Chapter 35 Minimally Invasive Percutaneous Lumbar Fusion Technique
It is estimated that more than 200,000 lumbar procedures are performed yearly for underlying discogenic disease and instability [1]. The degenerative process, often a result of normal aging, leads to degenerative disc disease, spondylolisthesis, and segmental instability. The traditional open posterior lumbar fusion yields an acceptable efficacy with a high fusion rate [2]. However, the exposure that is needed to achieve optimal visualization of the transverse processes, lamina, and facet joints often requires a midline incision of more than 5 to 7 cm, depending on the number of levels involved. Disruption and retraction of the deep paraspinal musculature has been shown to cause localized denervation, which may lead to continued back pain and spasm after the procedure [3]. Multiple studies in this area have shown deleterious histologic effects, including regional ischemia from increased intramuscular pressure secondary to the use of retractor blades [4,5].
Less invasive techniques and instrumentation have been and continue to be developed to address the effects of extensive soft tissue disruption. In 1995, Mathews and Long [6] published their experience with the use of percutaneous pedicle screws. Unfortunately, they noted a high nonunion rate. Lowery and Kulkarni [7] published a review of eight cases in which they utilize a similar technique but with the use of rods instead of longitudinal connectors; they reported a 96% fusion rate for a mini–open anterior approach supported with a minimally invasive posterior fusion. Advances to the procedure described by Harms and Rollinger [8] have produced a method of achieving a solid arthrodesis through a posterior interbody approach.
Subsequently, the transforaminal lumbar interbody fusion (TLIF) approach was developed as a means of addressing the pathology in the disc space while minimizing the risk of iatrogenic injury to nerve roots and soft tissue. Foley and colleagues [11] described a minimally invasive method of performing TLIF that has become popular with trained surgeons [9–11]. The TLIF procedure continues to evolve as a minimal access procedure that is ideal for a patient who has clinically defined symptoms that correlate with results of diagnostic studies related to radiculopathy pain secondary to degenerative disc disease, recurrent herniated nucleus pulposus, or segmental instability. The approach allows for adequate access to the affected facet joint and lamina to perform a laminotomy and discectomy. Furthermore, through a mini–open and percutaneous approach, restoration of disc height, sagittal balance, and stabilization of the vertebral segments can be effectively performed. A study by Schwender and colleagues [12] reported clinically significant improvements in visual analog scale and Oswestry Disability Index scores along with a 100% fusion rate in a cohort of patients who underwent a minimally invasive TLIF procedure [12].
Presurgical treatments
No clearly defined time frames exist for the consideration of nonemergency spinal fusion surgery. The natural history of acute low back pain with or without radiculopathy resulting from a disc herniation is favorable. According to the literature and our experience, more than 90% of patients recover within 3 months [13,14]. The same holds true for those who suffer from lumbar spinal stenosis, a large percentage of whom can be managed with conservative care and no need for spinal surgery. Patients in whom nonsurgical treatment fails, who have documented clinical and diagnostic findings consistent with their symptoms, or who have neurologic deficits, surgical management may be warranted.
Preoperative preparations
Assessment
The presurgical evaluation should include a thorough history and physical examination. A detailed patient history, including the onset of complaints, aggravating and mitigating factors, and response to any treatments tried, should be entered into the medical record. This step allows the surgeon or practitioner to better target the physical examination. A comprehensive review of the patient’s medical co-morbidities is necessary to assess and identify potential contraindications to spinal surgery. Symptoms of spinal disorders must be differentiated from other causes, including infection, fracture, and neoplasms. A physical examination to include assessment of gait and range of motion, as well as motor, reflex, and sensory testing is necessary to establish a baseline for the patient’s neurologic status. Provocative testing should also be conducted to determine the source of pain. Nonorganic or psychological signs as described by Waddell and colleagues [15] should be noted during the examination. Combined, the history and physical examination provide a basis on which a diagnosis can be made and are fundamental to making decisions about further testing and treatment.
Diagnostic Testing
Radiography should be the initial imaging study and can performed during the presurgical evaluation if radiographs have not already been obtained. We advocate the use of standing radiographs that include an anteroposterior (AP) view and lateral flexion and extension views. The rationale is that most other diagnostic studies are performed in a supine position, which may not detect changes that occur in the spine in an erect position. Although no standard definition of radiographic instability exists, it is generally accepted that 3 to 4 mm of translation or greater than 11 degrees of angular motion can suggest segmental instability. Flexion and extension radiographs allow for identification and quantification of abnormal motion. Another important component to identify is the presence of a transitional vertebra. In a normal spine, the 25th vertebral body below the occiput is S1. A review of the literature shows that transitional vertebrae occur in as much a 4% to 21% of the population [16]. Sacralization of the last lumbar vertebra results in four non–rib-bearing vertebrae, whereas lumbarization results in six rib-bearing segments. Additionally, congenital anomalies are also commonly seen. It is imperative that prior to surgery, the levels are properly identified and are consistent with further diagnostic studies that may be ordered. Evaluation of the disc space for decreased disc height and sclerosis surrounding the end plates is often indicative of advancing degenerative disc disease. Assessment of the sagittal curve for irregularity should be noted. The examiner should also scrutinize the films for lytic and blastic lesions.
