17 Metastatic Tumors in the Lung
A Practical Approach to Diagnosis
The most common form of pulmonary neoplasm is a metastasis from outside the lungs. Based on autopsy data, the lungs are involved by metastatic lesions in 25% to 55% of malignant diseases,1–5 and, in up to one fourth of those cases, the pulmonary parenchyma and pleura are the only sites of distant spread.4 On the other hand, the most common lung tumor encountered by a practicing surgical pathologist or cytopathologist is primary bronchogenic carcinoma; distinguishing primary from secondary pulmonary neoplasms is a major challenge. This chapter provides a concise background discussion of the pathobiologic principles and clinicoradiologic findings of metastases in the lungs and offers a framework for the practitioner to identify such lesions with an optimal level of certainty.
Routes of Spread for Intrapulmonary Metastases
Vascular Metastases
Most metastatic tumors in the lungs have arrived at that destination hematogenously. There are two principal reasons for this: The lungs receive the entire cardiac output, and they contain a rich vascular network, comprising a huge capillary bed. The detailed principles underlying vascular metastasis have been outlined elsewhere.6–8 Malignant tumors may contain subclones9 of cells with differing metastatic potential, and some of them acquire the ability to enter the bloodstream as microemboli. At selected distant sites, they adhere to endothelial basement membranes, and through a process known as extravasation, the tumor cells move through the