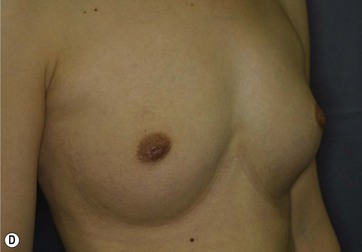
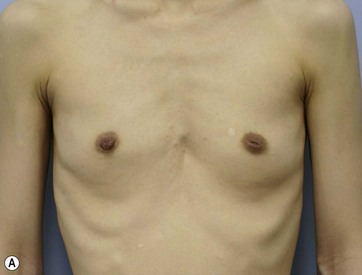
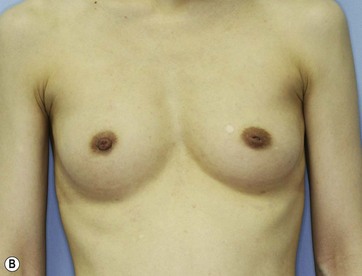
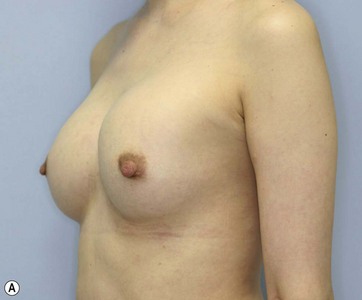
Chapter 48 Minimal scar breast augmentation using autologous fat grafting
• Moderate augmentation (100–200 ml) of the breast is successfully achieved by autologous lipoinjection without major complications if appropriately performed in selected patients.
• Breasts augmented with fat injection are soft and show natural texture and appearance, and patients are free from concerns associated with foreign materials (and related complications and potential implant replacement or removal).
• The surviving fat volume varies substantially among patients, and multiple factors likely affect clinical results; patient factors include skin redundancy of the breast and availability of fat.
• Surgeons need to improve quality (such as tissue viability and progenitor/adipocyte ratio) of fat grafts and infiltration techniques.
• Aspirated fat tissue should be appropriately harvested and stored, and quickly processed and infiltrated to place aliquots or noodles of fat grafts as diffusely as possible.
• Supplementation of vascular stromal fraction containing adipose progenitor cells may boost the efficacy and safety of lipoinjection to the breasts.
Introduction
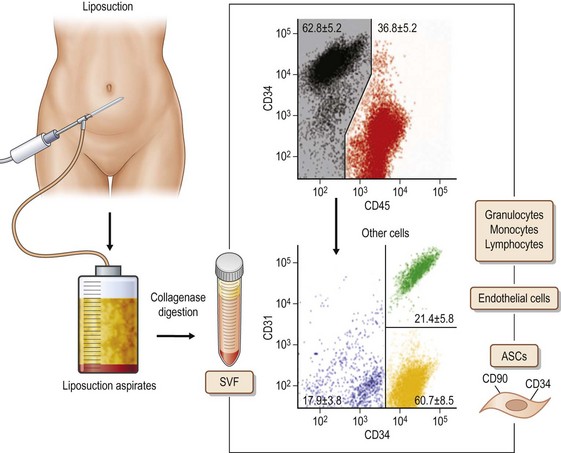
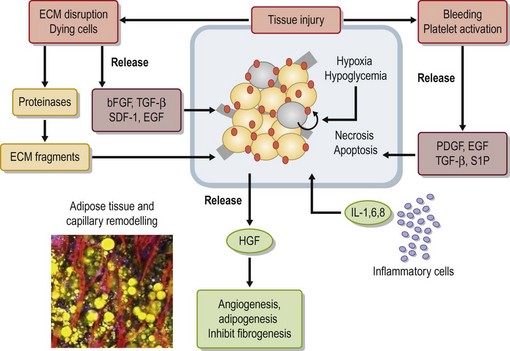
Adipose tissue has been considered an organ of energy storage, the largest endocrine organ, a soft tissue filler (augmentation by micro-fat grafting), and a cosmetically unnecessary tissue discarded by liposuction. Adipose tissue physiologically turns over very slowly. Adipocytes have a life span of 10 years;1 several thousands of adipocytes die and are replaced with new adipocytes every second in our body. Adipose tissue has many cells other than adipocytes, though adipocytes constitute more than 90% of adipose tissue volume. Through collagenase digestion, cellular components other than adipocytes are extracted as a cell pellet, which is called stromal vascular fraction (SVF). SVF contains adipose stromal cells (ASC), vascular endothelial cells (VEC), pericytes, adipose-resident macrophages, etc. (Fig. 48.1). It was found that the adipose stromal cell population contains not only monopotent progenitor cells responsible for the tissue turnover, but also multipotent mesenchymal stem cells,2 which are now called adipose (-derived) stem cells. These are regarded as a potent tool for cell-based therapies, comparable to bone marrow-derived mesenchymal stem cells, because they can be obtained in a large amount through a less invasive approach. For adipose tissue engineering/regeneration, it is a critical point to understand the physiological roles and functions of ASC; this population, working together with VEC and stem/progenitor cells recruited from bone marrow, is a main player for adipose tissue engineering, which means adipogenesis and angiogenesis.
