Chapter 90E Minimally invasive techniques in hepatic resection
Overview
Over the latter portion of the twentieth century, liver surgery has rapidly progressed from a risky procedure to a safe therapy for benign and malignant liver disease. As with many surgery disciplines, minimally invasive approaches were pursued in the 1990s. Gagner is credited for the first laparoscopic partial hepatectomy in 1992 (Gagner et al, 1992), and in a prospective series of 30 patients published in 2000, we demonstrated the safety and reproducibility of laparoscopic partial hepatectomy (Cherqui et al, 2000), which was followed by the safe application of these techniques to living donor left lateral sectionectomy in 2002 (Cherqui et al, 2002). Despite close to 20 years of development and nearly 3000 reported cases internationally, minimally invasive liver surgery remains an emerging field that should be approached with caution by surgeons experienced in the perioperative care and planning of both liver and laparoscopic surgery (Nguyen et al, 2009). Six centers have reported series of more than 100 patients, and most perform fewer than half of their total cases laparoscopically (Bryant et al, 2009; Buell et al, 2008; Chen et al, 2008; Cho et al, 2008; Koffron et al, 2006, 2007; Topal et al, 2008;). We have performed more than 200 laparoscopic partial hepatectomies since 1996, which represents 23% of our hepatectomy volume.
Terminology
In 2008, leaders in the field met to discuss the current status of laparoscopic liver surgery (Buell et al, 2009). In the so-called Louisville Statement that resulted, those in attendance agreed on three procedural definitions: 1) pure, 2) hand-assisted, and 3) hybrid laparoscopy. Pure laparoscopy involves complete mobilization and resection via laparoscopic ports, with an incision for specimen extraction only. Hand-assisted laparoscopy involves the elective placement of a hand port for mobilization or resection, which is then used for specimen extraction. Hybrid laparoscopy refers to a procedure in which the resection and extraction are performed through a minilaparotomy, but laparoscopy, with or without hand assistance, is utilized for mobilization.
According to a comprehensive international review performed by Nguyen and colleagues (2009), 75% of reported laparoscopic resections were pure laparoscopy, 17% were hand-assisted procedures, and 2% used the hybrid technique. In addition, 4.8% were conversions to laparotomy or hand-assisted procedures, with the remaining 2% using less common techniques, such as a thoracoscopic approach.
Laparoscopic resections should be categorized no differently than open resections, based on the Brisbane 2000 terminology of liver anatomy and resections (Pang, 2002). Anterior and lateral segments II, III, IVb, V, and VI are most amenable to laparoscopic resection. Isolated resections of segments I, IVa, VII, and VIII have been reported, as surgeons extend the limits after safe mastery of anterior resections. Of the nearly 3000 internationally reported laparoscopic liver resections, the majority (44.9%) were wedge resections or segmentectomies, followed by left lateral sectionectomies (20.3%). Major resections, consisting of three or more Couinaud segments, included right hemihepatectomy (9%) and left hemihepatectomy (6.8%). Extended right hepatectomy, caudate lobectomy, central hepatectomy, and extended left hepatectomy each made up less than 1% of the total reported cases (Nguyen et al 2009).
We performed 96% of our resections using pure laparoscopy, with hand assistance (4%) selectively used for right hepatectomies or posterior segmentectomies. Major resections comprise 19% of our experience, and limited resections comprise 81% (Bryant et al 2009).
Indications
Liver pathology under consideration for open partial hepatectomy should be evaluated for the feasibility of a laparoscopic resection (see Chapter 90A); however, the potential benefits of a minimally invasive resection do not confer a prescription to resect all hepatic lesions for the purpose of providing a definitive diagnosis. Specifically, the laparoscopic approach should not be used to resect incidental, asymptomatic lesions convincingly recognized by imaging and markers to be benign and without harmful potential (cysts, hemangiomas, focal nodular hyperplasia), nor should laparoscopic resection be performed for the purpose of diagnosis, when lesions are safely amenable to percutaneous biopsy.
