23 Microdermabrasion
Microdermabrasion (MDA) is a superficial skin resurfacing procedure that utilizes gentle mechanical abrasion to remove the outermost layers of the epidermis.1 Removal of outer skin layers, also called exfoliation, has been used for skin rejuvenation since 1500 BC, when the ancient Egyptians used sandpaper and sour milk baths containing lactic acid. Microdermabrasion uses refined abrasive elements, such as diamond-tipped pads or a constant flow of crystals swept across the skin, to remove the stratum corneum.2 Due to popular marketing, patients are aware of MDA and it is often considered as an initial treatment for aesthetic rejuvenation. It is one of the most commonly performed aesthetic procedures in the United States today, with more than half a million MDA treatments performed annually, according to data from the American Society for Aesthetic Plastic Surgery,3 and it is one of the most common aesthetic procedures incorporated into office practice.4,5
Skin rejuvenation with microdermabrasion is based on the principles of wound healing. By wounding and removing superficial skin layers in a controlled manner, cell renewal is stimulated with regeneration of a healthier epidermis and dermis.1 After a series of MDA treatments, histologic changes in the skin are evident. These changes include a compacted stratum corneum and smoother epidermis, increased dermal thickness with fibroblast production of new collagen and elastin,6 and increased skin hydration with improved epidermal barrier function.7,8 Clinical improvements can be seen in hyperpigmentation9 and rough skin texture.10 Some studies also show improvements in fine lines, pore size, superficial acne scars, and acne vulgaris.7,9,11 MDA can be readily combined with other minimally invasive aesthetic procedures, such as chemical peels and nonablative laser treatments, many of which can be performed in the same visit, to enhance skin rejuvenation results.12
Patient Selection
While almost any patient will benefit from exfoliation with MDA, patients with mild to moderate photoaging changes of solar lentigines, rough texture, and fine lines (e.g., Glogau types I and II) typically derive the most noticeable benefits (see Chapter 19, Aesthetics Principles and Consultation, for a description of Glogau types). Results with MDA are slow and progressive, requiring a series of treatments for visible improvements. Assessment of patients’ expectations at the time of consultation and commitment to a series of treatments is essential to the success of these treatments.
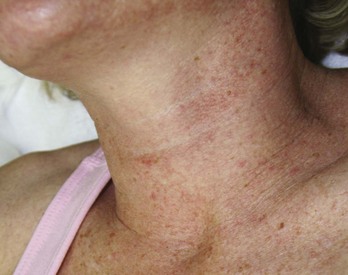
Patients of all Fitzpatrick skin types may be treated with MDA (see Chapter 19 for a description of Fitzpatrick skin types). It is advisable to treat darker skin types (IV through VI) conservatively because they have increased risks of pigmentary changes such as postinflammatory hyperpigmentation. Elderly patients with thin and friable skin and patients with erythematous conditions such as rosacea, telangiectasias, and poikiloderma of Civatte are also treated conservatively, because they have greater risks of abrasion and worsening of erythema respectively.