Autologous fat transplantation is one of the promising cosmetic treatments for facial rejuvenation and soft-tissue augmentation due to minimal incisional scars and lack of complications associated with foreign materials. However, certain problems remain, such as unpredictability and a low rate of graft survival due to partial necrosis. Implantation of prostheses has been a golden standard for breast augmentation, but complications such as capsular contracture remain to be resolved. Recently, autologous fat injection has been re-evaluated as a potential alternative to artificial implants for breast augmentation.3 This re-evaluation may reflect recent advances in autologous fat transfer and the radiological detection of breast cancer.
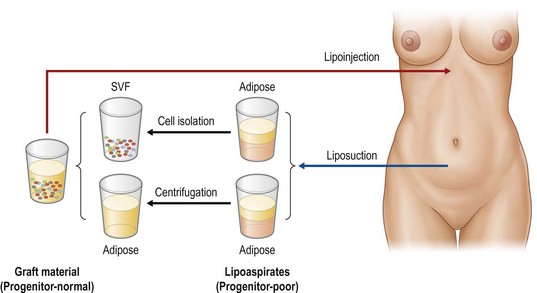
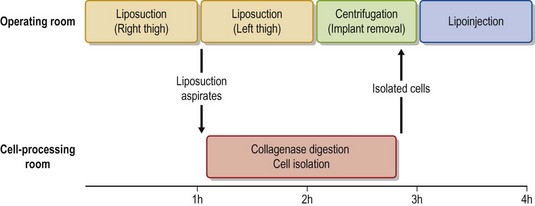
In this chapter, biological principles and surgical techniques of fat injection for breast augmentation are discussed as well as our novel approach of autologous fat grafting called cell-assisted lipotransfer (CAL),4,5 which is concurrent transplantation of aspirated fat tissue and adipose progenitor cells (Fig. 48.2).
Preoperative Preparation
There seem to be several patient factors that may affect the clinical result of lipoinjection; skin redundancy of the breasts, age, BMI, personal quality or character of fat tissue, adhesive scars, breast implant and its capsule, systemic disease such as autoimmune disease, oral corticosteroid, etc.4,6 Good candidates are those who have sufficient fat at donor sites and the redundant breast skin with healthy vascularity and without scars. Lipoinjection can be performed in any patients from teens to 70s, but patients with low BMI (less than 18) are not good candidates due to difficulty in harvesting a sufficient volume of fat tissue without severe morbidity. Patients who want a large-volume (250–400 ml) augmentation, are not good candidates because augmentation volume achieved by a single session of lipoinjection is limited (100–200 ml).
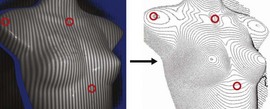
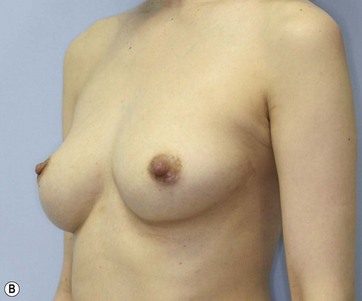
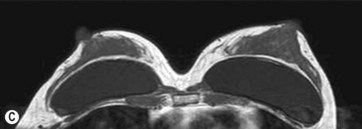
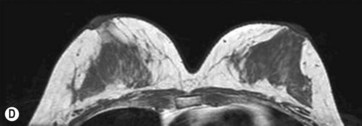
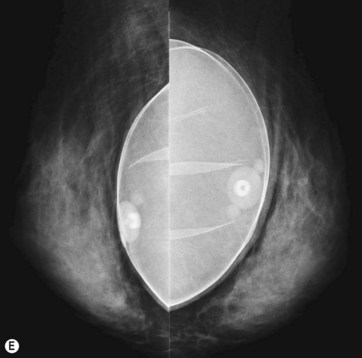
For preoperative examination, physical measurements (maximum and bottom breast circumferences, etc.), mammogram, MRI, echogram, photograph, and videograph are performed. We also adopted a 3-dimensional measurement system that enables a volumetric evaluation of the breast mound in a standing position (Fig. 48.3). An echogram is easy to perform at every patient’s visit and sensitive enough to detect small cyst formation. Long follow up with an annual mammogram up to 5 years is recommended to detect abnormal signs such as calcifications.