The indications for laparoscopic resection do not vary from those for open resection (Box 90E.1); however, lesion size and location are the most important determinants of when laparoscopic resection is appropriate. Resection of benign and malignant solid lesions and cystic and parasitic lesions are possible laparoscopically. The most favorable lesions for laparoscopic resection are solitary, 5 cm or less, and located in peripheral liver segments II to VI (Fig. 90E.1; Buell et al, 2009). Pedunculated tumors greater than 5 cm are often easily resectable laparoscopically. In experienced hands, laparoscopic left lateral sectionectomy should be the standard approach for pathology in segments II and III (Buell et al, 2009; Chang et al, 2007).
Box 90E.1 Indications and Contraindications for Laparoscopic Partial Hepatectomy
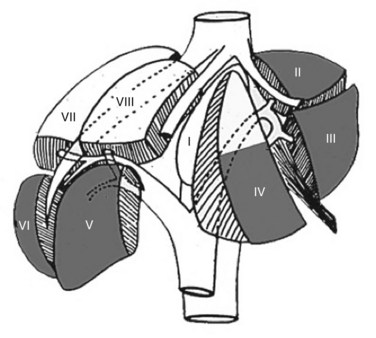
FIGURE 90E.1 The anterolateral liver—composed of segments II, III, IVb, V, and VI—is most amenable to the laparoscopic approach.
Relative contraindications include lesions isolated to segments VII and VIII, multiple or bilateral lesions, and lesions located in proximity to the major hepatic veins, the inferior vena cava, or the hepatic hilum. These contraindications are considered relative, because in experienced centers, these lesions are increasingly managed laparoscopically (Buell et al, 2008; Koffron et al, 2007; Vigano et al, 2009a). A more extensive laparoscopic resection than would otherwise be necessary through an open approach may be contraindicated. An example would be a laparoscopic right hemihepatectomy for a posterior lesion that could be resected by a more limited open posterior segmentectomy. For each patient, the surgeon must weigh the risks and benefits of the laparoscopic and open techniques with the benefits of parenchymal preservation.
Surgical margins for benign lesions do not vary between open and laparoscopic resections. If there is concern that an adequate margin cannot be obtained laparoscopically, but it is technically feasible by an open approach, the former is contraindicated. Other contraindications include gallbladder cancer and hilar cholangiocarcinoma, because of the risk of peritoneal and port-site seeding and the necessary complex hilar dissection and extensive resection, respectively (see Chapters 49, 50B, 90B, and 90C).
Safety and Benefits
Laparoscopic partial hepatectomy provides the benefits that laparoscopy has offered to patients undergoing many other abdominal operations. Case-control studies have demonstrated shorter operative times (Bryant et al, 2009; Buell et al, 2008; Koffron et al, 2007), shorter lengths of hospitalization (Buell et al, 2009; Cai et al, 2008; Koffron et al, 2007; Topal et al, 2008), less operative blood loss (Bryant et al, 2009; Buell et al, 2008; Koffron et al, 2007; Lesurtel et al, 2003), reduced transfusion requirements (Buell et al, 2008; Koffron et al, 2007; Topal et al, 2008), a reduced need for analgesia (Cai et al, 2008), quicker return to oral consumption (Cai et al, 2008), lower morbidity (Buell et al, 2008; Koffron et al, 2007; Topal et al, 2008), and fewer postoperative adhesions (Belli et al, 2009; Laurent et al, 2009). Studies have demonstrated decreased costs when accounting for shorter operative times and hospitalizations (Buell et al, 2008; Koffron et al, 2007; Polignano et al, 2008).
