Moraxella and Related Organisms
1. Identify the distinguishing characteristics of the species within the genera Moraxella and Neisseria.
2. Identify what species within this group of bacteria that are frequently isolated as pathogens and which are considered potential contaminants.
3. Explain the procedure the microbiologist can use to determine whether the bacteria in this grouping exist as true cocci and name these organisms.
4. Identify the species of Moraxella and Neisseria that may be isolated from human wounds resulting from a dog or cat bite.
5. Identify the species of Moraxella frequently isolated from cases of human conjunctivitis.
6. Explain the media used for culture for this group of organisms, including the chemical principle and composition.
7. List some of the conventional biochemical tests that can be used to distinguish these organisms from other bacteria, and explain the principle for each.
8. Correlate patient signs and symptoms with laboratory data, and identify the most likely etiologic agent.
Epidemiology, Spectrum of Disease, and Antimicrobial Therapy
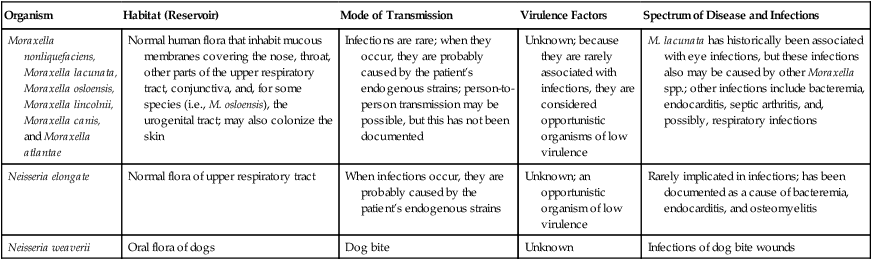
Infections caused by Moraxella spp. and Neisseria elongata most likely result when a breakdown of the patient’s mucosal or epidermal defensive barriers allows subsequent invasion of sterile sites by an organism that is part of the patient’s normal flora (i.e., an endogenous strain; Table 28-1). The fact that these organisms rarely cause infection indicates that they have low virulence. Whenever these organisms are encountered in clinical specimens, the possibility that they are contaminants should be seriously considered. This is especially the case when the specimen source may have come in contact with a mucosal surface.
TABLE 28-1
Epidemiology, Pathogenesis, and Spectrum of Disease
| Organism | Habitat (Reservoir) | Mode of Transmission | Virulence Factors | Spectrum of Disease and Infections |
| Moraxella nonliquefaciens, Moraxella lacunata, Moraxella osloensis, Moraxella lincolnii, Moraxella canis, and Moraxella atlantae | Normal human flora that inhabit mucous membranes covering the nose, throat, other parts of the upper respiratory tract, conjunctiva, and, for some species (i.e., M. osloensis), the urogenital tract; may also colonize the skin | Infections are rare; when they occur, they are probably caused by the patient’s endogenous strains; person-to-person transmission may be possible, but this has not been documented | Unknown; because they are rarely associated with infections, they are considered opportunistic organisms of low virulence | M. lacunata has historically been associated with eye infections, but these infections also may be caused by other Moraxella spp.; other infections include bacteremia, endocarditis, septic arthritis, and, possibly, respiratory infections |
| Neisseria elongate | Normal flora of upper respiratory tract | When infections occur, they are probably caused by the patient’s endogenous strains | Unknown; an opportunistic organism of low virulence | Rarely implicated in infections; has been documented as a cause of bacteremia, endocarditis, and osteomyelitis |
| Neisseria weaverii | Oral flora of dogs | Dog bite | Unknown | Infections of dog bite wounds |
Moraxella catarrhalis is the species most commonly associated with human infections, primarily of the respiratory tract. However, because the cellular morphology of this species is more similar to that of Neisseria spp. than that of the other Moraxella spp., details of this organism’s characteristics are discussed in Chapter 40.
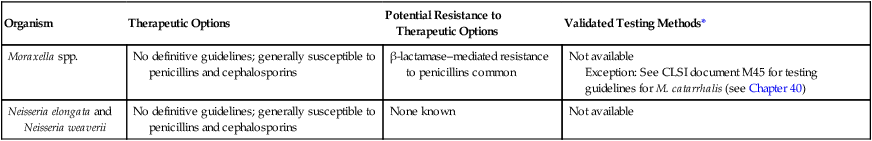
The rarity with which these organisms are encountered as the cause of infection and the lack of validated in vitro susceptibility testing methods does not allow definitive treatment guidelines to be given (Table 28-2). Although many of these organisms may grow on the media and under the conditions recommended for testing other bacteria, this does not necessarily mean that interpretable and reliable results will be produced. Chapter 12 should be reviewed for preferable strategies that can be used to provide susceptibility information when validated testing methods do not exist for a clinically important bacterial isolate.
