189 Methamphetamine, Ecstasy, and Other Street Drugs
 History
History
The first documented synthesis of methamphetamine, utilizing ephedrine as a substrate, occurred in 1918 in Japan. A closely related compound, d-amphetamine, had been created 20 years earlier in Germany (Figure 189-1). These compounds are not found naturally, although similar substances such as ephedra (ma-huang), cathine, and cathinone have stimulant properties and are extracted from certain plants. Both amphetamine and methamphetamine originally were used in nasal decongestants and bronchial inhalers beginning in the 1930s. A research report in 1937 claimed amphetamine enhanced work output and intellectual performance, and the legal use of amphetamine increased as a result.1 During this period, amphetamines were noted to reduce impulsive behavior and hyperactivity in children.2 In 1954, methylphenidate (Ritalin, Concerta, Methylin) was approved for this indication. There are a variety of these drugs with slight differences in chemical structure. For example, Adderall combines dextroamphetamine and amphetamine with d-amphetamine saccharate and d,l-amphetamine aspartate. In the fenethylline (Captagon) molecule, theophylline is covalently linked with amphetamine via an alkyl chain.
Millions of doses of amphetamine and methamphetamine were taken by military personnel of all nations during World War II.3 Thereafter, surplus supplies entered civilian markets, most notably Japan. Methamphetamine was widely prescribed for depression and obesity, reaching a peak of 31 million prescriptions in the United States in 1967. During this period, production and distribution was largely controlled by motorcycle gangs who hid the drugs in the crankcase of their motorcycles (hence the street name, “crank”). Amphetamines were outlawed after the United States Drug Abuse and Regulation Control Act of 1970. In the 1980s, a concentrated form of methamphetamine, known as “ice,” “glass,” or “crystal,” became popular.4 Production and trafficking in the United States continues to increase, with significant input from Mexican, Southeast Asian, West African, and European drug cartels. An estimated 12.3 million Americans have tried methamphetamine at least once, and 600,000 are weekly users.5 The global number of users is estimated to be over 50 million. Methamphetamine is the most prevalent illegally manufactured controlled substance in the United States and worldwide.6 Abuse of legally prescribed amphetamines represents a new problem among teenagers and young adults.7
 Pharmacology and Metabolism
Pharmacology and Metabolism
Methamphetamine can be ingested orally, snorted, injected, or smoked. It is lipid soluble and crosses the blood-brain barrier readily. Methamphetamine acts primarily on dopaminergic central nervous system (CNS) cells. In small doses, amphetamines cause release of dopamine from the cytoplasmic pool by exchange diffusion at the membrane dopamine uptake transporter locus.8 Methamphetamine also antagonizes the reuptake of catecholamines by competitive inhibition. As the dose increases, amphetamines diffuse through the presynaptic terminal membrane and bind to the neurotransmitter transporter on the vesicular membrane, resulting in the exchange release of dopamine into the cytoplasm (Figure 189-2). Dopamine is then released into the synapse by reverse transport at the dopamine uptake locus. At even higher doses, methamphetamine diffuses through the cellular and vesicular membranes and alkalinizes the vesicles. This results in dopamine release from the vesicles and delivery into the synapse by reverse transport. Chronic methamphetamine use results in down-regulation of dopamine D1 and D2 receptors.9
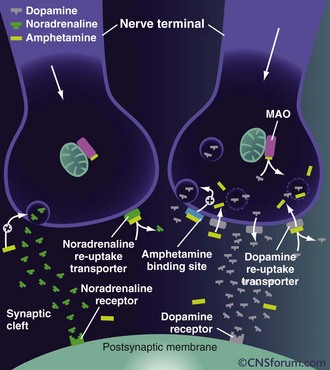
Figure 189-2 Mechanisms of action of high-dose amphetamine on central dopamine and norepinephrine transmission.
(From www.cnsforum.com, with permission.)
