Chapter 48 Middle Fossa Approach
 Videos corresponding to this chapter are available online at www.expertconsult.com.
Videos corresponding to this chapter are available online at www.expertconsult.com.
The middle fossa approach for vestibular nerve section was reported in 1904; however, hammer and chisel were used at that time, which put the facial nerve at risk.1 The middle fossa approach did not have widespread application until refined by the senior author (W.F.H.) in 1961.2 The approach was used initially for decompression of the internal auditory canal (IAC) in cases of extensive otosclerosis. That therapy was later abandoned, but it became evident that this approach was suitable for removal of acoustic tumors.
Initially, the middle fossa approach was used for tumors of all sizes. Further experience showed that it was most suitable for small tumors,3–5 however, and that preservation of hearing and facial nerve function was possible in a significant proportion of operated patients.6 The middle fossa approach was used infrequently until the development of gadolinium-enhanced magnetic resonance imaging (MRI). With this development, a larger number of acoustic tumors are diagnosed when they are small and before hearing has been significantly affected, making an attempt at hearing preservation desirable.
The middle fossa approach provides complete exposure of the contents of the IAC, allowing removal of laterally placed tumors without the need for blind dissection.7 This exposure ensures total removal and is well suited for the removal of very small acoustic tumors.8 The facial nerve can be located in its bony canal, allowing positive identification in a location not involved by tumor.
The middle fossa approach is technically difficult because of the lack of robust landmarks and the limited exposure. Bleeding in the posterior fossa can be difficult to control because of the limited access. Because of its location in the superior aspect of the IAC, the facial nerve is subjected to more manipulation in this approach than in other approaches.9,10 In the past, facial nerve results in middle fossa cases have not been as good as results from the translabyrinthine approach for similar-sized tumors.11 The routine use of the facial nerve monitor has helped improve these results, however, and now the results are comparable between the two approaches.12
Several authors use an extended middle fossa approach for large tumors.13–15 The tentorium is divided to give wider access to the posterior fossa. Some authors also perform a labyrinthectomy to enlarge the exposure when hearing preservation is not attempted.16–18
INDICATIONS
The primary indications for the middle fossa approach are a small acoustic tumor, in the IAC or with moderate extension into the cerebellopontine angle, and good preoperative hearing. In contrast, the retrosigmoid approach is used for patients with good hearing and small tumors located mostly in the cerebellopontine angle, without extension into the lateral aspect of the IAC. For hearing conservation surgery, we use the arbitrary audiometric criteria of speech reception threshold of better than 50 dB and speech discrimination score of better than 50%, although these indications must be individualized to the needs of the patient.19
Some patients have a subjective assessment about the usefulness of the preoperative hearing, such as the ability to use the telephone with the involved ear or the ability to localize a sound source. These subjective valuations should also be taken into consideration when determining hearing preservation candidacy. Some authors advocate attempting hearing preservation in the removal of small acoustic tumors if any measurable preoperative hearing exists.20 Patients older than 65 years do not tolerate the middle fossa approach as well as younger patients because of the fragility of the dura and retraction of the temporal lobe.
PREOPERATIVE EVALUATION
Several preoperative factors may predict postoperative hearing preservation. The most obvious is tumor size. Intuitively, the smaller the tumor, the easier it is to remove, and the more likely that hearing will be saved. This trend has been substantiated by several authors.11,21,22 Some authors have also found that the better the preoperative hearing, the more likely it will be preserved,23,24 whereas others have failed to identify such a relationship.11,22,25 Also, an intact preoperative stapedial reflex has been associated with successful postoperative hearing preservation.23
Several authors have reported a relationship between preoperative auditory brainstem response (ABR), and audiometry and hearing preservation.17,22 In one report, hearing was preserved in 78% of patients with an interaural wave V latency difference of 0.4 ms or less.11 For latency differences of 0.5 to 2 ms, the hearing preservation rate decreased to 58%. In patients with no response on the ABR, postoperative measurable hearing remained in only 50%. Patients with a more normal preoperative ABR result apparently have a greater success rate for postoperative hearing preservation. This result may reflect less tumor involvement of the cochlear nerve. Others do not find preoperative ABR to be predictive of hearing outcome.26,27
Tumors arising from the superior vestibular nerve have a higher rate of hearing preservation than tumors arising from the inferior vestibular nerve. Acoustic tumors developing in the inferior portion of the IAC may involve the cochlear nerve earlier and more severely.27,28 In a series of middle fossa acoustic tumor removals, 68% of patients whose tumors were found intraoperatively to arise from the superior vestibular nerve had measurable hearing preservation, whereas only 43% of patients whose tumors originated from the inferior vestibular nerve had measurable postoperative hearing.11 This difference was statistically significant.