The mortality and morbidity rates in these studies are at least equivalent to, if not better than, those of large case series of open liver resections. In their review of 127 published articles on laparoscopic hepatic resection, Nguyen and colleagues (2009) found a cumulative mortality rate of 0.3%. This compares favorably to the 0% to 5.4% reported in the open-resection literature from high-volume centers. All deaths were postoperative, and most often caused by liver and multiorgan system failure. Of 2804 patients, 295 morbid complications were reported (10.5%), with a range of 0% to 50% across the 127 studies (Nguyen et al 2009). Liver-specific complications included bile leaks (1.5%), transient liver failure and ascites (1%), and abscess (2%). The remaining 6% were complications common to all operations, including but not limited to hemorrhage, wound infection, hernia, bowel injury, arrhythmia, and urinary or respiratory tract infections.
Laparoscopy has been utilized with success in cirrhotic patients (see Chapter 90F), with multiple small case-control studies and series demonstrating lower morbidity and improved recovery. The laparoscopic approach also decreases the rate of postoperative ascites in this subgroup. Possible explanations include the preservation of venous and lymphatic collateral pathways in the abdominal wall, less intraoperative mobilization of the liver, and reduced intraoperative volume loading (Belli et al, 2007; Buell et al, 2009; Cherqui, 2006; Gigot et al, 2002; Kaneko et al, 2009; Laurent et al, 2003; Santambrogio et al, 2009; Vigano et al, 2009a). Nearly 40% of our laparoscopic experience has been with those patients who have chronic liver disease. These patients typically require longer operations and more pedicle clamping but globally have a better recovery than their open counterparts (Bryant et al, 2009).
As a consequence of decreased adhesions formed after an initial laparoscopic resection, reoperations, such as repeat hepatectomy and liver transplantation can often be performed more easily, with less blood loss, reduced transfusion requirements, and reduced operative time than following an initial, open partial hepatectomy (Belli et al, 2009; Bryant et al, 2009; Laurent et al, 2003). In our study comparing patients who had undergone a previous open or laparoscopic liver resection, the absence of adhesions at the time of liver transplantation in the laparoscopic group allowed for hepatectomy in a mean of 150 ± 52 minutes versus 247 ± 71 minutes in the open group. Additionally, a median of 2 U blood was transfused during hepatectomy in the open group versus zero in the laparoscopic group (Laurent et al, 2009). These are significant benefits, given that patients who require repeat operations often have diseased parenchyma because of cirrhosis or chemotherapy effects and the negative immune and oncologic impact of blood transfusion.
Barriers to the wide acceptance of laparoscopic surgery—such as threat of gas embolism, violation of oncologic principles, and significant risk of bleeding—have not been apparent in the literature (Vigano et al, 2009a). In addition, studies have consistently demonstrated that operative safety and postoperative morbidity improve with experience (Nguyen et al, 2009). When comparing our early and late groups, we found statistically significant reductions in operative time (210 to 150 minutes), blood loss (300 to 200 mL), conversion (16.9% to 2.4%), and morbidity (17.2% to 3.4%) (Vigano et al, 2009b).
Additional benefits include better cosmesis, improved maintenance of the sensorimotor integrity of the abdominal wall, and earlier access to adjuvant therapy because of quicker recovery. Despite these favorable results, it is important to acknowledge that these findings are from nonrandomized studies, and that laparoscopic cases remain highly selected for their potential for success. It can be inferred from the colorectal and bariatric literature that these benefits are real, especially for indicated cases (see Box 90E.1); however, the benefits may not be as robust for cases with relative contraindications (Castaing et al, 2009; Cho et al, 2008).
Oncologic Outcomes
Perhaps more important than demonstrating perioperative benefit is confirmation that oncologic principles are not dismissed. With regard to margins, recurrence, and survival, comparable results between open and laparoscopic resections have been well demonstrated in the literature (Nguyen et al, 2009; Vigano et al, 2009). In a meta-analysis of 165 laparoscopic liver resections and 244 open liver resections, Simillis and colleagues (2007) found no difference in tumor-free margin status, overall survival, and disease-free survival between laparoscopic and open liver resection for cancer. This has been demonstrated for both hepatocellular carcinoma (HCC) and colorectal metastatic disease (Castaing et al, 2009; Cherqui et al, 2009; Nguyen et al, 2009; Sarpel et al, 2009). Laparoscopic resection of other metastatic tumors has been reported in small numbers, mainly breast cancers and melanomas. Further, port-site metastases and peritoneal dissemination have not occurred with the use of closed-bag methods for specimen extraction.