Increased norepinephrine at the locus ceruleus results in anorectic and locomotor effects. Increased dopamine in the neostriatum results in glutamate release and inhibition of γ-aminobutyric acid (GABA)-ergic neurons.10 There is evidence that glutamate stimulation contributes significantly to the neurotoxicity of amphetamines.11 The serotonin transporter exhibits abnormal efflux in response to amphetamines.12 Elevated serotonin and dopamine levels within the mesolimbic system may cause hallucinations and psychosis. Release of dopamine in the ventral tegmental area is involved in reward and addiction.13 The rage reaction induced by amphetamines may result from increased release of dopamine in the limbic system.14
Metabolism of methamphetamine occurs in the liver. Dealkylation and demethylation are performed by cytochrome P450 isoenzymes. Metabolites include amphetamine, 4-hydroxymethamphetamine, and 4-hydroxyamphetamine, which are also biologically active stimulants.15 The pharmacokinetics are complex and nonlinear.16 The biological half-life varies from 6 to 15 hours after a single dose. However, the cellular effects may last for days. Excretion occurs primarily in the urine and is affected by pH. Amphetamines are basic, with a typical pKa range from 9 to 10, and renal elimination is enhanced with acidic urine. Approximately 60% of an oral dose is eliminated in the urine within the first 24 hours, with about one-third as intact drug and the remainder as metabolites.
 Acute Effects
Acute Effects
Methamphetamine abusers may present acutely with myriad symptoms including agitation, confusion, tremors, anxiety, hyperthermia, hypertension, tachycardia, and seizures. Less common effects include arrhythmias, cerebral and pulmonary edema, hepatotoxicity, and disseminated intravascular coagulation. Patients may present with atypical chest pain and be at risk for acute coronary syndrome.17 Compared to cocaine, methamphetamine is less likely to cause myocardial ischemia, as it does not interfere with thromboxane production and platelet aggregation.18 Severe abdominal pain may be the result of acute mesenteric vasoconstriction. Necrotizing vasculitis is associated with methamphetamine abuse and can involve multiple organ systems.19 Methamphetamine patients utilize prehospital, emergency department, and hospital resources at a much higher than average rate.20 Methamphetamine crosses the placenta, and use during pregnancy may result in fetal growth retardation, premature birth, developmental delay in neonates, and cognitive deficits in children.21
A dangerous stage of methamphetamine toxicity occurs when an abuser has not slept for days and is irritable and paranoid. This behavior is referred to as “tweaking.” The individual craves more methamphetamine, but it is impossible to achieve the original high, causing unpredictable, unstable, and violent behavior.22 Abusers frequently neglect nutrition and fluid intake during these periods, which can result in rhabdomyolysis when combined with periods of agitation or long periods of inactivity (Figure 189-3).23 The proliferation of clandestine methamphetamine laboratories has increased the incidence of chemical and thermal burn injuries.24 Lead and mercury contamination from illicit production of methamphetamine may result in acute toxicity.25
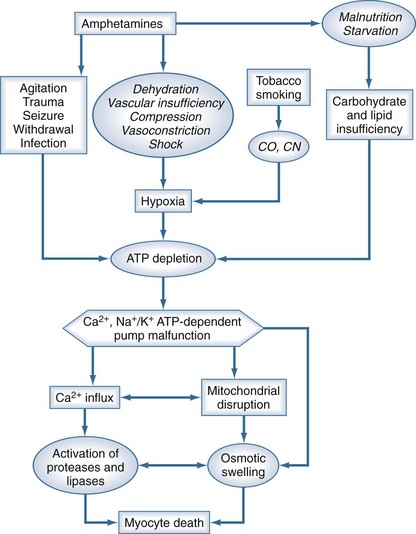
Figure 189-3 Putative relationship between amphetamines and other cofactors with development of rhabdomyolysis.