Provocative discography remains controversial in its value and approach. The role of discography is that it may help differentiate painful discs from other causes of back pain [17]. It is understood that as a part of the natural aging process, the disc can develop fissures with a loss of fluid content. For many people, this process does not cause pain, but for others such a disc can be a pain generator. Inflammatory mediators are believed to be involved in causing irritation of the nerve endings embedded within the anulus. Provocative discography represents a method of assessing whether a degenerated disc is a potential pain generator. A post-discography CT scan is used to identify the contrast dye pattern from the injection. An injection into another disc used as a control is necessary to confirm a concordant pain response. When the procedure is performed by a well-trained discographer, the findings can help identify both the disc integrity and the physiologic response to the defect. Like myelography, discography is used to delineate a disease process for surgical intervention.
Related anatomy and physiology
A thorough understanding of the patient’s specific anatomy, including orientation of the pedicles, is crucial to the consideration of minimally invasive spinal fusion. Coronal angulation of the pedicles typically increases from the top to the bottom of the lumbar spine, but anatomic variants of this pattern exist. The transverse pedicle width increases from L1 to the sacrum [18,19]. Pedicle width is more important than pedicle height in the determination of screw placement. Accurate and safe placement of pedicle screws is highly dependent on the surgeon’s understanding of this concept. As previously discussed, the presence of sacrilization or lumbarization should be clearly defined preoperatively on standard radiographs, which should compared with other diagnostic studies to ensure that surgery is being performed on the appropriate levels. The lordosis of the sagittal curve in a normal lumbar spine averages approximately 50 degrees. A large percentage of lumbar lordosis results from wedging of the intervertebral discs. Disease processes, including advanced degenerative disc disease and segmental instability, can negatively affect the overall alignment in the sagittal plane. Inclusion of the restoration of sagittal balance in the goals of the procedure improves the chances of a successful surgical outcome [20]. Other anatomically and physiologically related considerations are the presence of scar from previous surgery, bone quality, preoperative assessment of adjacent levels, and morphology of the pelvis for operations at L5-S1, as well as the presence of conjoined nerve roots [21].
Procedures and technique
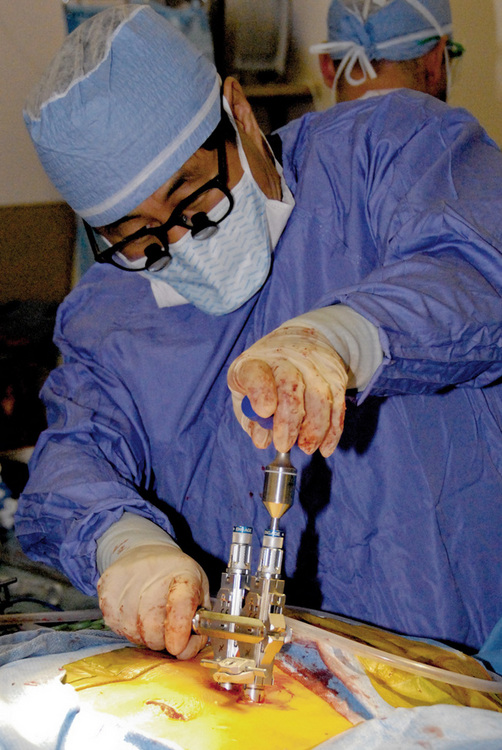
Fluoroscopic Pedicle Targeting
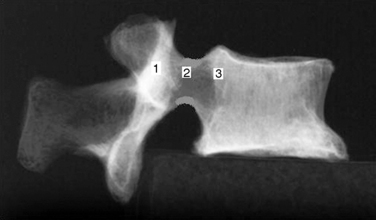
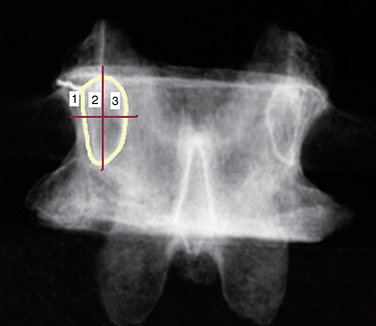
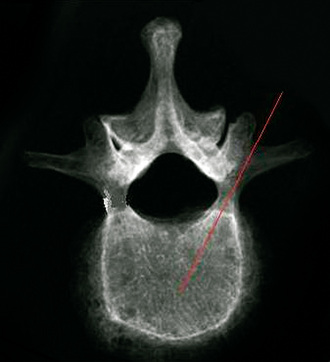
Figure 35–2 Lateral view of pedicle corresponding to Figure 35,1, showing the path for needle advancement.1, beginning point of pedicle; 2, center of pedicle; 3, posterior margin of vertebral body.
