CHAPTER 25 Midcarpal Instability
The concept of midcarpal instability (MCI) has unfolded over many years. It represents several distinct clinical entities that differ in their cause and direction of subluxation but share the common characteristic of abnormal force transmission at the midcarpal joint. Lichtman and Wroten consolidated the various forms into intrinsic and extrinsic types. The intrinsic types consist of palmar MCI (PMCI), dorsal MCI (DMCI), and combined forms.1 There are no arthroscopic findings that are characteristic of MCI, but wrist arthroscopy allows one to examine the chondral surfaces for abnormal wear and has a role in treatment for milder forms of MCI. Extrinsic MCI, as described by Taleisnik and Watson, is caused by a dorsally malunited distal radius fracture.2 This lesion is typically treated by a distal radius osteotomy to restore the normal palmar tilt and falls outside the scope of this discussion.
ANATOMY
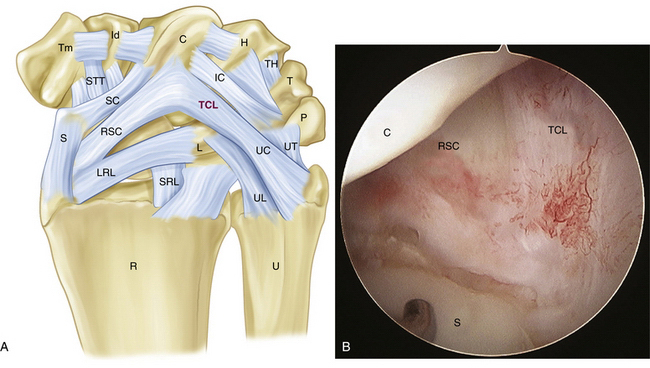
The palmar arcuate ligament complex comprises a radial arm, which is confluent with and distal to the radioscaphocapitate ligament, and an ulnar arm, the triquetrohamate-capitate ligament (TCL) (Fig. 25-1). In the normal situation, the proximal carpal row moves smoothly, from a flexed position when the wrist is in radial deviation to extension when the wrist is in ulnar deviation. This is possible because of the progressive tightening effect of the arcuate ligament as it stretches out to length, incrementally pulling the midcarpal row into extension, and the carpal bone geometry, which causes the triquetrum to translate dorsally along the helicoidal facet of the hamate. If the arcuate ligament is attenuated, this synchronous motion is lost.3
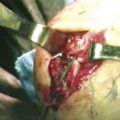
Studies by Trumble and colleagues4 and Viegas and coworkers5 showed that sectioning of the TCL or the dorsal radiocarpal ligament (DRCL), also known as the dorsal radiotriquetral ligament, could produce a volar intercalated segmental instability (VISI) deformity and simulate PMCI. Lichtman and colleagues showed in vivo that tightening the DRCL alone can stabilize the proximal carpal row and eliminate the clunk of PMCI, emphasizing the potential importance of dorsal ligament laxity in the pathogenesis of this disorder.6 Lichtman believed that PMCI is caused by laxity of the TCL and DRCL, which allows an excessive palmar sag of the heads of the capitate and hamate at the midcarpal joint. This produces a VISI pattern of the proximal row in the nonstressed wrist. This sag results in a loss of joint contact across the midcarpal joint, which manifests clinically as a loss of the smooth transition of the proximal row from flexion to extension as the wrist deviates ulnarward. The proximal carpal row stays in a flexed position until the terminal extent of ulnar deviation, when the helicoidal shape of the hamate facet suddenly forces the triquetrum to move dorsally. This snaps the lunate, and subsequently the scaphoid, into extension, causing a sudden reversal of the VISI. This sudden proximal row extension is responsible for the painful and rapid catch-up clunk that occurs. As the wrist moves back to neutral position, the triquetrum translates down the hamate facet, allowing the proximal row to drop back into VISI while the distal row again settles palmarly into its slightly subluxated starting point (Fig. 25-2).