Preoperative electronystagmography may predict tumor origin and hearing preservation. The caloric response reflects superior vestibular nerve function. In the presence of a small acoustic neuroma, a normal caloric response indicates an inferior vestibular nerve tumor, whereas a decreased response suggests a tumor arising from the superior vestibular nerve. Of a group of 54 patients who had preoperative electronystagmography, hearing was preserved in 64% with hypoactive caloric responses, whereas postoperative hearing remained in only 45% of patients with normal caloric responses.11 The association of normal caloric tests with nonpreservation of hearing has also been reported by others,10,17 although some series have not found this correlation.29 Vestibular evoked myogenic potentials may prove useful in determining the nerve of tumor origin. Early experience indicates that a combination of normal electronystagmography and a reduced vestibular evoked myogenic potentials predicts the inferior vestibular nerve as the nerve of origin.
For intracanalicular tumors, the radiographic appearance may predict success at hearing preservation. Small tumors that enlarge the IAC have a poorer prognosis for hearing preservation (Jackler RK, personal communication, 1990). In our experience, these small tumors that expand the canal are very adherent to the cochlear nerve, which adversely affects the hearing outcome. Fast spin echo MRI provides ultra-high-resolution images of IAC anatomy.30 With this imaging technique, it is possible to determine the nerve of origin for small tumors. Tumors that are impacted into the lateral end of the IAC, especially tumors that impinge on the cochlear nerve canal, have a lower rate of hearing preservation.31
PATIENT COUNSELING
After a thorough discussion of the relevant anatomy and the necessity to treat acoustic tumors, the options regarding surgical approaches to remove acoustic tumors are described to the patient. For patients with small tumors and good preoperative hearing, the issues of hearing preservation are discussed. We tell such patients that there is approximately a 50% chance of saving hearing. It is also important for patients to understand that hearing rarely improves after tumor removal.32 If the preoperative electronystagmography and ABR data are available and are favorable (see earlier), patients are informed that the prognosis for hearing preservation is above average.
Patients with preoperative tinnitus are counseled that the problem will likely get better, but probably will not disappear. Patients with no preoperative tinnitus have approximately a 25% chance of developing it postoperatively.33 Other important possible but rare complications are discussed, including cerebrospinal fluid leak, meningitis, serious brain complications, death, and blood transfusion options. The patient can donate 1 U of autologous blood before surgery, although transfusion is rarely needed.
SURGERY
Preoperative Preparation
Intraoperative furosemide and mannitol are given to allow easier temporal lobe retraction. A single dose of corticosteroid such as dexamethasone is routinely used intravenously at the beginning of surgery. This single dose of steroid does not seem to affect wound healing adversely. Long-acting muscle relaxants are avoided during surgery so as not to interfere with facial nerve monitoring. Preoperative antibiotics are administered. (Chapter 1 of this text details surgical site preparation and draping, along with the instruments used, including the House-Urban middle fossa retractor.)
