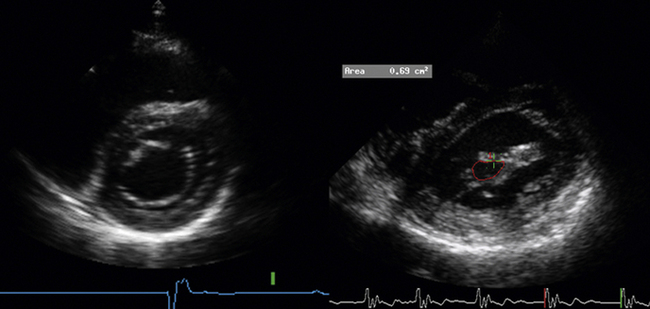
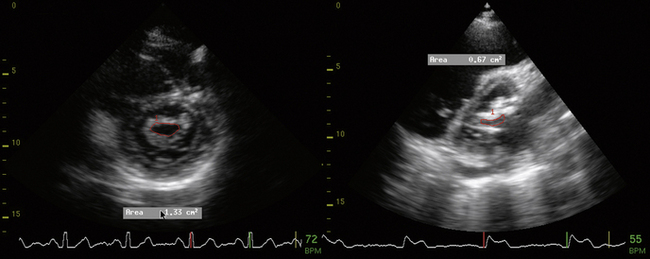
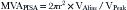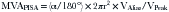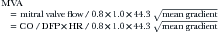5 Mitral Stenosis
Goals of Echocardiography in Mitral Stenosis
Scanning Issues
Required Parameters to Obtain from Scanning
 Right ventricle size and systolic function
Right ventricle size and systolic function
 Indirect pressure overload signs: left atrium (LA) size, RVSP
Indirect pressure overload signs: left atrium (LA) size, RVSP
 Left ventricular outflow tract (LVOT)–derived cardiac output and stroke volume. Note that in the presence of AI, the stroke volume measured in the LVOT is the total forward stroke volume, not the net forward stroke volume.
Left ventricular outflow tract (LVOT)–derived cardiac output and stroke volume. Note that in the presence of AI, the stroke volume measured in the LVOT is the total forward stroke volume, not the net forward stroke volume.
Valid Methods for Mitral Valve Area Determination (Need ≥2 Concordant)
Scanning Notes
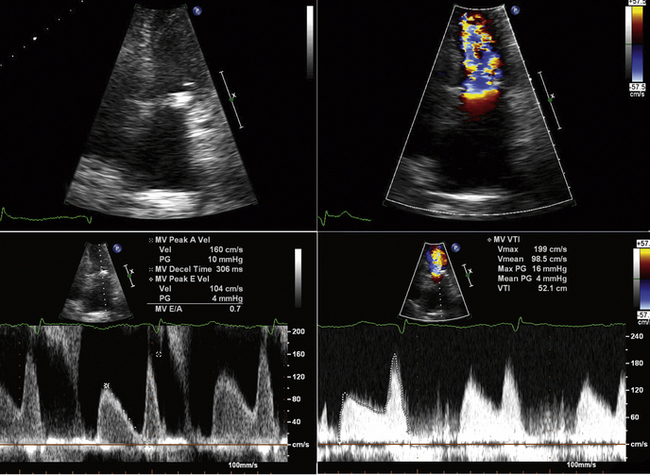
Gradient
 Ensure that transmitral Doppler sampling is correctly aligned, and that continuous wave Doppler is being used with adequate gain settings.
Ensure that transmitral Doppler sampling is correctly aligned, and that continuous wave Doppler is being used with adequate gain settings.
 If sinus rhythm is present, measure three spectral profiles.
If sinus rhythm is present, measure three spectral profiles.
 If atrial fibrillation is detected, measure five spectral profiles.
If atrial fibrillation is detected, measure five spectral profiles.
 Ideally, measure 10 spectral profiles.
Ideally, measure 10 spectral profiles.
 Spectral profiles should be displayed at two-thirds the height of the display, and wide enough so that there are two or three per display.
Spectral profiles should be displayed at two-thirds the height of the display, and wide enough so that there are two or three per display.
 If the gradient is not severe, but the area is, consider provocative maneuvers (e.g., sit-ups) to increase flow and (thereby the gradient).
If the gradient is not severe, but the area is, consider provocative maneuvers (e.g., sit-ups) to increase flow and (thereby the gradient).
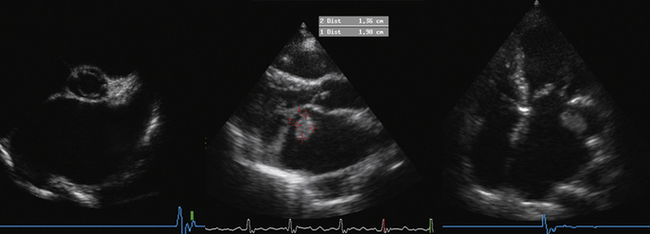
Planimetry
 Optimize the gain settings for planimetry—over-gain results in a falsely low estimation of mitral valve area (MVA) due to “blooming” of the margins.
Optimize the gain settings for planimetry—over-gain results in a falsely low estimation of mitral valve area (MVA) due to “blooming” of the margins.
 Measure the area at the narrowest part of the funnel-like orifice of MS—this may be at the level of the leaflet tips or at the subvalvar level.
Measure the area at the narrowest part of the funnel-like orifice of MS—this may be at the level of the leaflet tips or at the subvalvar level.
 Be cautious if there was a prior commissurotomy—planimetry underrepresents area if the orifice has deep “splits” into the commissures (or leaflets), which are difficult so see by ultrasound, especially if they extend off the plane of imaging.
Be cautious if there was a prior commissurotomy—planimetry underrepresents area if the orifice has deep “splits” into the commissures (or leaflets), which are difficult so see by ultrasound, especially if they extend off the plane of imaging.