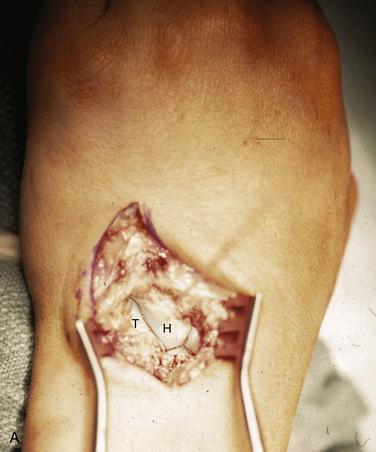
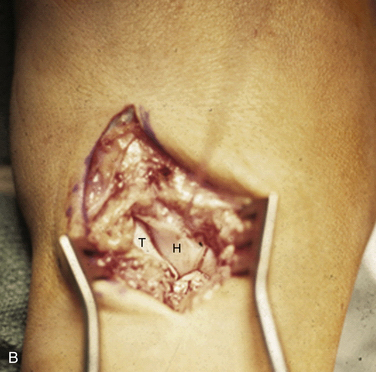
FIGURE 25-2 Dorsal exposure of the midcarpal joint in a left wrist. H, hamate, T, triquetrum. A, Triquetrohamate joint with the proximal row reduced. B, Subluxed triquetrohamate joint with volar sag of the proximal row. H, hamate, T, triquetrum.
(Courtesy of David J. Slutsky, MD, Torrance, California.)
The dorsal pattern of MCI has not been studied as extensively. It appears that laxity of the radial arm of the palmar arcuate ligament permits the capitate and hamate to translate dorsally to an excessive degree, especially with ulnar deviation of the wrist.7,8 In the palmar and dorsal patterns, the proximal row always moves into extension, and the distal row translates dorsally with ulnar deviation. It is the timing and force of this movement that differentiates the two patterns. In PMCI, the distal carpal row starts out in palmar subluxation with the wrist in neutral position. As the wrist moves into ulnar deviation, the subluxation suddenly corrects. In DMCI, the wrist starts out in a reduced position in neutral. Dorsal subluxation of the distal row then occurs with ulnar deviation. In either case, the instability is caused primarily by laxity of the selected extrinsic carpal ligaments that support the proximal row, which prevents them from controlling the complex kinematic relationships among the articular surfaces across the midcarpal joint.
PATIENT EVALUATION
History and Physical Examination
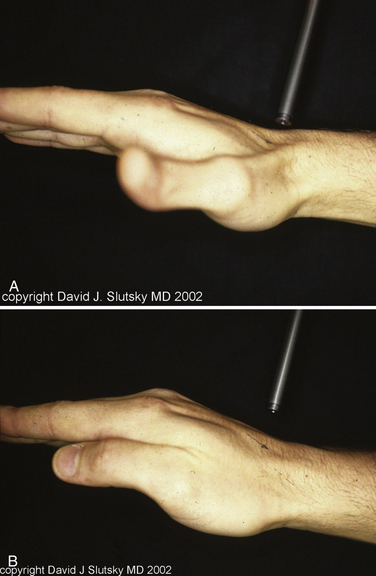
In PMCI, the patient presents with a history of clunking of the wrist. Patients can often reproduce the clunk on both sides, because generalized ligamentous laxity frequently coexists. The patient may have a trivial injury that accentuates this normal laxity, resulting in a painful clunk. On physical examination, close inspection reveals a sag of the midcarpal joint with the wrist in radial deviation, which is reduced with active or passive ulnar deviation (Fig. 25-3). The clunk may be reproduced by performing the midcarpal shift test.3 This test is performed by placing the patient’s wrist in neutral position with the forearm in pronation. A palmar force is then applied to the hand at the level of the distal capitate. The wrist is simultaneously loaded and deviated ulnarly. The test result is positive if a painful clunk occurs that reproduces the patient’s symptoms.
Diagnostic Imaging
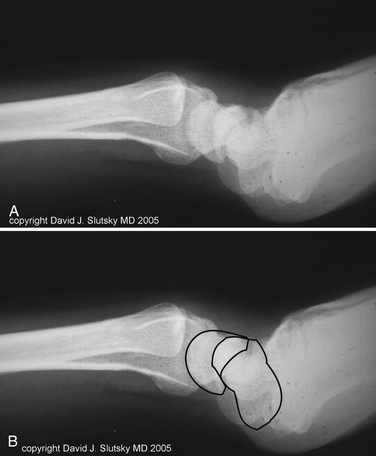
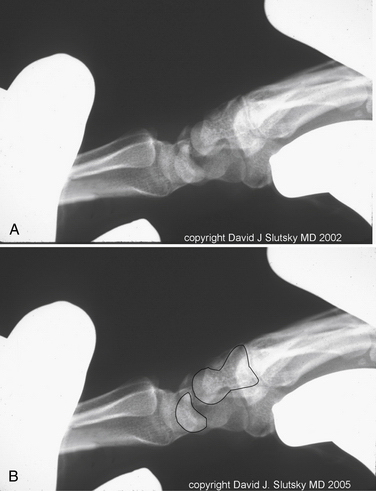
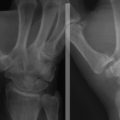
In dynamic DMCI, the radiographs are usually normal. In chronic cases, they often show a VISI pattern (Fig. 25-4). The capitolunate displacement test shows dorsal subluxation of the proximal carpal row in addition to dorsal subluxation of the capitate from the lunate (Fig. 25-5).9 This effect led Louis and colleagues to coin the term capitolunate instability pattern (i.e., CLIP wrist).10
Nonoperative Management
Nonsurgical treatment consists of activity modification, nonsteroidal anti-inflammatory medication (NSAIDs), and splinting.11 Various pisiform support splints have been described. They are based on the observation that application of dorsally directed pressure under the pisiform reduces the carpal sag along with the VISI position of the carpal row. Applying this principle, a three-point dynamic splint may maintain the reduction while permitting wrist motion in milder cases (Fig. 25-6). The splint may be worn full time for 6 to 8 weeks to reduce the midcarpal synovitis and then as needed.
