CHAPTER 163 Microvascular Decompression for Trigeminal Neuralgia
History
TN has been known since ancient times. Two thousand years ago, Aretaeus of Cappadocia referred to headache with “spasm and distortion of countenance,” and Persian scholar Avicenna described a similar syndrome of facial pain in the 10th century.1 In the 17th century, Fehr and Schmidt described the syndrome in a eulogy.2 John Locke described facial pain of the Countess of Northumberland, wife of the English Ambassador to France, as a “fit of such violent and exquisite torment that it forced her to … cries and shrieks … which extended itself all over the right side of her face and mouth.”1 In 1756, Nicolas Andre coined the term tic douloureux, and described it as “a cruel and obscure illness, which causes … in the face some violent motions, some hideous grimaces which are an insurmountable obstacle to the reception of food, [and] which put off sleep.”3 The first comprehensive clinical description occurred in 1773 when John Fothergill wrote to the medical society of London about 14 patients with TN, and his descriptions of triggerable lancinating pain are still considered accurate.4
Early surgical treatments for TN involved intentional lesioning of the trigeminal nerve. In 1934, Walter Dandy described the retrosigmoid approach to the trigeminal nerve and noted frequent vascular contact in patients with TN. He wrote, “In many instances the nerve is grooved or bent in an angle by the artery. This I believe is the cause of tic douloureux.”5 Despite his insight, Dandy did not attempt to decompress the nerve, believing the pathologic process to be irreversible. Microvascular decompression (MVD) was first performed by W. James Gardner in 1959, who described mobilizing a vessel from the trigeminal nerve and placing a piece of Gelfoam between them without any intentional damage to the nerve itself.6 The procedure was subsequently refined and popularized by Peter Jannetta, who, with the aid of the operating microscope, performed thousands of MVD operations and demonstrated that long-term relief of pain is possible in most of appropriately selected patients.7–9
Pathophysiology
The etiology of TN is believed to be related to abnormal conduction within the trigeminal nerve, possibly owing to changes in myelin induced by pulsatile mechanical trauma from an adjacent vessel. At the point just before it enters the brainstem, there is a short segment where nerve axons are still ensheathed in central myelin (produced by oligodendrocytes), but after a few millimeters, there is a transition to peripheral myelin (produced by Schwann cells). The region of this transition is called the Obersteiner-Redlich zone. It is thought that the area of the nerve containing the central form of myelin is especially susceptible to pathologic changes from vascular contact that result in demyelination and altered conduction. Pathologic studies from patients with TN have demonstrated severe damage to myelin as well as axon loss within the nerve adjacent to the site of compression.10 The resulting conduction abnormality may lead to nerve hyperactivity owing to ectopic impulse discharge, spontaneous and triggered afterdischarge, and cross-excitation among neighboring afferent fibers (ephaptic transmission).10,11
Separation of the nerve from the offending vessel appears to immediately reverse many of the physiologic changes. In Leandri and colleagues’12 study of 10 patients undergoing MVD who underwent nerve root and scalp electrode recordings, 7 showed signs of immediate improvement in neurophysiologic parameters after decompression. Others authors have reported improvement in sensory thresholds for touch, pinprick, and temperature sensations after MVD13 as well as resolution of asymmetric jaw motion.14,15 These findings suggest that the changes associated with neurovascular compression are likely to be reversible if the nerve is decompressed, at least in the early stages.
Radiographic and anatomic studies have demonstrated that vascular contact with the trigeminal nerve is common even in asymptomatic individuals but tends to be more severe and more proximal on the nerve ipsilateral to TN symptoms.16 Patients with TN are more likely to have contralateral arterial compression than asymptomatic people even though bilateral TN is distinctly rare.16,17 Symptomatic and asymptomatic arterial compression of the trigeminal nerve increases with age because of elongation of cisternal arteries, which explains why it is primarily a disease of older adults.16
Alternative Treatments
TN symptoms often improve with medications that exert a stabilizing effect on neural conduction such as antiepileptics. Medications that have been successfully used include carbamazepine, phenytoin, valproate, gabapentin, pregabalin, baclofen, and clonazepam. Most patients obtain good pain control initially,18,19 but the effect tends to diminish over time, and after 10 years about half will no longer respond.20 Because the clinical and pathologic changes associated with TN may be progressive over time, initial failure of pharmacologic therapy may represent an indication to proceed with more aggressive treatment.21 Nevertheless, medical therapy is recommended as a first-line treatment for patients with TN because some patients will require no further treatment.
