CHAPTER 192 Microtia Reconstruction
Microtia occurs with an overall frequency of approximately 1 to 3 cases per 10,000 population,1 although certain ethnic groups, such as Navaho Indians in Mexico and the Japanese, may have higher incidence of microtia.2 Microtia deformities appear to be more common on the right side and affect boys more often than girls at roughly a 2.5 : 1 ratio; unilateral cases outnumber bilateral cases by 4 : 1. Surgical management of microtia remains one of the greatest reconstructive challenges for an otolaryngologist.3–6 Auricular reconstruction of the microtia deformity is a complex and labor-intensive process that requires careful preoperative planning, surgical skill, and artistry. This chapter provides a basic overview of clinical issues in children with microtia and reconstruction with autologus rib cartilage. Discussion of aural atresia is presented elsewhere.
Embryology
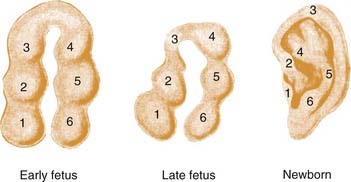
The inner ear begins as the otic placode at approximately the third week of gestation, and it then forms into the otic pit and otic cyst. The external ear begins to form in the fifth week of gestation. The auricle begins as six small buds of mesenchyme, classically known as the hillocks of His, surrounding the dorsal end of the first branchial cleft.7–10 On either side of the first branchial cleft are the first (mandibular) and second (hyoid) branchial arches. Hillocks 1 through 3 arise from the first branchial arch, and hillocks 4 through 6 arise from the second branchial arch. Conventional teaching holds that the first hillock forms the tragus, the second and third form the helix, the fourth and fifth form the antihelix, and the sixth forms the antitragus. The majority of the central ear is derived from hillocks 4 and 5, and the lobule appears to be one of the last parts of the ear to develop (Fig. 192-1). It has been estimated that the hyoid arch forms about 85% of the auricle.5 The exact embryology of each of these hillocks remains unclear. The position of the auricular complex begins at the anterior neck region. As the mandible develops during gestational weeks 8 through 12, the auricular complex moves in dorsal and cephalic directions. The etiology of microtia remains unknown in most cases, although the inciting event that arrests development of the ear must occur during the first trimester, and may be related to abnormal neural crest cell migration.
Known teratogens, such as the retinoic acid derivative isotretinoin (Accutane; Roche, Nutley, New Jersey), can produce forms of microtia in conjunction with craniofacial, cardiac, thymic, and CNS anomalies, a condition commonly known as retinoic embryopathy.11 Thalidomide and mycophenolate mofetil also have been reported to be associated with microtia.12,13 Microtia is associated with other anomalies about 50% of the time. Microtia is most commonly associated with other regional anomalies related to the first and second branchial arches, i.e., facioauriculovertebral syndrome. Within the facioauriculovertebral spectrum are malformations and syndromes that affect the first and second branchial arch derivatives, such as Goldenhar’s syndrome (preauricular nodes, epibulbar dermoids, mandibular hypoplasia), hemifacial microsomia,14 and oculoauricular vertebral dysplasia (microtia, cervical spine anomalies, and epibulbar dermoids).15,16 It may be that Goldenhar’s syndrome, hemifacial microsomia, oculoauriculovertebral dysplasia, and microtia are simply variants of the same entity.4 Microtia also may be seen in syndromes such as Treacher Collins syndrome, which has an autosomal dominant inheritance pattern.
Ear Anatomy
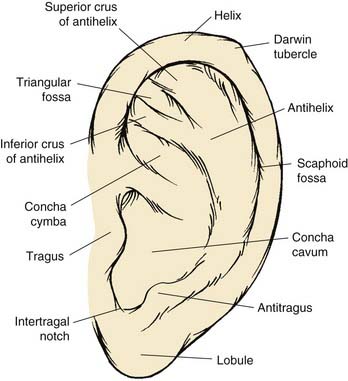
The anatomic contour of the normal ear must first be reviewed in order to understand the nature and extent of microtic ear deformities (Fig. 192-2). The auricle is an aesthetic sculpture of complex convexities, concavities, and curved lines such as the helix and the antihelix, which are smooth and uninterrupted. The elastic cartilage framework of the auricle is usually pliable yet structurally strong and extraordinarily resistant to trauma. The skin of the ear is fibrofatty and loose over the lobule and the margin of the helix but thin and fixed over the remaining cartilage framework.
