CHAPTER 366 Microsurgery of Paraclinoid Aneurysms
Paraclinoid aneurysms are defined as aneurysms arising from the internal carotid artery (ICA) in close proximity to the anterior clinoid process. Using the broadest definition, such aneurysms could include those arising from the intracavernous, clinoidal, ophthalmic, and posterior communicating ICA segments.1–3 The ensuing chapter, however, addresses only those lesions arising beyond the venous lumen of the cavernous sinus and proximal to the origin of the posterior communicating artery—the clinoidal and ophthalmic segments. More proximal and distal aneurysm variants are discussed in other chapters.
Anatomy
Osseous Anatomy
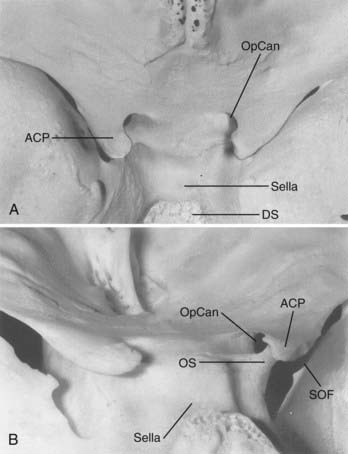
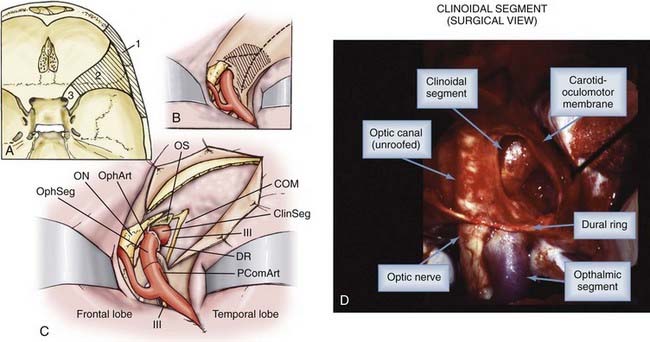
The anterior clinoid process (ACP), formed by the medial extension of the lesser wing of the sphenoid bone, provides a bony roof to the superior orbital fissure (SOF) and the anterior cavernous sinus (Fig. 366-1). The optic strut extends from the inferomedial surface of the ACP to the body of the sphenoid bone, separating the optic canal from the SOF. The ACP and optic strut define and obstruct access to the anterior and lateral borders of the ascending ICA as it exits the cavernous sinus to enter the subarachnoid space.
Dural Anatomy
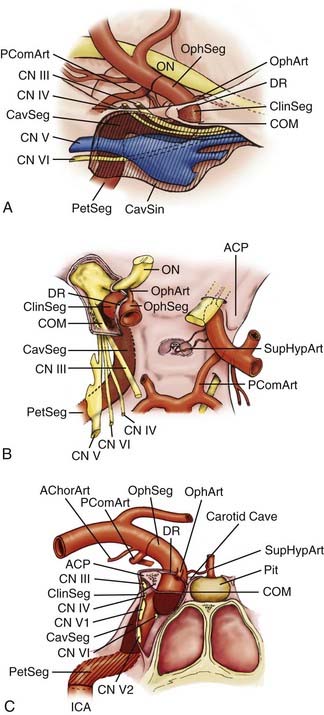
The relevant dural structures in this region represent dural reflections off the ACP, which primarily include the falciform ligament, the (distal) dural ring, and the carotid-oculomotor membrane (COM; Fig. 366-2).4 The dural ring represents the circular point of attachment of the dura to the ICA as the vessel penetrates the dura to enter the subarachnoid space. This dural attachment extends radially from the ICA and is continuous with the periosteum covering the superomedial aspect of the anterior clinoid process, the diaphragma sella, and the floor of the optic canal.5–9 The plane of the dural ring slopes downward from anterior-to-posterior and lateral-to-medial directions. The subarachnoid space diverticulum medial to the ICA created by this slant is called the carotid cave.6–10 The COM is formed by dura from the inferior surface of the ACP that extends from the ICA medially to the oculomotor nerve laterally.4 This membrane marks the point of exit of the ICA from the cavernous sinus main lumen.