MVD differs from the other treatments in that the primary cause of TN is treated so that long-term pain relief is possible. Also, because there is no intentional damage to the nerve, facial dysesthesia and numbness are rare. Although it is the most invasive and expensive treatment, MVD is associated with the lowest rate of pain recurrence and the highest rate of patient satisfaction among all surgical treatments for TN.22 MVD can also be safely performed after a lesioning procedure and appears to be no less effective so long as there is no evidence of trigeminal neuropathy.23
Patient Selection and Classification of Facial Pain
MVD is ideal for young healthy patients with TN because no other treatment offers a significant likelihood of long-term pain relief.24 However, advanced age is not by itself a contraindication because there is no difference in complication rate or outcome in elderly patients.25–27 The operation is in fact technically easier in older patients because cerebellar atrophy leads to less need for retraction and less risk for cerebellar swelling. If life expectancy is very short or general anesthesia cannot be tolerated, a less invasive destructive procedure may be more appropriate.28,29
A careful history is essential during preoperative evaluation. Patients generally report intense stabbing or electric shock–like sensation, although there may be an overlying constant pain that may be more severe than the stabbing pain. Any distribution within the trigeminal nerve innervation territory may be observed. V2 and V3 branches are more common,7 especially radiating out from near the mouth. V1 symptoms are sometimes associated with decreased corneal sensation. The pain is often worse during the day and may be positional with relief when supine, with the affected side up, or during sleep. Trigger points are present in most patients and are activated by light cutaneous stimuli such as wind, eating, talking, and shaving. Often, the triggers lead to guarding of the face and refusal to be touched, wash, apply makeup, shave, or brush the teeth because of fear of an attack. Pain-free intervals lasting weeks to months are common at first but become rare as the syndrome progresses. Initial onset of pain is frequently quite memorable. Many patients undergo dental procedures without relief before a diagnosis is made. If the patient is given antiepileptic medication, pain usually improves dramatically. Physical examination is usually normal, although about one third of patients have some degree of sensory loss in the affected area.
When evaluating a patient for surgery, it is helpful to classify facial pain according to the classification scheme reported by Burchiel.30 Patients with TN type 1 have predominantly shock-like pain, whereas patients with TN type 2 report that at least 50% is constant pain, although there still may be a component of lancinating pain. Pain relief after MVD is more strongly correlated with the lancinating pain component than with any other symptom, so although most patients with either type will have long-term pain control, patients with TN type 1 are more likely to do well than those with TN type 2 (Miller and coworkers, unpublished data). Facial pain diagnoses other than TN are unlikely to improve after MVD. TN with a history of multiple sclerosis (MS) is called symptomatic trigeminal neuralgia (STN). MS is present in 1% to 3% patients with TN, and 2% to 4% of patients with MS have TN, probably due to intrinsic demyelination within the nerve or increased sensitivity to vascular trauma.31,32 Although MS patients sometimes improve after MVD, the recurrence rate is higher, and long-term elimination of pain is rare,33,34 so destructive procedures may be more appropriate for these patients.35 Sensory loss with burning pain is a sign of trigeminal neuropathic pain (TNP); if it occurs after a previous destructive procedure, this is called trigeminal deafferentation pain (TDP). Allodynia and dysesthesia with a history of herpes zoster suggest postherpetic neuralgia (PHN). MVD is not a good option for any of these patients. Atypical facial pain (AFP), which refers to pain of psychological onset, requires neuropsychological testing for the diagnosis and is unlikely to improve after MVD.
Preoperative Imaging
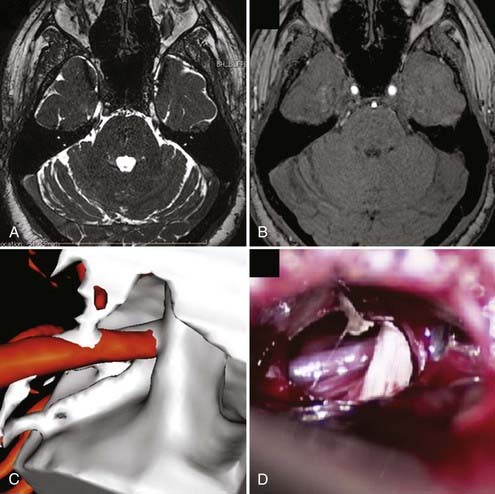
All patients should undergo magnetic resonance imaging (MRI) before MVD to verify that there is no intracranial mass lesion that may be responsible for the pain, such as a tumor, cyst, aneurysm, or arteriovenous malformation. MRI also can identify evidence of demyelinating disease or inflammatory changes and may reveal anatomic characteristics that would make MVD technically problematic, such as an ectopic basilar artery or bony abnormalities. High-resolution MRI using steady-state precession images can be used to identify neurovascular compression preoperatively with a high degree of accuracy, and fusion with magnetic resonance angiography followed by three-dimensional reconstruction can be used to simulate the intraoperative view for surgical planning (Fig. 163-1).36