As chemotherapeutic, biologic, and radiation options expand, patients with primary and metastatic liver cancer may anticipate longer survival despite the high possibility of recurrence. Resection of HCC as a bridge to future transplantation should also be a consideration (Cherqui et al, 2009; Laurent et al, 2009). As such, parenchyma-sparing procedures should be promoted in anticipation of repeat operative needs. Rather than performing a laparoscopic right or left hepatectomy because of relative ease technically, segmental resections along anatomic lines must be performed when possible.
General Principles
Incisions, Exploration, and Exposure
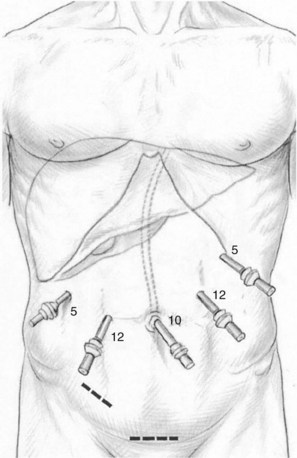
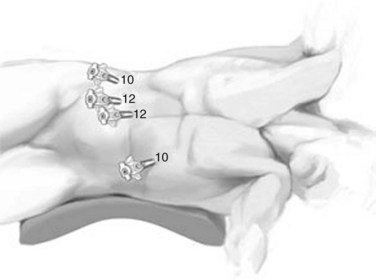
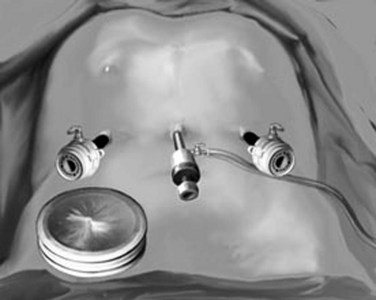
The open technique is used to gain access to the abdomen supraumbilically. The remaining four ports are placed under direct vision, with incisions sized to accommodate the necessary cannulas as pictured in Figure 90E.2. The median trocar is the camera port, the paramedian ones are the working ports, and the most lateral right and left ports are for retraction. For segment VI and VII resections, four ports are used: a 10-mm port in the left upper quadrant, a supraumbilical 12-mm port, a 12-mm paraumbilical port in the midclavicular line, and a 10-mm trocar in the anterior-axillary line (Fig. 90E.3).
After a thorough inspection of the peritoneal cavity for ascites, carcinomatosis, and signs of portal hypertension, attention is turned to the liver for signs of superficial lesions, steatosis, cholestasis, cirrhosis, or other gross pathology. Next, laparoscopic ultrasound is performed. A thorough knowledge of both B-mode and Doppler laparoscopic ultrasonography is mandatory for safe and accurate laparoscopic resections. A high-frequency laparoscopic probe is used to look for lesions less than 1 cm, which may be difficult to assess by extracorporeal imaging, and to confirm adequate margins (Foroutani et al, 2000; Milsom et al, 2000; Montorsi et al, 2001).
Transection Techniques
Parenchymal transection by repeated application of a linear stapler is practiced by some centers, with the cost of the devices said to be offset by reduced operating time and length of hospitalization (Buell et al, 2008). We prefer a method of complete visualization, believing that blind application of the linear stapler is a rougher and less precise technique.
Conversion to Laparotomy
Conversion rates in the literature range from 0% to 55%. The most cited reasons for conversion are hemorrhage, adhesions, and anatomic difficulties (Nguyen et al, 2009a). Many of these can be prospectively prevented by proper patient selection based on quality imaging and an understanding of laparoscopic principles. Conversion should not be considered failure and should be performed without hesitation when a patient’s well-being is at risk.