Neural Structures
The cranial nerves within the cavernous sinus (CN III, IV, V, and VI) are rarely clinically affected by paraclinoid aneurysms because these lesions generally project superiorly or medially away from the superior orbital fissure and lateral cavernous sinus wall (see Fig. 366-2). Nonetheless, their close proximity to the ACP makes knowledge of their anatomy mandatory so as to avoid injury during its removal.
Vascular Structures
Arterial Segments
The paraclinoid ICA is herein composed of two regions—the clinoidal and ophthalmic segments (see Fig. 366-2).11 The clinoidal segment, the distal portion of the anterior ascending vertical segment of the cavernous carotid artery, is located below and medial to the ACP, above the major venous lumen of the cavernous sinus. This region is a transitional ICA segment found between the COM and the dural ring.4,9,10 The clinoidal segment is located neither within the venous channels of the cavernous sinus nor within the subarachnoid space, and is essentially “interdural.”4,9,10 The ophthalmic segment, the distal portion of the paraclinoid ICA region, lies entirely within the subarachnoid space above and medial to the ACP. Beginning at the dural ring, the ophthalmic segment extends to the origin of the posterior communicating artery, and generally represents the longest subarachnoid ICA segment.11
Arterial Bends and Branches
Saccular aneurysms typically form at points of hemodynamic stress where a bend in the vessel and a branch site coincide.12 Two major bends in the paraclinoid ICA region predispose this region to aneurysm formation. The first bend, seen best on lateral angiogram, is the posteriorly projecting turn that begins at the anterior genu of the cavernous segment and continues as the vessel ascends through the dural ring. This bend creates a strong superior vector on the anterior and dorsal wall of the clinoidal and ophthalmic segments. A less conspicuous second bend, seen best from an anteroposterior or dorsal view, is the gentle lateral-to-medial-to-lateral curve beginning at the anterior genu of the cavernous segment and continuing as the ICA travels toward its terminal bifurcation. This medially projecting hemodynamic vector places significant stress upon the medial aspect of the clinoidal and ophthalmic ICA segments.
The ophthalmic segment harbors several prominent arterial branches that predispose this region to aneurysm formation. The ophthalmic artery is the most prominent and clinically significant branch, and typically arises from the dorsomedial carotid surface just above the dural ring and below the inferolateral portion of the optic nerve.13,14 This branch projects anterolaterally to run through the optic canal beneath the optic nerve, and supplies the optic nerve through perforating branches and the retina through the central retinal artery. The superior hypophyseal artery is the second major branch (or series of branches), usually arising from the medial or inferomedial ICA surface just distal to the dural ring, medial and unrelated to the take-off of the ophthalmic artery along the medial-to-lateral ICA bend within the ophthalmic segment.15,16 This vessel projects medially to supply the superior aspect of the pituitary stalk and gland, a portion of the cavernous sinus dura, and the posterior optic nerve and chiasm.