Chronic use of methamphetamine results in numerous harmful and irreversible cellular and end-organ effects. Long-term use has been shown to result in unique patterns of periodontal disease and tooth loss, a condition commonly referred to as “meth mouth” (Figure 189-4); the maxillary teeth tend to be most prominently affected.26 Chronic users also develop characteristic skin lesions such as prurigo nodularis, also known as “speed bumps,” from constant picking and scratching, usually from delusions of parasitosis. Methamphetamine is also neurotoxic to dopaminergic and serotoninergic cells.27 Chronic users may develop a syndrome similar to Parkinson’s disease.28 Unusual choreoathetoid movements result from increased dopaminergic activity within the striatal area.29 A magnetic resonance imaging (MRI) study of methamphetamine addicts demonstrated permanent gray-matter deficits of the cingulate, limbic, and paralimbic cortices and white matter hypertrophy, which correlated with memory and mood disorders exhibited by the subjects.30 Chronic methamphetamine use results in inhibition of tyrosine hydroxylase, deficits in mitochondrial energy production, and neuronal apoptosis from oxidative stress.31
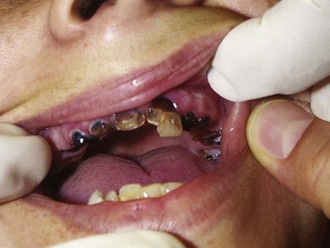
Figure 189-4 Anterior maxillary tooth loss associated with chronic methamphetamine abuse (ungloved finger is the patient’s).
Chronic use has been demonstrated to result in cardiomyopathy, congestive heart failure, and accelerated coronary artery disease.32 Methamphetamine use often results in impulsive behavior, which facilitates transmission of sexually transmitted diseases, viral hepatitis, and human immunodeficiency virus (HIV).33 Furthermore, for those injecting the drug, bacterial and foreign-body contamination may result in endocarditis, tetanus, wound botulism, osteomyelitis, and pulmonary and soft-tissue abscesses.34 Methamphetamine withdrawal syndrome is initially characterized by increased sleep, eating, and dysphoria. This pattern declines into a subacute phase lasting up to 2 weeks.35 These symptoms, coupled with increased risk-taking behavior, result in a higher incidence of traumatic injuries from motor vehicle accidents, falls, and assaults.36 Methamphetamine users often exhibit suspicion and paranoia and are rarely forthcoming about the details of their drug use.37 This pattern includes denial even when presented with a positive toxicology screen or other physical evidence.
 Clinical Management
Clinical Management
Acute methamphetamine toxicity is a true emergency. Airway, breathing, circulation, and temperature should be assessed, with continuous telemetry monitoring and frequent measurement of blood pressure. For patients with hyperthermia, a rectal thermometer is the most accurate method to trend core temperature. Agitated patients initially should be restrained physically, using a team approach (one person per limb) to protect both the patient and staff from harm. This form of restraint should immediately be followed by chemical restraint, using neuroleptic agents or benzodiazepines. Once adequate sedation has been achieved, physical restraints should be removed as soon as feasible. Neuroleptic agents may have a theoretical advantage over benzodiazepines, as these are CNS dopamine antagonists and may directly counteract the excess levels of dopamine in the CNS which result from methamphetamine abuse.37–41 Furthermore, neuroleptics do not affect respiratory drive, whereas multiple or large doses of benzodiazepines may result in respiratory depression. For benzodiazepines, typical dosages are 5 to 10 mg of diazepam or 1 to 4 mg of lorazepam by intravenous (IV) route.
The authors of a prospective study that compared lorazepam with droperidol for control of agitated methamphetamine users concluded that droperidol was longer acting than lorazepam and required fewer repeat doses to achieve sedation.37 Droperidol, or the longer-acting agent, haloperidol, may be administered by either the IV or intramuscular route. Typical doses required are 2.5 to 5 mg of droperidol or 5 to 10 mg of haloperidol. Of note, use of the butyrophenones for this particular indication is considered “off-label.” Droperidol, however, has been used safely for over 3 decades as an antiemetic, and millions of units have been dispensed; nevertheless, concern for QT-interval prolongation and development of torsades de pointes has resulted in a controversial U.S. Food and Drug Administration (FDA) Black Box warning for doses above 2.5 mg.42 Haloperidol is not FDA approved for IV use and also has a Black Box warning for use in elderly patients with dementia. Newer antipsychotics such as olanzapine and ziprasidone also may be effective, but they are not FDA approved for IV use and have not been studied in depth for this indication.43–45 For patients who do not respond to these pharmacologic interventions, rapid-sequence induction using paralytic agents and endotracheal intubation and mechanical ventilation may be required.