extracellular matrix to form metastatic deposits in various parenchymal structures. In this paradigm, the original micrometastasis then proliferates to yield a larger mass, which later (often as long as several years) may become visible clinically or radiographically. Most pulmonary metastases show nests of neoplastic cells that are surrounded by, and intercalated with, a variable quantity of fibrous stroma. At this stage, no cells typically remain inside the pulmonary arterial, venous, or capillary system.
The rate at which potentially metastasizing cellular subclones develop (if they do at all) in primary tumors varies considerably; the probability that such lesions will spread hematogenously depends on both tumor-related factors and conditions in the milieu of the host tissues. For reasons that are largely unknown, some tumors—such as osteosarcoma—often shed micrometastases before the primary tumor is detected clinically; other tumors may show metastasis only very late in their biologic evolution.10
The clinical presentation of patients with hematogenous pulmonary metastases is variable. Most patients have no symptoms,11 and their lesions are detected only through imaging studies that are undertaken for staging purposes or for surveillance during treatment. The radiographic appearance of metastatic pulmonary lesions may be that of a single central or peripheral mass, multiple central or peripheral masses, diffuse infiltrates, or a combination of the latter two possibilities. If a patient is symptomatic, the clinical findings reflect the location and extent of the metastatic deposits and commonly include chest pain, dyspnea, cough, hemoptysis, and wheezing, to name a few.