The clinoidal segment is usually devoid of named arterial perforators. On occasion, however, the ophthalmic or superior hypophyseal arteries originate from this segment, typically reaching their end organs through alternate anatomic pathways. The ophthalmic artery, for example, can originate from the distal cavernous segment or clinoidal segment in up to 10% of cadaveric specimens, usually entering the orbit through the SOF or an orifice within the optic strut.4,17,18
Aneurysm Classification
Clinoidal Segment Aneurysms
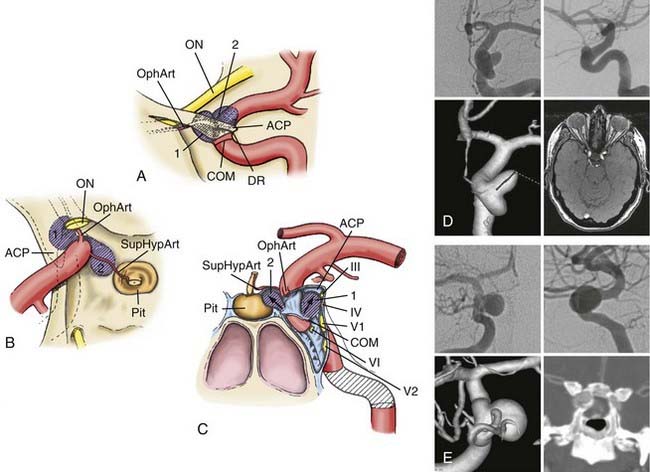
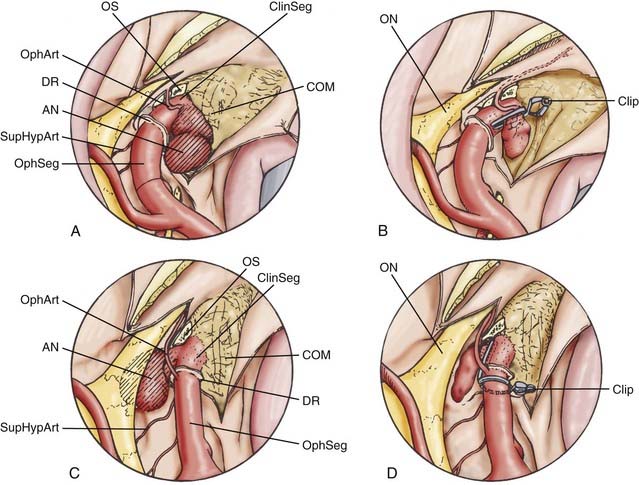
Two clinoidal segment aneurysm variants can be differentiated according to their site of origin, direction of projection, and relationships to arterial bends and branches and the adjacent dural and osseous structures within the segment (Fig. 366-3).
Anterolateral Variant
The anterolateral variant arises from the anterolateral surface of the clinoidal segment as it obliquely ascends toward the dural ring underneath the ACP.1 The superiorly and slightly medially directed course of the segment promotes an aneurysm that expands lateral and anterior to the ascending ICA, superiorly projecting toward and into the ACP. When small, the anterolateral variant may erode the optic strut and undersurface of the ACP to cause monocular visual loss from ipsilateral optic nerve compression within the optic canal. Larger lesions may secondarily compress the visual system (nerve or chiasm) within the subarachnoid space after extension through the dura medial to the ACP. These aneurysms can sometimes arise in association with a clinoidal segment origin of the ophthalmic artery but are more often associated just with the hemodynamic stress associated with the anterior ascending vertical segment of the cavernous ICA.
Medial Variant
The medial variant extends from the medial surface of the clinoidal segment and enlarges toward the sphenoid sinus and sella as the ICA turns from lateral to medial to lateral during its ascent toward and through the dural ring.1 Initially, this aneurysm type expands beneath the diaphragma sella into the pituitary fossa. Gradual enlargement may cause hypopituitarism; rarely, aneurysm rupture into the pituitary gland may simulate pituitary apoplexy. Extension and rupture into the sphenoid sinus may cause life-threatening epistaxis. Large or giant lesions may extend through the diaphragma sella to enter the subarachnoid space. Visual loss from this aneurysm type does not occur with small lesions, but field cuts resembling those of pituitary tumors may occur with large or giant lesions that extend into the suprasellar space.