Cosmetic Indications1
Microdermabrasion Devices Currently Available
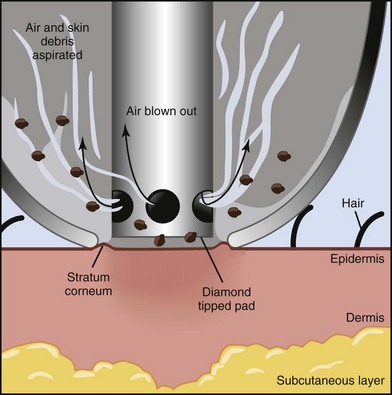
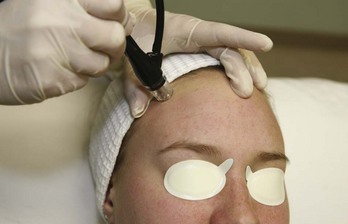
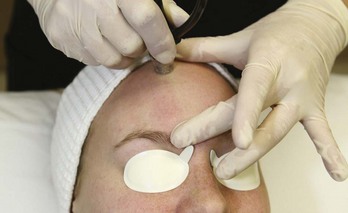
Most MDA devices utilize a closed-loop vacuum system, which draws the skin up to an abrasive element on the handpiece tip. The handpiece is passed across the skin and cellular debris is removed and suctioned up into a collection container and disposed of after treatment. The process of MDA exfoliation using a diamond-tipped device is shown in Figure 23-1. Abrasive elements used by MDA devices vary and the most commonly used elements are:
Crystal-free MDA devices have become popular because they lack these ocular and inhalation risks. Common crystal-free abrasive elements include diamond-tipped pads and bristles. Figure 23-2 shows a MDA handpiece with diamond-tipped treatment heads of various coarseness. Most of these devices have reusable treatment heads that can be sterilized following treatment. Some crystal-free devices combine simultaneous application of topical solutions during exfoliation, called infusion. The intent is to deliver topical products more effectively to the skin by taking advantage of the transient disruption of the epidermal barrier. Selection of topical solutions is based on the presenting condition. For example, hydroquinone or cosmeceuticals such as kojic acid and decapeptide-12 may be used for patients with hyperpigmentation; erythromycin and salicylic acid for acne and rosacea; and hyaluronic acid and glycerin for dehydrated skin.
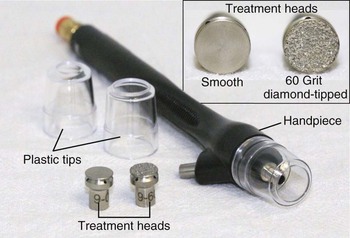
FIGURE 23-2 Microdermabrasion handpiece and diamond-tipped treatment heads.
(SilkPeel Envy, copyright Rebecca Small, MD.)
MDA devices are classified by the FDA as type I devices, which do not require the manufacturer to establish performance standards or perform clinical trials to demonstrate efficacy. With more than 30 MDA devices available, this presents a challenge to providers when selecting a device. Most MDA machines are either manufactured for estheticians (“esthetician grade”) or for clinical providers (“medical grade”), with the latter capable of deeper exfoliation with higher vacuum pressures and more abrasive treatment heads. Commonly used MDA devices are listed in the Resources section at the end of the chapter.
Contraindications1
Advantages of Microdermabrasion
Anatomy