In addition to the concept of microembolization as outlined earlier, tumors may also spread as macroscopic emboli that involve large or medium pulmonary arteries.12 Large-vessel tumor emboli may cause acute heart failure, sudden death, rapidly evolving pulmonary hypertension, and pulmonary infarction, as also seen with banal intravascular thromboemboli associated with deep venous thrombosis.13–15 The tumors that most commonly give rise to macroscopic emboli are those associated with major systemic veins (e.g., renal or hepatic carcinomas invading the renal and hepatic veins) and primary tumors of the heart (myxomas and sarcomas).16
In rare cases, hematogenous metastasis principally occurs in the lung with occlusive luminal tumor plugs in small vessels (arterioles and capillaries) without interstitial involvement or formation of masses.17 In a sense, those tumors have not gone through all of the biologic steps normally associated with the metastatic sequence, but they are nonetheless potentially lethal because they may cause severe pulmonary hypertension. Neoplasms that may show that pattern of spread (sometimes called tumor-related thrombotic pulmonary microangiopathy18) include carcinomas of the breast, gastrointestinal tract, liver, pancreas, uterus, gallbladder, prostate, and ovary.17 Soares et al. reported that most malignant tumors that occlude small pulmonary vascular channels also can be seen simultaneously within larger vessels.19 Patients with metastatic microvascular occlusion typically present with progressive dyspnea and cor pulmonale.17,18