Operative Technique
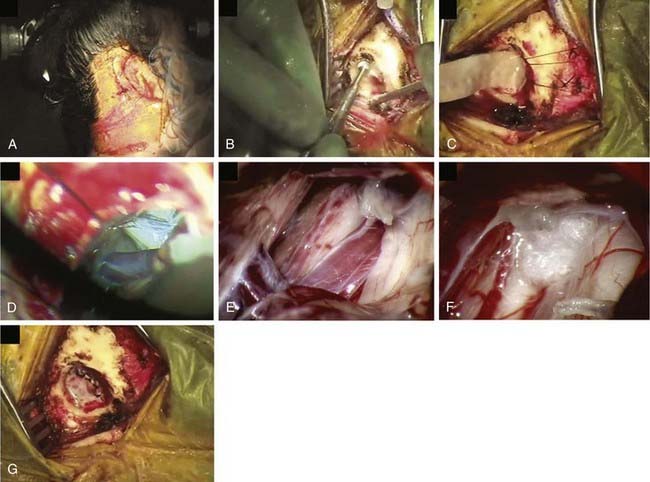
A cranial fixation device such as the three-pin Mayfield head holder is applied before positioning. There are several options for patient positioning for MVD. The simplest option is to place the patient in a flat supine position with the head rotated and flexed to the opposite side. Ideally, no shoulder roll should be used so that the ipsilateral shoulder does not obscure the operative approach. This position requires a flexible neck and may not work for obese or short-necked patients. Other options include the lateral decubitus or three-quarter prone position with the shoulder taped caudally and neck flexed, ensuring the chin is at least two fingerbreadths from the sternum. Regardless of the position chosen, it is generally best to place the vertex parallel to the floor so that the seventh and eighth cranial nerves are inferior relative to the trigeminal nerve, simplifying the approach.37 All pressure points are padded, and an axillary roll is used if necessary. The sitting position can also be used, although it is associated with complications such as air embolism and subdural hematoma.
The arachnoid over the nerve is carefully removed to allow complete inspection of the nerve. Thin, translucent arachnoid can be teased off, but thick, opaque bands must be sharply dissected to avoid injury to the trigeminal nerve, the trochlear nerve (a thin, delicate structure in the arachnoid above the trigeminal nerve), or small vascular branches in the subarachnoid space. Vascular compression is usually seen close to the brainstem, often anterior to the nerve. The site of compression may vary based on symptoms, with symptoms closer to V1 associated with compression of more caudolateral portions of the nerve. The arachnoid anterior to the nerve must be dissected to allow decompression. Endoscopic assistance has been advocated as a method of very thorough evaluation of the nerve with minimal cerebellar retraction.38–41 Some advocate use of a small mirror to visualize behind the nerve.
The artery that most frequently produces compression is the superior cerebellar artery (SCA), which often compresses the nerve anteromedially from within the axilla. Decompression requires elevation of the artery into a horizontal rather than vertical orientation, displacing it upward and away from the nerve. SCA often divides into two branches as it courses around the midbrain, either or both of which may compress the nerve. The anterior inferior cerebellar artery (AICA) may compress the nerve from below, requiring displacement more inferiorly away from the nerve. Sometimes both SCA and AICA are involved, surrounding the nerve like a pincer, in which case both must be mobilized and decompressed. Less commonly, the vertebral or basilar artery contacts the nerve, usually in hypertensive, elderly, and male patients.7 Small arterioles may be seen, more often in younger patients. Compression by a persistent trigeminal artery has also been described.42
Venous contact is frequently seen, often concomitantly with arterial compression. The vein may be anterior (transverse pontine vein), posterior (petrosal vein), or distal at the entrance to Meckel’s cave (trigeminal vein); frequently, compression is caused by one of the many unnamed veins that run along the brainstem.43,44 Posterior venous compression may be obscured by the ridge of the petrous bone. Compression from draining veins from a venous angioma or arteriovenous malformation is sometimes observed. If venous compression is identified, the vein is carefully dissected from the nerve and coagulated with low-voltage small bipolar forceps to prevent spread of current into the adjacent nerve, after which the vein is coagulated and divided.
After decompression, the vessels are carefully observed, and topical papaverine-soaked Gelfoam is used if there is any evidence of vasospasm. The operative field is liberally irrigated, retractors are removed, and a Valsalva maneuver is performed by anesthesia to ensure there is no bleeding. The dura is closed in a watertight manner using continuous or interrupted braided 4-0 sutures to prevent subsequent leakage of CSF. It is usually possible to close the dura primarily, but if necessary, a graft of cadaveric, synthetic, or autogenous tissue may be used. Fibrin dural sealant is helpful, especially in redo operations. A piece of Gelfoam is placed over the dura, and a cranioplasty of wire mesh and artificial bone material is fashioned, or the bone is replaced if a craniotomy was performed (Fig. 163-2). The fascia, subcutaneous tissue, and skin are closed in standard fashion using absorbable sutures.