Normal adult ear height ranges from 5.5 to 6.5 cm, which is typically attained by 13 years of age in boys and 12 years of age in girls. The ear goes through rapid growth between 2 and 3 years of age and reaches 95% of its adult size by about age 5. Thereafter it grows at a modest rate until it reaches its adult size during early teenage years. The horizontal width of the ear is achieved at a much earlier age. Protrusion of the ear from the surface of the mastoid should be 1.5 to 2.0 cm, thereby creating an angle between 15 and 20 degrees.17 The superior margin of the helix is at the level of the tail of the eyebrow in 85% of people. Because the tail of the eyebrow is also quite variable, the level of the upper eyelid also may be used as a landmark if the patient has bilateral microtia.18 The ear inclination is the angle formed by the vertical axis of the face and the longitudinal axis of the ear with the patient in the Frankfort horizontal position. This is approximately 24.8 degrees, with a standard deviation of 6.2 degrees.19 Therefore, the longitudinal axis of the ear is not quite parallel with the dorsum of the nose (as is commonly thought), because the ear is in a slightly more vertical orientation.
Microtia
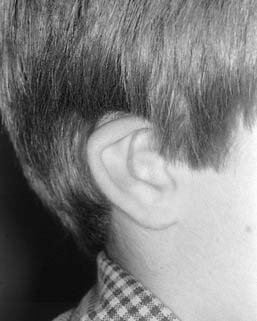
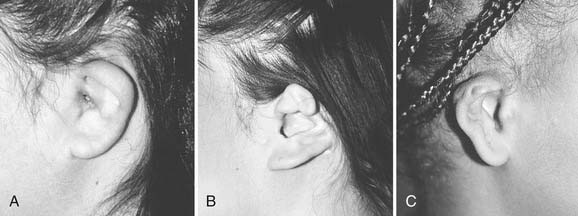
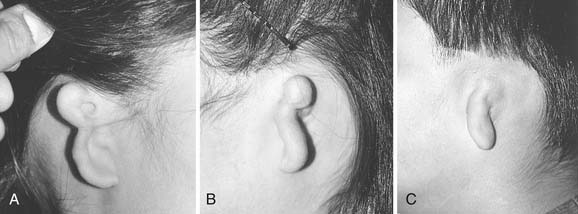
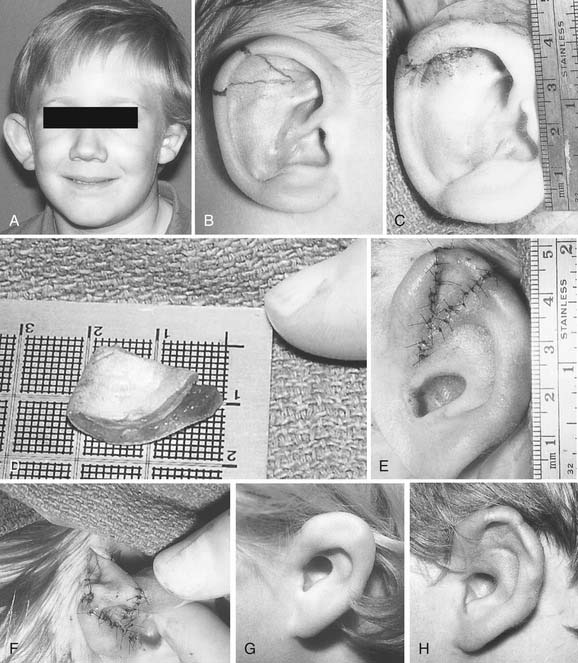
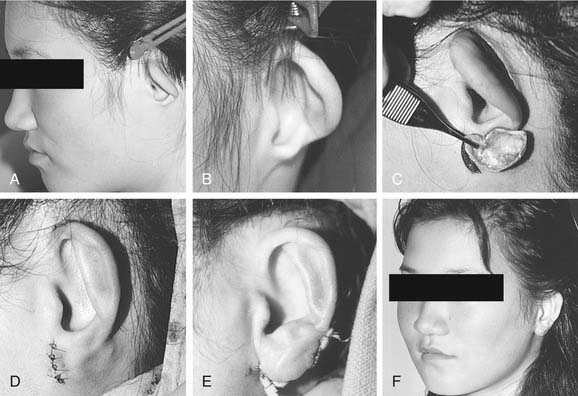
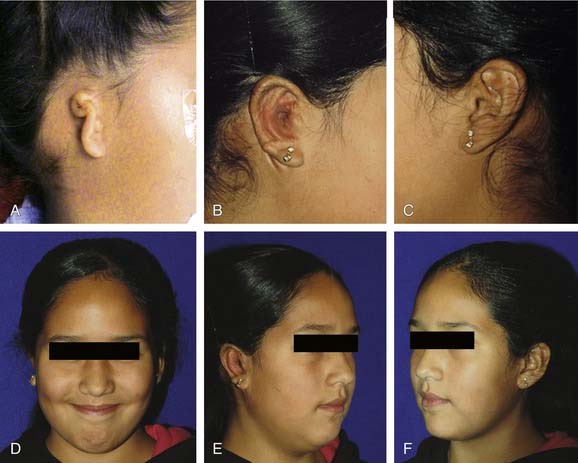
Microtia deformities come in various shapes and sizes, and unfortunately, there is currently no universal, precise, and accurate classification scheme that is clinically useful. Classification schemes are further complicated if the status of the external auditory canal (EAC) and middle ear contents and the presence or absence of an associated syndrome are included.5,20 Classification usually is simplified by evaluating the external auricular deformities alone. Perhaps dysplasia is a better term than microtia to use in describing ear deformities. Dysplasia deformities typically are divided into three broad and simple categories that have a great deal of overlap.21 Type I (Fig. 192-3) is used to designate ears with mild deformity. All major structures are present to some degree, and reconstruction does not require additional tissue. Type II (atypical microtia) dysplasia (Fig. 192-4A to C) includes ears that have all major structures present to some