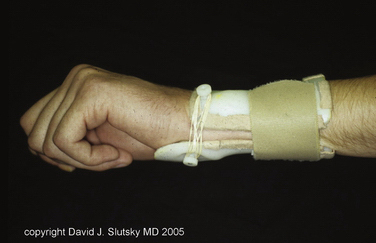
FIGURE 25-6 Three-point fixation with a dynamic splint.
(Courtesy of David J. Slutsky, MD, Torrance, California.)
Lichtman and Wroten observed that active co-contraction of the extensor carpi ulnaris, flexor carpi ulnaris, and hypothenar muscles can reduce the sagging of the midcarpal joint. Some patients can eliminate the catch-up clunk by contracting these muscles before ulnar deviation of the wrist. Patients are taught this isometric muscle contraction as a part of the therapy program.1 However, definitive treatment of this condition ultimately requires surgical treatment.
TREATMENT
Indications and Contraindications
A variety of open soft tissue procedures have been generally ineffective for severe forms of MCI12,13; hence, arthroscopic treatment should be approached with caution.
Arthroscopic Technique
Arthroscopic Capsular Shrinkage
Although thermal capsular shrinkage has not enjoyed great success in the shoulder, its role in the treatment of wrist disorders remains promising. Thermal energy unwinds the collagen triple helix in capsular and ligamentous structures, with subsequent healing in a shortened or tightened position. The temperature required to achieve the effect is about 70 to 80 degrees; to avoid tissue ablation, the temperature should not exceed 100 degrees. Tissue repair occurs by vascular invasion and fibroblastic activity. In vitro, capsular tissue has been shown to shrink by 9% to 50%, depending on the probe and the type of tissue. After shrinkage, collagen stiffness is reduced to approximately 20% of normal; it returns to normal by about 2 months.14 This concept has led to the use of these techniques as a treatment option for MCI of the wrist.
The patient is placed in a supine position on the operating table. After exsanguination of the limb, the tourniquet is inflated to 250 mm Hg. A 2.7-mm, 30-degree angle arthroscope, along with a fiberoptic light source and camera setup, are used. Some type of diathermy unit is used to create the thermal shrinkage. Large-bore outflow cannulas are desirable to provide rapid joint irrigation and minimize the risk of chondral damage through heat necrosis. With the use of a tower, 10 pounds of traction is applied to the index and long fingers. The radiocarpal joint is inflated 1 cm distal to Lister’s tubercle at the 3-4 portal, and the 2.7-mm arthroscope is introduced. Outflow is established through the 6-R portal. The standard dorsal portals, including a 3-4 and a 4-5 portal, are used for an arthroscopic survey. Any associated triangular fibrocartilage tears or lunotriquetral ligament tears are identified and treated by débridement or repair. The ulnar extrinsic ligaments are assessed for laxity. If laxity is observed, a 1.5-mm electrothermal probe (Arthrocare, Sunnyvale, CA; or Oratec, Menlo Park, CA) is introduced through the 6-R portal. The ulnolunate and ulnotriquetral ligaments are painted with the probe using a stripe technique, leaving sections of untouched ligament in between. The correction of any associated VISI deformity is assessed with a combination of arthroscopy and fluoroscopy.
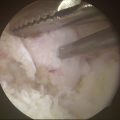
A midcarpal radial portal is then established. The scapholunate and lunotriquetral joints are inspected and probed for laxity. The TCL is identified as it runs obliquely from the triquetrum, across the proximal corner of the hamate, to the palmar neck of the capitate. A midcarpal ulnar portal is established and used for introduction of the thermal probe. The TCL is then shrunk while the tension is again adjusted with correction of any VISI deformity (Fig. 25-7).