Postoperative Care
After the operation, the patient must be observed overnight in the intensive care unit or stepdown unit.45 Blood pressure is monitored with an arterial line, and antihypertensives (labetalol or hydralazine) are administered if systolic blood pressure exceeds 160 mm Hg. Mild narcotics may be used for headache, which may persist for several weeks. Severe bifrontal headache warrants computed tomography (CT) to rule out posterior fossa hemorrhage, but postoperative imaging is otherwise not necessary. Nausea is common and usually responds to antiemetics. Steroids are not helpful. The patient is transferred to the ward on the first postoperative day, and diet and activity are gradually increased. Patients are generally discharged by the third postoperative day. After discharge, activity is gradually increased over a week, and most patients are able to return to work within 2 to 4 weeks. Half of all patients undergoing MVD return to baseline activity within 1 month, and 90% by 3 months.46
Complications
Complications from MVD are rare in experienced hands,7,24 and morbidity is lowest at high-volume centers.47 Cranial nerve damage is rare, and such complications as facial dysesthesia and anesthesia dolorosa are much less common than with the lesioning procedures. Many patients have a transient conductive hearing loss due to tracking of fluid from the mastoid into the middle ear that clears spontaneously within a few weeks. Sensorineural hearing loss tends to be permanent but can be prevented by gentle retraction, careful technique, and treatment of any AICA vasospasm that occurs. Brainstem auditory evoked potential monitoring is very helpful in preventing this complication and has diminished its incidence from 1.3% to 0.6%.37 Prolonged postoperative vertigo or tinnitus is reported in about 2% of patients,48 and some degree of facial nerve palsy occurs in up to 5% of patients.7 Both of these tend to improve spontaneously over a few weeks. Injury to the fourth cranial nerve produces a trochlear palsy that usually subsides after a few months.
CSF rhinorrhea, which occurs in 1.5% of cases,7 is caused by leakage of CSF through the dural opening, into the mastoid air cells, and through the pharyngotympanic tube into the nasopharynx. This complication occurs more often with reoperations and may be prevented by meticulous waxing of the mastoid, careful dural closure, and application of fibrin glue. CSF rhinorrhea is often successfully treated with 3 days of CSF drainage using a lumbar drain. If leakage continues, it may be necessary to reexplore the wound and close the fistula directly.
Results
Multiple investigators have found MVD to be an effective treatment for TN. In one study of 1204 patients, 75% had complete relief and 9% had partial relief after 1 year. After 10 years, 64% had complete relief, and 4% had partial relief. The annual rate of recurrence was less than 2% by 5 years and less than 1% by 10 years.7 Although initial results are similar, MVD offers a much higher likelihood than destructive procedures for a long-term cure. In one study comparing 378 MVD patients with 316 radiofrequency (RF) patients over 20 years, patients undergoing RF rhizotomy had a 75% chance of pain recurrence in the first 5 years. By contrast, 63% of MVD patients were pain free at 20 years.48
The most significant predictor of outcome after MVD appears to be type of TN pain. Patients with TN type 1 (mostly episodic, lancinating pain) tend to have much better immediate and long-term results than those with TN type 2 (mostly constant pain). This effect appears to be graded with the amount of lancinating pain, so that a greater proportion of lancinating pain leads to a better outcome.49 TN type is more predictive of outcome than any other clinical feature of TN, including duration of symptoms, presence of trigger points, response to antiepileptics, and type of compression (arterial versus venous) found at surgery.49 In one large study, 80% of patients with TN type 1 had some degree of pain relief at 5 years, compared with 51% of those with TN type 2.50 However, although outcomes for TN type 2 are less satisfactory than for TN type 1, MVD still benefits most patients, and no alternative interventions appear to offer a better chance of long-term relief.