Intraoperative ABR is routinely used. One of us (D.E.B.) also uses direct CN VIII recordings.34
Surgical Anatomy
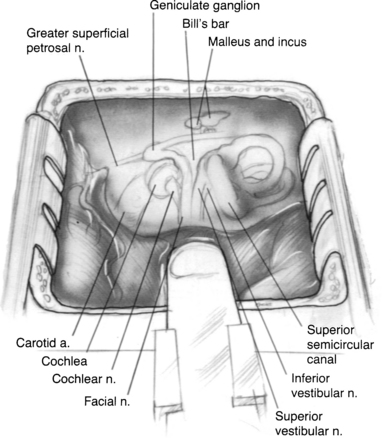
The surgical anatomy of the temporal bone from the middle fossa approach is compact and complex (Fig. 48-1). Landmarks are not as apparent as with other approaches through the temporal bone, so laboratory dissection is useful for the surgeon to become familiar with the anatomy from above.
Anteriorly, the limit of the dissection is the middle meningeal artery, which is lateral to the greater superficial petrosal nerve. The arcuate eminence marks the position of the superior semicircular canal and may be readily apparent in some patients, but obscure in others. Kartush and coworkers35 cautioned that the relationship between the arcuate eminence and the superior semicircular canal may be variable in some patients, but the superior canal tends to be perpendicular to the petrous ridge. Medially, the superior petrosal sinus runs along the petrous ridge.
The IAC lies approximately on the same axis as the external auditory canal; this relationship is useful in orienting the surgical field.12 The more medial one progresses along the IAC, the more space exists around it,36 allowing for safe dissection in this area.
Several methods can be used to locate the IAC, and are reviewed in detail elsewhere.12,35 The technique of Garcia-Ibanez and Garcia-Ibanez37 provides a reliable and safe method to locate the IAC. It involves drilling on the bisection of the angle formed by the superior semicircular canal and greater superficial petrosal nerve. The IAC can be initially located in the “safe” medial area of the temporal bone and followed laterally.
Surgical Technique
The patient is placed in the supine position with the head turned to the side. The surgeon is seated at the head of the table, and the anesthesiologist is at the foot. An incision is made in the pretragal area and extended superiorly in a gently curving fashion (Fig. 48-2). The midportion of the incision curves posteriorly, which keeps it in the hair of most patients with male pattern baldness. An inferiorly based U-shaped flap is fashioned of the temporalis muscle and fascia, and is reflected inferiorly.
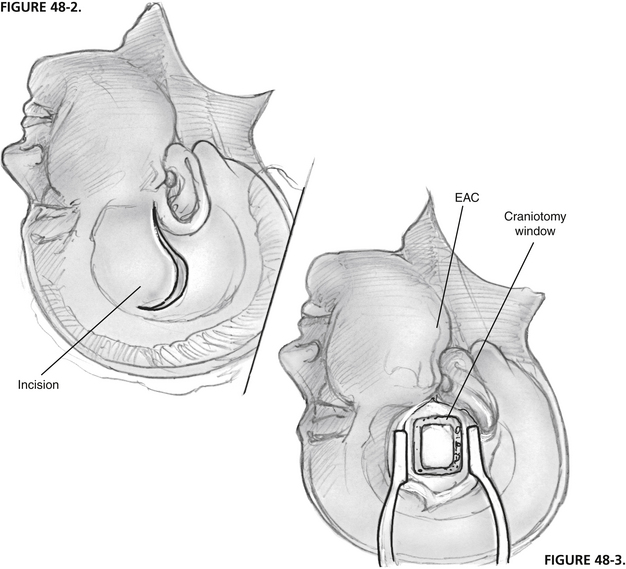
FIGURE 48-2 Incision begins in pretragal area and extends 7 to 8 cm superiorly in gently curving fashion.
FIGURE 48-3. Two thirds of craniotomy window is located anterior to external auditory canal (EAC).
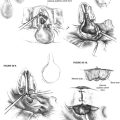
A craniotomy opening is made in the squamous portion of the temporal bone (Fig. 48-3). It is located approximately two thirds anterior and one third posterior to the external auditory canal and is approximately 5 cm by 5 cm. Anterior placement of the craniotomy is important for adequate exposure, especially for a left ear. This bone flap is based on the root of the zygoma as close to the floor of the middle fossa as possible. The craniotomy flap can be fashioned using a high-speed drill with a footplate attachment that protects the underlying dura.