A volar radial portal is established by making a 2-cm longitudinal incision in the proximal wrist crease, exposing the flexor carpi radialis tendon sheath. The sheath is divided, and the tendon is retracted ulnarly. The radiocarpal joint space is identified with a 22-gauge needle, and the joint is inflated with saline. A blunt trocar and cannula are introduced through the floor of the flexor carpi radialis sheath, which overlies the interligamentous sulcus between the radioscaphocapitate ligament and the long radiolunate ligament. A 2.7-mm, 30-degree arthroscope is inserted through the cannula. The DRCL can be seen ulnar to the 3-4 portal and underneath the lunate. If laxity is present, the electrothermal probe is introduced through the 3-4 and 4-5 portals and used to shrink the DRCL (Fig. 25-8). The tension of the DRCL can be adjusted by correcting any VISI deformity with a Kirschner wire in the lunate under fluoroscopic control. At the end of the procedure, 0.045-mm Kirschner wires are used to pin the triquetrum to the capitate and hamate in a neutral and reduced position.
Arthroscopic Findings
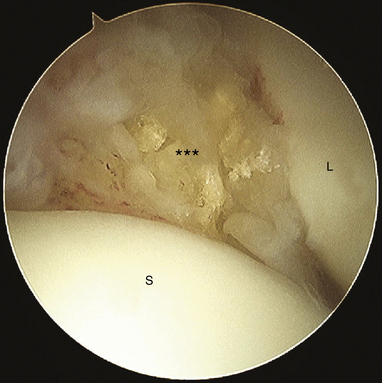
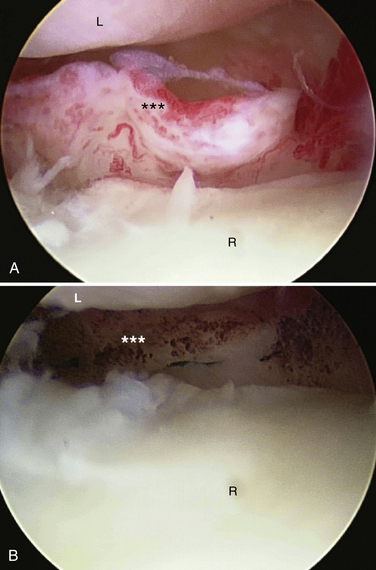
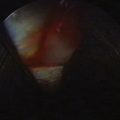
There are no arthroscopic findings that are diagnostic of MCI. Inspection of the radiocarpal joint may reveal a nonspecific synovitis. I have seen an associated tear of the DRCL (see Fig. 25-8). In this case, an arthroscopic DRCL repair failed to correct the MCI.15 Inspection of the midcarpal row may demonstrate erosive lesions along the triquetrum (Fig. 25-9) and the hamate, or both. Laxity of the lunotriquetral ligament may be seen though this is not invariable. Midcarpal arthroscopy may reveal laxity of the TCL (Fig. 25-10).
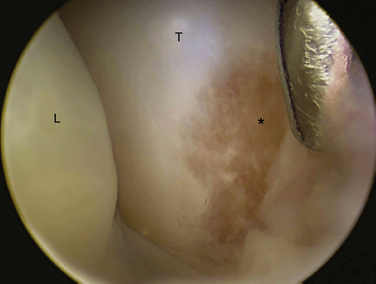
FIGURE 25-9 Erosion of the triquetrum (asterisk) is seen from the midcarpal ulnar portal. L, lunate; T, triquetrum.
(Courtesy of David J. Slutsky, MD, Torrance, California.)
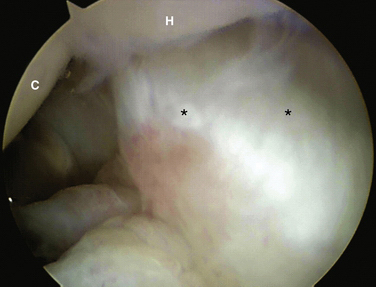
FIGURE 25-10 Laxity of the triquetrohamate-capitate ligament (asterisks). C, capitate; H, hamate.
(Courtesy of David J. Slutsky, MD, Torrance, California.)
CONCLUSIONS
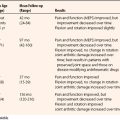
There is a paucity of literature on the use of arthroscopy for MCI. In 2003, Lichtman and associates reported their experience of five patients who underwent an arthroscopic capsular shrinkage.6 The average age was 33 years (range, 29 to 57 years). Follow-up averaged 9 months (range, 3 to 18 months). The midcarpal clunk resolved in six of the eight patients with pain resolution. Range of motion decreased by 20% in the flexion/extension plane, whereas grip strengths increased by an average of 15%.