Hemorrhage may be brisk, or it may be of a continuous, slow nature. Brisk hemorrhage may result from either arterial or venous bleeding. Arterial bleeding is controlled with instruments used to compress or grasp the vessel while clips or sutures are applied. The hepatic vein is the most common source of hemorrhage, and bleeding from the hepatic and portal veins and vena cava may be more difficult to manage because of vessel fragility and retraction (Vigano et al, 2009). Significant bleeding should ideally be managed laparoscopically, at least temporarily, because shock may ensue by the time the laparotomy is performed. This is a matter of urgent decision making by the surgeon, perfect collaboration with the anesthesiologist, and above all, mastering the advanced laparoscopic techniques required to embark on these procedures, such as intracorporeal suturing. Continuous, slow bleeding occurs during transection and may be due to elevated CVP, fragile liver parenchyma, or inappropriate technique. This slows the procedure and often interferes with visualization. In all situations, intermittent portal triad clamping may be utilized, and if rapid introduction of a hand port and conservative measures are ineffective to manage the bleeding, conversion to laparotomy should be performed without delay.
Specific Partial Hepatectomy Procedures
Left Lateral Sectionectomy
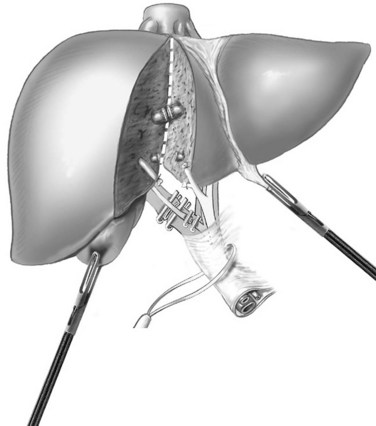
The parenchymal transection is performed to the left of the patient’s falciform ligament with the Harmonic or LigaSure. The plane of transection is better visualized by retracting the round ligament superiorly and to the right and the left lateral segment superiorly and to the left. The ultrasonic dissector can then be used to open the deeper parenchyma, progressing cranially from anterior to posterior, with small vessels and ducts controlled as previously discussed; however, transection can be completed with the Harmonic Scalpel or LigaSure device, because there are few bridging structures in this procedure. Transection is continued until the portal pedicles of segments II and III are visualized. These pedicles can then be divided with two to three firings of the linear stapler (Fig. 90E.4). Parenchymal transection continues cranially, until the left hepatic vein is visualized, at which point it is divided with the linear stapler (Fig. 90E.5).
Right Hemihepatectomy
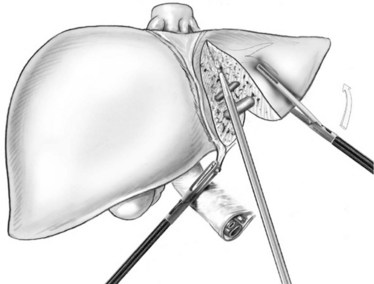
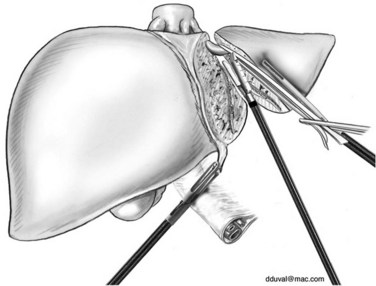
For the anterior approach, the right hepatic vein is not controlled prior to parenchymal transection. After ligation of the right hepatic artery and portal vein, parenchymal transection is started at the inferior edge of the liver in the anterior-posterior direction along the line of demarcation. The previously described techniques are utilized. As progress is made cranially, the proximal hepatic veins draining segments V and VIII toward the middle hepatic vein are exposed, clipped, and divided (Fig. 90E.6). At the level of the hilar plate, the right bile duct’s anterior and posterior branches are divided between clips or staples. Division of the intraparenchymal bile ducts and hilar plate allows the plane to be opened widely for easier parenchymal transection, and the junction between the right lobe and segment I is divided to allow exposure of the retrohepatic vena cava. The posterior Glisson capsule is now divided along the anterior surface of the vena cava, while systematically clipping and dividing the small bridging veins. The right hepatic vein is identified at its insertion into the vena cava and is transected with a linear stapler. The specimen is now retracted to the right, as the hepatocaval ligament and the attachments of the right hemiliver are freed from the diaphragm.