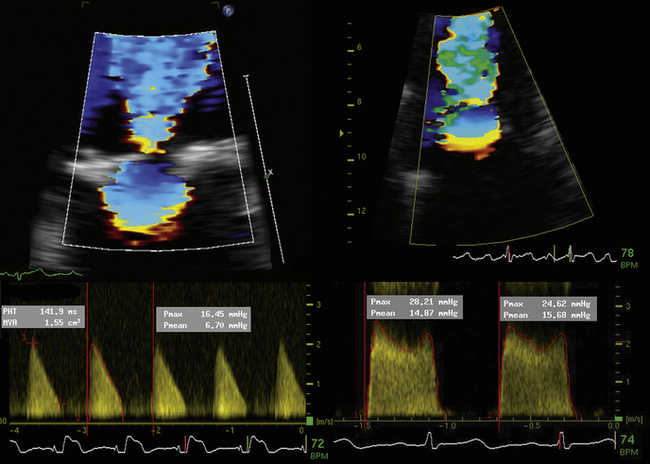
Pressure Half Time
All are factors that reduce the accuracy of the PHT relation to MVA.
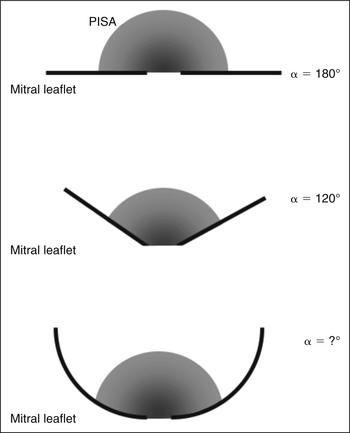
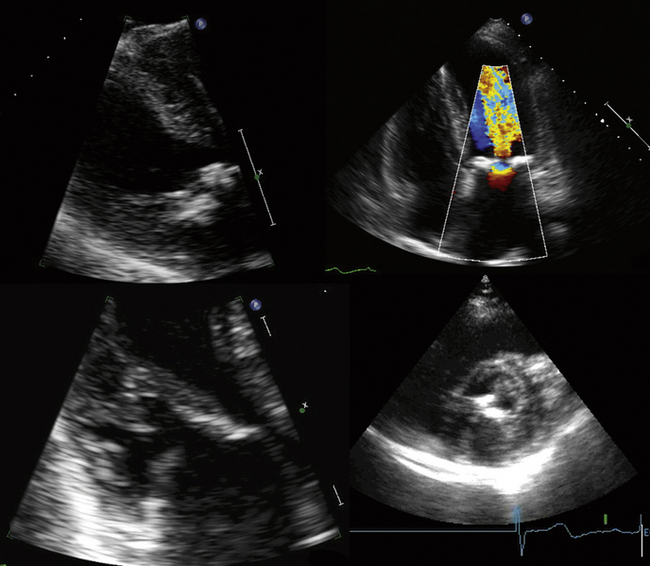
Proximal Isovelocity Surface Area
where r = PISA radius.
The PISA method is based on the assumption that there is a hemisphere of flow acceleration such as would occur with a planar orifice. This often is not the case in mitral stenosis, however, particularly when the greatest stenosis is subvalvar. To adjust for the effect of the orifice on the volume of the PISA, use of an angle correction factor has been suggested (Fig. 5-1), multiplying the preceding calculation by α/180°. Not all echo systems carry angle measurement software.
where α = the measured angle of the “plane” of the orifice and r = PISA radius.
Reporting Issues
Gradient Issues
 If there is a difference in gradient between current and previous echocardiographic determinations, then review the following variables:
If there is a difference in gradient between current and previous echocardiographic determinations, then review the following variables:
 Recall that the accepted gradients for “severe” assume the absence of factors provoking higher output, such as the following:
Recall that the accepted gradients for “severe” assume the absence of factors provoking higher output, such as the following:
 The 95% CI for mitral valve gradient is 3 mm Hg (vs. wedge:LV recordings).
The 95% CI for mitral valve gradient is 3 mm Hg (vs. wedge:LV recordings).
 Direct left atrial pressure recording (LA:LV) typically leads to a lower mitral gradient, as it is without the phase delay of pulmonary capillary wedge tracings.
Direct left atrial pressure recording (LA:LV) typically leads to a lower mitral gradient, as it is without the phase delay of pulmonary capillary wedge tracings.
Area Issues
 Place less emphasis on the valve area, which is invariably calculated (other than by the planimetry method) and, therefore, is subject to larger error.
Place less emphasis on the valve area, which is invariably calculated (other than by the planimetry method) and, therefore, is subject to larger error.
Pressure Half Time Method
 The PHT relation (220) was validated in a relatively small series and is best applied for MVA between 1 and 1.5 cm2; r = 0.75.1 The actual precision of the correlation is less than has been thought.
The PHT relation (220) was validated in a relatively small series and is best applied for MVA between 1 and 1.5 cm2; r = 0.75.1 The actual precision of the correlation is less than has been thought.
 The 220 constant is actually different for both MVA <1 cm2 and MVA >1.5 cm2; therefore, use of the relationship at higher or lower areas is essentially an extrapolation of the known relationship, and assumes that a known variable (220) is a constant, which it is not.
The 220 constant is actually different for both MVA <1 cm2 and MVA >1.5 cm2; therefore, use of the relationship at higher or lower areas is essentially an extrapolation of the known relationship, and assumes that a known variable (220) is a constant, which it is not.
 In the presence of AI, there are two ventricular inflows determining the spectral inflow:diastolic relationship. The PHT relation, therefore, is less representative of MS alone, in the presence of more than moderate AI. Correlation and SEE for MVA by PHT (vs. Gorlin) for patients both without and with AI are as follows:
In the presence of AI, there are two ventricular inflows determining the spectral inflow:diastolic relationship. The PHT relation, therefore, is less representative of MS alone, in the presence of more than moderate AI. Correlation and SEE for MVA by PHT (vs. Gorlin) for patients both without and with AI are as follows:
 PHT is less accurate in patients older than 65 years of age, because of effects of hypertension on the ventricular diastolic properties, leading to overestimation of MVA.3
PHT is less accurate in patients older than 65 years of age, because of effects of hypertension on the ventricular diastolic properties, leading to overestimation of MVA.3
 PHT is less accurate for 3 months after CBV,4 because passive ventricular diastolic properties and atrial diastolic properties are adapting to the changes in load (i.e., less atrial/more ventricular).