Ophthalmic Segment Aneurysms
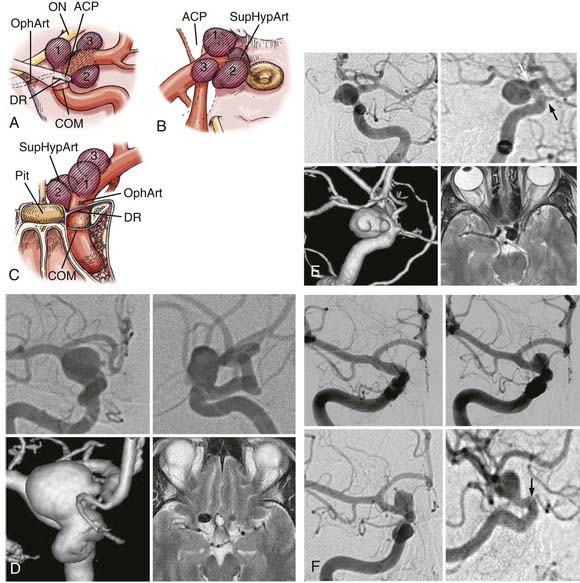
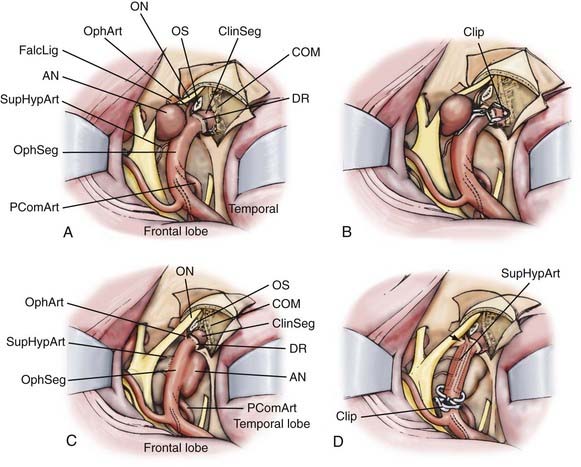
Three ophthalmic segment aneurysm variants can be differentiated according to their site of origin, direction of projection, and relationships to arterial bends and branches and the adjacent dural and osseous structures within the segment (Fig. 366-4).
Ophthalmic Artery Aneurysms
Ophthalmic artery aneurysms arise immediately distal to and in relation to the origin of the ophthalmic artery.1,2,19–22 These lesions typically arise along the posterior bend of the ICA just distal to the ophthalmic artery and dural ring, project dorsally or dorsomedially, and elevate the lateral edge of the nerve against the falciform ligament. The pressure from the falciform ligament against the superior surface of the nerve results in an inferior nasal field defect. A monocular inferior nasal field defect is initially produced; as the lesion enlarges, however, the entire nasal field can become affected, followed by a superior temporal field loss in the contralateral eye. If the clinical course is protracted, ipsilateral blindness with marked contralateral deficits can occur. Larger ophthalmic artery aneurysms tend to thicken or even calcify along their anterior portion, a factor that must be accommodated during clip placement.
Superior Hypophyseal Artery Aneurysms
Superior hypophyseal artery aneurysms arise in association with the superior hypophyseal artery along the medial surface of the ICA, usually just distal to the dural ring.2,20 There are two main variants. The parasellar variant projects inferiorly toward the sella into the carotid cave, whereas the suprasellar variant projects superiorly into the suprasellar space. Parasellar superior hypophysal artery aneurysms are usually asymptomatic. Because they project into the dura of the sella or cavernous sinus, purely parasellar aneurysms are unlikely to rupture.1 Their medial projection and proximity to ICA perforators also makes them more difficult to treat. Suprasellar or large lesions have a much higher tendency to rupture. When large or giant, these aneurysms tend to elevate the optic chiasm and produce visual changes and relationships to the visual system similar to those seen with pituitary tumors. Larger superior hypophyseal artery aneurysms tend to develop thickenings or calcifications along the anterior-medial aspect, near their origin from the ICA, similar to ophthalmic artery aneurysms. Because these lesions extend medially underneath the optic chiasm, they can result in bilateral visual field defects.
Many small lesions burrow inferiorly and medially toward and beneath the diaphragma sella, expanding the carotid cave. These lesions, termed parasellar variant superior hypophyseal artery aneurysms, have also been called carotid cave aneurysms.1,2,7,20 When small, the fundus of this variant is invested by adjacent lateral parasellar dura, and their risk for subarachnoid hemorrhage is quite low. With growth, however, these lesions expand superomedially above the diaphragma sella into the suprasellar space, where the hemorrhage risk becomes greater. A second type, the suprasellar variant superior hypophyseal artery aneurysm, projects medially above a shallow carotid cave and expands into the medial suprasellar space above the diaphragm, without investment by the parasellar dura. Superior hypophyseal artery lesions cause their visual compression by expansion into the suprasellar space and elevation of the optic chiasm, producing superior bitemporal or other patterns of visual loss more suggestive of pituitary tumors.