Venous compression appears to predict worse outcome, possibly because of regrowth of veins. In one study of 393 patients treated by MVD for venous compression, the 1-year recurrence rate was 31%, and 28 of 32 undergoing re-exploration had evidence of recurrent venous contact.43 Venous compression is also more common in pediatric patients with TN. In one study, venous contact was found in 86% of pediatric TN patients undergoing MVD, and a vein was the sole offending vessel in 18%. This may explain why pediatric TN patients have worse results after MVD, with 10-year recurrence rates as high as 43%.51 Other predictors of recurrence include female gender, prolonged preoperative symptoms (>8 years), and failure of immediate postoperative relief.7,52–58 Severity of compression and focal arachnoiditis have also been associated with worse outcome.59
It is unclear whether MVD for recurrent TN after a previous destructive procedure is associated with a worse prognosis. The likelihood of pain relief appears to be similar,23 but patients who have undergone a lesioning procedure before MVD have a higher likelihood of postoperative trigeminal neuropathic symptoms (burning, aching pain).7 Some investigators have noted that lesioning procedures before an initial MVD may increase the likelihood of negative exploration during subsequent MVD operations for recurrent TN.60
Recurrent disease after a prolonged postoperative pain-free interval may be effectively treated with medication, but repeat MVD has also been advocated, even though it is associated with a much higher rate of negative exploration. Reoperations are technically more difficult, and if the first MVD was unsuccessful for technical reasons, it is unlikely that repeating the operation will yield better results. When neurovascular compression is seen during a repeat MVD, it is nearly always because of growth of veins or expansion of arteries rather than dislodging of the Teflon felt placed during the first operation, although scarring produced by the felt can produce neural compression. Reports of operative findings during repeat MVD vary widely; most investigators report recurrent vascular compression in about half of patients.61,62 Outcome after the second operation is generally good.53
Other Neurovascular Facial Pain Syndromes
Glossopharyngeal Neuralgia
Glossopharyngeal neuralgia presents as sharp severe pain in the throat or neck, sometimes radiating to or from the upper neck or ear. It is much less common than TN and more likely to be bilateral.63 Vagal involvement can lead to bradycardia, syncope, and even asystole. Like TN, it may be triggerable, and common triggers include cold beverages, yawning, chewing, coughing, sneezing, and touching the tragus. Because the lower cranial nerves exit the brainstem as a series of rootlets, attempted decompression with Teflon may lead only to worsening of the compression. Instead, the nerve is exposed through a retrosigmoid craniectomy (as with MVD for TN), and the glossopharyngeal nerve and the upper few fascicles of the vagus nerve are sectioned. Patients should be warned that postoperative temporary dysphagia is often seen. Potential complications include permanent diminished gag reflex and vocal cord paralysis. There are few long-term studies, but most reports indicate successful long-term outcome in most patients.64,65 In one study, 58% of patients had complete relief of symptoms, and another 18% had some relief, after a mean follow-up time of 4 years.45 Isolated throat pain appears to predict a greater likelihood of postoperative success.
Anderson VC, Berryhill PC, Sandquist MA, et al. High-resolution three-dimensional magnetic resonance angiography and three-dimensional spoiled gradient-recalled imaging in the evaluation of neurovascular compression in patients with trigeminal neuralgia: a double-blind pilot study. Neurosurgery. 2006;58:666-673.
Apfelbaum RI. Neurovascular decompression: the procedure of choice? Clin Neurosurg. 2000;46:473-498.
Ashkan K, Marsh H. Microvascular decompression for trigeminal neuralgia in the elderly: a review of the safety and efficacy. [see comment]. Neurosurgery. 2004;55;:840-848.
Barker FG2nd, Jannetta PJ, Bissonette DJ, et al. The long-term outcome of microvascular decompression for trigeminal neuralgia. [see comment]. N Engl J Med. 1996;334;:1077-1083.
Bonicalzi V, Canavero S. Role of microvascular decompression in trigeminal neuralgia [comment]. Lancet. 2000;355:928-929.
Broggi G, Ferroli P, Franzini A, et al. Microvascular decompression for trigeminal neuralgia: comments on a series of 250 cases, including 10 patients with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2000;68:59-64.
Broggi G, Ferroli P, Franzini A, et al. Operative findings and outcomes of microvascular decompression for trigeminal neuralgia in 35 patients affected by multiple sclerosis. Neurosurgery. 2004;55:830-838.
Burchiel KJ. A new classification for facial pain. Neurosurgery. 2003;53:1164-1166.
Burchiel KJ, Clarke H, Haglund M, Loeser JD. Long-term efficacy of microvascular decompression in trigeminal neuralgia. J Neurosurg. 1988;69:35-38.
Burchiel KJ, Slavin KV. On the natural history of trigeminal neuralgia. Neurosurgery. 2000;46:152-154.
Cho DY, Chang CG, Wang YC, et al. Repeat operations in failed microvascular decompression for trigeminal neuralgia. Neurosurgery. 1994;35:665-669.
Devor M, Govrin-Lippmann R, Rappaport ZH. Mechanism of trigeminal neuralgia: an ultrastructural analysis of trigeminal root specimens obtained during microvascular decompression surgery. J Neurosurg. 2002;96:532-543.
El-Garem HF, Badr-El-Dine M, Talaat AM, Magnan J. Endoscopy as a tool in minimally invasive trigeminal neuralgia surgery. Otol Neurotol. 2002;23:132-135.
Kalkanis SN, Eskandar EN, Carter BS, Barker FG2nd. Microvascular decompression surgery in the United States, 1996 to 2000: mortality rates, morbidity rates, and the effects of hospital and surgeon volumes. Neurosurgery. 2003;52:1251-1261.
Lee SH, Levy EI, Scarrow AM, et al. Recurrent trigeminal neuralgia attributable to veins after microvascular decompression. Neurosurgery. 2000;46:356-361.