Surgical Technique
Breast Augmentation
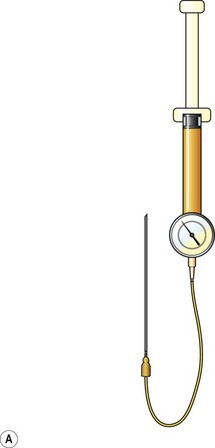
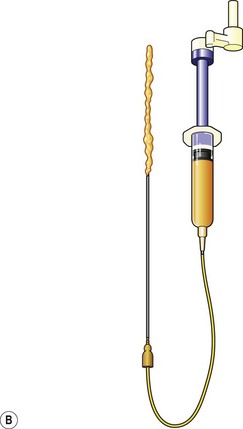
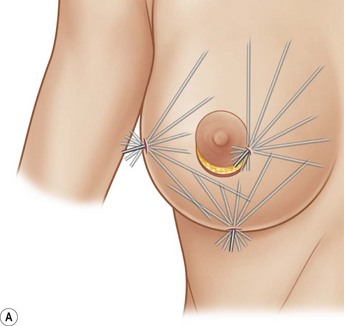
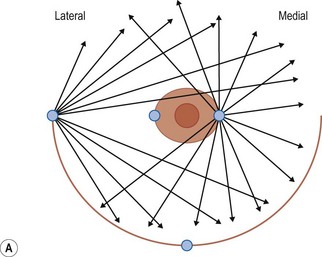
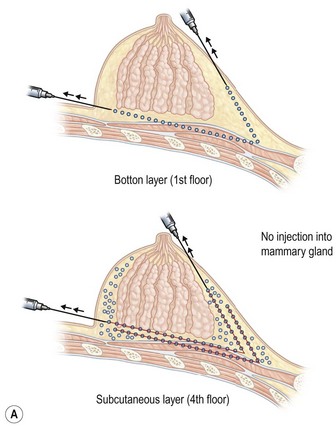
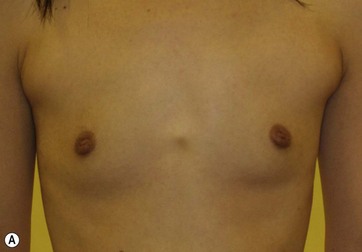
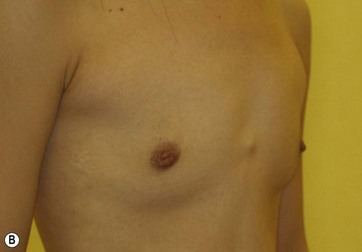
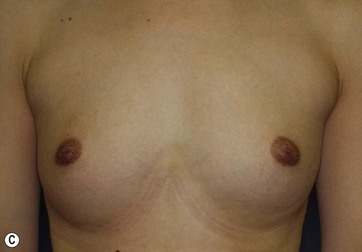
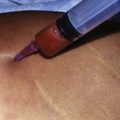
Donor sites are usually the thighs alone or the thighs and the abdomen or flanks, decided according to the patient’s preference and BMI. After the liposuction site is infiltrated with saline solution with epinephrine (0.001%) under general anesthesia, adipose tissue is suctioned using a cannula with 2–3 mm inner diameter and a conventional liposuction machine. We use screw-type syringes (with a threaded plunger) to allow for precise control and avoid a flushing injection (Fig. 48.4). To reduce the time of the procedure, two syringes are used; while one syringe is being used for an injection, the other is filled with the graft material in preparation for the next injection. A 16- or 18-gauge needle (150 mm long) is used for lipoinjection and inserted subcutaneously from the inframammary fold or areolar margin (Fig. 48.5A). The operator takes care to insert and place the needle horizontally (parallel to the body), in order to avoid causing a pneumothorax. The needle is inserted in several layers and directions, and is continuously and gradually retracted as the plunger is advanced (Fig. 48.5B). This technique is used to obtain a diffuse distribution of the graft material. The grafts are placed into the fatty layers on, around, and under the mammary glands (but not into the mammary glands), and also into the pectoralis muscles. After training, it is not hard for an operator to recognize the mammary gland or pectoralis fascia as a harder tissue than the fat or muscle tissue. Injection is discontinued when the skin tension becomes tense; injection volume is usually 200–300 ml for each breast in Asian patients.
