CHAPTER 35 Middle Fossa Meningiomas
INTRODUCTION
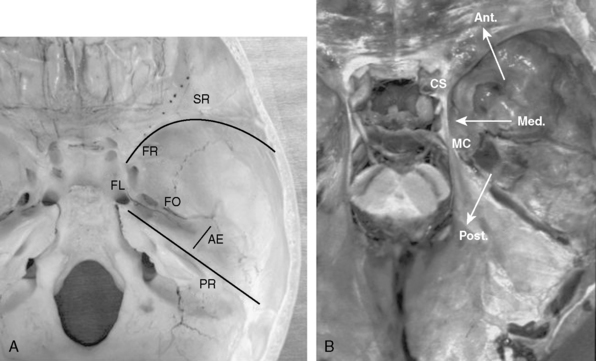
The middle fossa is a common localization for intracranial meningiomas. The floor of the middle cranial fossa is formed by the greater wing of the sphenoid bone joining the squamosal part of the temporal bone.1 Medially, it articulates with the clival portion of the occipital bone at the petroclival fissure. The foramen lacerum is located at the junction of the temporal, sphenoid, and occipital bones. The floor of the middle cranial fossa is bound anteriorly by the greater wing of the sphenoid (sphenoid ridge), posteriorly by the superior petrosal sinus (petrous ridge), and medially by the lateral wall of the cavernous sinus and Meckel’s cave (Fig. 35-1A, B). The bone and the dura mater of the middle fossa floor are supplied by branches of the middle meningeal artery and by caroticocavernous branches.
Meningiomas can involve the middle fossa primarily or secondarily. Pure middle fossa meningiomas originate anterolaterally and posterolaterally at the floor of the middle cranial fossa and extend to the vicinity of the superior petrosal sinus and the exit of the vein of Labbé.2 However, the majority of meningiomas encountered at the middle fossa are secondary extensions and involve the sphenoid wing, superior orbital fissure, cavernous sinus, Meckel’s cave, petroclival area, and the cerebellopontine angle. Some extend extradurally to the pterygopalatine fossa and infratemporal fossa through the foramen rotundum and ovale. Other authors reported unusual cases of the middle fossa meningiomas arising from the geniculate ganglion,3 the middle ear,4 and other parts of the temporal bone.5 Meningiomas of the middle fossa can also occasionally invade along the temporal base in en plaque fashion. Therefore, knowledge of microsurgical anatomy and variations of lateral skull-base approaches are essential for planning a surgical treatment in patients with middle fossa meningiomas.
SURGICAL APPROACHES FOR MIDDLE FOSSA MENINGIOMAS
For resection of most pure middle fossa meningiomas, a subtemporal and/or middle fossa approach or suprapetrosal approaches are adequate.6 In contrast, for meningiomas of the middle fossa that extend into surrounding skull-base structures (Fig. 35-1B), appropriate skull-base approaches should be tailored based on the preoperative imaging studies. Zygomatic osteotomies facilitate these approaches by widening the surgical corridor in exposure of the middle fossa, and enable access to the infratemporal fossa. In case of anterior extension to the frontal fossa, sphenoid wing, and superior orbital fissure (SOF), orbitozygomatic approaches may be planned.7,8 In case of posterior extension to the cerebellopontine angle, anterior and/or posterior petrosal approaches may be appropriate.9,10 In case of inferior extension to the infratemporal fossa, the zygomatic infratemporal approach may be chosen.11 Depending on the tumor location and extent, combinations of these approaches may be used to achieve safe and total removal of widely invasive tumors.