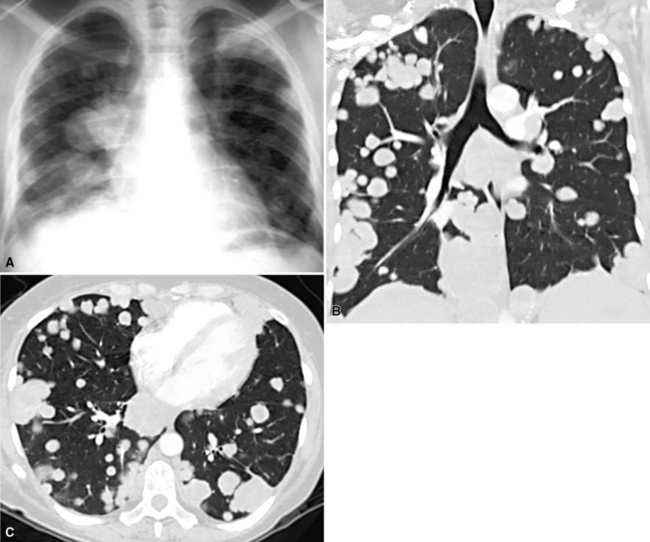
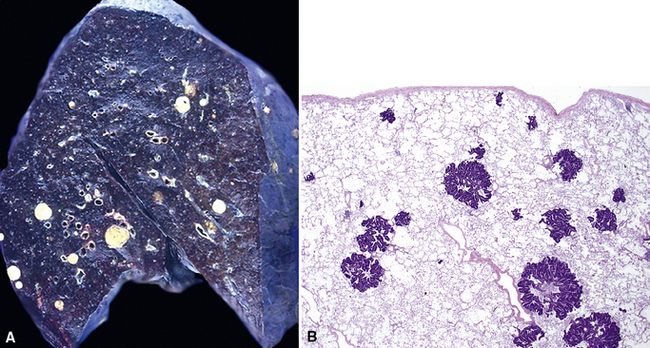
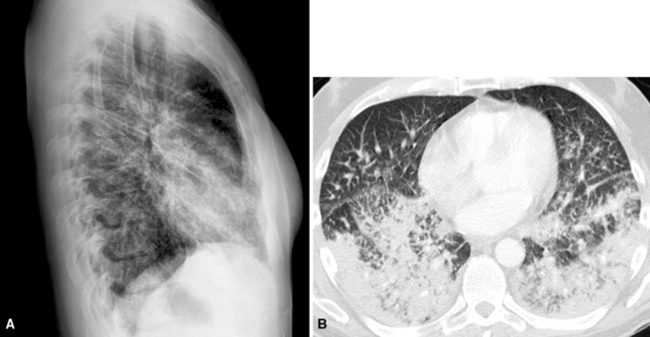
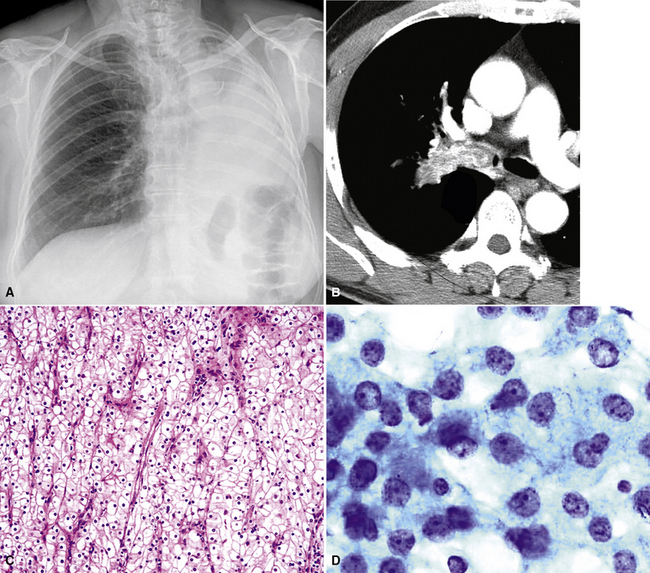
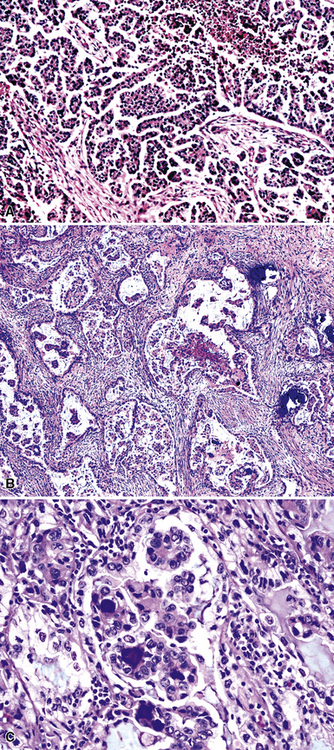
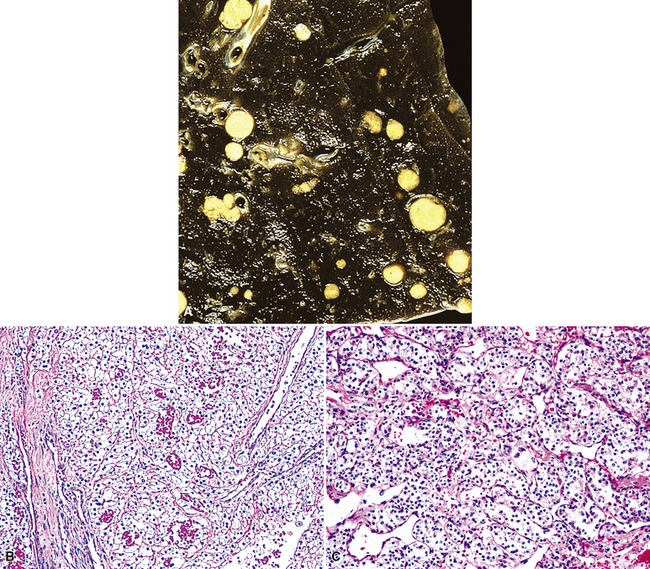
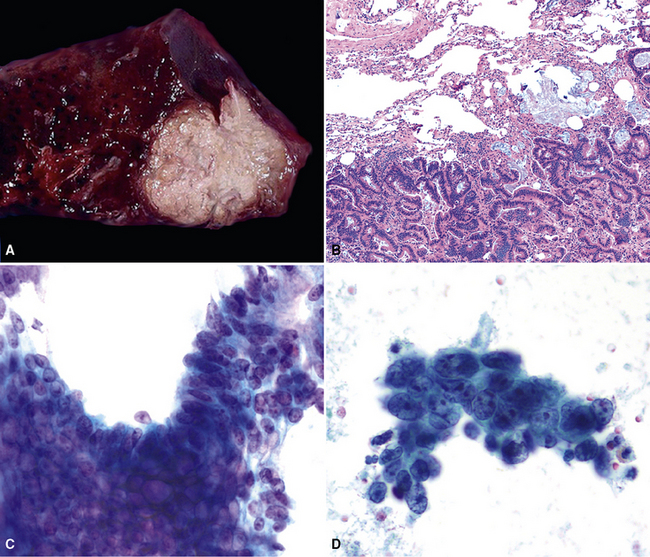
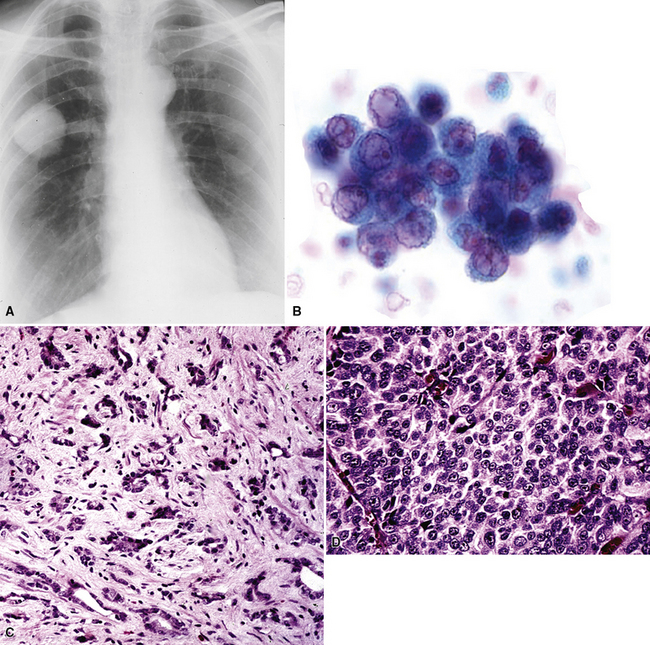
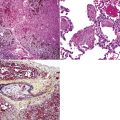
Hematogenous metastasis in the lung is associated with a spectrum of radiologic findings. The most common roentgenographic appearance is that of multiple, bilateral, variably sized masses (Figs. 17-1 to 17-3); occasionally, a solitary intraparenchymal nodule is seen. The metastatic implants usually appear in the mid- to lower lung field because that is where the greatest parenchymal perfusion occurs. In up to 90% of patients with bilateral secondary disease, the lesions are peripheral and subpleural (Fig. 17-4).1,20 Variability in the size of the metastatic nodules is related to the different “ages” of the lesions, dissimilar growth rates, and other factors. Such lesions are usually smaller than primary pulmonary carcinomas, measuring less than 3 to 4 cm in maximum diameter. Metastases also enlarge more rapidly than bronchogenic carcinomas do.
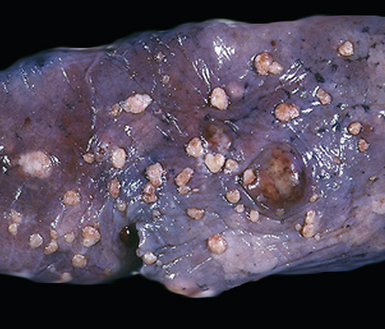
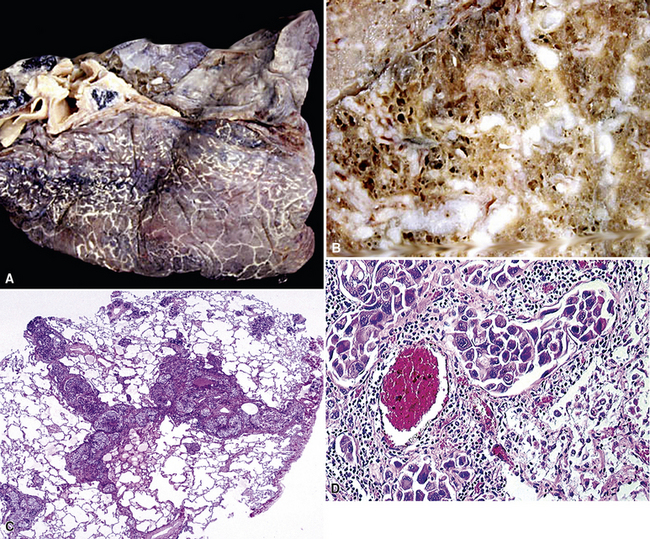
Figure 17-2 Gross photograph of metastatic carcinoma in the lungs showing several subpleural nodules of varying size.