The surgeon must be aware of differences in pedicle morphology in different disease states and among subpopulations. [19,22] Efficacy of the pedicle screw is highly dependent on proper placement, including matching the pedicle angle (Fig. 35-3). Care should be taken to avoid placing the needle too far medially and inferiorly.
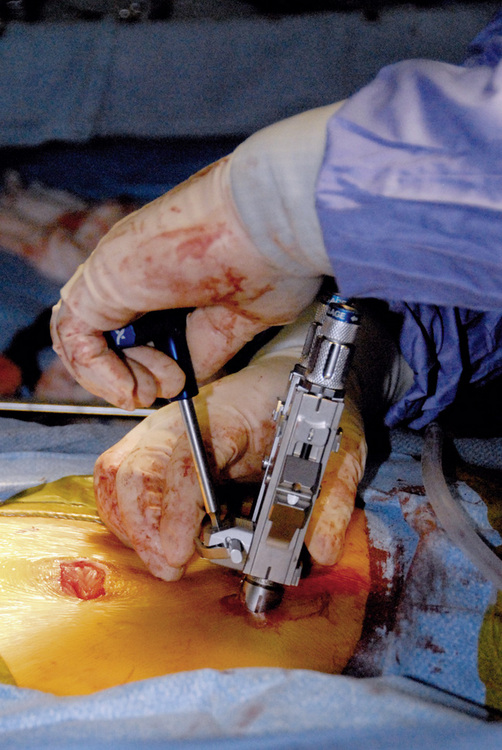
Percutaneous Approach

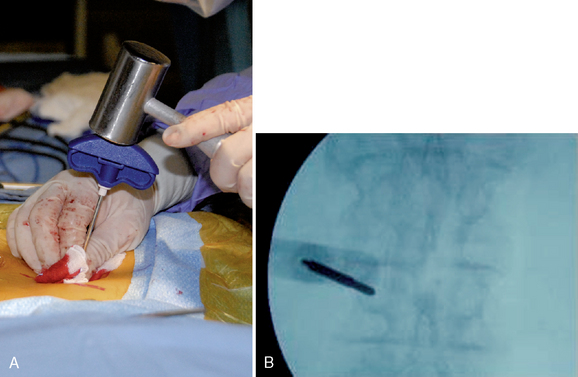
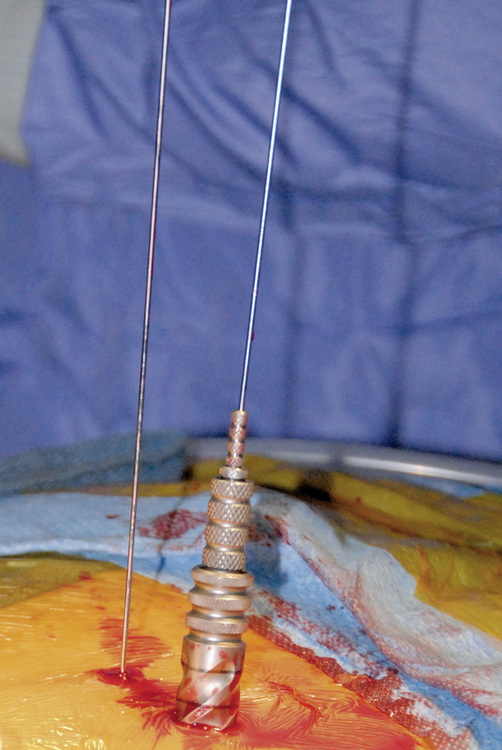
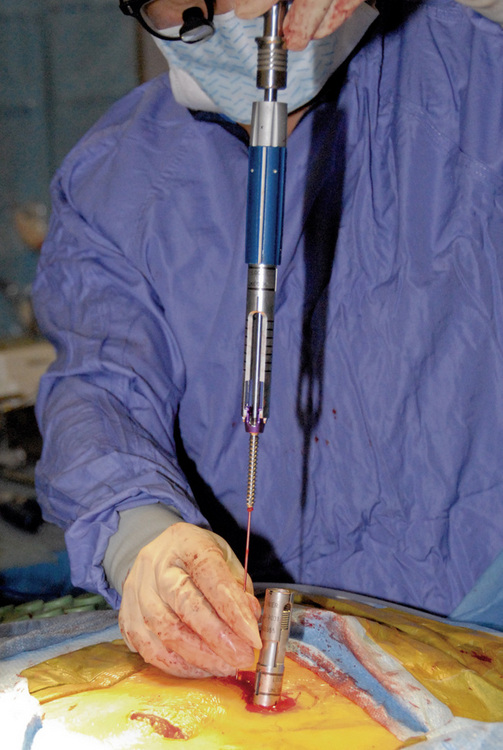
Figure 35–5 Insertion of guidewire following confirmation of correct needle placement within the pedicle.