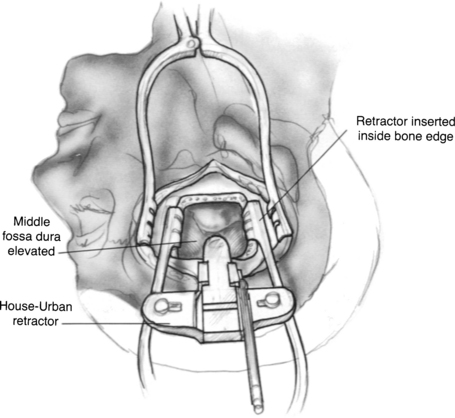
The arcuate eminence and greater superficial petrosal nerve are identified. These are the major landmarks to the subsequent intratemporal dissection. When the dura has been elevated, typically with a suction-irrigator and a blunt dural elevator, the House-Urban retractor is placed over the medial ridge of the superior petrosal sinus (true ridge) to support the temporal lobe. The tip of the retractor is placed beneath the true petrous ridge. To maintain a secure position, the teeth of the retaining retractor should be locked against the bony margins of the craniotomy window, and the tip of the retractor should be placed beneath the true petrous ridge (Fig. 48-4). One of us (C.S.) uses a Layla retractor system, which offers a lower profile than the House-Urban. Two retractor blades are employed to support the widely elevated temporal lobe.38 The tip of the anterior blade is placed into the groove of the fifth cranial nerve, and the tip of the posterior blade is placed medial to the petrous ridge near the arcuate eminence.
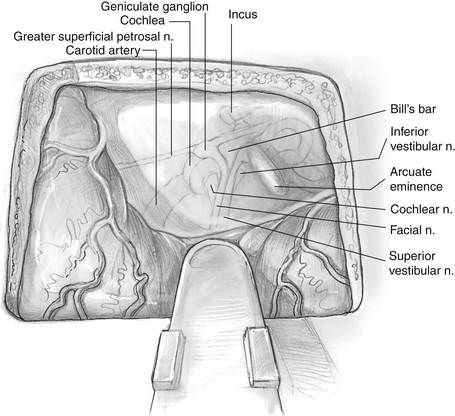
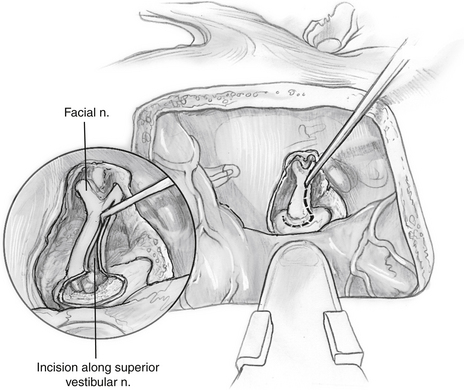
The greater superficial petrosal nerve is located medial to the middle meningeal artery (Fig. 48-5). Using a large diamond drill and continuous suction-irrigation, the superior semicircular canal is identified. As the superior semicircular canal is skeletonized and followed anteriorly, the geniculate ganglion can be identified. Bone is removed at the medial aspect of the petrous ridge at the bisection of the angle formed by the greater superficial petrosal nerve and the superior semicircular canal. The IAC is identified in this medial location and traced laterally. The dura of the posterior fossa is widely exposed (2 cm), and the circumference of the porus acusticus is exposed for approximately 270 degrees (Fig. 48-6). The posterior fossa dura can be opened with a microblade (No. 59; Beaver Company) to release the cerebrospinal fluid. This relaxes the temporal lobe and facilitates the removal of the retractors to allow an unimpeded view of the lateral end of the IAC. As the dissection proceeds laterally, it must narrow to about 90 degrees because of the encroachment of the cochlea and superior semicircular canal. At the lateral end of the IAC, Bill’s bar and the labyrinthine facial nerve are exposed.
