CHAPTER 2 Minimally invasive esophagectomy
Step 1. Surgical anatomy
♦ Adenocarcinoma is the most common type of esophageal cancer in Western society, and esophagectomy is the primary therapy for resectable tumors. Traditional “open” esophagectomy has been associated with significant morbidity and mortality rates. In an attempt to lower these rates, minimally invasive techniques were introduced. Two laparoscopic approaches, each indicated for different stages of disease, are described in this chapter.
♦ Laparoscopic transhiatal inversion esophagectomy (LIE) with gastric substitution can be employed for treatment of end-stage benign disease (Barrett’s high-grade dysplasia; achalasia) and early malignancy confined to the mucosa (T1a stage).
♦ Esophageal cancer is known for early and rapid dissemination because of the longitudinally oriented lymphatic plexus within the submucosa with direct transmural lymphatic connections and the lack of a serosal lining. Although lymph node involvement is infrequent in T1a-stage adenocarcinoma of the esophagus, lymph node involvement increases nearly 10-fold in T1b-stage (submucosal) disease.
♦ The combined laparoscopic-thoracoscopic (two-cavity) approach with en bloc lymph-adenectomy is indicated for treatment of resectable advanced locoregional disease.
Step 2. Preoperative considerations
Patient preparation
♦ Preoperative evaluation and staging includes endoscopy, bronchoscopy, endosonography and positron emission tomography combined with computed tomography (PET-CT) scanning.
♦ Preoperative evaluation of comorbid conditions should include at least an evaluation of a patient’s cardiopulmonary reserve. In selected cases with severe peripheral occlusive arterial disease, a visceral angiogram is obtained.
♦ A preoperative exercise program, smoking cessation, and optimization of nutritional status should be endeavored.
♦ Preoperative mechanical bowel preparation is performed when colon interposition may be required.
Equipment and instrumentation
♦ Blunt port (Covidien, Mansfield, Massachusetts) 5 to 12 mm
♦ 30-degree and 45-degree 10 mm endoscope and a 5-mm 30-degree endoscope
♦ Needle feeding jejunostomy kit (Compat Biosystems, Minneapolis, Minnesota)
♦ Autosonix ultrasonic scalpel (Covidien, Mansfield, Massachusetts)
♦ Diamond-flex liver retractor or Nathanson liver retractor for left lobe of liver
♦ Endoscopic retractor (10 mm) for retracting lung
Anesthesia
♦ Prior to induction, a thoracic epidural is placed for postoperative pain control, and antibiotic prophylaxis (second-generation cephalosporin) is administered.
♦ Endotracheal intubation is performed with a single lumen tube in LIE. In combined laparoscopic-thoracoscopic (two-cavity)-approach, a double lumen endotracheal tube is required for single-lung ventilation.
♦ A nasogastric tube and a urinary catheter are placed, and an arterial catheter for continuous blood pressure monitoring is instituted.
Step 3. Operative steps
Laparoscopic transhiatal inversion esophagectomy
Patient positioning
♦ After induction, an intraoperative bronchoscopy and esophagogastroduodenoscopy are performed for assessment of anatomic relationships and tumor location.
♦ Skin preparation and draping of the abdomen, chest, and left side of the neck is performed in a single field.
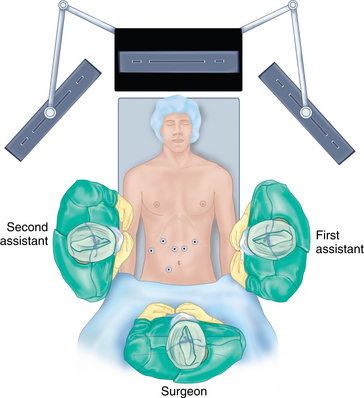
♦ For the abdominal portion of laparoscopic esophagectomy, the patient is placed in a supine, split-legged position and secured to the operation table with supportive padding for all pressure zones. A cushion can be placed at scapula level to induce slight neck extension for cervical exposure, if a neck anastomosis is planned.
♦ The surgeon stands between the patient’s legs (French position); the first assistant is positioned at the patient’s left and the second assistant at the patient’s right.
Port placement
♦ After pneumoperitoneum is obtained by using Veress needle technique, the primary site of access is approximately 15 cm below the left costal margin, 3 cm out of the midline. A 45-degree laparoscope is introduced through a 10-mm port and, before secondary port placements, a staging laparoscopy is performed.