degree. There is a deficiency of tissue, and surgical correction requires the addition of cartilage and skin. Mini-ear and severe cup-ear deformities are included in this category. The external auditory meatus is present, but it may demonstrate some degree of stenosis. Type III (classic microtia) dysplasia (Fig. 192-5A to C) deformities have few or no recognizable landmarks of the auricle, although the lobule usually is present and positioned anteriorly (see Fig. 192-16). Anotia is the complete absence of auricle and lobule. Reconstructive surgeons may find it more useful to simply note the status of the helix and antihelix, the conchal bowl, the lobule, the tragus, and the EAC, because these landmarks dictate the stages of ear reconstruction and the degree of surgical difficulty.
Planning
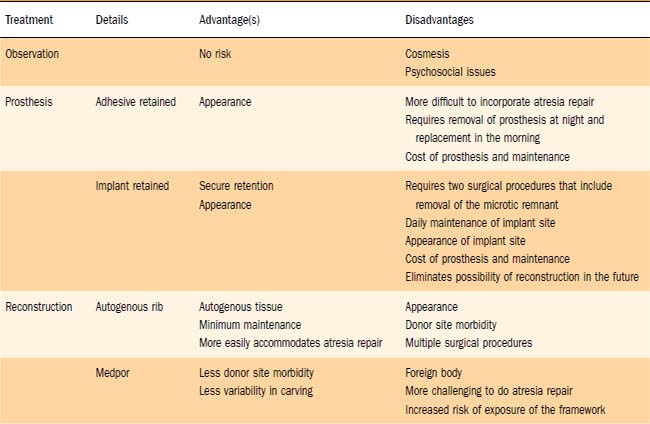
With regard to the pinna, the patient and family are presented with three options: observation; prosthetic management, either adhesive- or implant-retained; and reconstructive options including staged reconstruction with autologous rib graft, and reconstruction with linear high-density polyethylene (Medpor) surgical implant material and temporoparietal fascial flap. Generally, observation is recommended until the child is at least 5 to 8 years old. This allows both growth of the donor rib cartilage and development of the contralateral normal ear, which will act as a template for the microtic side. Waiting until the child is older may reduce the risk of the development of a thoracic deformity as a result of harvesting of cartilaginous rib graft, although we feel this risk is minimal.22 Finally, waiting until the child is school-aged allows the child to participate in these important decisions. We feel that it is important for the child to understand the options and participate in the decision making. The general health of the child is assessed, with particular attention paid to growth, maturation, and lung function. It is important to assess lung function, because the risk of pneumothorax and postoperative atelectasis and pneumonia is significant. This assessment usually is performed by the child’s pediatrician. It is important to question the child about his or her personal concerns and to inquire about the effects of the microtia on social interaction with friends and classmates. Each child and parent will have different concerns that should be explored preoperatively.