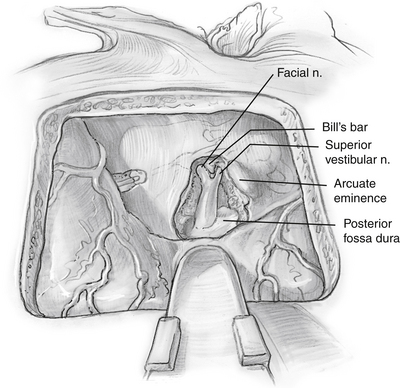
The dura of the IAC is divided along the posterior aspect (Fig. 48-7). The facial nerve is clearly identified in the anterior portion of the IAC.
The superior vestibular nerve is divided at its lateral end, and the tumor is separated from the facial nerve using high magnification (Fig. 48-8). The tumor is separated initially at Bill’s bar, but all dissection along the facial nerve is from medial to lateral. The arachnoid is divided, and the edge of the facial nerve is identified. The facial-vestibular anastomosis is sharply cut. Following the border of the facial nerve can facilitate tracing its course. Intracapsular debulking is performed, if needed, using microscissors and cup forceps. Tumor removal is accomplished from a medial-to-lateral direction to prevent traction on the cochlear nerve and blood supply as it enters the modiolus. If uninvolved, the inferior vestibular nerve can be left in an effort to preserve the labyrinthine artery. Persistent unsteadiness from a partial vestibulopathy can occur in patients with a retained inferior vestibular nerve. To avoid persistent unsteadiness, we recommend cutting the inferior vestibular nerve medial to Scarpa’s ganglion, but not dissecting it at the fundus of the IAC.
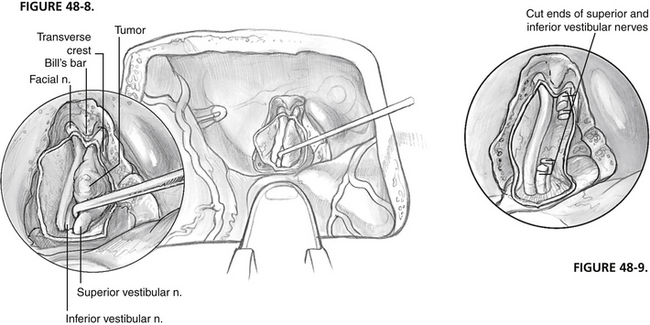
FIGURE 48-8 Intracanalicular acoustic tumor is dissected from facial and cochlear nerves.
FIGURE 48-9. Divided vestibular nerves are visible after tumor removal.
After irrigation of the tumor bed (Fig. 48-9) and establishment of hemostasis, papaverine-soaked Gelfoam is placed on the cochlear nerve to prevent vasospasm. The first author (C.S.) also places methylprednisolone (Solu-Medrol)–soaked Gelfoam on the facial nerve to decrease postoperative edema. Abdominal fat is used to close the defect in the IAC. The retractor is removed, and the temporal lobe is allowed to re-expand. The craniotomy flap is replaced. The wound is closed with absorbable subcutaneous sutures. A mastoid-type pressure dressing is maintained for 4 days postoperatively.
RESULTS
Success Rate
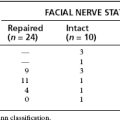
In a series of 106 patients over 25 years with tumors removed through the middle fossa approach, hearing was preserved in 59% and preserved near the preoperative level in 35% of cases.11 Hearing preservation rates have improved owing to technical improvements (wider bony exposure of medial IAC, medial-to-lateral tumor dissection, use of high magnification, and use of topical papaverine)39 and increased experience because of more frequent diagnosis of smaller tumors owing to improved imaging. A more recent series of 333 middle fossa acoustic tumor removals performed over a 7 year period yielded a hearing preservation rate of 80% with hearing preserved near the preoperative level (within 15 dB pure tone average and 15% speech discrimination score) in 50%.29 At 1 year, 95% of patients had normal or near-normal facial nerve results (House-Brackmann grade I or II).27
Complications
Postoperative complications are uncommon in reported middle fossa series. Postoperative cerebrospinal fluid leakage occurs in 2% to 7% of patients, and meningitis occurs in 2% to 5%.11,27 Not all of the postoperative meningitis cases were culture proven. There was a single patient with an epidural hematoma reported in one series.39
Postoperative seizures have been reported in only two patients from one series.22 Electroencephalographic studies in both of these patients were consistent with an ipsilateral temporal lobe source for the epileptic activity, which was believed to result from temporal lobe retraction. These complications are best avoided by limited temporal lobe retraction of short duration (1 to 1.5 hours).