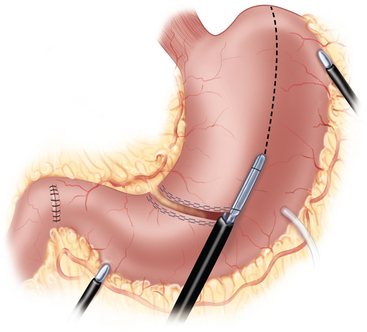
♦ A six-port approach is used with the remaining ports in the following locations: second port (12 mm, surgeon’s right hand) 12 cm from the xiphoid process, 2 cm below the left costal margin; third port (5 mm, first assistant) left anterior axillary line along the costal margin; fourth port (5 mm, liver retractor) left of the xiphoid process; fifth port (12 mm, surgeon’s left hand, access endoscopic stapling device) inferior to the right costal margin and immediately to the right of the falciform ligament; sixth port (5 mm, second assistant) right midabdominal position, based on internal anatomy (Figure 2-1).
Hiatal dissection
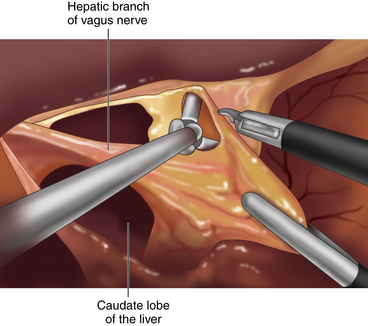
♦ Using a liver retractor, the hiatal opening and the gastrohepatic omentum are exposed and divided, along with the hepatic branch of the anterior vagus trunk, using the Harmonic scalpel (Figure 2-2).
♦ A replaced left hepatic artery in the lesser omentum, which arises from the left gastric artery in about 30% of population, should be spared.
♦ The phrenoesophageal membrane is opened around the esophagus until both the crural pillars are dissected and the esophagus can be encompassed.
♦ In LIE indicated for benign disease or early malignancies, the vagal nerves are preserved to maintain gastric physiology.
Gastric mobilization
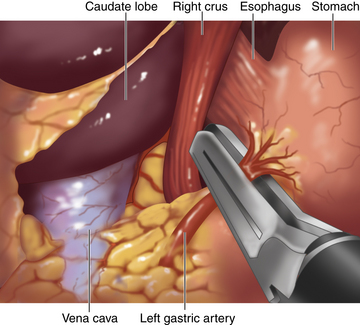
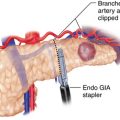
♦ The epiphrenic fat is retracted toward the left anterior abdominal wall to provide tension on the left gastric vessels. The overlying peritoneum is opened, using the Harmonic scalpel, and the left gastric vessels are identified and divided using an endoscopic vascular stapler (Figure 2-3).
♦ The gastric fundus is mobilized with the division of the short vessels.
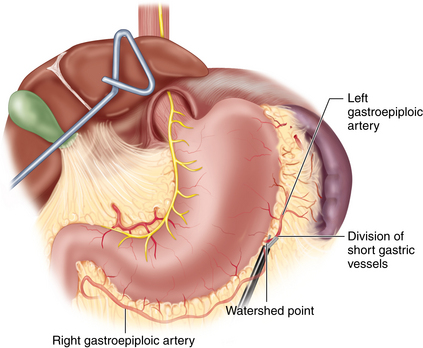
♦ The gastrocolic omentum is divided along the greater curvature. At Demel’s (watershed) point, the union of left and right gastroepiploic vessels is preserved to protect the blood supply to the gastric conduit (Figure 2-4).
♦ The gastrosplenic ligament is divided, including the intervening short gastric vessels, and the gastric fundus is further mobilized along the pancreaticogastric fold, where the posterior gastric artery is identified and divided.
♦ The distal stomach is mobilized by the division of the gastrocolic ligament at the greater curvature and then the attachments between the posterior gastric wall and pancreas. When the gastric duodenal artery is identified, Kocher’s maneuver is performed. The duodenum and pancreas head are detached from retroperitoneum and retracted to the patient’s left side. Care is taken to protect the gastroduodenal artery, to maintain blood flow to the right gastroepiploic artery to supply the gastric conduit.
Gastric conduit creation
♦ The nasogastric tube is retracted to an intrathoracic position.
♦ The gastric conduit is created using an endostapler (45 mm). The first load is fired 5 to 6 cm proximal to the pylorus and, stepwise, the conduit is created 5 cm from the lesser curvature. Care is taken to preserve the right gastric vessels (Figure 2-5).
♦ The distal margin of the lesser curvature, which is maintained in continuity with the esophagus, is resected and sent for frozen section examination intraoperatively to confirm clear resection margin.