Breast Implant Replacement
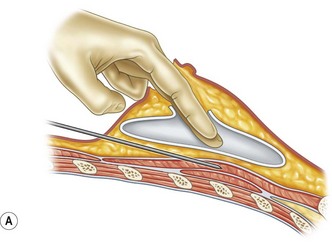
For patients with implants, lipoinjection can be performed simultaneously with implant removal.7 Breast implants are removed through a periareolar incision, which is placed at a caudal third of the areolar margin. The lipoinjection is begun at the deepest layer (under the implant capsule when the implant was subglandular) and completed with the injection into the most superficial subcutaneous layer. During the injection, the operator can insert fingers into the implant capsule and recognize the location of the injection needle (Fig. 48.6). Injection into the mammary glands or into the capsular cavity is not performed. Finally, the capsular cavity is irrigated with saline to remove any fat tissue injected and the periareolar incision is closed.
Preparation Procedures of Graft Materials
Conventional Lipoinjection
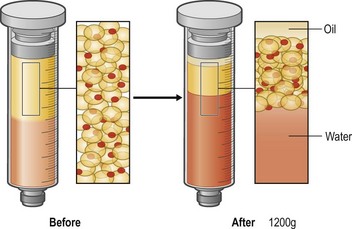
Lipoaspirates are harvested by suction-assisted liposuction and centrifuged (1200 g, 3 min), and put into a metal jar (1000 ml), which is placed in water with crushed ice. As the centrifugation reduces the adipose volume by 25–35%, the volume reduction should be taken into account in tissue harvesting (Fig. 48.7).
Cell-Assisted Lipotransfer (CAL)
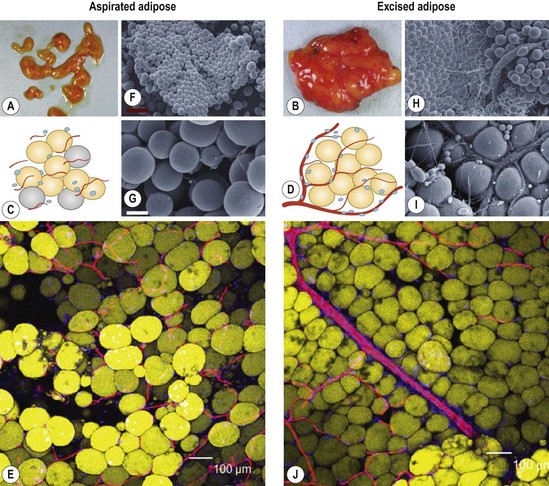
Aspirated fat tissue has a significantly lower progenitor/mature-cell ratio,5 and this low ASC/adipocyte ratio may be one of reasons for long-term atrophy of transplanted adipose tissue as demonstrated before4,5,8 (Fig. 48.8). Enrichment of adipose progenitor cells by supplementation of SVF improves/normalizes progenitor/adipocyte ratio and is also suggested to augment final volume retention. In this strategy, progenitor-poor aspirated fat tissue is converted to progenitor-enriched (but not more than normal) fat tissue.