Epidural or Subdural Subtemporal Approach (Middle Fossa Approach)
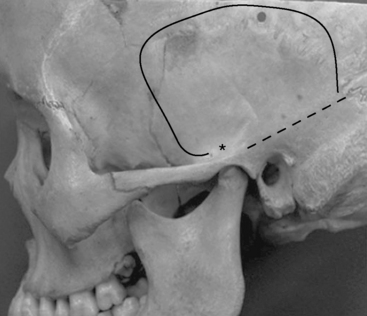
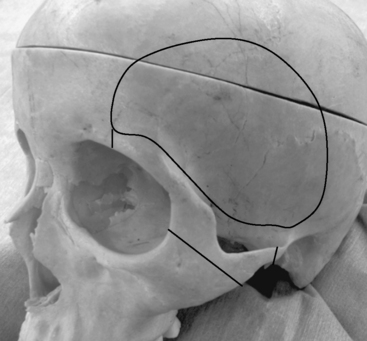
Most meningiomas of the middle fossa can be resected with the subtemporal approach. The lowest point of the middle cranial fossa is roughly at the level with the upper border of the zygomatic arch and is slightly posterior to the articular tubercle of the temporomandibular joint.12 Zygomatic osteotomies therefore facilitate the exposure of the anterior part of the middle cranial fossa. The surgical landmark of the base of the middle cranial fossa is the line between just above the root of the zygoma and the supramastoid crest (Fig. 35-2).
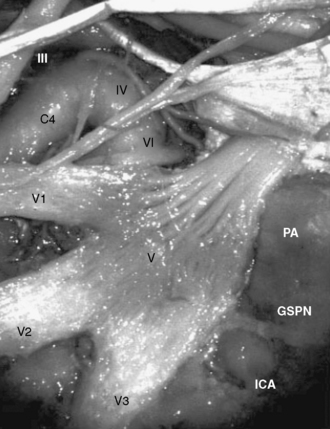
The petrous portion of the internal carotid artery (ICA) courses posterolaterally toward Meckel’s cave, and the transition between petrous and cavernous portion of ICA occurs below the trigeminal ganglion (Fig. 35-3). The Glasscock triangle is limited by the posterior rim of the foramen ovale, foramen spinosum, and the posterior border of V3. The GSPN usually runs just above this triangle, which is an important landmark of the lateral border for preserving the petrous ICA while drilling the petrous apex.9 When the GSPN is adherent to the dura of the middle fossa, it can be dissected together with the middle fossa dura.
During epidural dissection of the divisions of the trigeminal nerve, it is quite important to respect the microsurgical anatomy of the cavernous sinus and Meckel’s cave. The dura of the middle fossa has two layers: the outer periosteal layer and the inner meningeal layer. At its exit from the dura, the meningeal layer of the dura envelopes the epineurium of the nerve and after its entry into the bony foramens of the middle fossa the nerve is covered by the periosteal layer. Therefore, by cutting the periosteal layer of the dura and dissecting between the meningeal layer of the dura and the epineurium in the lateral wall of the cavernous sinus and Meckel’s cave, the divisions of the trigeminal nerve can be exposed extradurally. Peeling off the meningeal layer of the V3 and Meckel’s cave will also facilitate the exposure of the petrous apex for the anterior petrosal approach.9 This technique will also provide a wide exposure from the SOF to the foramen ovale and Meckel’s cave. The disadvantage of the subtemporal approach is the need for significant temporal lobe retraction and resultant posterior venous congestion. Removal of the zygoma and lumbar cerebrospinal fluid (CSF) drainage may minimize the need for temporal lobe retraction. The epidural subtemporal approach provides limited exposure of the tumor, because V2 and V3 may limit the exposure. In addition, traction injury of GSPN and of the geniculate ganglion may result in facial nerve palsy. Preservation of the arachnoid planes and the surrounding neurovascular structures should be attempted. However, some meningiomas may invade the arachnoid and even the pial sheets. In these cases, the operating surgeon will decide how aggressive the surgery will be, depending on the structures involved and the patient’s clinical situation.13
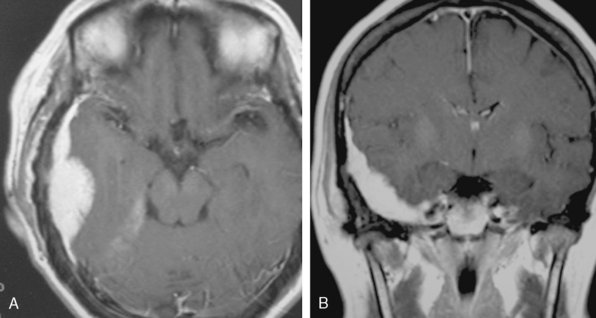
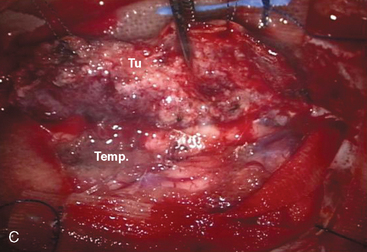
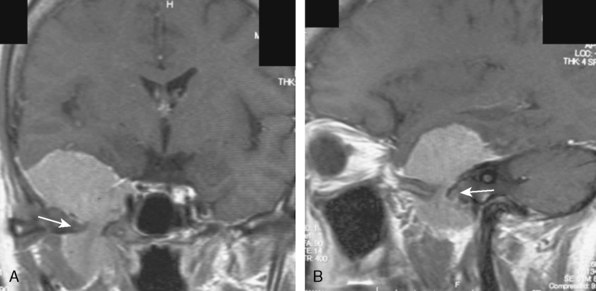