 Amphetamine Derivatives
Amphetamine Derivatives
A large group of drugs have been developed from amphetamine, either by clandestine or legitimate chemists; they include agents with enhanced euphoric and hallucinogenic effects, drugs used for weight loss and attention deficit hyperactivity syndromes, and drugs to overcome narcolepsy or fatigue. These drugs share many of the aforementioned acute or chronic physiologic and toxic effects of methamphetamine.46
MDMA, MDA, and Congeners
The first synthesis of 3,4-methylenedioxymethamphetamine (MDMA), alias “Ecstasy,” “X,” or “Adam,” occurred in 1912 by chemists at the German pharmaceutical company, Merck. It was patented in 1914 then shelved. The drug’s source compound and longer-acting metabolite, 3,4-methylenedioxyamphetamine (MDA), had been synthesized earlier in 1910, but was marketed decades later for use as a sedative and appetite suppressant in 1960. However, MDMA, MDA, and 5-dimethoxy-4-methylamphetamine (DOM) instead became popular as “love drugs” in the 1960s. In the 1980s, these drugs were used as pharmaceutical adjuncts to psychotherapy and couples’ therapy to promote empathy, introspection, and open communication. The use of these drugs in this way was heralded in the press, and widespread public misuse ensued. In 1985, MDMA and related drugs became U.S. Drug Enforcement Agency (DEA) Schedule I compounds. Less commonly available hallucinogenic amphetamine derivatives include methylenedioxyethylamphetamine (MDEA), alias “Eve,” 2,5-dimethoxyamphetamine (DMA), 2,4-DMA, 4-bromo-DMA (DOB), propyl-DMA (DPO), p-methoxyamphetamine (PMA), and p-methoxymethylamphetamine (PMMA). The 2C family, which refers to the two carbon atoms that separate the amine from the phenyl ring, are derivatives of the naturally occurring compound, β-phenethylamine. They contain methoxy groups in positions 2 and 5 of the phenyl ring and a hydrophobic 4-phenyl substituent such as iodine (2C-I), bromine (2C-B), and many others.47 Their affinity for CNS serotonin receptors has been demonstrated, and these compounds act as mixed agonists/antagonists with resultant hallucinogenic effects. A toxicology study of patients presenting from nightclubs in Ibiza, Spain, revealed a wide range of detected compounds.48 The most prevalent was MDMA, but others included the aforementioned compounds as well as ketamine and γ-hydroxybutyric acid. Nearly half of the subjects also tested positive for methamphetamine. The most dangerous of these compounds appears to be PMA, alias “Death,” with several deaths reported worldwide.49
Methcathinone
Methcathinone (“Cat”) is an amphetamine derivative that appeared on the clandestine market in the United States in the 1990s and is now classified as a DEA Schedule I substance. It is easily synthesized using recipes found on the Internet. Related compounds, cathine and cathinone, are naturally occurring compounds found in the plant, Cathula edulis (“khat,” “qat,” “chat,” “jaad”), which is endemic to the Middle East and Africa. The clinical use of khat was described in the 11th century in Pharmacy and Therapeutic Art.50 The fresh leaves or stems are chewed, as the stored product loses activity as it dries.