Full-CAL
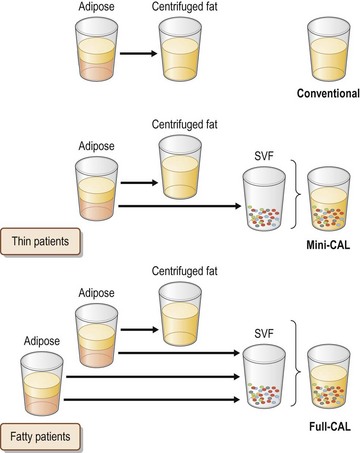
In full-CAL, SVF is isolated from additionally harvested lipoaspirates (Fig. 48.9). The SVF is isolated as described below. During the processing period, liposuction is continued and lipoaspirates are centrifuged (700 g, 3 min) for preparation of graft materials. The freshly isolated SVF was added to the centrifuged fat tissue, followed by gentle mixing and a 5–10 min incubation to achieve appropriate cell adhesion to the centrifuged fat tissue (Fig. 48.10).
SVF Isolation Procedure
Processed lipoaspirate cells (PLA) cells and liposuction aspirate fluid (LAF) cells are both so-called SVF; they are separated from the fatty and fluid portions of liposuction aspirates, respectively.9 PLA or both cells are used in full-CAL, while only LAF cells are used in mini-CAL (Fig. 48.9). For PLA cells, suctioned fat tissue is digested with 0.075% collagenase in phosphate-buffered saline (PBS) for 30 min on a shaker at 37°C. After centrifugation (800 g, 10 min), mature adipocytes and connective tissues (floating tissue) are discarded. The cell pellets are resuspended in Dulbecco’s modified Eagle’s medium (DMEM) and passed through a 100 mm mesh filter. To eliminate remaining collagenase, the cell pellets are repeatedly washed with resuspension in DMEM and following centrifugation at least three times. The process takes about 80 min (Fig. 48.10). For LAF cells, the suctioned fluid is centrifuged (400 g, 10 min) and the pellets are resuspended in distilled water (for 30 s) for erythrocyte lysis, followed by osmic normalization by adding 10% volume of 10× PBS (or 9% NaCl solution). After centrifugation (and filtration), cell pellets are obtained as SVF; the process takes about 20 min. For both PLA and LAF cells, cell counting for erythrocytes and nucleated cells is performed using a blood test cell counter; this takes 1 min.
Optimizing Outcomes
Understanding of Cellular Events in Fat Grafting
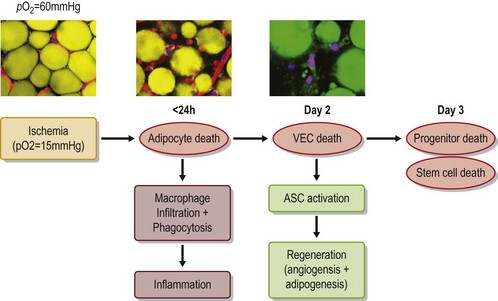
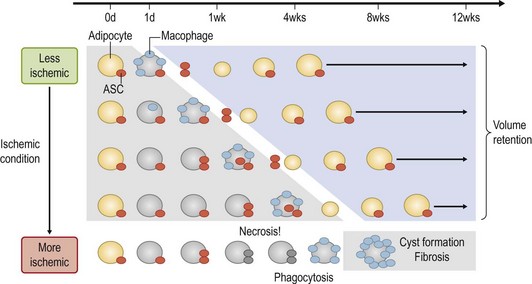
The grafted nonvascularized adipose tissue is placed under ischemia (hypoxia with low nutrition) and is temporarily nourished only by plasmatic diffusion from the surrounding host tissue for a few days until direct capillary supply is formed.9 In response to injury and cell death, many growth factors and proteinases are released from damaged tissue and activated platelets10 (Fig. 48.14). Inflammatory cells are infiltrated and inflammatory cytokines such as interleukins are secreted. During the repair process, adipocytes, known to be very sensitive to hypoxia, can die within 24 hours and subsequently endothelial cells start to die when the ischemia is prolonged. ASC are likely to be as resistant to ischemia as bone marrow-derived mesenchymal stem cells,11 which can be functional up to 72 hours under severe ischemia (Fig. 48.15).