Dorsal Variant Aneurysms
Dorsal variant aneurysms are rarer, and arise along the dorsal surface of the ICA distinctly distal to the ophthalmic artery origin, unrelated to any arterial branch point.1,2,19,20 Many are the result of hemodynamic forces produced by an accentuated bend in the ICA as the vessel courses laterally to become the communicating segment. Others appear as “blisters” on the dorsal carotid surface of the ophthalmic segment. With a genesis likely related to dissection, blister aneurysms are very fragile and rupture easily during surgery.23 Visual loss or a reliable relationship with the optic nerve or chiasm is not consistently seen with the dorsal variant.14,17
Indications for Treatment
Aneurysms from both of these carotid segments are much more common in women, with ratios as high as 9 : 1 female-to-male predominance.20,22,24 Most present during the fifth and sixth decades of life, usually as either incidental lesions or with mass effect.20,24,25 These segments have a propensity for aneurysm multiplicity; up to half of ophthalmic segment aneurysm patients harbor at least one additional intracranial aneurysm.2,26,27 With the exception of the dorsal variant, which often represents an unstable dissection, most paraclinoid aneurysms have a lower rupture risk compared with those at other intracranial subarachnoid sites.
Operative Technique
See Figures 366-5 through 366-7 for illustrations of the operative techniques described next. Video 366-1 ![]() demonstrates anterior clinoidectomy and clipping of an ophthalmic artery.
demonstrates anterior clinoidectomy and clipping of an ophthalmic artery.
Aneurysm Clipping
Giant and Complex Aneurysms
Many larger paraclinoid aneurysms have significant thickening within the aneurysm wall or neck, which can cause an initial aneurysm clip to migrate downward and partially occlude the parent vessel lumen. In such circumstances, a temporary clip is often placed proximally on the neck, in the desired plane for final clipping, to serve as a place-holder for the parent vessel to ensure its patency. Then, sequential clips are added distally in the same plane until the aneurysm is completely obliterated. The proximal temporary clip is then removed. Fenestrated clips facilitate closure of calcified lesions by encircling the affected segment of the aneurysm neck. An intraoperative angiogram through direct common carotid artery puncture (if the cervical carotid is already exposed) or through a preoperatively placed transfemoral catheter is highly recommended before closure to ascertain parent vessel patency and complete aneurysm obliteration. The use of indocyanine green injected into the venous system allows visualization of perforator patency and can substitute for arteriography in some instances.28
Surgical Complications and Outcome
Patients who undergo surgical treatment of paraclinoid aneurysms usually have good or excellent outcomes, particularly when the surgery is done by experienced surgeons and the secondary effects of vasospasm are negated.2,29–31
Visual deterioration following paraclinoid aneurysm surgery is usually attributable to excessive optic nerve manipulation or arterial perforator compromise during aneurysm exposure. In patients who present with visual field defects, visual symptoms improved in 50% to 74%, remained stable in 26% to 42%, and are worsened in 0% to 8% after surgical clipping.2,29 The cause of worsened vision (or failure of improvement) can be mechanical, thermal, or vascular. Mechanical injury may occur from manipulation of the optic nerve against the falciform ligament, which can be reduced by sectioning the ligament and unroofing the optic canal before manipulation of the aneurysm or optic nerve. Thermal injury can occur during drilling of the anterior clinoid process and is prevented by generous irrigation to dissipate the heat or by using the newer ultrasonic bone aspirators. Finally, perforator injury can be avoided by wide exposure and dural ring sectioning, to see the perforators clearly and avoid inadvertent clipping or kinking of their origins. Re-exploration and clip adjustment should be considered if intraoperative events do not adequately explain any postoperative visual deficit.
Batjer HH, Kopitnik TA, Giller CA, et al. Surgery for paraclinoidal carotid artery aneurysms. J Neurosurg. 1994;80:650.
Cawley CM, Zipfel GJ, Day AL. Surgical treatment of paraclinoid and ophthalmic aneurysms. Neurosurg Clin N Am. 1998;9:765.
Dawson BH. The blood vessels of the human optic chiasma and their relation to those of the hypophysis and hypothalamus. Brain. 1958;81:207.
Day A. Clinicoanatomic features of supraclinoid aneurysms. Clin Neurosurg. 1990;36:256.