During the planning stage of reconstruction, the surgical benefits, risks, and alternatives are discussed with the patient and the parents (Table 192-1). Complications such as infection, bleeding, scarring, graft loss, displacement or resorption, asymmetry, and pneumothorax should be discussed in detail. The possibility of a less-than-optimal aesthetic result also is discussed, and the expectations of the patient, parents, and surgeon must be realistic and clearly defined. Providing postoperative photographs of previous patients often is helpful to patients if the pictures depict a realistic result of a similar deformity.
Autogenous Costochondral Reconstruction
The use of rib cartilage in auricular reconstruction was pioneered by Tanzer23 and refined by the meticulous work and accomplishments of Brent.24,25 Reconstruction using the standard rib-grafting technique requires three to four stages, each separated by 2 to 6 months, beginning when the patient is approximately 6 to 8 years old.
Classic Microtia Reconstruction
Stage I: Cartilage Implantation
The first stage of microtia reconstruction is undoubtedly the most tedious and crucial of the entire process.24 One of the most important objectives during the first stage is to place the auricular framework in a position that achieves the best symmetry for the patient’s face. Placement of the reconstructed ear is determined by the position of the normal ear, and we do not alter this position to accommodate future reconstruction of the canal atresia or a low-lying hairline. If the hairline is low, the initial ear reconstruction is destined to have hair along the helix that will require depilation or excision at a later date. Preoperative tissue expansion to increase the amount of non–hair-bearing skin is not recommended, because the expanded skin capsule does not contour well over the implanted cartilaginous framework.
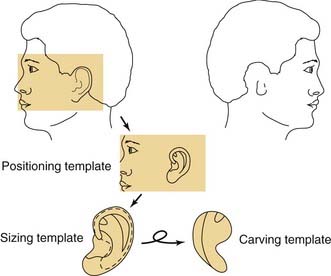
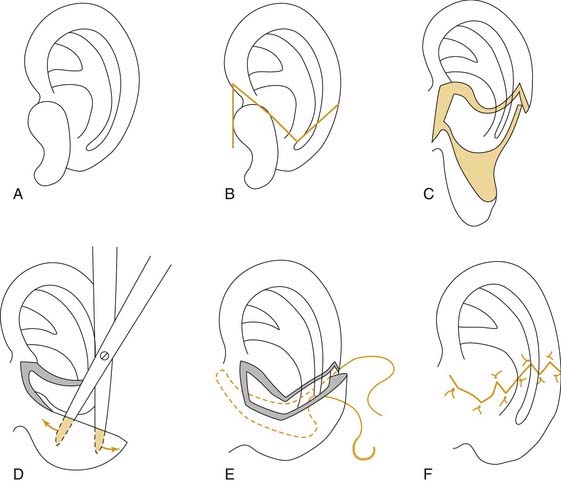
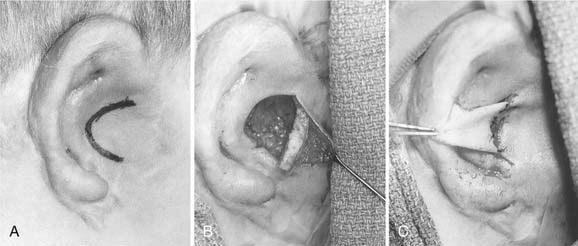
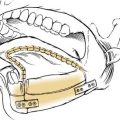
Three templates are made using radiographic film: a positioning template, a sizing template, and a carving template. The positioning template is made by carefully tracing the contour of the normal ear. It has the outline of the normal ear, with the landmarks of the lateral palpebral fissure and the lateral oral commissure for reference points. The landmarks are etched onto the radiographic film with an 18-gauge needle or scalpel to avoid losing these reference points (Fig. 192-6). The distance from the lateral palpebral fissure to the root of the helix is also measured on the template and should approximate 6.5 cm, or the length of the normal ear. The sizing template, which is a tracing of the entire ear, is used to test the size of the skin pocket in which the cartilaginous framework is placed. The carving template is drawn from the sizing template to include the landmarks of the antihelix, the triangular fossa, the scaphoid fossa, the medial aspect of the helix, and the antitragus. It is drawn 1 to 2 mm smaller than the sizing template to compensate for the thickness of the overlying skin. It is also used to determine the best donor site during rib harvesting and as a template for sculpting the cartilaginous framework. After the landmarks are permanently etched, the templates are autoclaved. The entire head and neck region is prepared so that the contralateral normal side can be easily visualized to evaluate symmetry. The eyes and mouth are covered with a transparent drape to allow visualization of the lateral palpebral fissure and the lateral commissure and to facilitate placement using the positioning template. The recipient ear site is injected with lidocaine with epinephrine to assist with hemostasis, and the incision is drawn along the anterior and superior margin of the rudimentary lobule. The contralateral side of the chest is prepared, because the contralateral rib graft has better contour characteristics. The chest incision is drawn just superior to the margin of the rib cage.