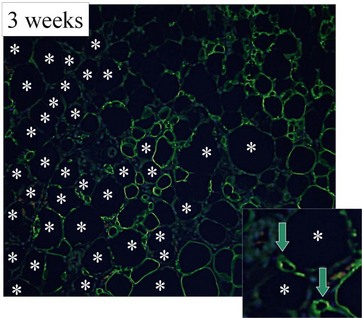
Our recent studies showed that most adipocytes are likely to die within a few days (Fig. 48.16) and some of them are replaced with next generation adipocytes by 12 weeks.11 From the surface of grafted fat tissue, adipocyte remodeling starts and progresses slowly toward the center and the rate of the adipocyte regeneration determines the final volume retention of fat grafting. In the case that stem/progenitor cells die due to prolonged severe conditions, the area is not properly replaced with new adipocytes and rather filled with fibrosis and calcification (Fig. 48.17). Oil cysts are being absorbed even after 3 months; the time course of the absorption depends on the size of lipid droplets, but takes weeks or months. Thus, nonvascularized adipose grafting can be called adipose tissue regeneration rather than adipose tissue survival, and we believe that this is seen in other nonvascularized tissue grafting such as skin or composite tissue grafting (Fig. 48.17).
Key Factors of Graft Materials
• One is to improve the viable cell number per volume. This means concentrating needed components (viable adipocytes, ASCs, and other cellular components) and discarding unneeded components such as water, oil (ruptured adipocytes) and erythrocytes. This is usually performed through centrifugation or filtration with negative pressure (Fig. 48.7). Centrifugation concentrates cellular components and separates water and oil with variable degrees, depending on the centrifugation force and time.12
• The other key point is to improve the cellular quality of the graft. This means improving (normalizing) the progenitor (ASC)/adipocyte ratio; aspirated adipose tissue has been suggested to have a lower ratio than intact adipose tissue.5 In general, stem/progenitor cells and activating signals are crucial factors for tissue regeneration. In fat grafting, there are many signals to activate stem/progenitor cells such as injury or bleeding, but dying adipocytes seem to be crucial signals to induce ASC differentiation into adipogenic lineage. If the adipose graft did not contain enough stem/progenitor cells, the tissue regeneration would not be efficient. On the other hand, if only stem/progenitor cells are transplanted, stem cells would not be either activated or differentiated into the expected direction. So, if the tissue is stem-cell-deficient in number, it would be reasonable to normalize stem cell density in the tissue by supplementing the stem/progenitor cells as performed in CAL strategy (Fig. 48.2) or by reducing the adipocyte number. Centrifugation can relatively reduce the viable adipocyte number without losing ASC12 (Fig. 48.7).
1 Spalding KL, Arner E, Westermark PO, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787.
2 Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295.
3 Coleman SR, Saboeiro AP. Fat grafting to the breast revisited: safety and efficacy. Plast Reconstr Surg. 2007;119:775–785.
4 Yoshimura K, Sato K, Aoi N, et al. Cell-assisted lipotransfer (CAL) for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells-. Aesth Plast Surg. 2008;32:48–55.
5 Matsumoto D, Sato K, Gonda K, et al. Cell-assisted lipotransfer: supportive use of human adipose-derived cells for soft tissue augmentation with lipoinjection. Tissue Eng. 2006;12:3375–3382.
6 Yoshimura K, Sato K, Aoi N, et al. Cell-assisted lipotransfer for facial lipoatrophy: efficacy of clinical use of adipose-derived stem cells. Dermatol Surg. 2008;34:1178–1185.
7 Yoshimura K, Asano Y, Aoi N, et al. Progenitor-enriched adipose tissue transplantation as rescue for breast implant complications. Breast J. 2010;16:169–175.
8 Yoshimura K, Suga H, Eto H. Adipose-derived stem/progenitor cells: roles in adipose tissue remodeling and potential use for soft tissue augmentation. Regen Med. 2009;4:265–273.
9 Yoshimura K, Shigeura T, Matsumoto D, et al. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J Cell Physiol. 2006;208:64–76.
10 Aiba-Kojima E, Tsuno NH, Inoue K, et al. Characterization of wound drainage fluids as a source of soluble factors associated with wound healing: comparison with platelet-rich plasma and potential use in cell culture. Wound Repair Regen. 2007;15:511–520.
11 Suga H, Eto H, Aoi N, et al. Adipose tissue remodeling under ischemia: Death of adipocytes and activation of stem/progenitor cells. Plast Reconstr Surg. 2010;126:1911–1923.
12 Kurita M, Matsumoto D, Shigeura T, et al. Influences of centrifugation on cells and tissues in liposuction aspirates: optimized centrifugation for lipotransfer and cell isolation. Plast Reconstr Surg. 2008;121:1033–1041.