PHT is less accurate for 3 months after CBV,4 because passive ventricular diastolic properties and atrial diastolic properties are adapting to the changes in load (i.e., less atrial/more ventricular).
Planimetry Method
 When images are amenable to planimetry, it is an accurate technique. It requires attention to scan extensively along the short axis of the orifice, to ensure sampling at the narrowest part. This may be at the leaflet tips, or in the subvalvar zone, which is important not to miss.
When images are amenable to planimetry, it is an accurate technique. It requires attention to scan extensively along the short axis of the orifice, to ensure sampling at the narrowest part. This may be at the leaflet tips, or in the subvalvar zone, which is important not to miss.
 Correlation for MVA by planimetry (vs. cardiac catheterization): r = 0.92 – 0.95.5,6
Correlation for MVA by planimetry (vs. cardiac catheterization): r = 0.92 – 0.95.5,6
 Planimetry of MVA is an AI-independent technique.
Planimetry of MVA is an AI-independent technique.
 Planimetry is less accurate with prior commissurotomy,3,7,8 because the split often is eccentric: it may lead out onto another plane of imaging and be missed, or may go into a bright commissure that is difficult to visualize.
Planimetry is less accurate with prior commissurotomy,3,7,8 because the split often is eccentric: it may lead out onto another plane of imaging and be missed, or may go into a bright commissure that is difficult to visualize.
Continuity Method
 MVA by the continuity method is less accurate when the ideal reference site (the LVOT) has more than mild AI. The tricuspid reference site is harder to use, and is at least as likely to have TR. For all-comers, the correlation of continuity is very good: r = 0.91, SEE = 0.24 cm2,2 especially in the absence of AI: r = 0.93.2 However, in the presence of AI it is less: r = 0.84.2
MVA by the continuity method is less accurate when the ideal reference site (the LVOT) has more than mild AI. The tricuspid reference site is harder to use, and is at least as likely to have TR. For all-comers, the correlation of continuity is very good: r = 0.91, SEE = 0.24 cm2,2 especially in the absence of AI: r = 0.93.2 However, in the presence of AI it is less: r = 0.84.2
Proximal Isovelocity Surface Area Method
Real-Time Three-Dimensional Echocardiography
The real-time 3D technique allows depiction of the mitral orifice on any plane, and thus should assist with the task of planimetry. Although studies are limited to date, real-time 3D echocardiography shows very good correlation with Gorlin (average difference of 0.08 cm2), and low inter- and intraobserver variability (κ of 0.84 and 0.96, respectively), which in comparative studies was better than with other methods.10
Descriptors of Mitral Stenosis Severity
 After it has been established that MS is present, the next important task is to determine its severity. This involves a composite assessment of symptoms, physical findings, and hemodynamics. It must be recalled that MVA < 1 cm2 always indicates severe MS, but larger patients or those with higher cardiac output (CO) needs are commonly “severe” above an area of 1 cm2 and receive intervention for average MVAs of 1.2 or 1.3 cm2.
After it has been established that MS is present, the next important task is to determine its severity. This involves a composite assessment of symptoms, physical findings, and hemodynamics. It must be recalled that MVA < 1 cm2 always indicates severe MS, but larger patients or those with higher cardiac output (CO) needs are commonly “severe” above an area of 1 cm2 and receive intervention for average MVAs of 1.2 or 1.3 cm2.
 Distinguishing moderate from mild is really of little consequence, as both are “nonsevere” and would not prompt intervention.
Distinguishing moderate from mild is really of little consequence, as both are “nonsevere” and would not prompt intervention.
 “Severe” = mean gradient >12 mm Hg at rest, and an area of <1.3 cm2.
“Severe” = mean gradient >12 mm Hg at rest, and an area of <1.3 cm2.
 “Critical” = mean gradient > 12 mm Hg at rest, and an area of <1.0 cm2
“Critical” = mean gradient > 12 mm Hg at rest, and an area of <1.0 cm2
 Moderate = mean gradient < 12 mm Hg at rest, and an area of ≥1.3 cm2
Moderate = mean gradient < 12 mm Hg at rest, and an area of ≥1.3 cm2
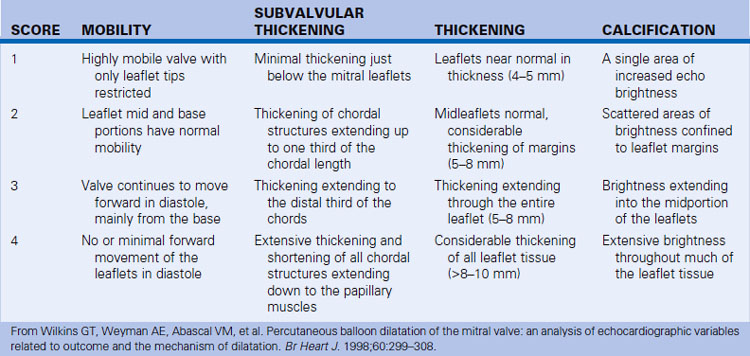
Suitability for Valvuloplasty and Commissurotomy Issues
 Suitability for commissurotomy is determined by the following:
Suitability for commissurotomy is determined by the following:
 Calcification, especially of commissures, is a marker of higher short- and long-term mortality with CBV.
Calcification, especially of commissures, is a marker of higher short- and long-term mortality with CBV.
 Commissurotomy is generally unsuitable if there is MR >2+.
Commissurotomy is generally unsuitable if there is MR >2+.
 There is substantial risk of arterial embolism if there is clot within the body of the left atrium, because valvuloplasty wires invariably are in the body of the atrium. There is a smaller risk of embolism if clot is within the LAA, because the wires that must traverse the LA body may avoid the LAA. TEE is clearly superior to evaluate for the presence of LA and LAA clot.
There is substantial risk of arterial embolism if there is clot within the body of the left atrium, because valvuloplasty wires invariably are in the body of the atrium. There is a smaller risk of embolism if clot is within the LAA, because the wires that must traverse the LA body may avoid the LAA. TEE is clearly superior to evaluate for the presence of LA and LAA clot.