Liao JJ, Cheng WC, Chang CN, et al. Reoperation for recurrent trigeminal neuralgia after microvascular decompression. Surg Neurol. 1997;47:562-568.
Matsushima T, Huynh-Le P, Miyazono M. Trigeminal neuralgia caused by venous compression. Neurosurgery. 2004;55:334-337.
McLaughlin MR, Jannetta PJ, Clyde BL, et al. Microvascular decompression of cranial nerves: lessons learned after 4400 operations. [see comment]. J Neurosurg. 1999;90;:1-8.
Miller J, Acar F, Hamilton B, Burchiel K. Preoperative visualization of neurovascular anatomy in trigeminal neuralgia. J Neurosurg. 2008;108:477-482.
Ogungbo BI, Kelly P, Kane PJ, Nath FP. Microvascular decompression for trigeminal neuralgia: report of outcome in patients over 65 years of age. [see comment][erratum appears in Br J Neurosurg 2000;14:504]. Br J Neurosurg. 2000;14;:23-27.
Rath SA, Klein HJ, Richter HP. Findings and long-term results of subsequent operations after failed microvascular decompression for trigeminal neuralgia. Neurosurgery. 1996;39:933-938.
Sindou M, Leston J, Decullier E, Chapuis F. Microvascular decompression for primary trigeminal neuralgia: long-term effectiveness and prognostic factors in a series of 362 consecutive patients with clear-cut neurovascular conflicts who underwent pure decompression. J Neurosurg. 2007;107:1144-1153.
Tatli M, Satici O, Kanpolat Y, Sindou M. Various surgical modalities for trigeminal neuralgia: literature study of respective long-term outcomes. Acta Neurochir (Wien). 2008;150:243-255.
Tronnier VM, Rasche D, Hamer J, et al. Treatment of idiopathic trigeminal neuralgia: comparison of long-term outcome after radiofrequency rhizotomy and microvascular decompression. Neurosurgery. 2001;48:1261-1267.
Tyler-Kabara EC, Kassam AB, Horowitz MH, et al. Predictors of outcome in surgically managed patients with typical and atypical trigeminal neuralgia: comparison of results following microvascular decompression. [see comment]. J Neurosurg. 2002;96;:527-531.
1 Stookey B, Ransohoff J. Trigerminal Neuralgia: Its History and Treatment. Springfield, IL: Charles C. Thomas; 1959.
2 Schmidt J. Medical Discoveries. Springfield, IL: Charles C. Thomas; 1959. Vol 503
3 Brown JA, Coursaget C, Preul MC, et al. Mercury water and cauterizing stones: Nicolas Andre and tic douloureux. J Neurosurg. 1999;90:977.
4 Fothergill J. Of a painful affection of the face. In: Medical Observation and Inquiries by a Society of Physicians in London. London: Society of Physicians; 1776:129.
5 Dandy W. Concerning the cause of trigeminal neuralgia. Am J Surg. 1934;24:447.
6 Gardner WJ. Concerning the mechanism of trigeminal neuralgia and hemifacial spasm. J Neurosurg. 1962;19:947.
7 Barker FG2nd, Jannetta PJ, Bissonette DJ, et al. The long-term outcome of microvascular decompression for trigeminal neuralgia. [see comment]. N Engl J Med. 1996;334;:1077.
8 Jannetta PJ. Arterial compression of the trigeminal nerve at the pons in patients with trigeminal neuralgia. J Neurosurg. Suppl 26. 1967:159.
9 Jannetta PJ, Rand RW. Transtentorial retrogasserian rhizotomy in trigeminal neuralgia by microneurosurgical technique. Bull Los Angeles Neurol Soc. 1966;31:93.
10 Devor M, Govrin-Lippmann R, Rappaport ZH. Mechanism of trigeminal neuralgia: an ultrastructural analysis of trigeminal root specimens obtained during microvascular decompression surgery. J Neurosurg. 2002;96:532.
11 Burchiel KJ. Abnormal impulse generation in focally demyelinated trigeminal roots. J Neurosurg. 1980;53:674.
12 Leandri M, Eldridge P, Miles J. Recovery of nerve conduction following microvascular decompression for trigeminal neuralgia. Neurology. 1998;51:1641.
13 Miles JB, Eldridge PR, Haggett CE, et al. Sensory effects of microvascular decompression in trigeminal neuralgia. J Neurosurg. 1997;86:193.
14 Jannetta PJ. Treatment of trigeminal neuralgia by suboccipital and transtentorial cranial operations. Clin Neurosurg. 1977;24:538.
15 Saunders RL, Krout R, Sachs EJr. Masticator electromyography in trigeminal neuralgia. Neurology. 1971;21:1221.
16 Miller JP, Acar F, Burchiel KJ. Radiographic evaluation of trigeminal neurovascular compression in patients with and without trigeminal neuralgia. J Neurosurg. 2009;110:627-632.