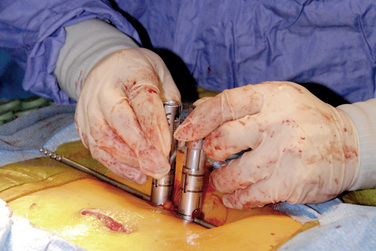
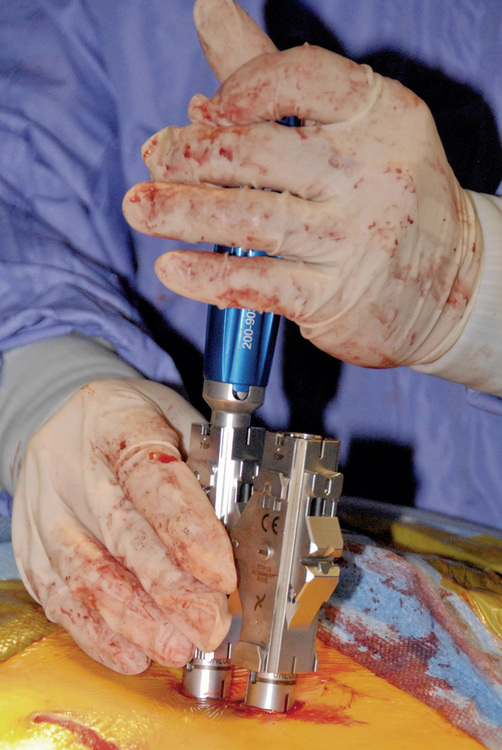
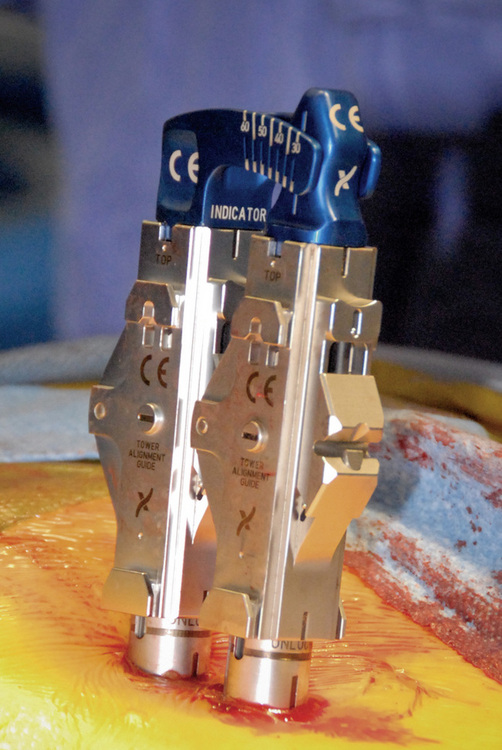
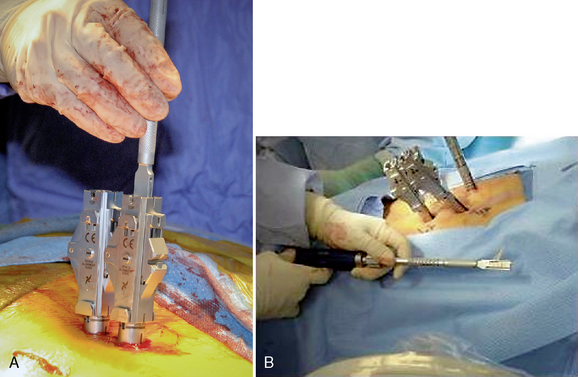
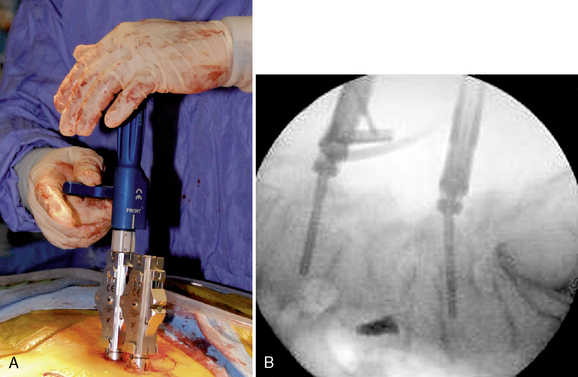
Subsequent pedicle screws are placed with this same technique. It is important to note that all screw tower assemblies should line up in the same orientation and height before on the next step of the procedure (Fig. 35-8).