Surgical Issues
 Establish the degree of submitral (annular) calcification. There is a greater risk of periprosthesis (paravalvar) MR when submitral (annular) calcification is severe.
Establish the degree of submitral (annular) calcification. There is a greater risk of periprosthesis (paravalvar) MR when submitral (annular) calcification is severe.
 Determine the degree of AI: in severe MR, IABP should not be used if there is concurrent AI ≥2+.
Determine the degree of AI: in severe MR, IABP should not be used if there is concurrent AI ≥2+.
 Describe the RV in detail (RVH/systolic dysfunction/size), as well as the RVSP.
Describe the RV in detail (RVH/systolic dysfunction/size), as well as the RVSP.
 RA size is a reasonable descriptor of RV diastolic function, if neither TR nor atrial fibrillation is present.
RA size is a reasonable descriptor of RV diastolic function, if neither TR nor atrial fibrillation is present.
Provocative Testing to Enhance Transmitral Gradients and Right Ventricular Systolic Pressure
Simple bedside maneuvers (e.g., a few sit-ups) or more sophisticated ones (e.g., supine bicycle, upright treadmill testing, or dobutamine) may be used to increase the heart rate and cardiac output, and thereby enhance the mitral gradient and RVSP. The role of such testing is unclear: in mitral stenosis the gradient would be expected to increase, and generally it does, about two-fold, when patients are subjected to a Bruce or modified Bruce protocol: from 9 ± 7 mm Hg at rest to 17 ± 8 mm Hg, as does the RVSP, from 41 ±1 9 mm Hg to 70 ± 32 mm Hg.11 The optimal interpretation of the provoked hemodynamics is unclear. Advocates suggest that it better describes the basis of exertional symptoms and may be contributory when the resting hemodynamics are less striking than the (exertional) symptoms and findings.
Dobutamine stress echo (a gradient cut-off of 18 mm Hg) has been shown to predict clinical events (90% sensitivity, 87% specificity, and 90% accuracy) over a 5-year period, and increases the detection of medium- and higher-risk cases.12 Dobutamine stress echocardiography may assist with the evaluation of patients whose symptoms are out of proportion to the calculated valve area, and who may benefit from “medical or invasive intervention.”13
Notes on Mitral Stenosis
Etiologies of Mitral Stenosis
Acquired
 Rheumatic origin is by far the most common cause of MS (>99% of all cases).
Rheumatic origin is by far the most common cause of MS (>99% of all cases).
 Severe mitral annular calcification may cause mild/moderate MS but very rarely causes severe MS.
Severe mitral annular calcification may cause mild/moderate MS but very rarely causes severe MS.
 LA myxoma/thrombus is an uncommon cause of MS (<1%).
LA myxoma/thrombus is an uncommon cause of MS (<1%).
 A mitral annuloplasty ring may overly narrow the orifice.
A mitral annuloplasty ring may overly narrow the orifice.
 Malignant carcinoid, rheumatoid arthritis, systemic lupus erythematosus, and other tumors are very rare causes.
Malignant carcinoid, rheumatoid arthritis, systemic lupus erythematosus, and other tumors are very rare causes.
Pathophysiology
 The normal mitral valve area is 4.5 to 6 cm2. A diastolic transmitral valve gradient occurs with loss of about half of the original area. There is increased left atrial pressure at rest with MVA of 1.5 cm2.
The normal mitral valve area is 4.5 to 6 cm2. A diastolic transmitral valve gradient occurs with loss of about half of the original area. There is increased left atrial pressure at rest with MVA of 1.5 cm2.
 Two thirds (at least) of MS cases occur in women. Of all cases of rheumatic heart disease, 25% are pure MS and 40% are mixed MS/mitral insufficiency.
Two thirds (at least) of MS cases occur in women. Of all cases of rheumatic heart disease, 25% are pure MS and 40% are mixed MS/mitral insufficiency.
 The course of disease proceeds as follows:
The course of disease proceeds as follows:
Role of Transthoracic and Transesophageal Electrocardiography in Mitral Stenosis
Transthoracic Echocardiography
TEE is better than TTE for the following:
 Detection of LAA and LA thrombus, which is necessary information when contemplating CBV
Detection of LAA and LA thrombus, which is necessary information when contemplating CBV
 Estimation of MR severity (TTE may underestimate MR severity)
Estimation of MR severity (TTE may underestimate MR severity)
TEE is not superior for the evaluation of subvalvar disease. The thickened and often calcified leaflets impair subvalvar imaging from the esophagus. Transgastric views are helpful to circumvent this imaging problem.
Cardiac Catheterization for Assessment of Mitral Stenosis
Usual Technique
Gorlin Equation
 The Gorlin equation is employed, using the following variables: CO, DFP, HR, gradient. These variables are readily obtained at cardiac catheterization.
The Gorlin equation is employed, using the following variables: CO, DFP, HR, gradient. These variables are readily obtained at cardiac catheterization.
 Historically, the Gorlin equation constant was developed for MS, because there is less flow dependence with MS than for AS.14
Historically, the Gorlin equation constant was developed for MS, because there is less flow dependence with MS than for AS.14
 In a small initial validation (11 cases), variation of serial measurements averaged ±0.0 cm2, but ranged from +0.2 to –0.4 cm2.15
In a small initial validation (11 cases), variation of serial measurements averaged ±0.0 cm2, but ranged from +0.2 to –0.4 cm2.15
 In the initial series, autopsy standard was ±0.5 to ±0.1 cm2 and the variation against autopsy was ≤0.2 cm2.14
In the initial series, autopsy standard was ±0.5 to ±0.1 cm2 and the variation against autopsy was ≤0.2 cm2.14
 The Gorlin equation purportedly estimates anatomic, not effective, orifice, because it was validated against surgery and autopsy, but there is evidence that it does not truly reflect anatomic area.
The Gorlin equation purportedly estimates anatomic, not effective, orifice, because it was validated against surgery and autopsy, but there is evidence that it does not truly reflect anatomic area.
 The Gorlin equation is less accurate in the presence of the following:
The Gorlin equation is less accurate in the presence of the following:
 Gorlin works best in the context of normal LV systolic function.