TABLE 28-2
Antimicrobial Therapy and Susceptibility Testing
| Organism | Therapeutic Options | Potential Resistance to Therapeutic Options | Validated Testing Methods* |
| Moraxella spp. | No definitive guidelines; generally susceptible to penicillins and cephalosporins | β-lactamase–mediated resistance to penicillins common | Not available Exception: See CLSI document M45 for testing guidelines for M. catarrhalis (see Chapter 40) |
| Neisseria elongata and Neisseria weaverii | No definitive guidelines; generally susceptible to penicillins and cephalosporins | None known | Not available |
*Validated testing methods include those standard methods recommended by the Clinical and Laboratory Standards Institute (CLSI) and those commercial methods approved by the Food and Drug Administration (FDA).
Laboratory Diagnosis
Specimen Collection and Transport
No special considerations are required for specimen collection and transport of the organisms discussed in this chapter. Refer to Table 5-1 for general information on specimen collection and transport.
Specimen Processing
No special considerations are required for processing of the organisms discussed in this chapter. Refer to Table 5-1 for general information on specimen processing.
Cultivation
Media of Choice
Colonial Appearance
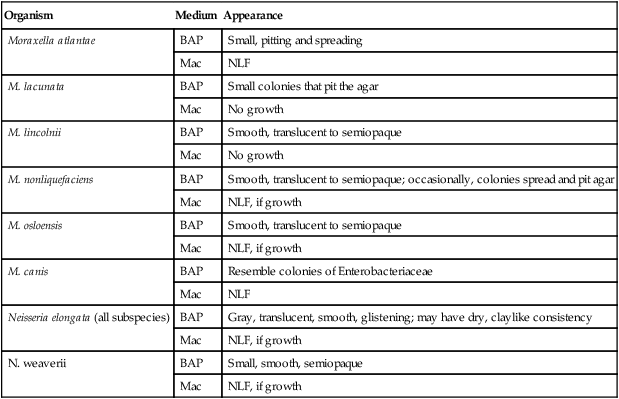
Table 28-3 describes the colonial appearance and other distinguishing characteristics (e.g., pitting) of each species on 5% sheep blood and MacConkey agars. The ability of most commercial identification systems to accurately identify the organisms discussed in this chapter is limited or uncertain. Table 28-4 lists some conventional biochemical tests that can be used to presumptively differentiate the species in this chapter. This is a simplified scheme; clinically important isolates should be sent to a reference laboratory for definitive identification.
TABLE 28-3
Colonial Appearance and Characteristics
| Organism | Medium | Appearance |
| Moraxella atlantae | BAP | Small, pitting and spreading |
| Mac | NLF | |
| M. lacunata | BAP | Small colonies that pit the agar |
| Mac | No growth | |
| M. lincolnii | BAP | Smooth, translucent to semiopaque |
| Mac | No growth | |
| M. nonliquefaciens | BAP | Smooth, translucent to semiopaque; occasionally, colonies spread and pit agar |
| Mac | NLF, if growth | |
| M. osloensis | BAP | Smooth, translucent to semiopaque |
| Mac | NLF, if growth | |
| M. canis | BAP | Resemble colonies of Enterobacteriaceae |
| Mac | NLF | |
| Neisseria elongata (all subspecies) | BAP | Gray, translucent, smooth, glistening; may have dry, claylike consistency |
| Mac | NLF, if growth | |
| N. weaverii | BAP | Small, smooth, semiopaque |
| Mac | NLF, if growth |
BAP, 5% sheep blood agar; Mac, MacConkey agar; NLF, non-lactose-fermenter.