♦ At this point, a pyloroplasty can be performed to prevent gastric outlet obstruction in the case of vagotomy in en bloc lymph node dissection.
Transhiatal mobilization and esophagogastric division
♦ The distal esophagus is mobilized circumferentially in the mediastinum through the hiatus. The right crus can be divided to improve exposure. The dissection of the mediastinal portion of the esophagus from the surrounding intrathoracic structures is performed as proximally as is safe. Care is taken to prevent damage to the trachea, inferior pulmonary veins, azygos vein, and aortic arch.
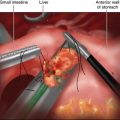
♦ To determine the location of the anatomic esophagogastric junction, an intraoperative endoscopy is performed. The gastric fundus is divided with an endoscopic stapler, distal to the esophagogastric junction, placed in an Endobag and removed at the end of the procedure (Figure 2-6).
♦ A needle jejunostomy is placed for postoperative nutrition.
♦ In advanced disease stage, the greater curve side of the conduit tip is secured to the staple line in the distal esophagus to facilitate the gastric pull-up and the procedure is converted to a right-sided thoracoscopy for the laparoscopic-thoracoscopic (two-cavity) approach.
Cervical access
♦ Access to the cervical esophagus is obtained through a 5-cm incision along the medial border of the sternocleidomastoid muscle. The platysma and omohyoid muscles are divided. Through the avascular plane, the carotid sheath is exposed and retracted laterally. The thyroid gland and the trachea are retracted medially, while attention is paid to preserve the recurrent laryngeal nerve. The deep cervical fascia is opened, and blunt fingertip dissection is used to surround the esophagus with fascia and access the upper mediastinum.
Inversion technique
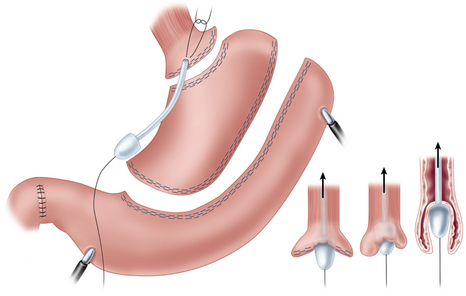
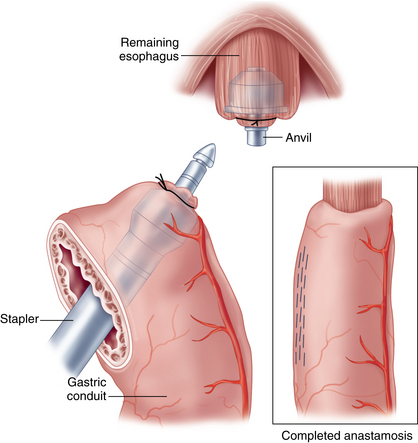
♦ The cervical esophagus is opened and a vein stripper is introduced retrograde into the esophagus. Through a small gastrotomy at staple-line level, the vein stripper is retrieved and taken out of the abdomen through the right upper abdominal 12-mm port site. A medium-sized anvil and a 60-cm trailing suture are placed on the end of the vein stripper.
♦ After retraction into the abdominal cavity, the anvil is secured with a horizontal mattress suture to the gastrotomy and the esophagus is inverted by drawing back the cervical end of the vein stripper.
♦ The inverted border of the esophagus is grasped and gentle traction at the cervical end of the vein stripper provides counter-tension to facilitate mobilization during division of mediastinal attachments and blood vessels.
♦ The dissection is continued until the esophagus is extracted through the cervical wound and transected at the proximal margin.
Conduit pull-through and esophagogastrostomy
♦ The 60-cm trailing suture, which was fixed at the anvil site of the vein stripper and pulled through the cervical wound with the specimen, is used to bring a 26 French thoracic drainage tube down into the abdominal cavity from the cervical incision. The proximal gastric conduit is sutured to the tube, carefully pulled through the mediastinum, and brought to the surface in the cervical wound without rotation.
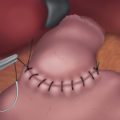
♦ A vertical gastrotomy is made in the gastric conduit to allow a 3.5-mm Endo GIA stapler (Covidien, Mansfield, Massachusetts) to be inserted in the conduit and the proximal esophagus to create a stapled end-to-side anastomosis. The gastrostomy and enterotomy are closed in two layers (Figure 2-7).
Closing
♦ A nasogastric tube is introduced into the gastric conduit.
♦ In the cervical opening, a closed suction drain is placed alongside the anastomosis, and the fascia layers and platysma muscle are closed.
♦ Final inspection of the abdominal cavity is performed, and the specimen retrieval bag containing the gastric fundus and all trocars are removed under direct visualization. The fascia is closed using Vicryl sutures and the skin is closed using resorbable sutures.