Derivatives Affecting Sleep
Amphetamine-related agents used for narcolepsy and to increase wakefulness are growing in use. In humans, alteration of CNS dopaminergic transmission affects alertness, performance, and quality of sleep. A case-control study of narcoleptic patients showed that methamphetamine caused a dose-dependent decrease in daytime sleep tendency and improvement in task performance in both narcoleptics and controls.51 Amphetamine derivatives have been studied most extensively by the military as a countermeasure to fatigue induced by circadian desynchronosis. Modafinil is an amphetamine derivative that enhances wakefulness, vigilance, and memory. It may have effects within the anterior hypothalamus.52 Its dopamine-releasing action in the nucleus accumbens reward center is weak, and thus its abuse potential is limited. Modafinil is a central α1-adrenergic agonist. It inhibits reuptake of norepinephrine by axon terminals on sleep-promoting neurons of ventrolateral preoptic nucleus and increases excitatory glutamine transmission. This effect in turn reduces GABA transmission. Modafinil is safe and well tolerated and less likely to cause anxiety, agitation, or result in a hypersomnolent rebound effect. It is being studied for attention deficit disorders, Alzheimer’s disease, depression, myotonic dystrophy, multiple sclerosis, schizophrenia, cerebral palsy, and memory decline related to aging. In September 2003, the FDA approved modafinil for the additional indications of treating sleep disorders due to shift work and obstructive sleep apnea. Other similar agents include adrafinil and armodafinil.
Key Points
Cruickshank CC, Dyer KR. A review of the clinical pharmacology of methamphetamine. Addiction. 2009;104:1085-1099.
Hall AP, Henry JA. Acute toxic effects of “Ecstasy” (MDMA) and related compounds: overview of pathophysiology and clinical management. Br J Anaesth. 2006;96:678-685.
Richards JR, Derlet RW, Duncan DR. Methamphetamine toxicity: treatment with a benzodiazepine versus a butyrophenone. Eur J Emerg Med. 1997;4:130-135.
Shoptaw SJ, Kao U, Ling W. Treatment for amphetamine psychosis. Cochrane Database Syst Rev 2009;(1):CD003026.
Yamamoto BK, Moszczynska A, Gudelsky GA. Amphetamine toxicities: classical and emerging mechanisms. Ann N Y Acad Sci. 2010;1187:101-121.
This article examines the myriad toxic effects of methamphetamine and other amphetamine derivatives.
1 Derlet RW, Heischober B. Methamphetamine. Stimulant of the 1990s? West J Med. 1990;153:625-628.
2 Gainetdinov RR, Wetsel WC, Jones SR, Levin ED, Jaber M, Caron MG. Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science. 1999;283:397-401.
3 Rasmussen N. On speed: the many lives of amphetamine. New York: NYU Press; 2008.
4 Anglin MD, Burke C, Perrochet B, Stamper E, Dawud-Noursi S. History of the methamphetamine problem. J Psychoactive Drugs. 2000;32:137-141.
5 Roehr B. Half a million Americans use methamphetamine every week. BMJ. 2005;331:476.
6 Amphetamines and ecstasy. http://www.unodc.org/documents/scientific/ATS/Global-ATS-Assessment-2008-Web.pdf, 2008. accessed April 1, 2010
7 Garnier LM, Arria AM, Caldeira KM, Vincent KB, O’Grady KE, Wish ED. Sharing and selling of prescription medications in a college student sample. J Clin Psychiatry. 2010;71:262-269.
8 Schuldiner S, Steiner-Mordoch S, Yelin R, Wall SC, Rudnick G. Amphetamine derivatives interact with both plasma membrane and secretory vesicle biogenic amine transporters. Mol Pharmacol. 1993;44:1227-1231.
9 Richards TL, Zahniser NR. Rapid substrate-induced down-regulation in function and surface localization of dopamine transporters: rat dorsal striatum versus nucleus accumbens. J Neurochem. 2009;108:1575-1584.
10 Mark KA, Quinton MS, Russek SJ, Yamamoto BK. Dynamic changes in vesicular glutamate transporter 1 function and expression related to methamphetamine-induced glutamate release. J Neurosci. 2007;27:6823-6831.
11 Miyatake M, Narita M, Shibasaki M, Nakamura A, Suzuki T. Glutamatergic neurotransmission and protein kinase C play a role in neuron-glia communication during the development of methamphetamine-induced psychological dependence. Eur J Neurosci. 2005;22:1476-1488.