1. Parry R.H. A case of tinnitus and vertigo treated by division of the auditory nerve. J Laryngol Otol. 1991;19:402.
2. House W.F. Surgical exposure of the internal auditory canal and its contents through the middle cranial fossa. Laryngoscope. 1961;71:1363.
3. House W.F. Middle cranial fossa approach to the petrous pyramid: Report of 50 cases. Arch Otolaryngol Head Neck Surg. 1963;78:460.
4. House W.F., Gardner G., Hughes R.L. Middle cranial fossa approach to acoustic tumor surgery. Arch Otolaryngol Head Neck Surg. 1968;88:631.
5. Kurze T., Doyle J.B.Jr. Extradural intracranial (middle fossa) approach to the internal auditory canal. J Neurosurg. 1962;19:1033.
6. House F., Hitselberger W.E. The middle fossa approach for removal of small acoustic tumors. Acta Otolaryngol (Stockh). 1969;67:413.
7. Wade P.J., House W.F. Hearing preservation in patients with acoustic neuromas via the middle fossa approach. Otolaryngol Head Neck Surg. 1984;92:184.
8. Shelton C., Hitselberger W.E. The treatment of small acoustic tumors: Now or later? Laryngoscope. 1991;101:925.
9. Brackmann D.E. Middle cranial fossa approach. In: House W.F., Luetje C.M., editors. Acoustic Tumors Management. Baltimore: University Park Press; 1979:15. vol. 2
10. Glasscock M.E., Poe D.S., Johnson G.D. Hearing preservation in surgery of cerebellopontine angle tumors. In: Fisch U., Valavanis A., Yasargil M.G., editors. Neurological Surgery of the Ear and Skullbase. Amsterdam: Kugler & Ghedini; 1989:207.
11. Shelton C., Brackmann D.E., House W.F., et al. Middle fossa acoustic tumor surgery: Results in 106 cases. Laryngoscope. 1989;99:405.
12. Arriaga M.A., Luxford W.M., Berliner K.I. Facial nerve function following middle fossa and translabyrinthine acoustic tumor surgery: A comparison. Am J Otol. 1994;15:620-624.
13. Dautheribes M., Migueis A., Vital J.M., et al. Anatomical basis of the extended subtemporal approach to the cerebellopontine angle: Its value and limitations. Surg Radiol Anat. 1989;11:187.
14. Rosomoff H.L. The subtemporal transtentorial approach to the cerebellopontine angle. Laryngoscope. 1971;81:1448.
15. Wigand M.E., Haid T., Berg M. The enlarged middle cranial fossa approach for surgery of the temporal bone and of the cerebellopontine angle. Arch Otorhinolaryngol. 1989;246:299.
16. Bochenek Z., Kukwa A. An extended approach through the middle cranial fossa to the internal auditory meatus and the cerebellopontine angle. Acta Otolaryngol (Stockh). 1975;80:410.
17. Kanzaki J., Ogawa K., Shiobara R., et al. Hearing preservation in acoustic neuroma surgery and postoperative findings. Acta Otolaryngol (Stockh). 1989;107:474.
18. Shiobara R., Ohira T., Kanzaki J., et al. A modified extended middle cranial fossa approach for acoustic nerve tumors: Results of 125 operations. J Neurosurg. 1988;68:358.