Solitary metastases in the lungs are seen in up to 10% of all malignant tumors involving those organs.21–23 Filderman and coworkers suggested that solitary lesions larger than 5 cm in diameter most likely originate in the breast, kidney, or soft tissue.6 Metastasis in the form of a solitary mass on plain films may appear as multiple contiguous or coalescent masses on computed tomograms. Quint and colleagues reported that, statistically, patients with carcinomas of the head and neck, bladder, breast, cervix, bile ducts, esophagus, ovary, prostate, or stomach were more likely to have a solitary metastasis to the lung than a new primary bronchogenic tumor, even when a significant period had elapsed after diagnosis of the extrapulmonary neoplasms.24 On the other hand, patients with a history of malignant melanoma, sarcoma, or malignant germ cell tumor were more likely to have a second primary malignancy of the lung under the same circumstances.24
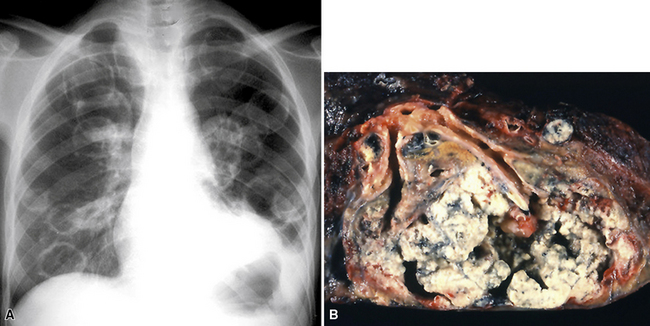
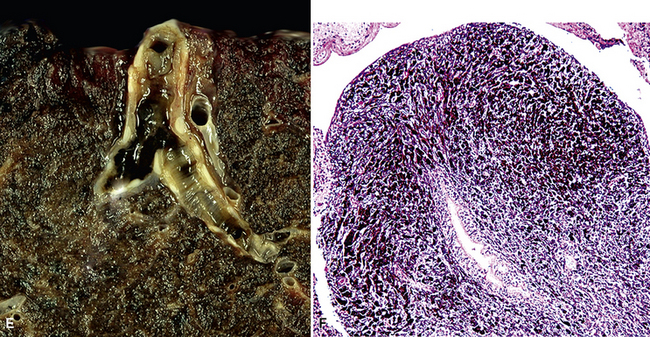
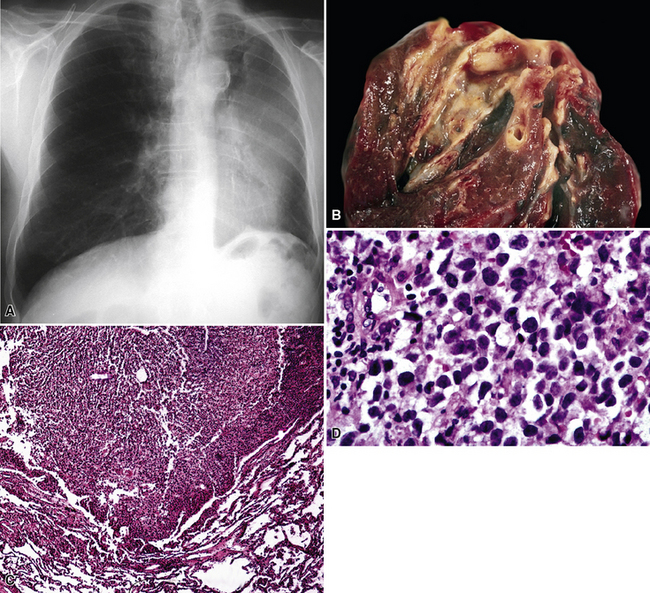
Large metastatic foci may undergo cavitation or result in pneumothorax or bronchopleural fistulization (Fig. 17-5). The most common secondary tumor that cavitates is squamous cell carcinoma, often originating in the head and neck.25 Metastatic sarcoma and adenocarcinoma also may exhibit that feature.25 Pneumothoraces and transpleural fistulae result from erosion by the metastatic tumor through the pleura, as seen most frequently in pediatric mesenchymal malignancies (e.g., osteosarcoma).26
Lymphogenous Metastases
One study27 reported that up to 56% of pulmonary metastases were lymphogenously mediated, although a more generally accepted figure is 5% to 8%.28,29 Most patients with lymphatic-borne lung metastases have a poor prognosis, with 90% dying within 6 months.29
The radiographic appearance of lymphangitic metastasis is variable; in 50% of cases, plain chest films show no apparent abnormality.30 Yang and Lin described four radiologic patterns for this condition29:
With the first two patterns representing secondary tumors, pleural, or less frequently, hilar lymph nodal involvement may be seen. More than 90% of these cases are metastatic adenocarcinomas.29 It is estimated that fewer than 1% of patients with pulmonary metastases from tumors arising outside the thorax also have hilar adenopathy.31 Although this figure may appear low, hilar adenopathy in patients with secondary pulmonary malignancies is not uncommon in absolute terms because of the high prevalence of patients with metastatic disease.