Histologic evaluation of skin immediately after treatment with MDA demonstrates smoothing of the stratum corneum.13 Each pass of an aluminum oxide crystal MDA handpiece removes approximately 10 to 15 µm of skin, two passes fully remove the stratum corneum, and four passes penetrate to the stratum granulosum layer of the epidermis.14 The epidermal barrier function is transiently disrupted for 2 to 3 days after treatment with increased transepidermal water loss. These effects, however, are reversed 1 week after treatment, and a more compacted stratum corneum is regenerated with improved barrier function and increased skin hydration compared to pretreatment skin.15,16
In addition to short-term improvements in epidermal barrier function, longer term improvements have also been demonstrated in the epidermis and dermis following repeated MDA treatments. Epidermal thickness increases by up to 40% due to increased cellularity,7 and dermal collagen and elastin deposition increase, which can be seen clinically as a reduction of coarse pores and fine lines.6
Equipment
Procedure Preparation
Microdermabrasion: Steps and Principles
General Treatment Technique
Planning and Designing
Safety Zone
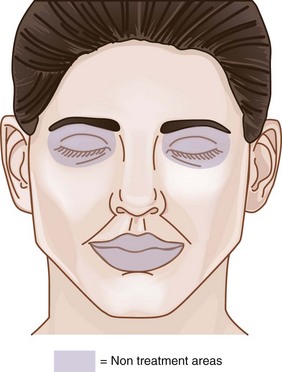
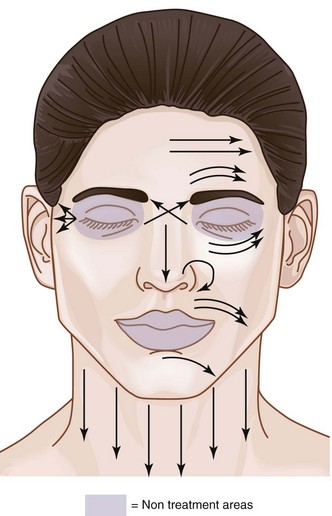
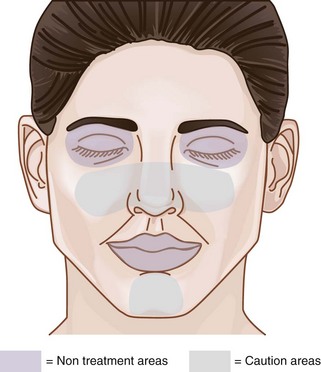
The area within which MDA treatments can be safely performed on the face, called the Microdermabrasion Safety Zone, is shown in Figure 23-3. All areas of the face may be treated, apart from the area within the bony orbital rim and, for most devices, the lips. Some MDA devices have nonabrasive smooth treatment tips that may be used on the lips (Figure 23-2). MDA can be used on the face, neck, chest, hands, back, and almost any area of the body requiring exfoliation.
Performing Microdermabrasion
TABLE 23-1 Treatment Parameters for SilkPeel Microdermabrasion
|
Treatment Intensity |
Tip Coarseness |
Vacuum Pressure (psi) |
|---|---|---|
| Mild | 140 grit | 3.0–4.0 |
| Moderate | 120 grit | 3.5–4.3 |
| Aggressive | 100 grit | 4.4–5.0 |
Results
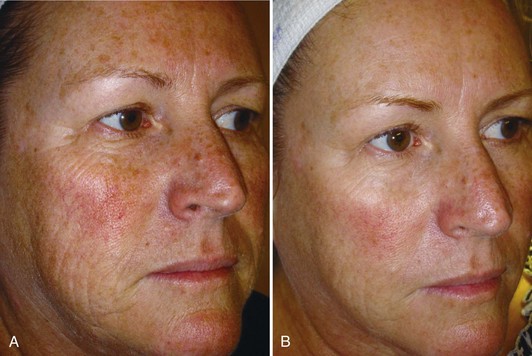
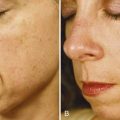
Figure 23-8 shows a patient demonstrating signs of moderate photodamage with solar lentigines, dullness, and fine lines (A) before and (B) after a series of six treatments with a bristle MDA using an infusion of topical solutions containing glycolic and lactic acid, and cosmeceutical lightening agents of kojic and azelaic acids, bearberry, and licorice.
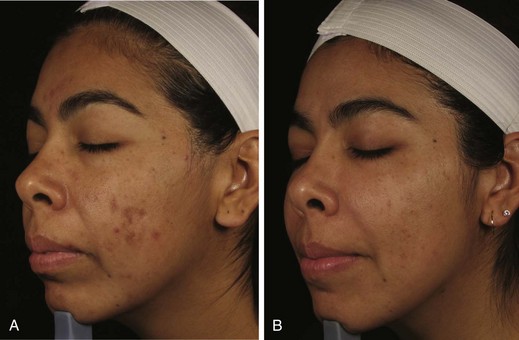
Figure 23-9 shows a patient with papulopustular acne (A) before and (B) after a series of six MDA treatments with a diamond-tipped MDA using an infusion of topical solutions containing salicylic, glycolic and kojic acids and arbutin.