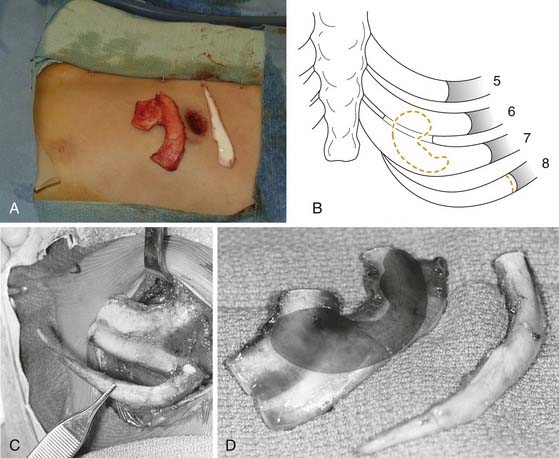
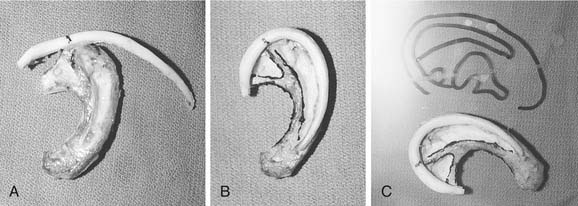
We generally use two surgical teams to reduce operating time. The donor site incision is made on the contralateral side of the chest, and the inferior margin of the rib cage is exposed. We have refined a minimally invasive technique, using a 2-cm skin incision (Fig. 192-7A). Two cartilaginous segments are required: a segment of free-floating cartilage to create the helical rim and a portion of synchondrosis to create the auricular framework.
The floating eighth rib is isolated, and dissection is continued posterolaterally to the bony-cartilaginous junction. Ideally this segment is 8 to 9 cm in length. Then the synchondrosis, usually between sixth and seventh ribs, is exposed. The carving template is used to determine the best donor site for the synchondrosis (see Fig. 192-7B to D). The rib cartilage is harvested with the superficial perichondrium intact. After removal of both segments, the donor site is checked for violation of the pleura. The chest wound is filled with saline and the anesthesiologist is asked to deliver positive-pressure ventilation to sustain a pressure of 40 cm H2O. If a defect in the pleura is identified, a red rubber catheter is introduced into the pleural space and placed on continuous suction. The catheter is removed during positive-pressure ventilation, and the wound is closed with an airtight closure. A suction drain is placed in the subcutaneous space after closure of the rectus muscle. Intercostal bupivacaine blocks are placed at the end of the procedure to reduce postoperative pain.
The framework and helix are carved using Nos. 10, 11, and 15 scalpel blades. The 6700 Beaver blade can also be used (Fig. 192-8). As much perichondrium as possible is left intact over the lateral surface to enhance revascularization and tissue fixation. Suture or wire fixation is needed occasionally to reinforce the synchondrosis and to provide additional support to the framework. Additional cartilage grafts can be sutured to the posterior surface of the synchondrosis for more rigidity. The helix is carved from the eighth rib using the thicker part of the graft for the root of the helix and the thin distal tip of the graft for the caudal helix. The graft is carefully thinned until it is pliable enough to bend around the framework, and it is sutured into position with 4-0 clear nylon sutures. Additional thinning of the scaphoid and triangular fossa can be done after the helix is adequately fixated (Fig. 192-9A to C).