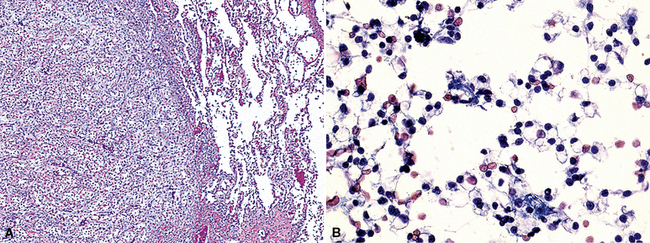
Carcinomas may gain access to the pulmonary lymphatic system by retrograde spread, direct invasion of the pulmonary lymphatics, and passage through adjacent blood vessels. The most common route of spread is the last of these three possibilities32; tumor first spreads hematogenously to the lung and results in small areas of interstitial growth. The neoplastic cells are then absorbed into the lymphatics and permeate further throughout the lungs (Fig. 17-7). Tumor in alveolar spaces may likewise be absorbed through the lymphatics adjacent to terminal bronchioles. Therefore, patients who have lymphangitic intrapulmonary metastases generally also have had previous hematogenous spread.32 Direct lymphatic invasion is most often associated with tumors arising in the breast or stomach29,33,34; in this mode, metastases may be seen exclusively in the pulmonary lymphatic spaces, without the formation of mass lesions. Other metastatic carcinomas capable of showing the same pattern of spread are those arising in the ovary, thyroid, bladder, esophagus, and liver.29,32
Pleural Metastases
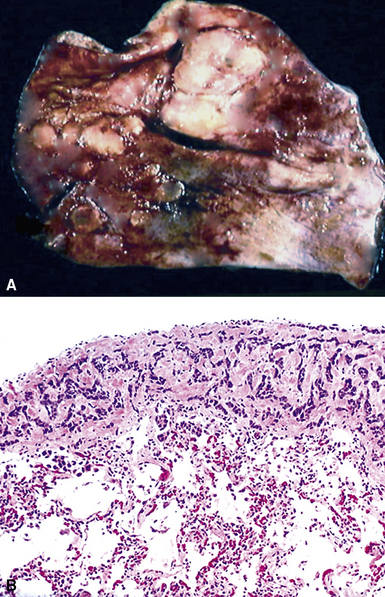
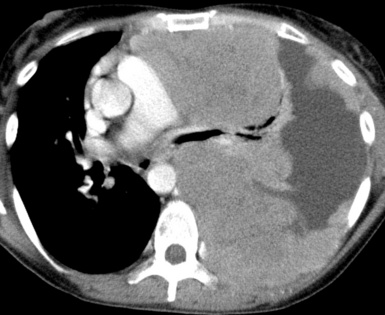
Metastases are the most common form of pleural malignancy. Most derive from primary neoplasms of the chest wall, mediastinum, or lungs,6 although extrathoracic primary malignancies are also well represented. The largest tumor nodules tend to be basally situated in the chest35 (Fig. 17-8).
