurol India. 2002;50:262.
98 Katz TM, Miller JH, Hebert AA. Insect repellents: Historical perspectives and new developments. J Am Acad Dermatol. 2008;58:865.
99 Kay B, Vu SN. New strategy against Aedes aegypti in Vietnam. Lancet. 2005;365:613.
100 Kelly-Hope LA, Purdie DM, Kay BH. Differences in climatic factors between Ross River virus disease outbreak and non-outbreak years. J Med Entomol. 2004;41:1116.
101 Kelly-Hope LA, Purdie DM, Kay BH. Ross River virus disease in Australia, 1886-1998, with analysis of risk factors associated with outbreaks. J Med Entomol. 2004;41:133.
102 Khairallah M, Ben Yahia S, Ladjimi A, et al. Chorioretinal involvement in patients with West Nile virus infection. Ophthalmology. 2004;111:2065.
103 Kienzle N, Boyes L. Murray Valley encephalitis: Case report and review of neuroradiological features. Australas Radiol. 2003;47:61.
104 Klapsing P, MacLean JD, Glaze S, et al. Ross River virus disease reemergence, Fiji, 2003-2004. Emerg Infect Dis. 2005;11:613.
105 Klempner MS, Unnasch TR, Hu LT. Taking a bite out of vector-transmitted infectious diseases. N Engl J Med. 2007;356:2567.
106 Koren G, Matsui D, Bailey B. DEET-based insect repellents: Safety implications for children and pregnant and lactating women. CMAJ. 2003;169:209.
107 Kumar R, Mathur A, Kumar A, et al. Clinical features and prognostic indicators of Japanese encephalitis in children in Lucknow (India). Indian J Med Res. 1990;91:321.
108 Kumar R, Tripathi P, Baranwal M, et al. Randomized, controlled trial of oral ribavirin for Japanese encephalitis in children in Uttar Pradesh, India. Clin Infect Dis. 2009;48:400.
109 Kumar R, Tripathi P, Singh S, et al. Clinical features in children hospitalized during the 2005 epidemic of Japanese encephalitis in Uttar Pradesh, India. Clin Infect Dis. 2006;43:123.
110 Kuno G. Review of the factors modulating dengue transmission. Epidemiol Rev. 1995;17:321.
111 LaDeau SL, Kilpatrick AM, Marra PP. West Nile virus emergence and large-scale declines of North American bird populations. Nature. 2007;447:710.
112 Leber SM, Brunberg JA, Pavkovic IM. Infarction of basal ganglia associated with California encephalitis virus. Pediatr Neurol. 1995;12:346.
113 Lincoln AF, Sivertson SE. Acute phase of Japanese B encephalitis: Two hundred and one cases in American soldiers, Korea, 1950. JAMA. 1952;150:268.
114 Lum LC, Goh AY, Chan PW, et al. Risk factors for hemorrhage in severe dengue infections. J Pediatr. 2002;140:629.
115 Mackenzie JS, Lindsay MD, Coelen RJ, et al. Arboviruses causing human disease in the Australasian zoogeographic region. Arch Virol. 1994;136:447.
116 Malinoski FJ, Hasty SE, Ussery MA, et al. Prophylactic ribavirin treatment of dengue type 1 infection in rhesus monkeys. Antiviral Res. 1990;13:139.
117 Marfin AA, Eidex RS, Kozarsky PE, et al. Yellow fever and Japanese encephalitis vaccines: Indications and complications. Infect Dis Clin North Am. 2005;19:151.
118 Markoff L. Alphaviruses. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and practice of infectious diseases. Philadelphia: Churchill Livingstone; 2000:1704-1707.
119 Martin DA, Noga A, Kosoy O, et al. Evaluation of a diagnostic algorithm using immunoglobulin M enzyme-linked immunosorbent assay to differentiate human West Nile Virus and St Louis Encephalitis virus infections during the 2002 West Nile virus epidemic in the United States. Clin Diagn Lab Immunol. 2004;11:1130.
120 Martin JE, Pierson TC, Hubka S, et al. A West Nile virus DNA vaccine induces neutralizing antibody in healthy adults during a phase 1 clinical trial. J Infect Dis. 2007;196:1732.