Combined laparoscopic-thoracoscopic (two-cavity) approach
Positioning
♦ In this approach, the patient is intubated with a double-lumen tube for single-lung ventilation during the thoracic part of the procedure.
♦ The abdominal portion of the procedure is carried out similar to that described above for the LIE, but the inversion technique is not performed; instead, lymphatic tissue and vagal nerve branches are resected en bloc with the esophagus and esophagogastric junction.
♦ For thoracoscopic mobilization of the esophagus (after the abdominal portion of the procedure), the patient is repositioned into a left lateral decubitus position and supported with a beanbag. The right hemithorax is disinfected, drapes are placed, and single (right) lung ventilation is applied. The surgeon stands on the patient’s right, the first assistant stands on the patient’s left.
Port placement
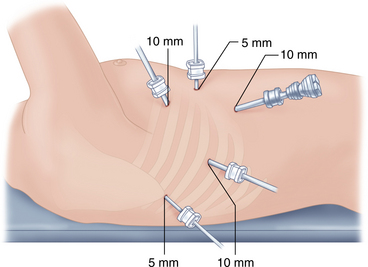
♦ Five ports are introduced: a 10-mm camera port in the 7th-8th intercostal space, midaxillary line; a 5-mm port in the 8th-9th intercostal space, posterior axillary line; a 10-mm port in the 4th intercostal space on the anterior axillary line; and a 5-mm port posterior to the scapula tip (Figure 2-8). A single 5-mm port is placed anteriorly for suction.
♦ A staging thoracoscopy and laparoscopy is performed to evaluate the presence of gastric extension, liver metastases, peritoneal carcinomatosis, or T4 tumor.
Esophageal mobilization
♦ The central tendon of the diaphragm is retracted inferior-anteriorly using a percutaneous suture.
♦ The inferior pulmonary ligament is divided to the level of the inferior pulmonary vein and artery, and the esophagus with attached lymphatic tissues is dissected away from the pericardium and developed toward both cranial and caudal sides.
♦ The mediastinal pleura are opened until the azygos arch can be identified and divided using a reticulating endoscopic vascular stapling device.
♦ Proximal to the azygos vein, dissection must be in close proximity to the esophagus to avoid recurrent laryngeal nerve injury. The subcarinal space is developed, and the esophagus is dissected away from the right and left mainstem bronchi (Figure 2-9).
♦ Division of the aortoesophageal vessels is performed with special attention to the preservation of the contralateral pleura and the thoracic duct.
Conduit pull-through and esophagogastrostomy
♦ The esophagus is mobilized into the abdominal-thoracic inlet and separated from the hiatus.
♦ The conduit that is attached via a suture to the specimen is pulled intramediastinally through the hiatus. The suture is cut and the specimen is retracted in a cranial direction, thereby exposing the most medial aspect of the mediastinal dissection (e.g., contralateral pleura).
♦ The upper esophagus is divided 3 cm cranially to the azygos vein level using endoscopic scissors, and the specimen is removed through the extended surgeon right-hand port using a wound protector. The specimen is opened and checked for gross margins.
Intrathoracic anastomosis
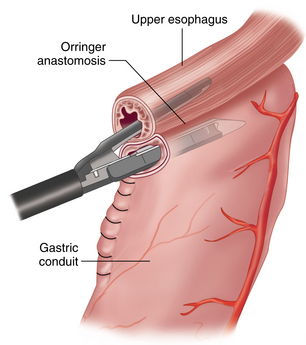
♦ The esophageal-conduit anastomosis is created using a 28 mm endoscopic end-to-end stapler (Ivor-Lewis approach). The anvil is sutured into the proximal esophagus, and the conduit staple line is opened to allow access of a stapler that is introduced intrathoracically through the extended inferior posterolateral port (Figure 2-9).
♦ Rotation must be prevented and the length of conduit necessary to achieve a tensionless anastomosis must be determined. The presence of tissue “doughnuts” in the stapler after firing is confirmed, as this indicates a complete circumferential anastomosis. Closure of the gastrotomy is performed by dividing the surplus conduit with a roticulating endoscopic stapler and removed. This specimen will serve as the final distal margin for histologic examination.
♦ A nasogastric tube is placed in the conduit proximal to the hiatal opening and a Jackson-Pratt drain is placed near the anastomosis opposite from the staple line. After the thoracic cavity is irrigated with warmed antibiotic saline, hemostasis is achieved and the right lung is re-ventilated after placement of a 28 French thoracostomy tube.