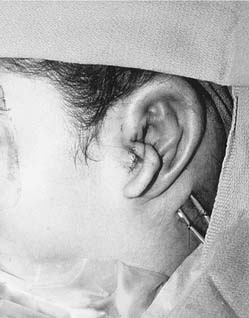
The reconstructed framework with attached helix is then placed into the pocket in a rotational manner, beginning with the root of the helix and then rotating the framework clockwise for the right side and counterclockwise for the left. If the pocket is excessively tight and the overlying skin remains blanched with poor vascular refill, the implant is removed and the pocket enlarged. After the graft is properly positioned, a small round suction drain is placed deep to the framework and brought out through the skin in the hairline. After the wound is sealed, the skin should conform to the framework, and the details of the helix and antihelix should be clearly visible (Fig. 192-10). The concavities of the ear are packed with petroleum gauze and secured with 4-0 chromic bolster sutures. A Glasscock dressing is applied using fluffed gauze to avoid unnecessary pressure. The drains are placed on intermediate suction. One of us (CSM) prefers continuous suction for 24 hours before placing the drains on bulb suction; another (VQ) recommends the continuous use of butterfly drains connected to vacuum-sealed red rubber–topped tubes.
Stage II: Lobule Transfer
The second surgery is performed 2 to 3 months after the first stage. A transposition flap is designed to move the vertically oriented lobule to the tail of the framework. After the incisions are made, a pocket is created within the lobule to accommodate the tail of the framework. The tail is elevated from the mastoid cortex so that the lobule can be wrapped around the posteroinferior margin of the cartilage framework. Care is taken to evert the wound margins in an attempt to avoid a stepoff at the junction of the lobule and framework. One to two Z-plasties are performed across the transverse portion of the incision (Fig. 192-11A to F).
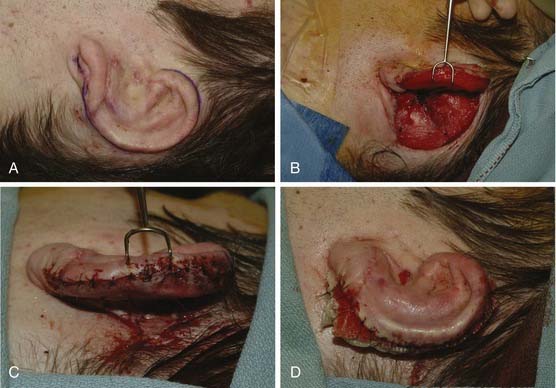
Stage III: Creation of Postauricular Sulcus
This procedure is performed approximately 3 months after stage II (Fig. 192-12A to D). An incision is made around the helical rim. However, if there is hair on the helix, the incision is made slightly lower on its lateral surface to minimize the hair-bearing skin over the framework. The non–hair-bearing auricular skin is advanced 2 to 3 mm superiorly over the helical rim, and absorbable tacking sutures are placed between the subcutaneous tissue and the cartilaginous framework. The ear is released by dissecting into the postauricular sulcus and leaving the perichondrium intact to protect the cartilage graft. The postauricular skin over the mastoid is undermined and advanced into the postauricular sulcus, to help project the ear laterally away from the mastoid. After the postauricular skin is advanced and secured, the remaining defect is measured. It usually measures approximately 4 × 8 cm2. An elliptical thick split-thickness graft is harvested freehand from the left groin. The donor site is closed primarily. The graft is secured into the newly created postauricular sulcus with 5-0 chromic sutures. A bolster is secured into the sulcus with a 4-0 nylon. The ear is covered with a Glasscock dressing for protection for 1 to 2 weeks. The bolster is removed after 5 to 7 days, and the skin graft is kept moist for 2 to 3 weeks.
Stage IV: Tragus Reconstruction
The fourth stage of reconstruction is to form a tragus and conchal bowl. If the conchal bowl is well defined, a composite graft can be used to reconstruct the tragus. An elliptical composite graft is removed from the concha cymba of the contralateral ear. An incision is made along the posterior border of the future tragus, and the composite graft is placed on the posterior surface to give the tragus some projection. The shadowing effect gives the illusion of an external auditory meatus. If the conchal bowl is not defined, an incision is made along the posterior border of the future conchal bowl, and an anterior-based cutaneous circular flap is created. The conchal bowl is then debulked of scar tissue and any rudimentary skin and cartilage that might remain. Because of facial nerve anomalies, a facial nerve monitor is recommended. The flap is folded onto itself to create a tragus that is then reinforced with a small piece of auricular or rib cartilage, and the surface of the conchal bowl is covered with a small skin graft (Fig. 192-13A to C). The bolster is removed in 3 to 5 days. Occasionally, an additional operation is needed to further elevate the reconstructed ear away from the mastoid using grafts of cartilage implanted medial to the framework.