At least two thirds of malignant pleural effusions can be diagnosed by cytologic sampling of the pleural fluid. More than 90% of cases are recognized on the first specimen,36,37 but sensitivity predictably increases with successive sampling; three specimens are routinely recommended if the clinical suspicion of pleural metastasis is high.38
Malignancy is second only to congestive heart failure as a cause of pleural effusions.39 Neoplastic effusions are typically described as “massive” or “copious,” ranging up to 2500 mL in volume,40 and they are often bloody. Nevertheless, malignant involvement of the pleura may also be associated with scant fluid production and a serous character.35 Obviously, not all pleural effusions in patients with a history of malignancy contain tumor cells41; benign effusions in such cases may be secondary to lymphatic obstruction, altered lymphatic drainage as a result of chemotherapy or radiation therapy,42 heart failure, or other causes. Because most sarcomas do not spread via lymphatic channels, metastases in the lung and pleura are not usually accompanied by a tumorous effusion.43 Up to 90% of malignant pleural effusions caused by metastatic lung or breast cancers are ipsilateral with regard to the site of the original tumor.35 Patients with malignant pleural effusions typically have a dismal prognosis, and most die within a few months of diagnosis.44–46 Selected subgroups of patients, such as those with lymphoma, breast cancer, or some pediatric malignancies, may fare somewhat better.
Chretien and Jaubert reported that 42% of cytologically sampled pleural effusions contained malignant cells.47 The likely site of tumor origin in such cases appears to depend on patient demographics, although most series have reported that primary pulmonary carcinomas are the most common.45,48 Squamous cell carcinomas of the lung do not usually involve the pleural fluid; however, adenocarcinomas usually do (Fig. 17-9), followed by small cell neuroendocrine carcinoma.49,50 Almost any other extrathoracic malignancy may metastasize to the pleural space, but the most common tumors that do so are carcinomas arising in the breast, gastrointestinal tract, and ovary; non-Hodgkin lymphoma is also well represented.45,48,51 Up to 7% of metastatic carcinomas in the pleura must be classified as originating in an unknown primary location.48 In one analysis of malignant pleural effusions, women predominated by a ratio of 2:1,40 but no sex preferences were seen in other series.45
Endobronchial Metastases
Endobronchial metastases are considered in a separate category because of their distinctive clinical findings, principally the syndrome of “adult-onset asthma.” The reported incidence of endobronchial and endotracheal metastatic disease is 1% to 18% of patients who also have intrapulmonary metastases.11,52,53 The most common sites of tumor origin in patients from North America and Western Europe are the breast, bone, soft tissue, large intestine, kidney, and skin (melanoma).11,54–58 More than one third of endobronchial metastases are sarcomatous.53,57 In populations with a high prevalence of acquired immune deficiency syndrome, the most common secondary malignancies of the bronchi are Kaposi sarcoma and malignant lymphoma.59 Nasopharyngeal and laryngeal carcinomas are frequent sources of endobronchial metastasis in Asia.60
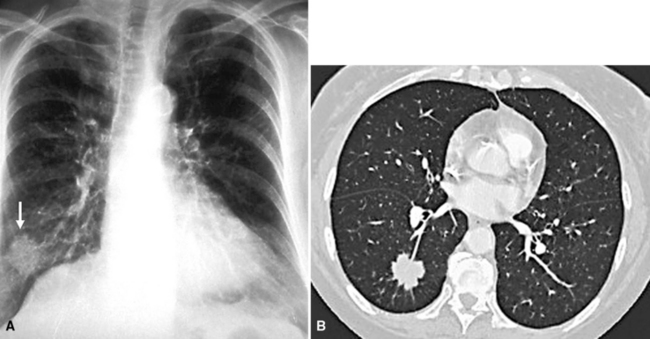
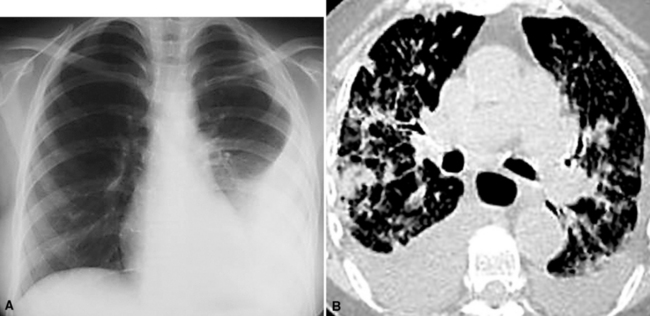
Endobronchial metastases may be either hematogenous or lymphogenous.11,53,57 Aerogenous spread of an upper-airway malignancy has also been suggested as a possibility. Tumors that originate in the lung, hilar lymph nodes, or mediastinum may spread by direct extension into the bronchial system. Endobronchial lesions cause symptoms early in their course of growth, namely, cough with sputum production, dyspnea, wheezing, infection, and hemoptysis.60,61 However, up to 25% of patients are asymptomatic.58 Radiographically, an endobronchial mass is typically visible only on computed tomography or magnetic resonance scan; plain film studies commonly show only postobstructive consolidation or atelectasis (Fig. 17-10). The mean interval between diagnosis of the original tumor and the appearance of endobronchial metastasis is 4 to 5 years.61 These patients have a poor survival, with a median of 11 months61; patients with breast cancer may have a better prognosis.54,57,58