Mini-Open Approach
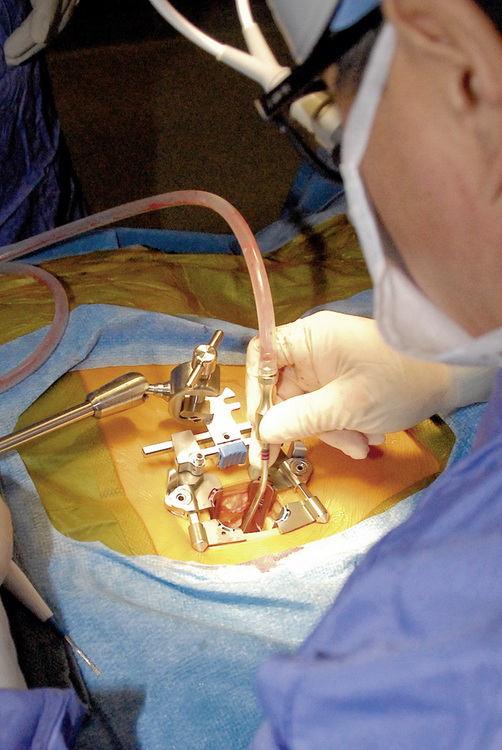
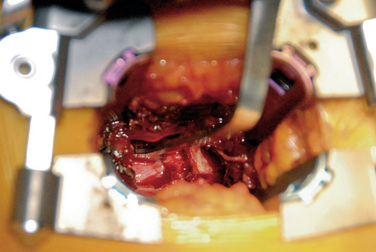
A mini-open approach can be performed unilaterally for the purposes of decompression with or without interbody fusion. If interbody fusion is being considered, the incision site should be made just lateral to the pedicles to facilitate exposure to the disc space for cage placement. A table-mounted retractor such as the VertiFlex Oracle Expandable Retractor is used to create a working channel (Figs. 35-16 to 35-18):
 An illuminated headlight and loupe magnification are beneficial to the surgeon during the initial part of the procedure.
An illuminated headlight and loupe magnification are beneficial to the surgeon during the initial part of the procedure. Decortication and placement of bone grafting are performed in the lateral gutter. Pedicle screws are placed on the opposing side by means of the percutaneous technique already described.
Decortication and placement of bone grafting are performed in the lateral gutter. Pedicle screws are placed on the opposing side by means of the percutaneous technique already described.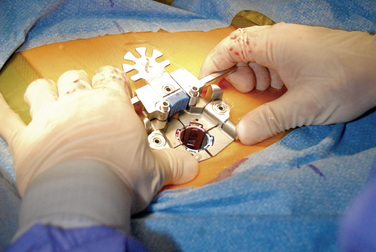
Figure 35–16 Placement of Oracle Expandable Retractor (VertiFlex, Inc., San Clemente, CA) after tissue dilation.
Postoperative care
The usual precautions during the acute postoperative phase include avoidance of heavy lifting, bending, and high-impact activities. Generally, patients should be encouraged to ambulate to avoid deconditioning. Nonsteroidal anti-inflammatory medications should be avoided because they are known to decrease the fusion rate. [23] Instructions for surgical wound care should be given to and understood by the patient prior to discharge. Patients should be told to contact the surgeon or support staff if they have persistent fevers, chills, motor weakness, increasing pain, headaches, or wound problems. Follow-up examinations to determine neurologic status and radiographs should be performed at intervals defined by the treating surgeon. Assessment of the patient’s complaints, pain level, function, and satisfaction should be ascertained at subsequent follow-up visits.