A 47-year-old woman presented with left temporal pain, which was present for the last few years. Magnetic resonance imaging (MRI) showed an en plaque meningioma along the right middle fossa, extending to the lateral wall of the cavernous sinus and Meckel’s cave (Fig. 35-4). A right cerebral angiogram revealed the tumor blush on the venous phase and indicated that the tumor was mainly fed by the middle meningeal artery. Computed tomography (CT) showed hyperostosis of the temporal bone. Gross total removal was achieved with a right zygomatic subtemporal approach. Wide exposure of the floor of the middle fossa made it possible to safely resect the hyperostotic bone as far as medially as the lateral aspect of the foramen ovale and foramen rotundum. The middle fossa dura was also widely resected after peeling off the outer layer of the lateral wall of the posterior cavernous sinus. Cavernous sinus involvement may represent a limit to achieving total resection. Skull-base reconstruction is performed using a vascularized temporal fascial and muscle flap. In case of a large bone defect, artificial materials such as bone cement or ceramic material can be used for cranioplasty. Close observation under imaging studies is essential because of the high frequency of recurrence in patients with en plaque meningioma.14
Venous considerations in the subtemporal approach
Knowledge of the microsurgical anatomy of the temporal bridging veins is essential while planning a subtemporal route for removal of a middle fossa meningioma. Sakata and colleagues15 analyzed temporal venous drainage patterns, presented a classification scheme, and described common surgical pitfalls. Attention should be paid not only to the vein of Labbé, but also to other temporobasal bridging veins, especially the petrosal group of the temporal bridging veins. The petrosal group of the bridging veins are defined as the temporal veins terminating anterior to the transverse–sigmoid junction, which tethers the temporal lobe to the cerebellar tentorium or middle fossa dura and limits surgical mobilization of the temporal lobe. This group of the temporal bridging veins is, therefore, very important surgically. For cases in which a large petrosal bridging vein is encountered, we recommend use of Sugita’s technique, which involves detachment of the temporal vein from the temporal lobe surface to create a surgical working corridor between the temporal lobe and temporal base.10,16 In the petrosal approach, division of the tentorium in front of its termination facilitates the mobilization of the temporal lobe with the bridging veins. This technique provides us more working space around the temporal base, while enabling venous preservation. While planning a surgical strategy for middle fossa meningiomas, it is essential to evaluate the temporal venous drainage pattern in detail before surgery, as the only way to reduce venous complications is to preserve the venous drainage as much as possible. New generation multislice CT (Fig. 35-5) scanners can provide detailed anatomic information regarding the tumor and surrounding arteries and veins.17
Orbitozygomatic Approach
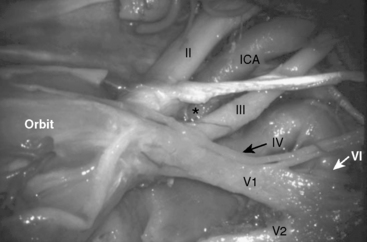
For middle fossa meningiomas extending to the anterior fossa, we may choose the pterional approach with or without orbitozygomatic osteotomies (Fig. 35-6). If necessary, optic canal decompression or anterior clinoidectomy could be added depending on the tumor extension. Extradural anterior clinoid resection is one of the key maneuvers in this approach. The anterior clinoid process (ACP) has a triangular conical shape and resembles a “shark’s tooth.” However, it is important to respect the surrounding anatomic structures during anterior clinoidectomy. The optic nerve, enveloped by the optic nerve sheath, runs just medial to the ACP. The oculomotor nerve and the carotico-oculomotor membrane lie lateral to the ACP, and the C3 portion of the ICA lies just below the ACP in the so-called “anteromedial (Dolenc’s) triangle” (Fig. 35-7). A successful removal of the ACP can be performed by skeletonizing the shark tooth and elevating the bony shell with the help of a microdissector.