121 Mayo D, Karabatsos N, Scarano FJ, et al. Jamestown Canyon virus: Seroprevalence in Connecticut. Emerg Infect Dis. 2001;7:911.
122 McJunkin JE, de los Reyes EC, Irazuzta JE, et al. La Crosse encephalitis in children. N Engl J Med. 2001;344:801.
123 McJunkin JE, Khan R, de los Reyes EC, et al. Treatment of severe La Crosse encephalitis with intravenous ribavirin following diagnosis by brain biopsy. Pediatrics. 1997;99:261.
124 McJunkin JE, Khan RR, Tsai TF. California-La Crosse encephalitis. Infect Dis Clin North Am. 1998;12:83.
125 Misra UK, Kalita J. A comparative study of Japanese and herpes simplex encephalitides. Electromyogr Clin Neurophysiol. 1998;38:41.
126 Misra UK, Kalita J. Seizures in Japanese encephalitis. J Neurol Sci. 2001;190:57.
127 Misra UK, Kalita J, Jain SK, et al. Radiological and neurophysiological changes in Japanese encephalitis. J Neurol Neurosurg Psychiatry. 1994;57:1484.
128 Monath TP. Yellow fever: An update. Lancet Infect Dis. 2001;1:11.
129 Monath TP. Dengue and yellow fever: Challenges for the development and use of vaccines. N Engl J Med. 2007;357:2222.
130 Monath TP, Liu J, Kanesa-Thasan N, et al. A live, attenuated recombinant West Nile virus vaccine. Proceed Nat Acad Sci U S A. 2006;103:6694.
131 Monath TP, Nichols R, Archambault WT, et al. Comparative safety and immunogenicity of two yellow fever 17D vaccines (ARILVAX and YF-VAX) in a phase III multicenter, double-blind clinical trial. Am J Trop Med Hyg. 2002;66:533.
132 Mondini A, Cardeal IL, Lazaro E, et al. Saint Louis encephalitis virus, Brazil. Emerg Infect Dis. 2007;13:176.
133 Morrey JD, Day CW, Julander JG, et al. Effect of interferon-alpha and interferon-inducers on West Nile virus in mouse and hamster animal models. Antivir Chem Chemother. 2004;15:101.
134 Morrey JD, Smee DF, Sidwell RW, et al. Identification of active antiviral compounds against a New York isolate of West Nile virus. Antiviral Res. 2002;55:107.
135 Myint KS, Gibbons RV, Perng GC, et al. Unravelling the neuropathogenesis of Japanese encephalitis. Trans R Soc Trop Med Hyg. 2007;101:955.
136 Mylonas AD, Brown AM, Carthew TL, et al. Natural history of Ross River virus-induced epidemic polyarthritis. Med J Aust. 2002;177:356.
137 Nash D, Mostashari F, Fine A, et al. The outbreak of West Nile virus infection in the New York City area in 1999. N Engl J Med. 2001;344:1807.
138 Panpanich R, Sornchai P, Kanjanaratanakorn K: Corticosteroids for treating dengue shock syndrome, Cochrane Database Syst Rev 3:CD003488, 2006.
139 Patnaik JL, Harmon H, Vogt RL. Follow-up of 2003 human West Nile virus infections, Denver, Colorado. Emerg Infect Dis. 2006;12:1129.
140 Paulke-Korinek M, Kollaritsch H. Japanese encephalitis and vaccines: Past and future prospects. Wien klin Wochenschr. 2008;120:15.
141 Pealer LN, Marfin AA, Petersen LR, et al. Transmission of West Nile virus through blood transfusion in the United States in 2002. N Engl J Med. 2003;349:1236.
142 Petersen LR, Hayes EB. West Nile virus in the Americas. Med Clin North Am. 2008;92:1307.
143 Petersen LR, Marfin AA. West Nile virus: A primer for the clinician. Ann Intern Med. 2002;137:173.
144 Pickett JA, Birkett MA, Logan JG. DEET repels ORNery mosquitoes. Proceed Nat Acad Sci U S A. 2008;105:13195.
145 Pinheiro FP, Corber SJ. Global situation of dengue and dengue haemorrhagic fever, and its emergence in the Americas. World Health Stat Q. 1997;50:161.