When parenchymal transection is to be performed after mobilization of the right hemiliver, the right glissonian pedicle is dissected and divided as discussed for the anterior approach. This mobilization approach may require the use of a hand port (Fig. 90E.7). Next, the right triangular and coronary ligaments are divided. The liver is retracted to the left and anteriorly, and the accessory veins between the right hemiliver and vena cava are clipped and divided. As progress is made cranially, the hepatocaval ligament is divided between clips, and the right hepatic vein is subsequently divided with a linear stapler. The liver is now both completely mobilized and devascularized, and parenchymal transection is performed along the line of demarcation according to previously discussed principles.
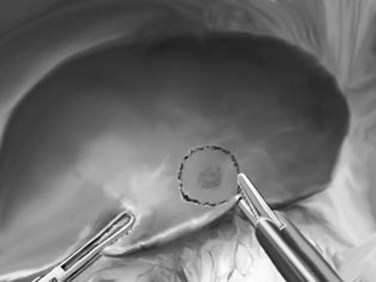
Peripheral Partial Hepatectomies: Segmentectomy, Metastasectomy, or Tumorectomy
Peripheral partial hepatectomies are performed for lesions in the anterior or lateral segments. They can be anatomic, such as segmentectomies along theoretic segmental borders, or they can be nonanatomic, as with metastasectomy or tumorectomy (Fig. 90E.8). They usually do not require liver mobilization, especially for anterior lesions. For malignant lesions, 10- to 20-mm margins are determined using ultrasonography and are marked using diathermy. For benign lesions, a wide margin is unnecessary. Parenchymal transection can be performed with the Harmonic Scalpel or LigaSure, and it follows the margins marked on the liver surface. Hemostasis is achieved with bipolar diathermy and clips. If significant veins or pedicles are within the resection margins, these are clipped and divided; the use of a linear stapler is rarely necessary.
Belli G, et al. Laparoscopic versus open liver resection for hepatocellular carcinoma in patients with histologically proven cirrhosis: short- and middle-term results. Surg Endosc. 2007;21(11):2004-2011.
Belli G, et al. Laparoscopic redo surgery for recurrent hepatocellular carcinoma in cirrhotic patients: feasibility, safety, and results. Surg Endosc. 2009;23(8):1807-1811.
Bryant R, et al. Laparoscopic liver resection: understanding its role in current practice: the Henri Mondor Hospital experience. Ann Surg. 2009;250(1):103-111.
Buell JF, et al. Experience with more than 500 minimally invasive hepatic procedures. Ann Surg. 2008;248(3):475-486.
Buell JF, et al. The international position on laparoscopic liver surgery: the Louisville Statement, 2008. Ann Surg. 2009;250(5):825-830.
Cai XJ, et al. Clinical study of laparoscopic versus open hepatectomy for malignant liver tumors. Surg Endosc. 2008;22(11):2350-2356.
Castaing D, et al. Oncologic results of laparoscopic versus open hepatectomy for colorectal liver metastases in two specialized centers. Ann Surg. 2009;250(5):849-855.
Chang S, et al. Laparoscopy as a routine approach for left lateral sectionectomy. Br J Surg. 2007;94(1):58-63.
Chen HY, Juan CC, Ker CG. Laparoscopic liver surgery for patients with hepatocellular carcinoma. Ann Surg Oncol. 2008;15(3):800-806.
Cherqui D. Surgery for hepatocellular carcinoma in patients with chronic liver disease. Br J Surg. 2006;93(10):1179-1181.
Cherqui D, et al. Laparoscopic liver resections: a feasibility study in 30 patients. Ann Surg. 2000;232(6):753-762.