Day AL. Aneurysms of the ophthalmic segment. A clinical and anatomical analysis. J Neurosurg. 1990;72:677.
Day AL, Morcos JJ, Revilla R. Management of aneurysms of the anterior circulation, 4th ed. Youmans JR, editor. Neurological Surgery, Vol 2. Philadelphia: Saunders. 1996:1272-1309.
Dolenc VV. A combined epi- and subdural direct approach to carotid-ophthalmic artery aneurysms. J Neurosurg. 1985;62:667.
Drake CG, Vanderlinden RG, Amacher AL. Carotid-ophthalmic aneurysms. J Neurosurg. 1968;29:24.
Gibo H, Kobayashi S, Kyoshima K, et al. Microsurgical anatomy of the arteries of the pituitary stalk and gland as viewed from above. Acta Neurochir (Wien). 1988;90:60.
Gibo H, Lenkey C, Rhoton ALJr. Microsurgical anatomy of the supraclinoid portion of the internal carotid artery. J Neurosurg. 1981;55:560.
Harris FS, Rhoton AL. Anatomy of the cavernous sinus. A microsurgical study. J Neurosurg. 1976;45:169.
Inoue T, Rhoton ALJr, Theele D, et al. Surgical approaches to the cavernous sinus: a microsurgical study. Neurosurgery. 1990;26:903.
Knosp E, Muller G, Perneczky A. The paraclinoid carotid artery: anatomical aspects of a microneurosurgical approach. Neurosurgery. 1988;22:896.
Kobayashi S, Kyoshima K, Gibo H, et al. Carotid cave aneurysms of the internal carotid artery. J Neurosurg. 1989;70:216.
Meling TR, Sorteberg A, Bakke SJ, et al. Blood blister–like aneurysms of the internal carotid artery trunk causing subarachnoid hemorrhage: treatment and outcome. J Neurosurg. 2008;108:662.
Nutik SL. Removal of the anterior clinoid process for exposure of the proximal intracranial carotid artery. J Neurosurg. 1988;69:529.
Nutik SL. Ventral paraclinoid carotid aneurysms. J Neurosurg. 1988;69:340.
Perneczky A, Knosp E, Vorkapic P, et al. Direct surgical approach to infraclinoidal aneurysms. Acta Neurochir (Wien). 1985;76:36.
Raco A, Frati A, Santoro A, et al. Long-term surgical results with aneurysms involving the ophthalmic segment of the carotid artery. J Neurosurg. 2008;108:1200.
Rhoton ALJr. Anatomy of saccular aneurysms. Surg Neurol. 1980;14:59.
Yasargil MG, Gasser JC, Hodosh RM, et al. Carotid-ophthalmic aneurysms: direct microsurgical approach. Surg Neurol. 1977;8:155.
1 Cawley CM, Zipfel GJ, Day AL. Surgical treatment of paraclinoid and ophthalmic aneurysms. Neurosurg Clin N Am. 1998;9:765.
2 Day AL. Aneurysms of the ophthalmic segment. A clinical and anatomical analysis. J Neurosurg. 1990;72:677.
3 Day AL, Morcos JJ, Revilla R. Management of aneurysms of the anterior circulation, 4th ed. Youmans JR, editor. Neurological Surgery, Vol 2. Philadelphia: Saunders. 1996:1272-1309.
4 Inoue T, Rhoton ALJr, Theele D, et al. Surgical approaches to the cavernous sinus: a microsurgical study. Neurosurgery. 1990;26:903.
5 Dolenc VV. A combined epi- and subdural direct approach to carotid-ophthalmic artery aneurysms. J Neurosurg. 1985;62:667.
6 Knosp E, Muller G, Perneczky A. The paraclinoid carotid artery: anatomical aspects of a microneurosurgical approach. Neurosurgery. 1988;22:896.
7 Kobayashi S, Kyoshima K, Gibo H, et al. Carotid cave aneurysms of the internal carotid artery. J Neurosurg. 1989;70:216.
8 Nutik SL. Removal of the anterior clinoid process for exposure of the proximal intracranial carotid artery. J Neurosurg. 1988;69:529.