146 Plesner AM. Allergic reactions to Japanese encephalitis vaccine. Immunol Allergy Clin North Am. 2003;23:665.
147 Potts JA, Rothman AL. Clinical and laboratory features that distinguish dengue from other febrile illnesses in endemic populations. Trop Med Int Health. 2008;13:1328.
148 Rahal JJ, Anderson J, Rosenberg C, et al. Effect of interferon-alpha2b therapy on St Louis viral meningoencephalitis: Clinical and laboratory results of a pilot study. J Infect Dis. 2004;190:1084.
149 Reisen WK, Lothrop HD, Wheeler SS, et al. Persistent West Nile virus transmission and the apparent displacement St. Louis encephalitis virus in southeastern California, 2003-2006. J Med Entomol. 2008;45:494.
150 Robertson SE, Hull BP, Tomori O, et al. Yellow fever: A decade of reemergence. JAMA. 1996;276:1157.
151 Russell RC. Ross River virus: Ecology and distribution. Annu Rev Entomol. 2002;47:1.
152 Rust RS, Thompson WH, Matthews CG, et al. La Crosse and other forms of California encephalitis. J Child Neurol. 1999;14:1.
153 Schioler KL, Samuel M, Wai KL: Vaccines for preventing Japanese encephalitis, Cochrane Database Syst Rev CD004263, 2007.
154 Schmidt CW. Outsmarting olfaction: The next generation of mosquito repellents. Environ Health Perspect. 2005;113:A468.
155 Sejvar JJ, Bode AV, Marfin AA, et al. West Nile virus–associated flaccid paralysis. Emerg Infect Dis. 2005;11:1021.
156 Sejvar JJ, Haddad MB, Tierney BC, et al. Neurologic manifestations and outcome of West Nile virus infection. JAMA. 2003;290:511.
157 Shiff C. Integrated approach to malaria control. Clin Microbiol Rev. 2002;15:278.
158 Shirtcliffe P, Cameron E, Nicholson KG, et al. Don’t forget dengue! Clinical features of dengue fever in returning travellers. J R Coll Physicians London. 1998;32:235.
159 Shlim DR, Solomon T. Japanese encephalitis vaccine for travelers: Exploring the limits of risk. Clin Infect Dis. 2002;35:183.
160 Shoji H, Murakami T, Murai I, et al. A follow-up study by CT and MRI in 3 cases of Japanese encephalitis. Neuroradiology. 1990;32:215.
161 Snodgrass R. The anatomical life of the mosquito. Washington, DC: Smithsonian Institution Press; 1959.
162 Solomon T. Control of Japanese encephalitis—within our grasp? N Engl J Med. 2006;355:869.
163 Solomon T, Dung NM, Kneen R, et al. Japanese encephalitis. J Neurol Neurosurg Psychiatry. 2000;68:405.
164 Solomon T, Dung NM, Kneen R, et al. Seizures and raised intracranial pressure in Vietnamese patients with Japanese encephalitis. Brain. 2002;125:1084.
165 Solomon T, Dung NM, Vaughn DW, et al. Neurological manifestations of dengue infection. Lancet. 2000;355:1053.
166 Solomon T, Kneen R, Dung NM, et al. Poliomyelitis-like illness due to Japanese encephalitis virus. Lancet. 1998;351:1094.
167 Spinsanti LI, Diaz LA, Glatstein N, et al. Human outbreak of St Louis encephalitis detected in Argentina, 2005. J Clin Virol. 2008;42:27.
168 Steen CJ, Carbonaro PA, Schwartz RA. Arthropods in dermatology. J Am Acad Dermatol. 2004;50:819.
169 Stramer SL, Fang CT, Foster GA, et al. West Nile virus among blood donors in the United States, 2003 and 2004. N Engl J Med. 2005;353:451.
170 Suhrbier A, La Linn M. Clinical and pathologic aspects of arthritis due to Ross River virus and other alphaviruses. Curr Opin Rheumatol. 2004;16:374.
171 Tanner L, Schreiber M, Low JG, et al. Decision tree algorithms predict the diagnosis and outcome of dengue fever in the early phase of illness. PLoS Negl Trop Dis. 2008;2:e196.