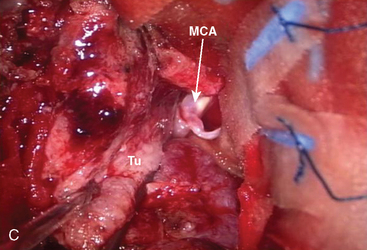
A 63-year-old man presented with decreased activity and mild left hemiparesis. MRI examination revealed a middle fossa tumor, severely compressing the frontal and temporal lobes. The preliminary diagnosis was that of a sphenoid wing meningioma (Fig. 35-8). An orbitozygomatic approach was planned. Surgical exploration revealed that the tumor was attached to the middle fossa dura rather than to the sphenoid ridge. The tumor capsule could not be dissected from the lateral wall of the cavernous sinus. It is quite critical in surgery for this type of meningioma to dissect the middle cerebral artery (MCA) and lenticulostriate artery from the tumor. This dissection is easier in middle fossa meningiomas with anterior extension, when compared to large sphenoid wing meningiomas, due to the presence of an arachnoid layer surrounding the MCA. However; it is sometimes difficult to distinguish middle fossa meningioma or sphenoid ridge meningioma on preoperative imaging studies. In giant meningiomas, preoperative embolization can help to reduce bleeding form the tumor.
Zygomatic Infratemporal Approach
For middle fossa meningiomas extending to the infratemporal fossa, the zygomatic infratemporal approach provides the necessary exposure for total surgical resection. Zygomatic osteotomies and middle fossa drilling are essential for the management of the infratemporal extension (Fig. 35-9). After temporal craniotomy and epidural dissection as described previously, drilling the base of the middle fossa around the maxillary nerve (V2) and V3 exposes the infratemporal fossa and the pterygoid process of the sphenoid bone. The various branches of V3, maxillary artery, and Vidian nerve come into view in this area.18
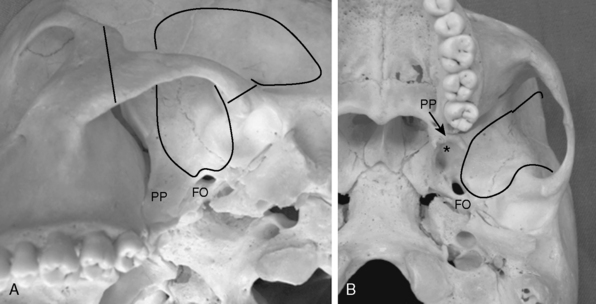
FIGURE 35-9 Extent of craniotomy and middle fossa drilling for zygomatic infratemporal approach (solid line).
A, Inferior oblique view of the left infratemporal fossa. PP, pterygoid process; FO, foramen ovale.
B, Inferior view of the left infratemporal fossa. The asterisk indicates the pterygoid fossa.
Temporalis muscle retraction may be performed in two ways: superiorly or inferiorly. Superior retraction after resection of mandibular coronoid process provides the widest exposure of the infratemporal fossa, but at the expense of denervation and devascularization of the temporal muscle.11 The more commonly performed inferior retraction results in a more limited infratemporal exposure but has significant cosmetic benefits. This technique also preserved the muscle to be used as a vascularized flap for complex reconstructions.18 The need for caudal exposure should be determined preoperatively based on a detailed imaging studies and an appropriate surgical approach should be chosen. It should be emphasized again that the knowledge of surgical anatomy of the middle fossa and various options of lateral skull base approaches are essential for surgery of the middle fossa meningiomas extending to the infratemporal fossa.