9 Perneczky A, Knosp E, Vorkapic P, et al. Direct surgical approach to infraclinoidal aneurysms. Acta Neurochir (Wien). 1985;76:36.
10 Nutik SL. Ventral paraclinoid carotid aneurysms. J Neurosurg. 1988;69:340.
11 Gibo H, Lenkey C, Rhoton ALJr. Microsurgical anatomy of the supraclinoid portion of the internal carotid artery. J Neurosurg. 1981;55:560.
12 Rhoton ALJr. Anatomy of saccular aneurysms. Surg Neurol. 1980;14:59.
13 Hayreh S. The ophthalmic artery. In: Newton T, Potts D, editors. Radiology of the Skull and Brain. St. Louis: Mosby; 1974:1333-1350.
14 Nishio S, Matsushima T, Fukui M, et al. Microsurgical anatomy around the origin of the ophthalmic artery with reference to contralateral pterional surgical approach to the carotid-ophthalmic aneurysm. Acta Neurochir (Wien). 1985;76:82.
15 Dawson BH. The blood vessels of the human optic chiasma and their relation to those of the hypophysis and hypothalamus. Brain. 1958;81:207.
16 Gibo H, Kobayashi S, Kyoshima K, et al. Microsurgical anatomy of the arteries of the pituitary stalk and gland as viewed from above. Acta Neurochir (Wien). 1988;90:60.
17 Harris FS, Rhoton AL. Anatomy of the cavernous sinus. A microsurgical study. J Neurosurg. 1976;45:169.
18 Kyoshima K, Oikawa S, Kobayashi S. Interdural origin of the ophthalmic artery at the dural ring of the internal carotid artery. Report of two cases. J Neurosurg. 2000;92:488.
19 Batjer HH, Kopitnik TA, Giller CA, et al. Surgery for paraclinoidal carotid artery aneurysms. J Neurosurg. 1994;80:650.
20 Day A. Clinicoanatomic features of supraclinoid aneurysms. Clin Neurosurg. 1990;36:256.
21 Drake CG, Vanderlinden RG, Amacher AL. Carotid-ophthalmic aneurysms. J Neurosurg. 1968;29:24.
22 Yasargil MG, Gasser JC, Hodosh RM, et al. Carotid-ophthalmic aneurysms: direct microsurgical approach. Surg Neurol. 1977;8:155.
23 Meling TR, Sorteberg A, Bakke SJ, et al. Blood blister–like aneurysms of the internal carotid artery trunk causing subarachnoid hemorrhage: treatment and outcome. J Neurosurg. 2008;108:662.
24 Kupersmith MJ, Hurst R, Berenstein A, et al. The benign course of cavernous carotid artery aneurysms. J Neurosurg. 1992;77:690.
25 Linskey ME, Sekhar LN, Hirsch WLJr, et al. Aneurysms of the intracavernous carotid artery: natural history and indications for treatment. Neurosurgery. 1990;26:933.
26 al-Rodhan N. Aneurysms within the cavernous sinus and transitional cavernous aneurysms. In: Wilkins R, Rengachary S, editors. Neurosurgery. 3rd ed. New York: McGraw-Hill; 1996:2283-2289.
27 al-Rodhan NR, Piepgras DG, Sundt TMJr. Transitional cavernous aneurysms of the internal carotid artery. Neurosurgery. 1993;33:993.
28 de Oliveira JG, Beck J, Seifert V, et al. Assessment of flow in perforating arteries during intracranial aneurysm surgery using intraoperative near-infrared indocyanine green videoangiography. Neurosurgery. 2008;62:1300.
29 Baccin CE, Krings T, Alvarez H, et al. Multiple mirror-like intracranial aneurysms. Report of a case and review of the literature. Acta Neurochir (Wien). 2006;148:1091.
30 Nonaka T, Haraguchi K, Baba T, et al. Clinical manifestations and surgical results for paraclinoid cerebral aneurysms presenting with visual symptoms. Surg Neurol. 2007;67:612.
31 Raco A, Frati A, Santoro A, et al. Long-term surgical results with aneurysms involving the ophthalmic segment of the carotid artery. J Neurosurg. 2008;108:1200.