172 Tauber E, Kollaritsch H, Korinek M, et al. Safety and immunogenicity of a Vero-cell-derived, inactivated Japanese encephalitis vaccine: A non-inferiority, phase III, randomised controlled trial. Lancet. 2007;370:1847.
173 Tilley PA, Fox JD, Jayaraman GC, et al. Nucleic acid testing for West Nile virus RNA in plasma enhances rapid diagnosis of acute infection in symptomatic patients. J Infect Dis. 2006;193:1361.
174 Tiroumourougane SV, Raghava P, Srinivasan S. Japanese viral encephalitis. Postgrad Med J. 2002;78:205.
175 Tong S. Ross River virus disease in Australia: Epidemiology, socioecology and public health response. Intern Med J. 2004;34:58.
176 Tong S, Dale P, Nicholls N, et al. Climate variability, social and environmental factors, and Ross River virus transmission: Research development and future research needs. Environ Health Perspect. 2008;116:1591.
177 Tsai TF. Flaviviruses. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and practice of infectious diseases. Philadelphia: Churchill Livingstone; 2000:1714-1732.
178 Tsai TF, Khan AS, McJunkin JE. Togaviridae, Flaviviridae, and Bunyaviridae. In: Long SS, Pickering LK, Prober CG, editors. Principles and practice of pediatric infectious diseases. ed 2. New York: Elsevier; 2003:1714-1732.
179 U.S. Environmental Protection Agency. Pesticides: Mosquito control, April 4, http://www.epa.gov/pesticides/health/mosquitoes/about_mosquitos.htm, 2007.
180 Utzinger J, Tozan Y, Singer BH. Efficacy and cost-effectiveness of environmental management for malaria control. Trop Med Int Health. 2001;6:677.
181 Watson JT, Pertel PE, Jones RC, et al. Clinical characteristics and functional outcomes of West Nile Fever. Ann Intern Med. 2004;141:360.
182 Weir E, Haider S. Yellow fever: Readily prevented but difficult to treat. CMAJ. 2004;170:1909.
183 WHO position statement on integrated vector management. Wkly Epidemiol Rec. 2008;83:177.
184 Wilder-Smith A, Schwartz E. Dengue in travelers. N Engl J Med. 2005;353:924.
185 Wills BA, Nguyen MD, Ha TL, et al. Comparison of three fluid solutions for resuscitation in dengue shock syndrome. N Engl J Med. 2005;353:877.
186 Woodruff RE, Guest CS, Garner MG, et al. Early warning of Ross River virus epidemics: Combining surveillance data on climate and mosquitoes. Epidemiology. 2006;17:569.
187 World Health Organization. Dengue haemorrhagic fever: Diagnosis, treatment, prevention and control, ed 2. Geneva: WHO; 1997.
188 World Health Organization. Vector-borne viral infections: West Nile virus. January 25 http://www.who.int/vaccine_research/diseases/vector/en/index3.html, 2008.
189 World Health Organization. Dengue and dengue hemorrhagic fever. Fact sheet No. 117 http://www.who.int/mediacentre/factsheets/fs117/en/, March 2009.
190 World Health Organization. Yellow fever. Fact sheet No. 100 www.who.int/mediacentre/factsheets/fs100/en
191 Yahia SB, Khairallah M. Ocular manifestations of West Nile virus infection. Int J Med Sci. 2009;6:114.
192 Yang SE, Pan MJ, Tseng HF, et al. The efficacy of mouse-brain inactivated Nakayama strain Japanese encephalitis vaccine: Results from 30 years experience in Taiwan. Vaccine. 2006;24:2669.
193 Yellow fever in Africa and South America, 2007. Wkly Epidemiol Rec. 2009;84:97.
194 The yellow fever situation in Africa and South America in 2004. Wkly Epidemiol Rec. 2005;80:250.
195 Yosipovitch G. Itching in the new millennium: Highlights of the second international workshop for the study of itch, Toyoma, Japan. J Am Acad Dermatol. 2004;51:625.
196 Zak IT, Zak IT, Altinok D, Merline JR, et al. West Nile virus infection. AJR Am J Roentgenol. 2005;184:957.