Gorlin works best in the context of normal LV systolic function.
Gorlin Equation Considerations
Cardiac Output Issues
 By any technique, CO is not reproducible within 15%.
By any technique, CO is not reproducible within 15%.
 Thermodilution is rendered less accurate by significant TR, which may not be known to be present, and is fairly common in advanced mitral disease.
Thermodilution is rendered less accurate by significant TR, which may not be known to be present, and is fairly common in advanced mitral disease.
 The Fick technique is not likely more accurate in the real world, but does offer a “cross-check.” 3 mL/kg is an estimate of oxygen consumption, and has its own variance.
The Fick technique is not likely more accurate in the real world, but does offer a “cross-check.” 3 mL/kg is an estimate of oxygen consumption, and has its own variance.
Gradient Issues
 Ideally, the mitral gradient is recorded by catheters directly on either side of the valve (LA catheter via transseptal puncture and LV catheter), but this is not actual practice, and would confer additional risk if performed universally. Instead of an LA catheter, a pulmonary capillary wedge pressure (PCWP) recording is generally used, because it is easier and carries lower risk, but it is subject to artifacts that tend to increase the measured gradient.
Ideally, the mitral gradient is recorded by catheters directly on either side of the valve (LA catheter via transseptal puncture and LV catheter), but this is not actual practice, and would confer additional risk if performed universally. Instead of an LA catheter, a pulmonary capillary wedge pressure (PCWP) recording is generally used, because it is easier and carries lower risk, but it is subject to artifacts that tend to increase the measured gradient.
 In most patients, the PCWP is the same as the left atrial pressure, but delayed by 40 to 120 msec. To attempt to account for this, the peak of the V-wave is shifted (“phase corrected”) onto the downslope of the LV pressure tracing.
In most patients, the PCWP is the same as the left atrial pressure, but delayed by 40 to 120 msec. To attempt to account for this, the peak of the V-wave is shifted (“phase corrected”) onto the downslope of the LV pressure tracing.
 When the data by PCWP tracings are in doubt, a transseptal puncture for direct LA pressure should be considered.
When the data by PCWP tracings are in doubt, a transseptal puncture for direct LA pressure should be considered.
 Gradients will vary with atrial fibrillation, which is common with MS, and more beats than usual should be averaged, ideally 10.16
Gradients will vary with atrial fibrillation, which is common with MS, and more beats than usual should be averaged, ideally 10.16
Variables and Constants
 DFP is an accurately measured variable with little error.
DFP is an accurately measured variable with little error.
 HR must be averaged to account for sinus variation/arrhythmia and especially for atrial fibrillation.
HR must be averaged to account for sinus variation/arrhythmia and especially for atrial fibrillation.
Catheterization–Echocardiographic Discordance of Hemodynamic Parameters of Mitral Stenosis
Some cases of MS have discordance with catheter-derived estimates. The most common scenario that begets case discussion occurs when the case is not (quite) severe by echocardiography, but is severe as assessed by the wedge technique at catheterization. In most cases, this type of discordance reflects only lack of familiarity with wedge technique errors.
Reasons for Discordance with Catheterization–Echocardiography
The 95% CI Doppler mitral valve gradient versus catheter (wedge technique) gradient is 3 mm Hg.
Catheterization Estimates Transmitral Gradient Higher Than Does Echocardiography
 Use of PCWP (not direct LAP) is the most likely cause of overestimation of MVG by catheterization. Use of PCWP tracing overestimates LAP–LVP by 3.3 ± 3.5 mm Hg (53%).22
Use of PCWP (not direct LAP) is the most likely cause of overestimation of MVG by catheterization. Use of PCWP tracing overestimates LAP–LVP by 3.3 ± 3.5 mm Hg (53%).22
 Use of phase-“corrected” PCWP improves correlation, but still leads to overestimation: 2.5 ± 2.9 mm Hg (43%) in one study22 and a lesser mean difference in another study (1.7 mm Hg), as well as lesser difference in area.23
Use of phase-“corrected” PCWP improves correlation, but still leads to overestimation: 2.5 ± 2.9 mm Hg (43%) in one study22 and a lesser mean difference in another study (1.7 mm Hg), as well as lesser difference in area.23
 Use of direct LAP results in a negligible mean difference between Doppler and catheterization gradient: only 0.2 ± 1.2 mm Hg.22
Use of direct LAP results in a negligible mean difference between Doppler and catheterization gradient: only 0.2 ± 1.2 mm Hg.22
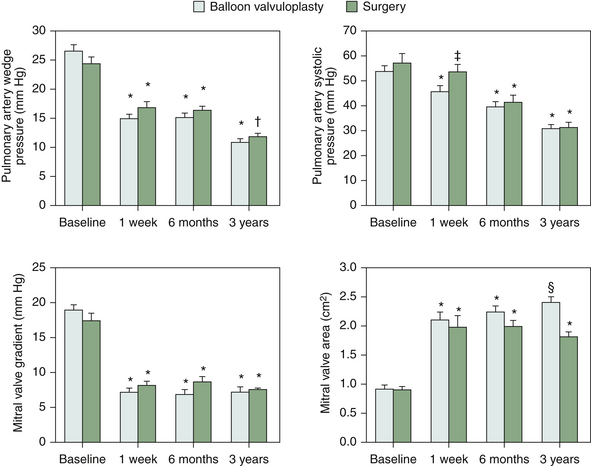
Mitral Valve Catheter Balloon Valvuloplasty
Summary
For MS, echocardiography is able to determine the following:
BOX 5-1 Indications for Percutaneous Mitral Balloon Valvotomy: ACC/AHA 2006 Recommendations
Class I
 Percutaneous mitral balloon valvotomy is effective for symptomatic patients (New York Heart Association [NYHA] functional class II, III, or IV), with moderate or severe mitral stenosis (MS) and valve morphology favorable for percutaneous mitral balloon valvotomy in the absence of left atrial thrombus or moderate to severe mitral regurgitation (MR). (Level of evidence: A)
Percutaneous mitral balloon valvotomy is effective for symptomatic patients (New York Heart Association [NYHA] functional class II, III, or IV), with moderate or severe mitral stenosis (MS) and valve morphology favorable for percutaneous mitral balloon valvotomy in the absence of left atrial thrombus or moderate to severe mitral regurgitation (MR). (Level of evidence: A)
 Percutaneous mitral balloon valvotomy is effective for asymptomatic patients with moderate or severe MS and valve morphology that is favorable for percutaneous mitral balloon valvotomy who have pulmonary hypertension (pulmonary artery systolic pressure > 50 mm Hg at rest or > 60 mm Hg with exercise) in the absence of left atrial thrombus or moderate to severe MR. (Level of evidence: C)