A 52-year-old woman presented with right retrobulbar pain. MRI revealed the middle fossa tumor extending to the infratemporal fossa through the foramen ovale (Fig. 35-10). A zygomatic infratemporal approach was planned. After scalp incision around the right ear, the fascial flap and temporal muscular flap (two-layer flap) were prepared for middle fossa reconstruction. After temporal craniotomy with zygomatic osteotomy, the middle fossa dura was elevated and the MMA was coagulated and divided to control the bleeding from the tumor. The subdural component of the tumor was removed using the techniques as described previously. Then, complete drilling the middle fossa around the foramen ovale was done to access to the infratemporal component of the tumor. After removal of the tumor, the middle fossa was reconstructed with vascularized temporal muscular and fascial flaps in separate layers. Spinal drainage was placed to prevent postoperative CSF leakage. Stereotactic radiosurgery was performed for the small extracranial residual tumor six months after surgery.
[1] Rhoton A.L.Jr. The temporal bone and transtemporal approach. Neurosurgery. 2003;53(Rhoton’s anatomy):643-697.
[2] Yasargil M.G. Microneurosurgery IVB. New York: Thieme, 1996.
[3] Luetje C.M., Syms C.A.III, Luxford W.E., et al. Meningioma intrinsic to the geniculate ganglion. Am J Otol. 1997;18:393-397.
[4] Prayson R.A. Middle ear meningioma. Ann Dian Pathol. 2000;4:149-153.
[5] Laws E.R. Meningiomas of the temporal bone. In: Al-Mefty O., editor. Meningiomas. New York: Raven Press; 1991:539-541.
[6] Ribas G.C., Rodrigues A.J. The suprapetrosal craniotomy. J Neurosurg. 2007;106:449-454.
[7] Fujitsu K., Kuwabara T. Zygomatic approach for lesions in the interpeduncular cisterns. J Neurosurg. 1985;62:340-343.
[8] Hakuba A., Liu S., Nishimura S. The orbitozygomatic infratemporal approach: a new surgical technique. Surg Neurol. 1986;26:271-276.
[9] Kawase T., Shiobara R., Toya S. Anterior transpetrosal – transtentorial approach for sphenopetroclival meningiomas: Surgical method and results in 10 patients. Neurosurgery. 1991;28:869-976.
[10] Al-Mefty O., Fox J.L., Smith R.R. Petrosal approach for petroclival meningiomas. Neurosurgery. 1988;22:510-517.
[11] Al-Mefty O., Anad V.K. Zygomatic approach to the skull-base lesions. J Neurosurg. 1990;73:668-673.
[12] Lang J. Anatomy of the middle cranial fossa with reference to the subtemporal approach, transtentorial approach, and middle fossa approach. In: Sammi M., Draf W., editors. Surgery of the Skull Base. Berlin: Springer-Verlag; 1989:72-89.
[13] Samii M., Gustavo C., Marcos T., Cordula M. Surgical management of meningiomas originating in Meckel’s cave. Neurosurgery. 1997;41:767-775.
[14] Honeybul S., Neil-Dwyer G., Lang D.A., et al. Sphenoid wing meningioma en plaque: A clinical review. Acta Neurochir. 2001;143:749-758.
[15] Sakata K., Al-Mefty O., Yamamoto I. Venous consideration in petrosal approach: Microsurgical anatomy of the temporal bridging vein. Neurosurg. 2000;47:153-161.
[16] Sugita K., Kobayashi S., Yokoo A. Preservation of large veins during brain retraction. J Neurosurg. 1982;57:856-858.
[17] Sakata K., Murata H., Tanabe Y., et al. Preoperative simulation using a new generation 3DCTA in brain tumor surgery CT preoperative surgical simulation. In: Kuroiwa T., editor. Brain Tumor Surgery. Osaka, Japan: Medica Shuppan; 2007:136-141.
[18] Fukushima T. Manual of Skull Base Dissection. Pittsburgh: AF NeuroVideo, 1996.