Step 4. Postoperative care
General rules for postoperative care after minimally invasive esophagectomy:
♦ Early ambulation and pulmonary physiotherapy are initiated.
♦ The nasogastric tube is removed and jejunostomy tube feeding is started around the third postoperative day if there is no sign of ileus.
♦ Dietary education is provided and is focused on small, frequent meals.
♦ Before discharge, an upper GI-tract contrast x-ray is performed (on the 6th to 7th postoperative day) to verify the integrity of the anastomosis and pyloroplasty.
♦ An evaluation visit is scheduled at 2 to 3 weeks, and the jejunostomy is removed at 6 weeks if the patient’s oral intake and weight are sufficient.
♦ The closed suction drain is pulled back by 2 cm prior to discharge. This drain is removed at the first postoperative clinic visit after the patient undergoes an upper gastrointestinal contrast radiographic examination.
Step 5. Pearls and pitfalls
♦ In addition to the techniques described in this chapter, other successful minimally invasive approaches have been reported. The key to success for minimally invasive esophagectomy is a high patient volume and a well-trained multidisciplinary surgical team.
♦ Neoadjuvant chemotherapy is not seen as a contraindication to the minimally invasive approach.
♦ LIE can be used for lesions across the esophagogastric junction by the use of an antegrade (proximal to distal) inversion technique. The inversion starts in the proximal esophagus and the esophagus is extracted through an abdominal port site.
♦ If vagotomy is performed, a gastric drainage procedure (pyloroplasty, pyloromyotomy, or pyloric finger disruption) is performed, at the surgeon’s discretion, to prevent delayed gastric emptying and associated complications (e.g., aspiration pneumonia). We routinely perform pyloroplasty following a vagotomy; however, this procedure is controversial because it could induce bile reflux into the conduit and contribute to anastomotic stricture development.
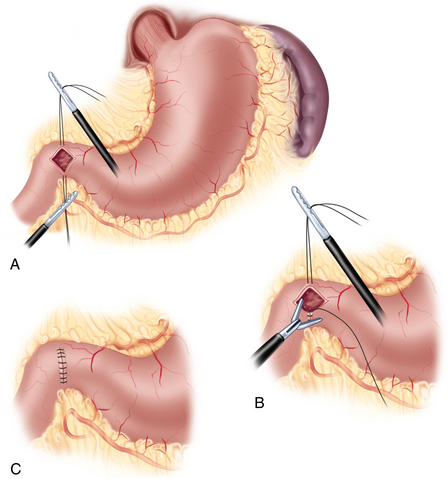
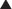 Pyloriplasty—Heineke and Mikulicz technique: superior and inferior tension sutures are placed. The muscular layer is incised using ultrasonic scissors and subsequently closed transversally with interrupted sutures (Figure 2-10).
Pyloriplasty—Heineke and Mikulicz technique: superior and inferior tension sutures are placed. The muscular layer is incised using ultrasonic scissors and subsequently closed transversally with interrupted sutures (Figure 2-10).♦ The Ivor-Lewis approach in the combined laparoscopic-thoracoscopic (two-cavity) esophagectomy with thoracoscopic intrathoracic anastomosis can be employed only for lesions in the distal third of the esophagus. For more proximal lesions, a cervical anastomosis is advised.
Akiyama H, Tsurumaru M, Ono Y, et al. Esophagectomy without thoracotomy with vagal preservation. J Am Coll Surg. 1994;178;1:83-85.
Banki F, Mason RJ, DeMeester SR, et al. Vagal-sparing esophagectomy: a more physiologic alternative. Ann Surg. 2002;236;3:324-335. discussion 335-36
Luketich JD, Alvelo-Rivera M, Buenaventura PO, et al. Minimally invasive esophagectomy: outcomes in 222 patients. Ann Surg. 2003;238;4:486-494. discussion 494-95
Luketich JD, Nguyen NT, Weigel T, et al. Minimally invasive approach to esophagectomy. JSLS. 1998;2;3:243-247.
Jobe BA, Kim CY, Minjarez RC, et al. Simplifying minimally invasive transhiatal esophagectomy with the inversion approach: lessons learned from the first 20 cases. Arch Surg. 2006;141;9:857-865. discussion 865-66
Orringer MB, Marshall B, Iannettoni MD. Eliminating the cervical esophagogastric anastomotic leak with a side-to-side stapled anastomosis. J Thorac Cardiovasc Surg. 2000;119;2:277-288.
Swanstrom LL, Hansen P. Laparoscopic total esophagectomy. Arch Surg. 1997;132;9:943-947. discussion 947-9