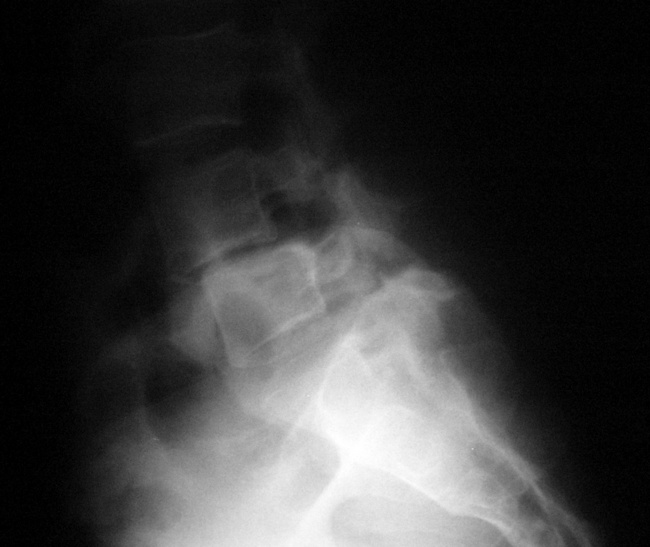
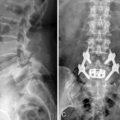
A focused physical examination of the back and lower extremities was conducted. It revealed mild tenderness over the midline, a slight list, and mild weakness of the right anterior tibialis anterior (4+/5). Straight-leg raising on the right side reproduced the left-sided low back symptoms. No long tract signs were identified. Standing radiographs, including flexion and extension views, were taken. The radiographs showed a grade I spondylolisthesis at L4-L5 with moderate loss of disc height (Fig. 35-19). Furthermore, a flexion-extension instability was noted.
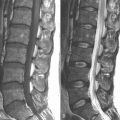
The patient was referred for MRI because some conservative measures had already been tried and had failed and because she presented with significant physical findings. MRI showed 6 mm of anterior displacement of L4 on L5 (Fig. 35-20). No definite pars defect was identified. A central disc herniation was present at L4-L5 with neural encroachment. Disc desiccation was also prominent at the L4-L5 level. Following a detailed review of the MRI findings, conservative and surgical treatment options were discussed with the patient. She elected to pursue surgical management of her problem secondary to increasing pain and limited ability to perform daily activities.
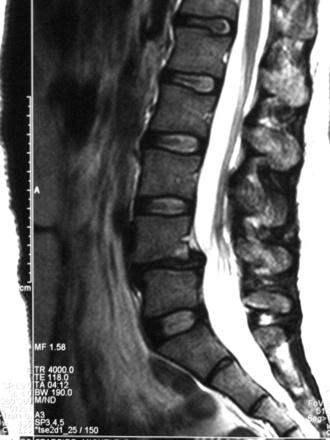
Figure 35–20 Case Study 35.1: Preoperative sagittal magnetic resonance image showing 6 mm of anterior displacement of L4 on L5.
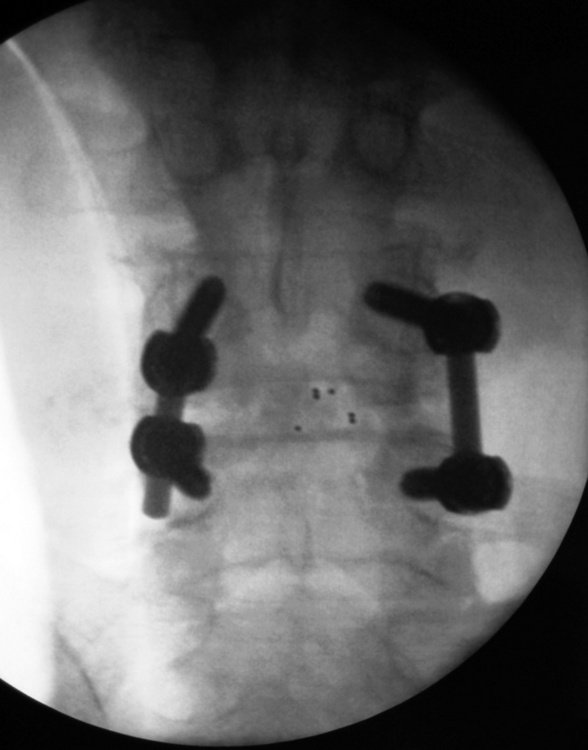
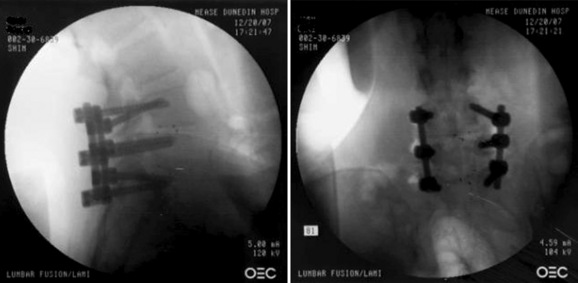
Attention was then turned to the right side. The pedicles of L4 and L5 were identified and marked with fluoroscopic guidance. A Jamshidi needle was placed at the same orientation as the L5 pedicle; placement and orientation were confirmed with biplanar fluoroscopy. A guidewire was placed through the needle and advanced through the pedicle into the vertebral body. The pedicle was tapped with a cannulated tap. A pedicle screw was placed percutaneously at the L5 level. The same procedure was carried out at the L4 level. The appropriate-size rod was then delivered to the screws. Final tightening of the screws was performed, and final fluoroscopic images were obtained (Fig. 35-21).