19. Shelton C., Brackmann D.E., House W.F., et al. Acoustic tumor surgery: Prognostic factors in hearing conservation. Arch Otolaryngol Head Neck Surg. 1989;115:1213.
20. Gantz B.J., Parnes L.S., Harker L.A., et al. Middle cranial fossa acoustic neuroma excision: Results and complications. Ann Otol Rhinol Laryngol. 1986;95:454.
21. Frerebeau P.H., Benezech J., Uziel A., et al. Hearing preservation after acoustic neurinoma operation. Neurosurgery. 1987;21:197.
22. Glasscock M.III, McKennan K.X., Levine S.C. Acoustic neuroma surgery: The results of hearing conservation surgery. Laryngoscope. 1987;97:785.
23. Josey A.F., Glasscock M.E., Jackson C.G. Preservation of hearing in acoustic tumor surgery: Audiologic indicators. Ann Otol Rhinol Laryngol. 1988;97:626.
24. Nadol J.B.Jr., Levine R., Ojemann R.G., et al. Preservation of hearing in surgical removal of acoustic neuromas of the internal auditory canal and cerebellar pontine angle. Laryngoscope. 1987;97:1287.
25. Mangham CA, Skalabrin TA: Indications for hearing preservation in acoustic tumor surgery. Kona, Hawaii, Presented at the Annual Meeting of the American Neurotology Society, May 4, 1991.
26. Kemink J.L., LaRouere M.J., Kileny R.P., et al. Hearing preservation following suboccipital removal of acoustic neuromas. Laryngoscope. 1990;100:597.
27. Slattery W.H., Brackmann D.E., Hitselberger W.E. Middle fossa approach for hearing preservation with acoustic neuromas. Am J Otol. 1997;18:596-601.
28. Glasscock M.E., Woods C.I., Jackson C.G., et al. Management of bilateral acoustic tumors. Laryngoscope. 1989;99:475.
29. Brackmann D.E., Owens R.M., Friedman R.A., et al. Prognostic factors for hearing preservation in vestibular schwannoma surgery. Am J Otol. 2000;21:417-424.
30. Shelton C., Harnsberger H.R., Allen R., King B. Fast spin echo magnetic resonance imaging: Clinical application in screening for acoustic neuroma. Otolaryngol Head Neck Surg. 1996;114:71-76.
31. Dubrulle F., Ernst O., Vincent C., et al. Cochlear fossa enhancement at MR evaluation of vestibular schwannoma: Correlation with success at hearing-preservation surgery. Radiology. 2000;215:458-462.
32. Shelton C., House W.F. Hearing improvement after acoustic tumor removal. Otolaryngol Head Neck Surg. 1990;103:963-965.
33. Berliner K.I., Shelton C., Hitselberger W.E., Luxford W.M. Acoustic tumors: Effect of surgical removal on tinnitus. Am J Otol. 1992;13:13.
34. Roberson J., Senne A., Brackmann D., et al. Direct cochlear nerve action potentials as an aid to hearing preservation in middle fossa acoustic neuroma resection. Am J Otol. 1996;17:653-657.
35. Kartush J.M., Kemink J.L., Graham M.D. The arcuate eminence: Topographic orientation in middle cranial fossa surgery. Ann Otol Rhinol Laryngol. 1985;94:25.
36. Parisier S.C. The middle cranial fossa approach to the internal auditory canal: An anatomical study stressing critical distances between surgical landmarks. Laryngoscope. 1977;87(Suppl 4):1.
37. Garcia-Ibanez E., Garcia-Ibanez J.L. Middle fossa vestibular neurectomy: A report of 373 cases. Otolaryngol Head Neck Surg. 1980;88:486.
38. Chen D., Arriaga M., Fukushima T. Technical refinements in retraction for middle fossa surgery. Am J Otol. 1998;19:208-211.
39. Brackmann D.E., House J.R., Hitselberger W.E. Technical modifications to the middle fossa craniotomy approach in removal of acoustic neuromas. Am J Otol. 1994;15:614-619.