TABLE 28-4
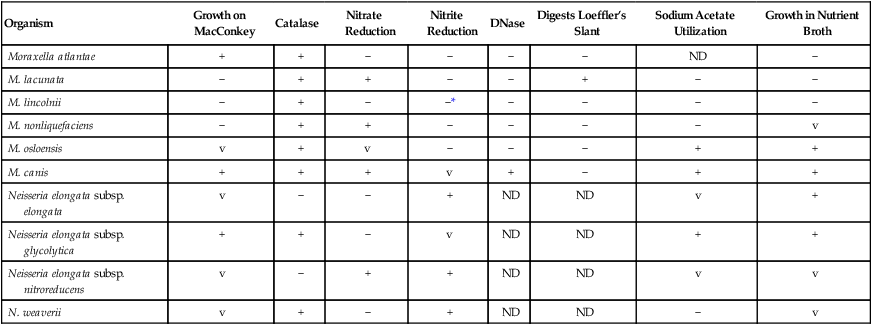
Key Biochemical and Physiologic Characteristics
| Organism | Growth on MacConkey | Catalase | Nitrate Reduction | Nitrite Reduction | DNase | Digests Loeffler’s Slant | Sodium Acetate Utilization | Growth in Nutrient Broth |
| Moraxella atlantae | + | + | − | − | − | − | ND | − |
| M. lacunata | − | + | + | − | − | + | − | − |
| M. lincolnii | − | + | − | −* | − | − | − | − |
| M. nonliquefaciens | − | + | + | − | − | − | − | v |
| M. osloensis | v | + | v | − | − | − | + | + |
| M. canis | + | + | + | v | + | − | + | + |
| Neisseria elongata subsp. elongata | v | − | − | + | ND | ND | v | + |
| Neisseria elongata subsp. glycolytica | + | + | − | v | ND | ND | + | + |
| Neisseria elongata subsp. nitroreducens | v | − | + | + | ND | ND | v | v |
| N. weaverii | v | + | − | + | ND | ND | − | v |
ND, No data; v, variable; +, >90% of strains positive; −, >90% of strains negative.
Note: Organisms listed are generally indole-negative.
Approach to Identification
As previously mentioned, these organisms can be difficult to differentiate from gram-negative diplococci (see Chapter 40 for more information about gram-negative diplococci). In addition, these organisms are relatively biochemically inert. Elongation in the presence of penicillin is a useful criterion for differentiating them from true cocci. The effect of penicillin is determined by streaking a blood agar plate, placing a 10-unit penicillin disk in the first quadrant and overnight incubation at 35° C. A Gram stain of the growth taken from around the edge of the zone of inhibition readily demonstrates whether the isolate in question is a true cocci or has elongated.
Comments Regarding Specific Organisms
M. nonliquefaciens and M. osloensis, the two most frequently isolated species, can be differentiated by the ability of M. osloensis to utilize acetate. M. lacunata is able to liquefy serum, so depressions are formed on the surface of Loeffler’s serum agar slants. Most of the species considered in this chapter do not utilize glucose; Neisseria elongata subsp. glycolytica, which produces acid from glucose in the rapid sugar test used for Neisseria spp., is the only exception. Unlike Oligella spp. (see Chapter 25 for more information regarding this genus), none of the organisms considered here are motile.
1. Which of the following species of the gram-negative, non-fermenting group of bacteria is considered normal oropharyngeal flora in cats and dogs and is frequently isolated from the bite wounds of these animals?
2. Which of the following species of Moraxella is normal human flora inhabiting the urogenital tract?
3. Which of the following species of the Neisseria group of bacteria is able to utilize glucose?
4. Which of the species in the Moraxella group of bacteria does not pit BAP agar in culture?
5. Which of the following species of Moraxella is able to liquefy serum, causing depressions in the surface of Loeffler’s serum agar slants?
_____ The colonial appearance of the bacteria Neisseria elongata (all subspecies) on BAP agar is gray, translucent, smooth, glistening colonies, which may also have a dry, claylike consistency.
_____ Susceptibility testing is normally performed on isolates of Moraxella and Neisseria spp.
_____ In the family of bacteria Moraxella, the biochemical characteristics are oxidase positive and catalase negative.
_____ The test used to differentiate Moraxella nonliquefaciens and Moraxella osloensis is acetate, as M. osloensis utilizes acetate, whereas M. nonliquefaciens does not.
_____ Moraxella spp. and the elongated Neisseria spp. grow well on 5% sheep blood, chocolate, and MacConkey agar.
_____ The species of Moraxella most frequently associated with human infection, mostly respiratory, is Moraxella catarrhalis.
_____ The species of Moraxella known to cause conjunctivitis is Moraxella lacunata.