17 Anderson VC, Berryhill PC, Sandquist MA, et al. High-resolution three-dimensional magnetic resonance angiography and three-dimensional spoiled gradient-recalled imaging in the evaluation of neurovascular compression in patients with trigeminal neuralgia: a double-blind pilot study. Neurosurgery. 2006;58:666.
18 Campbell FG, Graham JG, Zilkha KJ. Clinical trial of carbazepine (Tegretol) in trigeminal neuralgia. J Neurol Neurosurg Psychiatry. 1966;29:265.
19 Rockliff BW, Davis EH. Controlled sequential trials of carbamazepine in trigeminal neuralgia. Arch Neurol. 1966;15:129.
20 Taylor JC, Brauer S, Espir ML. Long-term treatment of trigeminal neuralgia with carbamazepine. Postgrad Med J. 1981;57:16.
21 Burchiel KJ, Slavin KV. On the natural history of trigeminal neuralgia. Neurosurgery. 2000;46:152.
22 Tatli M, Satici O, Kanpolat Y, et al. Various surgical modalities for trigeminal neuralgia: literature study of respective long-term outcomes. Acta Neurochir. 2008;150:243.
23 Shetter AG, Zabramski JM, Speiser BL. Microvascular decompression after gamma knife surgery for trigeminal neuralgia: intraoperative findings and treatment outcomes. J Neurosurg. suppl. 2005:259.
24 Apfelbaum RI. Neurovascular decompression: the procedure of choice? Clin Neurosurg. 2000;46:473.
25 Ashkan K, Marsh H. Microvascular decompression for trigeminal neuralgia in the elderly: a review of the safety and efficacy. [see comment]. Neurosurgery. 2004;55;:840.
26 Jodicke A, Winking M, Deinsberger W, et al. Microvascular decompression as treatment of trigeminal neuralgia in the elderly patient. Minim Invasive Neurosurg. 1999;42:92.
27 Ogungbo BI, Kelly P, Kane PJ, et al. Microvascular decompression for trigeminal neuralgia: report of outcome in patients over 65 years of age. [see comment][erratum appears in Br J Neurosurg. 2000 Oct;14(5):504]. Br J Neurosurg. 2000;14;:23.
28 Mullan S, Lichtor T. Percutaneous microcompression of the trigeminal ganglion for trigeminal neuralgia. J Neurosurg. 1983;59:1007.
29 Sweet WH, Poletti CE. Problems with retrogasserian glycerol in the treatment of trigeminal neuralgia. Appl Neurophysiol. 1985;48:252.
30 Burchiel KJ. A new classification for facial pain. Neurosurgery. 2003;53:1164.
31 Jensen TS, Rasmussen P, Reske-Nielsen E. Association of trigeminal neuralgia with multiple sclerosis: clinical and pathological features. Acta Neurol Scand. 1982;65:182.
32 Love S, Gradidge T, Coakham HB. Trigeminal neuralgia due to multiple sclerosis: ultrastructural findings in trigeminal rhizotomy specimens. Neuropathol Applied Neurobiol. 2001;27:238.
33 Broggi G, Ferroli P, Franzini A, et al. Operative findings and outcomes of microvascular decompression for trigeminal neuralgia in 35 patients affected by multiple sclerosis. Neurosurgery. 2004;55:830.
34 Eldridge PR, Sinha AK, Javadpour M, et al. Microvascular decompression for trigeminal neuralgia in patients with multiple sclerosis. Stereotact Funct Neurosurg. 2003;81:57.
35 Bonicalzi V, Canavero S. Role of microvascular decompression in trigeminal neuralgia. [comment]. Lancet. 2000;355;:928.
36 Miller J, Acar F, Hamilton B, et al. Preoperative visualization of neurovascular anatomy in trigeminal neuralgia. J Neurosurg. 2008;108:477.
37 McLaughlin MR, Jannetta PJ, Clyde BL, et al. Microvascular decompression of cranial nerves: lessons learned after 4400 operations. [see comment]. J Neurosurg. 1999;90;:1.
38 Abdeen K, Kato Y, Kiya N, et al. Neuroendoscopy in microvascular decompression for trigeminal neuralgia and hemifacial spasm: technical note. Neurol Res. 2000;22:522.
39 El-Garem HF, Badr-El-Dine M, Talaat AM, et al. Endoscopy as a tool in minimally invasive trigeminal neuralgia surgery. Otol Neurotol. 2002;23:132.
40 Jarrahy R, Berci G, Shahinian HK. Endoscope-assisted microvascular decompression of the trigeminal nerve. Otolaryngol Head Neck Surg. 2000;123:218.