Percutaneous mitral balloon valvotomy is effective for asymptomatic patients with moderate or severe MS and valve morphology that is favorable for percutaneous mitral balloon valvotomy who have pulmonary hypertension (pulmonary artery systolic pressure > 50 mm Hg at rest or > 60 mm Hg with exercise) in the absence of left atrial thrombus or moderate to severe MR. (Level of evidence: C)
Class IIb
 Percutaneous mitral balloon valvotomy may be considered for asymptomatic patients with moderate or severe MS and valve morphology favorable for percutaneous mitral balloon valvotomy who have new onset of atrial fibrillation in the absence of left atrial thrombus or moderate to severe MR. (Level of evidence: C)
Percutaneous mitral balloon valvotomy may be considered for asymptomatic patients with moderate or severe MS and valve morphology favorable for percutaneous mitral balloon valvotomy who have new onset of atrial fibrillation in the absence of left atrial thrombus or moderate to severe MR. (Level of evidence: C)
 Percutaneous mitral balloon valvotomy may be considered for symptomatic patients (NYHA functional class II, III, or IV) with mitral valve area >1.5 cm2 if there is evidence of hemodynamically significant MS based on pulmonary artery systolic pressure >60 mm Hg, pulmonary artery wedge pressure ≥25 mm Hg, or mean MV gradient >15 mm Hg during exercise. (Level of evidence: C)
Percutaneous mitral balloon valvotomy may be considered for symptomatic patients (NYHA functional class II, III, or IV) with mitral valve area >1.5 cm2 if there is evidence of hemodynamically significant MS based on pulmonary artery systolic pressure >60 mm Hg, pulmonary artery wedge pressure ≥25 mm Hg, or mean MV gradient >15 mm Hg during exercise. (Level of evidence: C)
 Percutaneous mitral balloon valvotomy may be considered as an alternative to surgery for patients with moderate or severe MS who have a nonpliable calcified valve and are in NYHA class III–IV. (Level of evidence: C)
Percutaneous mitral balloon valvotomy may be considered as an alternative to surgery for patients with moderate or severe MS who have a nonpliable calcified valve and are in NYHA class III–IV. (Level of evidence: C)
From ACC/AHA 2006 guidelines for the management of patients with valvular heart disease. J Am Coll Cardiol. 2006;48(3):e1–e148.
BOX 5-2 Indications for Surgery for Mitral Stenosis: ACC/AHA 2006 Recommendations
Class I
 Mitral valve (MV) surgery (repair, if possible) is indicated in patients with symptomatic (NYHA functional class III–IV) moderate or severe MS when
Mitral valve (MV) surgery (repair, if possible) is indicated in patients with symptomatic (NYHA functional class III–IV) moderate or severe MS when
 Symptomatic patients with moderate to severe MS who also have moderate to severe MR should receive MV replacement, unless valve repair is possible at the time of surgery. (Level of evidence: C)
Symptomatic patients with moderate to severe MS who also have moderate to severe MR should receive MV replacement, unless valve repair is possible at the time of surgery. (Level of evidence: C)
Class IIa
MV replacement is reasonable for patients with severe MS and severe pulmonary hypertension (i.e., pulmonary artery systolic pressure >60 mm Hg) with NYHA functional class I–II symptoms who are not considered candidates for percutaneous mitral balloon valvotomy or surgical MV repair. (Level of evidence: C)
From ACC/AHA 2006 guidelines for the management of patients with valvular heart disease. J Am Coll Cardiol. 2006;48(3):e1–e148.
BOX 5-3 Valvular Disease: Focused Update on Endocarditis: ACC/AHA 2008 Guidelines
From ACC/AHA 2006 guidelines for the management of patients with valvular heart disease. J Am Coll Cardiol. 2006;48(3):e1–e148.
Transthoracic Echocardiography
ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 Appropriate Use Criteria for Echocardiography25
Native Valvular Stenosis with TTE
 Routine surveillance (<3 yr) of mild valvular stenosis without a change in clinical status or cardiac examinationAppropriateness criteria: I; median score: 3
Routine surveillance (<3 yr) of mild valvular stenosis without a change in clinical status or cardiac examinationAppropriateness criteria: I; median score: 3
 Routine surveillance (≥3 yr) of mild valvular stenosis without a change in clinical status or cardiac examinationAppropriateness criteria: A; median score: 7
Routine surveillance (≥3 yr) of mild valvular stenosis without a change in clinical status or cardiac examinationAppropriateness criteria: A; median score: 7
 Routine surveillance (<1 yr) of moderate or severe valvular stenosis without a change in clinical status or cardiac examinationAppropriateness criteria: I; median score: 3
Routine surveillance (<1 yr) of moderate or severe valvular stenosis without a change in clinical status or cardiac examinationAppropriateness criteria: I; median score: 3
Routine surveillance (≥1 yr) of moderate or severe valvular stenosis without a change in clinical status or cardiac examinationAppropriateness criteria: A; median score: 8
ACC/AHA 1997 Guidelines for the Clinical Application of Echocardiography27
Indications for Echocardiography in Valvular Stenosis
ACC/AHA 2006 Guidelines for the Management of Patients with Valvular Heart Disease28
Recommendations and Indications for Echocardiography in Mitral Stenosis
Transesophageal Echocardiography
ACC/AHA 2006 Guidelines for the Management of Patients with Valvular Heart Disease28
Recommendations Indications for Echocardiography in Mitral Stenosis
Cardiac Catheterization
ACC/AHA 2006 Guidelines for the Management of Patients with Valvular Heart Disease28
Indications for Invasive Hemodynamic Evaluation
Cardiac Computed Tomography
Cardiac Magnetic Resonance
Nuclear
TABLE 5-3 Utility of Different Imaging Modalities and Cardiac Catheterization in the Assessment of Mitral Stenosis
| MODALITY | PROS | CONS/CAVEATS |
|---|---|---|
| Transthoracic Echocardiography |
Spectral Doppler echocardiography
• CW Doppler determinations of mitral valve gradients are nearly equivalent to those determined by LA (trans-septal) to LV catheterization.