12 Hilber B, Scholze P, Dorostkar MM, Sandtner W, Holy M, Boehm S, et al. Serotonin-transporter mediated efflux: a pharmacological analysis of amphetamines and non-amphetamines. Neuropharmacology. 2005;49:811-819.
13 Drevets WC, Gautier C, Price JC, Kupfer DJ, Kinahan PE, Grace AA, et al. Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biol Psychiatry. 2001;49:81-96.
14 Xu JH, Shen H, Zhang YP. Amphetamine-induced rage reaction in mice and its mechanism. Yao Xue Xue Bao. 1992;27:566-571.
15 Li L, Everhart T, Jacob PIii, Jones R, Mendelson J. Stereoselectivity in the human metabolism of methamphetamine. Br J Clin Pharmacol. 2010;69:187-192.
16 Cruickshank CC, Dyer KR. A review of the clinical pharmacology of methamphetamine. Addiction. 2009;104:1085-1099.
17 Turnipseed SD, Richards JR, Kirk JD, Diercks DB, Amsterdam EA. Frequency of acute coronary syndrome in patients presenting to the emergency department with chest pain after methamphetamine use. J Emerg Med. 2003;24:369-373.
18 Singh S, Arora R, Khraisat A, Handa K, Bahekar A, Trivedi A, et al. Increased incidence of in-stent thrombosis related to cocaine use: case series and review of literature. J Cardiovasc Pharmacol Ther. 2007;12:298-303.
19 Ho EL, Josephson SA, Lee HS, Smith WS. Cerebrovascular complications of methamphetamine abuse. Neurocrit Care. 2009;10:295-305.
20 Richards JR, Bretz SW, Johnson EB, Turnipseed SD, Brofeldt BT, Derlet RW. Methamphetamine abuse and emergency department utilization. West J Med. 1999;170:198-202.
21 Salisbury AL, Ponder KL, Padbury JF, Lester BM. Fetal effects of psychoactive drugs. Clin Perinatol. 2009;36:595-619.
22 Donovan DM, Wells EA. “Tweaking 12-Step”: the potential role of 12-Step self-help group involvement in methamphetamine recovery. Addiction. 2007;102(Suppl 1):121-129.
23 Richards JR, Johnson EB, Stark RW, Derlet RW. Methamphetamine abuse and rhabdomyolysis in the ED: a 5-year study. Am J Emerg Med. 1999;17:681-685.
24 Santos AP, Wilson AK, Hornung CA, Polk HCJr, Rodriguez JL, Franklin GA. Methamphetamine laboratory explosions: a new and emerging burn injury. J Burn Care Rehabil. 2005;26:228-232.
25 Norton RL, Burton BT, McGirr J. Blood lead of intravenous drug users. J Toxicol Clin Toxicol. 1996;34:425-430.
26 Richards JR, Brofeldt BT. Patterns of tooth wear associated with methamphetamine use. J Periodontol. 2000;71:1371-1374.
27 Yamamoto BK, Moszczynska A, Gudelsky GA. Amphetamine toxicities: classical and emerging mechanisms. Ann N Y Acad Sci. 2010;1187:101-121.
28 Thrash B, Thiruchelvan K, Ahuja M, Suppiramaniam V, Dhanasekaran M. Methamphetamine-induced neurotoxicity: the road to Parkinson’s disease. Pharmacol Rep. 2009;61:966-977.
29 Sperling LS, Horowitz JL. Methamphetamine-induced choreoathetosis and rhabdomyolysis. Ann Intern Med. 1994;121:986.
30 Berman S, O’Neill J, Fears S, Bartzokis G, London ED. Abuse of amphetamines and structural abnormalities in the brain. Ann N Y Acad Sci. 2008;1141:195-220.
31 Cadet JL, Krasnova IN. Molecular bases of methamphetamine-induced neurodegeneration. Int Rev Neurobiol. 2009;88:101-119.