Atypical Microtia Repair
Second-degree dysplasia or atypical microtia presents a more difficult surgical challenge. As previously discussed, many components of the auricular structure are recognizable, but there is a distinct tissue deficiency that necessitates the transportation of cartilage and skin. The additional structure often eliminates the necessity for complete framework insertion. When the vertical height difference between the two ears is greater than 20 mm, consideration is given to using a rib framework. When the difference is less than 20 mm, the result is achieved by expanding the existing structure; this is done by a combination of unfurling, uncupping, and increasing the vertical height with the use of cartilage composite grafts (Fig. 192-14A to H).26 The helical root can also be advanced in a V-Y fashion into the helix to help augment the vertical height. Unlike classic microtia reconstruction, the use of vestigial remnants as a main framework does not lead to consistent results. Figure 192-15A to F shows a child with atypical microtia with an undeveloped lobule that was reconstructed by placing a cartilage implant that was harvested from the contralateral conchal bowl. This reconstruction was followed by a staged skin graft with an advancement flap of postauricular skin.
Aase JM, Tegtmeier RE. Microtia in New Mexico. Birth Defects. 1977;13:113.
Aguilar EA. Major congenital malformations of the auricle. In: Bailey BJ, Johnson JJ, Kohut RI, Pillsbury HCIII, Tardy ME, editors. Head and Neck Surgery–Otolaryngology. Philadelphia: JB Lippincott, 1993.
Bennun RD, Mulliken JB, Kaban LB, et al. Microtia: a microform of hemifacial microsomia. Plast Reconstr Surg. 1985;76:859.
Brent B. Correction of microtia with autogenous cartilage grafts: I. Classic deformity. Plast Reconstr Surg. 1980;66:1.
Brent B. Correction of microtia with autogenous cartilage grafts: II. Atypical and complex deformities. Plast Reconstr Surg. 1980;66:13.
Brent B. The Artistry of Reconstructive Surgery. St Louis: Mosby; 1987.
Farkas LG. Anthropometry of the normal and defective ear. Clin Plast Surg. 1990;17:213.
Fukuda O. Long-term evaluation of modified Tanzer ear reconstruction. Clin Plast Surg. 1990;17:241.
Gorlin RJ, Pindborg JJ, Cohen MMJr. Oculoauriculovertebral dysplasia. In: Gorlin RJ, Pindborg JJ, Cohen MMJr, editors. Syndromes of the Head and Neck. New York: McGraw-Hill, 1976.
Granstrom G. Retinoid-induced ear malformations. Otolaryngol Head Neck Surg. 1990;103:702.
His W. Austellung von entwickelungsnormen zweiter monat. Anat Mensch Embryonen. 55, 1883. Part II
His W. Die Formentwickelung des ausseren Ohres. Anat Mensch Embryonen. 1885;3:211.
Jarvis BL, Johnson MC, Sulik KK. Congenital malformations of the external, middle, and inner ear produced by isotretinoin exposure in mouse embryos. Otolaryngol Head Neck Surg. 1990;102:391.
Jones KL. Smith’s Recognizable Patterns of Human Malformation. Philadelphia: WB Saunders; 1988.
Karmody CS, Annino DJ. Embryology and anomalies of the external ear. Facial Plast Surg. 1995;11:251.
Kaye CI, Rollnick BR, Hauck WW, et al. Microtia and associated anomalies. Am J Med Genet. 1989;34:574.
Lammer E. Preliminary observations on isotretinoin-induced ear malformations and pattern formation of the external ear. J Craniofac Genet Dev Biol. 1991;11:292.
Lucente FE, Lawson W, Novick NL. The External Ear. Philadelphia: WB Saunders; 1995.
Marx H. Beitrag zur Morpholgie und Genese der Mittelohrmissbildungen mit Gehorgangsatresie. Z Hals Nasan Ohrenheilkd. 1922;2:230.
Mastroiacovo P, Corchia C, Botto LD. Epidemiology and genetics of microtia-anotia: a registry based study on over one million births. J Med Genet. 1995;32:453.
Melnick M, Myrianthopoulos NC. External Ear Malformations: Epidemiology, Genetics and Natural History. New York: Alan R Liss; 1979.
Moore KL. The Developing Human. Philadelphia: WB Saunders; 1977.
Posnick JC, Al-Qattan MM, Whitaker LA. Assessment of the preferred vertical position of the ear. Plast Reconstr Surg. 1993;91:1198.
Rosa FW. Teratogenicity of isotretinoin [letter]. Lancet. 1983;2:513.
Skiles MS, Randall P. The aesthetics of ear placement: an experimental study. Plast Reconstr Surg. 1983;72:133.