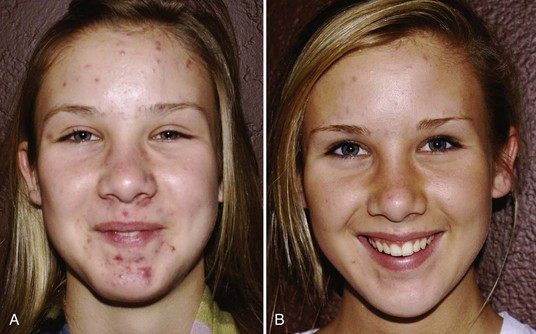
FIGURE 23-9 Acne vulgaris (A) before and (B) after six diamond-tipped (SilkPeel) microdermabrasion treatments.
(Courtesy of Envy Medical, Los Angeles, CA.)
Figure 23-10 shows a patient with darker Fitzpatrick skin type with solar lentigines and dullness (A) before and (B) after a series of six MDA treatments with a diamond-tipped MDA using an infusion of topical solutions containing a lightening peptide, decapeptide-12 (Lumixyl™).
Advanced Microdermabrasion
As providers gain experience and skill with microdermabrasion, they may choose to broaden its applications and combine it with other procedures to increase treatment intensity. Microdermabrasion can be used as part of skin preparation prior to the application of certain chemical peels to allow for more even peel penetration and to enhance the depth of penetration into the skin. Providers are advised to use established manufacturer protocols when combining these two exfoliation modalities because peel penetration can be significantly altered by the lack of the stratum corneum barrier that is removed by microdermabrasion.12
Microdermabrasion may also be used as part of photodynamic therapy (PDT), an FDA-approved treatment for nonhyperkeratotic actinic keratoses of the face and used off label to enhance photorejuvenation treatments with lasers (see Chapter 27, Photorejuvenation with Lasers, for more information about PDT). Prior to application of topical photosensitizing medications, such as 5-aminolevulinic acid (Levulan®), MDA can be performed which enhances product penetration and increases intensity of the treatment.17
Complications
MDA has minimal risks of side effects and complications.1 One study of more than 100 patients receiving MDA during a 2-year period reported no instances of infection, long-term hyperpigmentation, or scarring.18 However, complications are possible with any procedure, and knowledge of these is important to help ensure the best possible outcomes.
Hyperpigmentation can be treated with lightening agents such as hydroquinone or cosmeceutical agents such as kojic acid, arbutin, and licorice (see Chapter 24, Skin Care Products). In darker Fitzpatrick skin types, resolution of hyperpigmentation typically takes several months. Hyperpigmentation usually resolves, but in rare instances can be permanent.
Urticaria has been reported as a rare complication after MDA,19 and is presumed to be dermatographic or pressure-induced. Products applied during treatment may also trigger an allergic response. Ice may be applied, an oral antihistamine (e.g., cetirizine 10 mg) given to the patient, and a high-potency topical steroid applied to the treatment area. Although extremely unlikely, it is also advisable to assess patients for signs and symptoms of more severe allergic responses such as bronchospasm or anaphylaxis if an allergic reaction occurs.
Ocular injury due to crystal adherence to the conjunctiva or punctuate keratopathy can occur with crystal MDA devices. Symptoms include ocular pain, photophobia, and conjunctival erythema.11 Protective eye shields or moist gauze is advised with crystal MDA to cover patients’ eyes, appropriate eyewear and masks for providers, and removal of all crystals from the face following treatment.
Treating Specific Lesions
Financial Considerations
Insurance does not reimburse for microdermabrasion procedures. The charges for treatments vary and are largely determined by local prices. Patients may pay for individual treatments, which generally range from $75 to $100. However, MDA is most effective as a series of treatments, and packages of treatments (usually six) may be offered so that patients achieve the best possible results and satisfaction with the procedure. Box 23-1 lists codes for MDA procedures.
1. Grimes PE. Microdermabrasion. Derm Surg. 2005;31(9 Pt 2):1160-1165.
2. Small R. Microdermabrasion. In: Mayeaux E, editor. The Essential Guide to Primary Care Procedures. Philadelphia: Lippincott Williams & Wilkins; 2009:265-277.