32 Yeo KK, Wijetunga M, Ito H, Efird JT, Tay K, Seto TB, et al. The association of methamphetamine use and cardiomyopathy in young patients. Am J Med. 2007;120:165-171.
33 Cheng WS, Garfein RS, Semple SJ, Strathdee SA, Zians JK, Patterson TL. Binge use and sex and drug use behaviors among HIV(-), heterosexual methamphetamine users in San Diego. Subst Use Misuse. 2010;45:116-133.
34 Offerman SR, Schaefer M, Thundiyil JG, Cook MD, Holmes JF. Wound botulism in injection drug users: time to antitoxin correlates with intensive care unit length of stay. West J Emerg Med. 2009;10:251-256.
35 McGregor C, Srisurapanont M, Jittiwutikarn J, Laobhripatr S, Wongtan T, White JM. The nature, time course and severity of methamphetamine withdrawal. Addiction. 2005;100:1320-1329.
36 London JA, Utter GH, Battistella F, Wisner D. Methamphetamine use is associated with increased hospital resource consumption among minimally injured trauma patients. J Trauma. 2009;66:485-490.
37 Richards JR, Derlet RW, Duncan DR. Methamphetamine toxicity: treatment with a benzodiazepine versus a butyrophenone. Eur J Emerg Med. 1997;4:130-135.
38 Derlet RW, Albertson TE, Rice P. The effect of haloperidol in cocaine and amphetamine intoxication. J Emerg Med. 1989;7:633-637.
39 Derlet RW, Albertson TE, Rice P. Antagonism of cocaine, amphetamine, and methamphetamine toxicity. Pharmacol Biochem Behav. 1990;36:745-749.
40 Derlet RW, Rice P, Horowitz BZ, Lord RV. Amphetamine toxicity: experience with 127 cases. J Emerg Med. 1989;7:157-161.
41 Ruha AM, Yarema MC. Pharmacologic treatment of acute pediatric methamphetamine toxicity. Pediatr Emerg Care. 2006;22:782-785.
42 Richards JR, Schneir AB. Droperidol in the emergency department: is it safe? J Emerg Med. 2003;24:441-447.
43 Shoptaw SJ, Kao U, Ling W. Treatment for amphetamine psychosis. Cochrane Database Syst Rev 2009;(1):CD003026.
44 Misra LK, Kofoed L, Oesterheld JR, Richards GA. Olanzapine treatment of methamphetamine psychosis. J Clin Psychopharmacol. 2000;20:393-394.
45 Leelahanaj T, Kongsakon R, Netrakom P. A 4-week, double-blind comparison of olanzapine with haloperidol in the treatment of amphetamine psychosis. J Med Assoc Thailand. 2005;88(Suppl 3):43-52.
46 Hall AP, Henry JA. Acute toxic effects of ‘Ecstasy’ (MDMA) and related compounds: overview of pathophysiology and clinical management. Br J Anaesth. 2006;96:678-685.
47 Drees JC, Stone JA, Wu AH. Morbidity involving the hallucinogenic designer amines MDA and 2C-I. J Forensic Sci. 2009;54:1485-1487.
48 Lora-Tamayo C, Tena T, Rodríguez A, Moreno D, Sancho JR, Enseñat P, et al. The designer drug situation in Ibiza. Forensic Sci Int. 2004;140:195-206.
49 Kraner JC, McCoy DJ, Evans MA, Evans LE, Sweeney BJ. Fatalities caused by the MDMA-related drug paramethoxyamphetamine (PMA). J Anal Toxicol. 2001;25:645-648.
50 Al-Motarreb A, Baker K, Broadley KJ. Khat: pharmacological and medical aspects and its social use in Yemen. Phytother Res. 2002;16:403-413.
51 Mitler MM, Hajdukovic R, Erman MK. Treatment of narcolepsy with methamphetamine. Sleep. 1993;16:306-317.
52 Kumar R. Approved and investigational uses of modafinil: an evidence-based review. Drugs. 2008;68:1803-1839.