Mason and Hargreaves16 reported the results of a prospective study of 13 patients (15 wrists) with painful wrist clunking due to PMCI who underwent arthroscopic thermal capsulorrhaphy after at least 6 months of failed conservative treatment. The mean duration of symptoms was 5 years (range, 8 months to 20 years). Preoperatively, patients were evaluated clinically and by fluoroscopic examination, which confirmed a positive ulnar shift test. A thermal probe was applied to the ulnar arm of the arcuate ligament (i.e., the ulnocapitate, ulnotriquetral, and triquetrocapitate ligaments) and to the radial arm (i.e., radioscaphocapitate, long and short radiolunate ligaments, and accessible parts of the dorsal capsule in the radiocarpal and midcarpal joints). Postoperatively, the patients’ wrists were splinted for 6 weeks. Patients were evaluated at a mean follow-up of 42 months (range, 14 to 67 months) by means of the ulnar shift test; grip strength; range of motion; the Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire; and a structured questionnaire that included a question on their ability to pour a heavy kettle without wrist clunking. Complete resolution of symptoms occurred in 4 patients and almost complete resolution in the remaining 11 patients. Functional improvement was confirmed by an improvement in the mean DASH score from 34 (range, 13-67; SD 16) preoperatively to 12 (range, 0-48; SD 14) at final follow-up. The ulnar shift test was negative in 12 of 14 wrists that were available for examination. Wrist movement was reduced by a mean of 16 degrees in flexion and 10 degrees in extension in 9 wrists compared with the opposite side, but there was no reduction in grip strength.
1. Lichtman DM, Wroten ES. Understanding midcarpal instability. J Hand Surg Am. 2006;31:491-498.
2. Taleisnik J, Watson HK. Midcarpal instability caused by malunited fractures of the distal radius. J Hand Surg Am. 1984;9:350-357.
3. Lichtman DM, Schneider JR, Swafford AR, Mack GR. Ulnar midcarpal instability: clinical and laboratory analysis. J Hand Surg Am. 1981;6:515-523.
4. Trumble TE, Bour CJ, Smith RJ, Glisson RR. Kinematics of the ulnar carpus related to the volar intercalated segment instability pattern. J Hand Surg Am. 1990;15:384-392.
5. Viegas SF, Patterson RM, Peterson PD, et al. Ulnar-sided perilunate instability: an anatomic and biomechanic study. J Hand Surg Am. 1990;15:268-278.
6. Lichtman DM, Culp RW, Joshi A. Palmar midcarpal instability. In: McGinty E, editor. Operative Arthroscopy. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2003:737-742.
7. Johnson RP, Carrera GF. Chronic capitolunate instability. J Bone Joint Surg Am. 1986;68:1164-1176.
8. Apergis EP. The unstable capitolunate and radiolunate joints as a source of wrist pain in young women. J Hand Surg Br. 1996;21:501-506.
9. White SJ, Louis DS, Braunstein EM, et al. Capitate-lunate instability: recognition by manipulation under fluoroscopy. AJR Am J Roentgenol. 1984;143:361-364.
10. Louis DS, Hankin FM, Greene TL. Chronic capitolunate instability. J Bone Joint Surg Am. 1987;69:950-951.
11. Lichtman DM, Gaenslen ES, Pollock GR. Midcarpal and proximal carpal instabilities. In: Lichtman DM, Alexander AH, editors. The Wrist and Its Disorders. Philadelphia, PA: WB Saunders; 1997:316-328.
12. Goldfarb CA, Stern PJ, Kiefhaber TR. Palmar midcarpal instability: the results of treatment with 4-corner arthrodesis. J Hand Surg Am. 2004;29:258-263.
13. Lichtman DM, Bruckner JD, Culp RW, Alexander CE. Palmar midcarpal instability: results of surgical reconstruction. J Hand Surg Am. 1993;18:307-315.
14. Medvecky MJ, Ong BC, Rokito AS, Sherman OH. Thermal capsular shrinkage: basic science and clinical applications. Arthroscopy. 2001;17:624-635.
15. Slutsky D. Arthroscopic repair of dorsoradiocarpal ligament tears. J Arthrosc Rel Surg. 2005;21:1486e1-186e8.
16. Mason WT, Hargreaves DG. Arthroscopic thermal capsulorrhaphy for palmar midcarpal instability. J Hand Surg Eur. 2007;32:411-416.