• CW Doppler determinations of MVA by the PHT method are accurate within the range of 1–1.5 cm2, in the absence of variables that influence LV and LA compliance. The PHT relation is not confounded by the all-too-common presence of mitral insufficiency. The method is validated between 1 and 1.5 cm2.
• Many non–mitral valve factors influence the transmitral gradient. (E.g., tachycardia, fever, anemia, pregnancy, and large dialysis fistula increase the gradient, and bradycardia and severe pulmonary hypertension reduce the gradient.)
• Many factors reduce the accuracy of the PHT method: e.g., valvuloplasty within three months, LVH, AS, AI, and prior MI.
• Offers the best means to identify LA and LA appendage thrombi that are relevant to balloon valvuloplasty procedures
• Can guide catheter balloon valvuloplasty procedures (confirms safety of trans-septal punctures, and identifies significant increments in MI with successive balloon inflations)
• MS hemodynamics by TEE are similar to those of TTE, as long as Doppler alignment remains favorable.
• MS determination of PISA estimates of orifice area are generally better than those of TTE because PISAs are better depicted by TEE.
• The traditional test to assess gradient and area, and the traditional reference standard to which other tests to compare their determinations of transmitral gradient and of valve area
• The most reliable means by which to determine the pulmonary artery pressures, and the only means by which to determine the pulmonary vascular resistence
• The platform from which to provide catheter balloon valvuloplasty
2D, two-dimensional; AI, aortic insufficiency; AS, aortic stenosis; CMR, cardiac magnetic resonance; CW, continuous wave; LA, left atrium; LGE, late gadolinium enhancement; LV, left ventricle; LVH, left ventricular hypertrophy; MI, mitrial insufficiency; MS, mitral stenosis; MVA, mitral valve area; NA, not applicable; PHT, pressure half time; PISA, proximal isovelocity surface area; SSFP, steady-state free precession; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography; VEPC, velocity-enhanced phase contrast.
1. Ganau A., Devereux R.B., Roman M.J., et al. Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol. 1992;19(7):1550-1558.
2. Nakatani S., Masuyama T., Kodama K., et al. Value and limitations of Doppler echocardiography in the quantification of stenotic mitral valve area: comparison of the pressure half-time and the continuity equation methods. Circulation. 1988;77(1):78-85.
3. Abascal V.M., Moreno P.R., Rodriguez L., et al. Comparison of the usefulness of Doppler pressure half-time in mitral stenosis in patients ≤65 years of age. Am J Cardiol. 1996;78(12):1390-1393.
4. Nishimura R.A., Tajik A.J. Quantitative hemodynamics by Doppler echocardiography: a noninvasive alternative to cardiac catheterization. Prog Cardiovasc Dis. 1994;36(4):309-342.
5. Martin R.P., Rakowski H., Kleiman J.H., et al. Reliability and reproducibility of two dimensional echocardiograph measurement of the stenotic mitral valve orifice area. Am J Cardiol. 1979;43(3):560-568.
6. Nichol P.M., Gilbert B.W., Kisslo J.A. Two-dimensional echocardiographic assessment of mitral stenosis. Circulation. 1977;55(1):120-128.
7. Smith M.D., Handshoe R., Handshoe S., et al. Comparative accuracy of two-dimensional echocardiography and Doppler pressure half-time methods in assessing severity of mitral stenosis in patients with and without prior commissurotomy. Circulation. 1986;73(1):100-107.
8. Teirstein P.S., Yock P.G., Popp R.L. The accuracy of Doppler ultrasound measurement of pressure gradients across irregular, dual, and tunnellike obstructions to blood flow. Circulation. 1985;72(3):577-584.
9. Rodriguez L., Thomas J.D., Monterroso V., et al. Validation of the proximal flow convergence method. Calculation of orifice area in patients with mitral stenosis. Circulation. 1993;88(3):1157-1165.
10. Zamorano J., Cordeiro P., Sugeng L., et al. Real-time three-dimensional echocardiography for rheumatic mitral valve stenosis evaluation: an accurate and novel approach. J Am Coll Cardiol. 2004;43(11):2091-2096.
11. Leavitt J.I., Coats M.H., Falk R.H. Effects of exercise on transmitral gradient and pulmonary artery pressure in patients with mitral stenosis or a prosthetic mitral valve: a Doppler echocardiographic study. J Am Coll Cardiol. 1991;17(7):1520-1526.
12. Reis G., Motta M.S., Barbosa M.M., et al. Dobutamine stress echocardiography for noninvasive assessment and risk stratification of patients with rheumatic mitral stenosis. J Am Coll Cardiol. 2004;43(3):393-401.
13. Cheitlin M.D. Stress echocardiography in mitral stenosis: when is it useful? J Am Coll Cardiol. 2004;43(3):402-404.
14. Gorlin R., Gorlin S.G. Hydraulic formula for calculation of the area of the stenotic mitral valve, other cardiac valves, and central circulatory shunts. Am Heart J. 1951;41(1):1-21.
15. Gorlin R., Gorlin S.G. Hydrolic formula for calculation of the area of the stenotic mitral valve, other cardiac valves, and central circulatory shunts. Am Heart J. 1951;41(1):1-21.
16. Kern M.J. Hemodynamic Rounds. New York: Wiley-Liss; 1999.
17. Cannon S.R., Richards K.L., Crawford M.H., et al. Inadequacy of the Gorlin formula for predicting prosthetic valve area. Am J Cardiol. 1988;62(1):113-116.