Cherqui D, et al. Laparoscopic living donor hepatectomy for liver transplantation in children. Lancet. 2002;359(9304):392-396.
Cherqui D, et al. Liver resection for transplantable hepatocellular carcinoma: long-term survival and role of secondary liver transplantation. Ann Surg. 2009;250(5):738-746.
Cho JY, et al. Experiences of laparoscopic liver resection including lesions in the posterosuperior segments of the liver. Surg Endosc. 2008;22(11):2344-2349.
Foroutani A, et al. Laparoscopic ultrasound vs triphasic computed tomography for detecting liver tumors. Arch Surg. 2000;135(8):933-938.
Gagner M, Rheault M, Dubuc J. Laparoscopic partial hepatectomy for liver tumor. Surg Endosc. 1992;6:97-98.
Gigot JF, et al. Laparoscopic liver resection for malignant liver tumors: preliminary results of a multicenter European study. Ann Surg. 2002;236(1):90-97.
Kaneko H, et al. Laparoscopic hepatectomy for hepatocellular carcinoma in cirrhotic patients. J Hepatobiliary Pancreat Surg. 2009;16(4):433-438.
Koffron A, et al. Laparoscopic liver surgery: shifting the management of liver tumors. Hepatology. 2006;44(6):1694-1700.
Koffron AJ, et al. Evaluation of 300 minimally invasive liver resections at a single institution: less is more. Ann Surg. 2007;246(3):385-392.
Laurent A, et al. Laparoscopic liver resection for subcapsular hepatocellular carcinoma complicating chronic liver disease. Arch Surg. 2003;138(7):763-769.
Laurent A, et al. Laparoscopic liver resection facilitates salvage liver transplantation for hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2009;16(3):310-314.
Lesurtel M, et al. Laparoscopic versus open left lateral hepatic lobectomy: a case-control study. J Am Coll Surg. 2003;196(2):236-242.
Milsom JW, et al. Prospective, blinded comparison of laparoscopic ultrasonography vs. contrast-enhanced computerized tomography for liver assessment in patients undergoing colorectal carcinoma surgery. Dis Colon Rectum. 2000;43(1):44-49.
Montorsi M, et al. Laparoscopy with laparoscopic ultrasound for pretreatment staging of hepatocellular carcinoma: a prospective study. J Gastrointest Surg. 2001;5(3):312-315.
Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection: 2,804 patients. Ann Surg. 2009;250(5):831-841.
Nguyen KT, et al. Minimally invasive liver resection for metastatic colorectal cancer: a multi-institutional, international report of safety, feasibility, and early outcomes. Ann Surg. 2009;250(5):842-848.
Pang YY. The Brisbane 2000 terminology of liver anatomy and resections. HPB (Oxford). 2002;4(2):99-100.
Polignano FM, et al. Laparoscopic versus open liver segmentectomy: prospective, case-matched, intention-to-treat analysis of clinical outcomes and cost effectiveness. Surg Endosc. 2008;22(12):2564-2570.
Santambrogio R, et al. Laparoscopic liver resections for hepatocellular carcinoma: is it a feasible option for patients with liver cirrhosis? Langenbecks Arch Surg. 2009;394(2):255-264.
Sarpel U, et al. Outcome for patients treated with laparoscopic versus open resection of hepatocellular carcinoma: case-matched analysis. Ann Surg Oncol. 2009;16(6):1572-1577.
Simillis C, et al. Laparoscopic versus open hepatic resections for benign and malignant neoplasms: a meta-analysis. Surgery. 2007;141(2):203-211.
Topal B, et al. Laparoscopic versus open liver resection of hepatic neoplasms: comparative analysis of short-term results. Surg Endosc. 2008;22(10):2208-2213.
Vigano L, et al. Laparoscopic liver resection: a systematic review. J Hepatobiliary Pancreat Surg. 2009;16(4):410-421.
Vigano L, et al. The learning curve in laparoscopic liver resection: improved feasibility and reproducibility. Ann Surg. 2009;250(5):772-782.