Streeter GL. Development of the auricle in the human embryo. Control Embryol. 1922;14:111.
Sulik KK, Dehart DB. Retinoic-acid-induced limb malformations resulting from apical ectodermal ridge cell death. Teratology. 1988;37:527.
Tanzer R. Total reconstruction of the external ear. Plast Reconstr Surg. 1959;23:1.
Westerman ST, Gilbert LM, Schondel L. Vestibular dysfunction in a child with embryonic exposure to Accutane. Am J Otol. 1994;15:400.
1. Mastroiacovo P, Corchia C, Botto LD. Epidemiology and genetics of microtia-anotia: a registry based study on over one million births. J Med Genet. 1995;32:453.
2. Aase JM, Tegtmeier RE. Microtia in New Mexico. Birth Defects. 1977;13:113.
3. Karmody CS, Annino DJ. Embryology and anomalies of the external ear. Facial Plast Surg. 1995;11:251.
4. Kaye CI, Rollnick BR, Hauck WW, et al. Microtia and associated anomalies. Am J Med Genet. 1989;34:574.
5. Lucente FE, Lawson W, Novick NL. The External Ear. Philadelphia: WB Saunders; 1995.
6. Melnick M, Myrianthopoulos NC. External Ear Malformations: Epidemiology, Genetics and Natural History. New York: Alan R Liss; 1979.
7. His W. Austellung von entwickelungsnormen zweiter monat. Anat Mensch Embryonen. 55, 1883. Part II
8. His W. Die Formentwickelung des ausseren Ohres. Anat Mensch Embryonen. 1885;3:211.
9. Moore KL. The Developing Human. Philadelphia: WB Saunders; 1977.
10. Streeter GL. Development of the auricle in the human embryo. Control Embryol. 1922;14:111.
11. Lammer E. Preliminary observations on isotretinoin-induced ear malformations and pattern formation of the external ear. J Craniofac Genet Dev Biol. 1991;11:292.
12. Lammer EJ, Chen DT, Hoar RM. Retinoic acid embryopathy. N Engl J Med. 1985;313:837.
13. Sifontis NM, Coscia LA, Constantinescu S. Pregnancy outcomes in solid organ transplant recipients with exposure to mycophenolate mofetil or sirolimus. Transplantation. 2006;82:1698.
14. Bennun RD, Mulliken JB, Kaban LB, et al. Microtia: a microform of hemifacial microsomia. Plast Reconstr Surg. 1985;76:859.
15. Gorlin RJ, Pindborg JJ, Cohen MMJr. Oculoauriculovertebral dysplasia. In: Gorlin RJ, Pindborg JJ, Cohen MMJr, editors. Syndromes of the Head and Neck. New York: McGraw-Hill, 1976.
16. . Smith’s Recognizable Patterns of Human Malformation. KL Jones. Philadelphia: WB Saunders. 1988.
17. Farkas LG. Anthropometry of the normal and defective ear. Clin Plast Surg. 1990;17:213.
18. Posnick JC, Al-Qattan MM, Whitaker LA. Assessment of the preferred vertical position of the ear. Plast Reconstr Surg. 1993;91:1198.
19. Skiles MS, Randall P. The aesthetics of ear placement: an experimental study. Plast Reconstr Surg. 1983;72:133.
20. Marx H. Beitrag zur Morpholgie und Genese der Mittelohrmissbildungen mit Gehorgangsatresie. Z Hals Nasan Ohrenheilkd. 1922;2:230.
21. Aguilar EA. Major congenital malformations of the auricle. In: Bailey BJ, Johnson JJ, Kohut RI, Pillsbury HCIII, Tardy ME, editors. Head and Neck Surgery–Otolaryngology. Philadelphia: JB Lippincott, 1993.
22. Fukuda O. Long-term evaluation of modified Tanzer ear reconstruction. Clin Plast Surg. 1990;17:241.
23. Tanzer R. Total reconstruction of the external ear. Plast Reconstr Surg. 1959;23:1.
24. Brent B. Correction of microtia with autogenous cartilage grafts: I. Classic deformity. Plast Reconstr Surg. 1980;66:1.
25. Brent B. The Artistry of Reconstructive Surgery. St Louis: Mosby; 1987.
26. Brent B. Correction of microtia with autogenous cartilage grafts: II. Atypical and complex deformities. Plast Reconstr Surg. 1980;66:13.