A detailed examination identified a positive straight-leg raising test response on the right side and mild tenderness in the right sacroiliac region. No motor, reflex, or sensory deficits were apparent. Standing radiographs were obtained, which clearly documented a spondylolisthesis grade I/II at L4-L5, grade I slip at L5-S1 with disc space narrowing, and facet disease at both levels (Fig. 35-22). Flexion and extension radiographs confirmed segmental instability. Furthermore, MRI of the lumbar spine showed a right lateral disc protrusion at L4-L5 that was causing moderate right foraminal narrowing. A diffuse posterior disc bulge with mild foraminal narrowing was noted at L5-S1 with facet arthropathy at both levels (Fig. 35-23). The findings were reviewed at length with the patient. Surgical management was recommended in light of her complaints and the findings of the diagnostic workup. The patient consented to an L4-L5, L5-S1 laminectomy with interbody and intertransverse fusion with instrumentation, despite the risks, benefits, and alternatives presented to her.
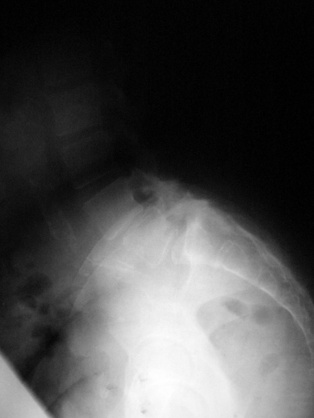
Figure 35–22 Case Study 35.2: Preoperative lateral radiograph showing spondylolisthesis at L4–L5 and L5–S1 with disc space narrowing.
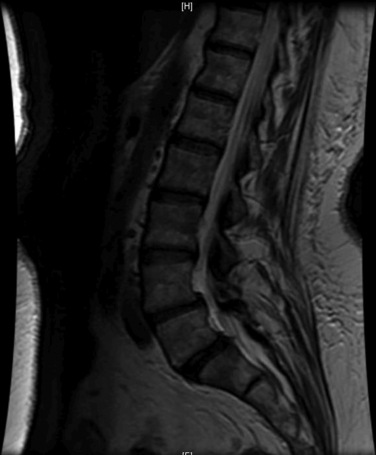
Figure 35–23 Case Study 35.2: Preoperative sagittal magnetic resonance image showing a diffuse posterior disc bulge with mild foraminal narrowing at L5–S1 with facet arthropathy at both levels.
The percutaneous approach was used to deliver the pedicle screws to the opposite side. Final fluoroscopic images obtained prior to wound closure showed significant reduction in the preoperative spondylolisthesis at L4-L5 and at L5-S1 (Fig. 35-24). The patient reported immediate relief of her leg complaints.
Key points
 Indications for minimally invasive lumbar fusion surgery mirror those for conventional, open surgery. Proper patient selection and surgical indications are necessary to avoid surgical failure.
Indications for minimally invasive lumbar fusion surgery mirror those for conventional, open surgery. Proper patient selection and surgical indications are necessary to avoid surgical failure. The learning curve for this procedure can be steep for surgeons who are accustomed to performing open spinal surgery. Complexities can relate to exposures through a narrow working channel, having to rely on biplanar fluoroscopy instead of direct three-dimensional visualization of anatomy, and using minimally invasive instrumentation that the surgeon may be unfamiliar with.
The learning curve for this procedure can be steep for surgeons who are accustomed to performing open spinal surgery. Complexities can relate to exposures through a narrow working channel, having to rely on biplanar fluoroscopy instead of direct three-dimensional visualization of anatomy, and using minimally invasive instrumentation that the surgeon may be unfamiliar with. Many minimally invasive systems are available. Each system features differences in design and instrumentation. However, the overall approach is the same. A thorough understanding of each patient’s anatomy and pedicle orientation is necessary to be able to safely direct pedicle screws utilizing a percutaneous approach
Many minimally invasive systems are available. Each system features differences in design and instrumentation. However, the overall approach is the same. A thorough understanding of each patient’s anatomy and pedicle orientation is necessary to be able to safely direct pedicle screws utilizing a percutaneous approachAcknowledgement
We wish to thank W.C. “Chief” Wright for his assistance with the intraoperative photographic images.