18. Segal J., Lerner D.J., Miller D.C., et al. When should Doppler-determined valve area be better than the Gorlin formula? Variation in hydraulic constants in low flow states. J Am Coll Cardiol. 1987;9(6):1294-1305.
19. Burwash I.G., Thomas D.D., Sadahiro M., et al. Dependence of Gorlin formula and continuity equation valve areas on transvalvular volume flow rate in valvular aortic stenosis. Circulation. 1994;89(2):827-835.
20. Cannon S.R., Richards K.L., Crawford M. Hydraulic estimation of stenotic orifice area: a correction of the Gorlin formula. Circulation. 1985;71(6):1170-1178.
21. Cannon J.D.Jr., Zile M.R., Crawford F.A.Jr., Carabello B.A. Aortic valve resistance as an adjunct to the Gorlin formula in assessing the severity of aortic stenosis in symptomatic patients. J Am Coll Cardiol. 1992;20(7):1517-1523.
22. Nishimura R.A., Rihal C.S., Tajik A.J., Holmes D.R.Jr. Accurate measurement of the transmitral gradient in patients with mitral stenosis: a simultaneous catheterization and Doppler echocardiographic study. J Am Coll Cardiol. 1994;24(1):152-158.
23. Lange R.A., Moore D.M.Jr., Cigarroa R.G., Hillis L.D. Use of pulmonary capillary wedge pressure to assess severity of mitral stenosis: is true left atrial pressure needed in this condition? J Am Coll Cardiol. 1989;13(4):825-831.
24. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease. J Am Coll Cardiol. 2006;48(3):e1-e148.
25. Douglas P.S., Garcia M.J., Haines D.E., et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 appropriate use criteria for echocardiography. J Am Coll Cardiol. 2011;57(9):1126-1166.
26. Cheitlin M.D., Armstrong W.F., Aurigemma G.P., et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). Circulation. 2003;108(9):1146-1162.
27. Cheitlin M.D., Chair J.S., Alpert J.S., et al. ACC/AHA guidelines for the clinical application of echocardiography: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Clinical Application of Echocardiography). Circulation. 1997;95:1686-1744.
28. Bonow R.O., Blase A.C., Chatterjee K., et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2006;114:e84-e231.
29. Douglas P.S., Khandheria B.K., Stainback R.F., Weissman N.J. ACCF/ASE/ACEP/AHA/ASNC/SCAI/SCCT/SCMR 2008 appropriateness criteria for stress echocardiography. Circulation. 2008;117(11):1478-1497.
30. Taylor A.J., Cerqueira M., Hodgson J.M., et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. J Am Coll Cardiol. 2010;56(22):1864-1894.
31. Hendel R.C., Manesh P.R., Kramer C.M., Poon M. ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging. J Am Coll Cardiol. 2006;48(7):1475-1497.
32. Pennell D.J., Sechtem U.P., Higgins C.B., et al. Clinical indications for cardiovascular magnetic resonance (CMR): consensus panel report. J Cardiovasc Magn Reson. 2004;6(4):727-765.
33. Hendel R.C., Berman D.S., Di Carli M.F., et al. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 appropriate use criteria for cardiac radionuclide imaging. J Am Coll Cardiol. 2009;53(23):2201-2229.
34. Klocke F.J., Baird M.G., Bateman T.M., et al. ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to revise the 1995 guidelines for the clinical use of cardiac radionuclide imaging). Circulation. 2003;108(11):1404-1418.
35. Nishimura R.A., Carabello B.A., Faxon D.P., et al. ACC/AHA 2008 guideline update on valvular heart disease: focused update on infective endocarditis. J Am Coll Cardiol. 2008;52(8):676-685.
36. Lin S.J., Brown P.A., Watkins M.P., et al. Quantification of stenotic mitral valve area with magnetic resonance imaging and comparison with Doppler ultrasound. J Am Coll Cardiol. 2004;44(1):133-137.
37. Djavidani B., Debl K., Lenhart M., et al. Planimetry of mitral valve stenosis by magnetic resonance imaging. J Am Coll Cardiol. 2005;45(12):2048-2053.
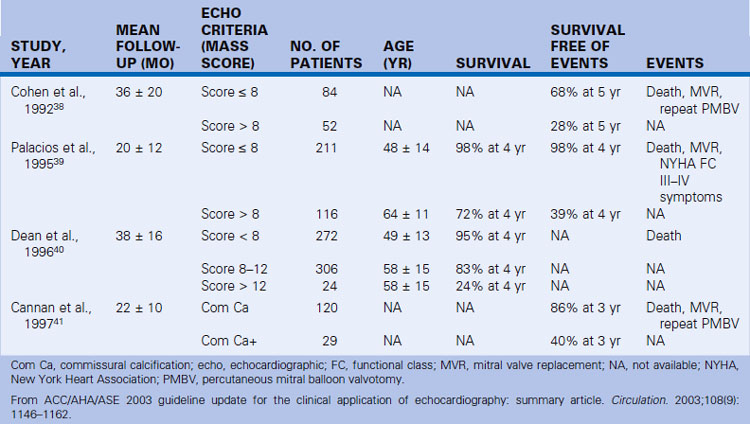
38. Cohen D.J., Kuntz R.E., Gordon S.P., et al. Predictors of long-term outcome after percutaneous balloon mitral valvuloplasty. N Engl J Med. 1992;327:1329-1335.
39. Palacios I.F., Tuzcu M.E., Weymen A.E., et al. Clinical follow-up of patients undergoing percutaneous mitral balloon valvotomy. Circulation. 1995;91:671-676.
40. Dean L.S., Mickel M., Bonan R., et al. Four-year follow-up of patients undergoing percutaneous balloon mitral commissurotomy: a report from the National Heart, Lung, and Blood Institute Balloon Valvuloplasty Registry. J Am Coll Cardiol. 1996;28:1452-1457.
41. Cannan C.R., Nishimura R.A., Reeder G.S., et al. Echocardiographic assessment of commissural calcium: a simple predictor of outcome after percutaneous mitral balloon valvotomy. J Am Coll Cardiol. 1997;29:175-180.