3. Cosmetic Surgery National Data Bank 2010 Statistics. American Society for Aesthetic Plastic Surgery; 2010.
4. Small R. Aesthetic procedures in office practice. Am Fam Physician. 2009;80(11):1231-1237.
5. Small R. Aesthetic procedures introduction. In: Mayeaux E, editor. The Essential Guide to Primary Care Procedures. Philadelphia: Lippincott Williams & Wilkins; 2009:195-199.
6. Rubin MG, Greenbaum S. Histologic effects of aluminum oxide microabrasion on facial skin. J Aesthet Dermatol Cosm Surg. 2000;1(4):237-239.
7. Coimbra M, Rohrich RJ, Chao J, et al. A prospective controlled assessment of microdermabrasion for damaged skin and fine rhytides. Plast Reconstr Surg. 2004;113(5):1438-1443.
8. Karimipour DJ, Kang S, Johnson TM, et al. Microdermabrasion with and without aluminum oxide crystal abrasion: a comparative molecular analysis of dermal remodeling. J Am Acad Dermatol. 2006;54(3):405-410.
9. Shim EK, Barnette D, Hughes K, et al. Microdermabrasion: a clinical and histopathologic study. Derm Surg. 2001;27(6):524-530.
10. Hernandez-Perez E, Ibiett EV. Gross and microscopic findings in patients undergoing microdermabrasion for facial rejuvenation. Derm Surg. 2001;27(7):637-640.
11. Tsai RY, Wang CN, Chan HL. Aluminum oxide crystal microdermabrasion. A new technique for treating facial scarring. Derm Surg. 1995;21(6):539-542.
12. Briden ME, Jacobsen E, Johnson C. Combining superficial glycolic acid (alpha-hydroxy acid) peels with microdermabrasion to maximize treatment results and patient satisfaction. Cutis. 2007;79(1 Suppl):13-16.
13. Koch RJ, Hanasono MM. Microdermabrasion. Facial Plast Surg Clin North Am. 2001;9(3):377-382.
14. Blome D. Microdermabrasion. In Pfenninger JL, Fowler GC, editors: Pfenninger and Fowler’s Procedures for Primary Care, 3rd ed, Philadelphia: Mosby/Elsevier, 2010.
15. Freedman BM, Rueda-Pedraza E, Waddell SP. The epidermal and dermal changes associated with microdermabrasion. Derm Surg. 2001;27(12):1031-1033.
16. Rajan P, Grimes PE. Skin barrier changes induced by aluminum oxide and sodium chloride microdermabrasion. Derm Surg. 2002;28(5):390-393.
17. Nootheti PK, Gold MH, Goldman MP. Photodynamic therapy for photorejuvenation. In: Goldman MP, editor. Photodynamic Therapy. Philadelphia: Saunders/Elsevier; 2008:125-135.
18. Freeman MS. Microdermabrasion. Facial Plast Surg Clin North Am. 2001;9(2):257-266.
19. Farris P, Rietschel R. An unusual response to microdermabrasion. Dermatol Surg. 2002;28:606-608.
20. Desai T, Moy RL. Evaluation of the SilkPeel system in treating erythematotelangectatic and papulopustular rosacea. Cosm Dermatol. 2006;19:51-57.
21. Taub A. Photodynamic therapy: other uses. Dermatol Clin. 2007;25(1):101-109.
Hantash BM. Microdermabrasion and dermal infusion. In Pfenninger JL, Fowler GC, editors: Pfenninger and Fowler’s Procedures for Primary Care, 3rd ed, Philadelphia: Mosby/Elsevier, 2011.
Small R, Linder J. Microdermabrasion. In: Small R, Linder J, editors. A Practical Guide to Skin Care Procedures and Products. Philadelphia: Lippincott Williams & Wilkins, 2011.