41 Rak R, Sekhar LN, Stimac D, et al. Endoscope-assisted microsurgery for microvascular compression syndromes. Neurosurgery. 2004;54:876.
42 Tamura Y, Shimano H, Kuroiwa T, et al. Trigeminal neuralgia associated with a primitive trigeminal artery variant: case report. [see comment]. Neurosurgery. 2003;52;:1217.
43 Lee SH, Levy EI, Scarrow AM, et al. Recurrent trigeminal neuralgia attributable to veins after microvascular decompression. Neurosurgery. 2000;46:356.
44 Matsushima T, Huynh-Le P, Miyazono M. Trigeminal neuralgia caused by venous compression. Neurosurgery. 2004;55:334.
45 Romansky K, Stoianchev N, Dinev E, et al. Results of treatment of trigeminal neuralgia by microvascular decompression of the Vth nerve at its root entry zone. Arch Physiol Biochem. 1998;106:392.
46 Lovely TJ, Lowry DW, Jannetta PJ. Functional outcome and the effect of cranioplasty after retromastoid craniectomy for microvascular decompression. Surg Neurol. 1999;51:191.
47 Kalkanis SN, Eskandar EN, Carter BS, et al. Microvascular decompression surgery in the United States, 1996 to 2000: mortality rates, morbidity rates, and the effects of hospital and surgeon volumes. Neurosurgery. 2003;52:1251.
48 Tronnier VM, Rasche D, Hamer J, et al. Treatment of idiopathic trigeminal neuralgia: comparison of long-term outcome after radiofrequency rhizotomy and microvascular decompression. Neurosurgery. 2001;48:1261.
49 Miller JP, Magill ST, Acar F, et al. Predictors of long-term success after microvascular decompression for trigeminal neuralgia. J Neurosurg. 2009;110:620-626.
50 Tyler-Kabara EC, Kassam AB, Horowitz MH, et al. Predictors of outcome in surgically managed patients with typical and atypical trigeminal neuralgia: comparison of results following microvascular decompression. [see comment]. J Neurosurg. 2002;96;:527.
51 Resnick DK, Levy EI, Jannetta PJ. Microvascular decompression for pediatric onset trigeminal neuralgia. Neurosurgery. 1998;43:804.
52 Barba D, Alksne JF. Success of microvascular decompression with and without prior surgical therapy for trigeminal neuralgia. J Neurosurg. 1984;60:104.
53 Bederson JB, Wilson CB. Evaluation of microvascular decompression and partial sensory rhizotomy in 252 cases of trigeminal neuralgia. J Neurosurg. 1989;71:359.
54 Broggi G, Ferroli P, Franzini A, et al. Microvascular decompression for trigeminal neuralgia: comments on a series of 250 cases, including 10 patients with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2000;68:59.
55 Burchiel KJ, Clarke H, Haglund M, et al. Long-term efficacy of microvascular decompression in trigeminal neuralgia. J Neurosurg. 1988;69:35.
56 Kolluri S, Heros RC. Microvascular decompression for trigeminal neuralgia. A five-year follow-up study. Surg Neurol. 1984;22:235.
57 Puca A, Meglio M, Cioni B, et al. Microvascular decompression for trigeminal neuralgia: prognostic factors. Acta Neurochir Suppl. 1993;58:165.
58 Sun T, Saito S, Nakai O, et al. Long-term results of microvascular decompression for trigeminal neuralgia with reference to probability of recurrence. Acta Neurochir. 1994;126:144.
59 Sindou M, Leston J, Decullier E, et al. Microvascular decompression for primary trigeminal neuralgia: long-term effectiveness and prognostic factors in a series of 362 consecutive patients with clear-cut neurovascular conflicts who underwent pure decompression. J Neurosurg. 2007;107:1144.
60 Rath SA, Klein HJ, Richter HP. Findings and long-term results of subsequent operations after failed microvascular decompression for trigeminal neuralgia. Neurosurgery. 1996;39:933.
61 Cho DY, Chang CG, Wang YC, et al. Repeat operations in failed microvascular decompression for trigeminal neuralgia. Neurosurgery. 1994;35:665.
62 Liao JJ, Cheng WC, Chang CN, et al. Reoperation for recurrent trigeminal neuralgia after microvascular decompression. Surg Neurol. 1997;47:562.
63 Patel NK, Aquilina K, Clarke Y, et al. How accurate is magnetic resonance angiography in predicting neurovascular compression in patients with trigeminal neuralgia? A prospective, single-blinded comparative study. Br J Neurosurg. 2003;17:60.
64 Kondo A. Follow-up results of using microvascular decompression for treatment of glossopharyngeal neuralgia. J Neurosurg. 1998;88:221.
65 Resnick DK, Jannetta PJ, Bissonnette D, et al. Microvascular decompression for glossopharyngeal neuralgia. Neurosurgery. 1995;36:64.