1 Katz J.N. Lumbar disc disorders and low back pain: socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88(Suppl. 2):21-24.
2 Gill K., Blumenthal S.L. Posterior lumbar interbody fusion: A 2-year follow-up of 238 patients. Acta Orthop Scand Suppl. 1993;251:108-110.
3 Sihvonen T., Herno A., Paljärvi L., et al. Local denervation atrophy of paraspinal muscles in postoperative failed back syndrome. Spine. 1993;18:575-581.
4 Styf J.R., Willén J. The effects of external compression by three different retractors on pressure in the erector spine muscles during and after posterior lumbar spine surgery in humans. Spine. 1998;23:354-358.
5 Gejo R., Matsui H., Kawaguchi Y., et al. Serial changes in trunk muscle performance after posterior lumbar surgery. Spine. 1999;24:1023-1028.
6 Mathews H., Long B. Endoscopy assisted percutaneous anterior interbody fusion with subcutaneous suprafascial internal fixation: evolution of technique and surgical considerations. Orthopaedics. 1995;3:496-500.
7 Lowery G.L., Kulkarni S.S. Posterior percutaneous spine instrumentation. Eur Spine J. 2000;9(Suppl. 1):S126-S130.
8 Harms J.G., Rollinger H. Die operative Behandlung der Spondylolisthese durch dorsal Aufrichtung und ventral Verblockkkung. Z Orthop. 1982;120:343-347.
9 Foley K.T., Gupta S.K., Justis J.R., Sherman M.C. Percutaneous pedicle screw fixation of the lumbar spine. Neurosurg Focus. 2001;10:E10.
10 Foley K.T., Holly L.T., Schwender J.D., Rouben D.P. Minimally invasive transforaminal lumbar interbody fusion: indications, technique, and complications. Neurosurg Focus. 2006;20:E6.
11 Foley K.T., Holly L.T., Schwender J.D. Minimally invasive lumbar fusion. Spine. 2003;28(Suppl.):S26-S35.
12 Schwender J.D., Holly L.T., Rouben D.P., Foley K.T. Minimally invasive transforaminal lumbar interbody fusion (TLIF): technical feasibility and initial results. J Spinal Disord Tech. 2005;18(Suppl.):S1-S6.
13 Weber H. The natural history of disc herniation and the influence of intervention. Spine. 1994;19(19):2234-2238. discussion 2233
14 Benoist M. The natural history of lumbar disc herniation and radiculopathy. Joint Bone Spine. 2002;69:155-160.
15 Waddell G., McCulloch J.A., Kummel E., Venner R.M. Nonorganic physical signs in low-back pain. Spine. 1980;5:117-125.
16 Castellvi A.E., Goldstein L.A., Chan D.P. Lumbosacral transitional vertebrae and their relationship with lumbar extradural defects. Spine. 1984;9:493-495.
17 Bini W., Yeung A.T., Calatayud V., et al. The role of provocative discography in minimally invasive selective endoscopic discectomy. Neurocirugia (Astur). 2002;13:27-31. discussion 32
18 Lien S.B., Liou N.H., Wu S.S. Analysis of anatomic morphometry of the pedicles and the safe zone for through-pedicle procedures in the thoracic and lumbar spine. Eur Spine J. 2007;16:1215-1222.
19 Kim N.H., Lee H.M., Chung I.H., et al. Morphometric study of the pedicles of thoracic and lumbar vertebrae in Koreans. Spine. 1994;19:1390-1394.
20 Brantigan J.W., Neidre A. Achievement of normal sagittal plane alignment using a wedged carbon fiber reinforced polymer fusion cage in treatment of spondylolisthesis. Spine J. 2003;3:186-196.
21 Scuderi G.J., Vaccaro A.R., Brusovanik G.V., et al. Conjoined lumbar nerve roots: a frequently underappreciated congenital abnormality. J Spinal Disord Tech. 2004;17:86-93.
22 Nojiri K., Matsumoto M., Chiba K., et al. Comparative assessment of pedicle morphology of the lumbar spine in various degenerative diseases. Surg Radiol Anat. 2005;27:317-321.
23 Deguchi M., Rapoff A.J., Zdeblick T.A. Posterolateral fusion for isthmic spondylolisthesis in adults: analysis of fusion rate and clinical results. J Spinal Disord. 1998;11:459-464.