Modalities for the Diagnosis of Pleuropulmonary Metastases
The diagnostic tests that are typically used for any suspected pleuropulmonary tumor are also applicable to the study of metastatic disease. These include sputum cytology,62,63 bronchoscopy with brushing, washing, alveolar lavage, transbronchial or transtracheal aspiration and biopsy,64–66 transthoracic fine-needle aspiration (FNA),67,68 thoracoscopic biopsy (video-assisted thoracic surgery),69–71 open thoracotomy and biopsy, and effusion cytology.46 In some cases, expectant management is undertaken, with sequential radiologic studies.72,73 Computed tomography scans may be obtained to further delineate abnormalities and possibly to aid in the distinction between primary and secondary malignancies; for example, mediastinal adenopathy favors a primary lung tumor.74 When multiple radiographic lesions of the lung or pleura are detected radiographically in some patients with well-documented histories of malignancy, further diagnostic evaluations may be eschewed.
For the most part, the diagnostic accuracy of tests that yield tissue for pathologic examination generally has not been determined in this specific context. The accuracy is believed to depend principally on the size and location of the lesion rather than its specific histologic nature. For example, the sensitivity of FNA is 93% if the lesion is larger than 2 cm in diameter and 60% for those smaller than 1 cm. Higher sensitivity is realized in the sampling of peripheral nodules compared with central lesions, regardless of whether they are primary or secondary.75 Pilotti et al. reported that the sensitivity of FNA in the detection of metastatic pleuropulmonary disease was 89%, whereas it was 92% for primary malignancies.76 Another interinstitutional study reported 96% specificity for transthoracic FNA.77 The sensitivity of bronchoscopy also depends on the location of the lesion; as expected, that technique is particularly well suited for the visualization and sampling of endobronchial metastases.64–66 Thoracoscopy is a sensitive means of accessing peripheral lesions and has a high level of accuracy overall. It may be viewed as a treatment option as well as a diagnostic test in patients who have limited metastatic disease, especially if lung function is compromised.69–71
Kern and Schweizer concluded that the sensitivity of sputum cytology for the detection of intrapulmonary metastasis was similar to that associated with primary lung cancers.78 However, that technique is much more likely to be productive if the metastatic lesions are large and centrally located.78
Practical Approach to Differential Diagnosis
A definite challenge in pulmonary pathology is determining whether a newly detected lung mass is primary or secondary in patients with or without a history of extrapulmonary malignancy. If no previous tumor has been seen and the lesion has the morphologic attributes of a nonpulmonary proliferation, it is necessary to search for a primary site. Malignancies that are clinically occult and present with pulmonary metastases are not unusual, and they account for approximately 2% to 5% of all metastatic carcinomas of unknown origin (MCUOs).79,80 Because of the treatment-related and prognostic issues concerning secondary malignancies of the lung, it may be decided that additional resources are not justified to determine the primary location of the tumor.81
With regard to the general distinction of primary and metastatic pulmonary tumors, one generally depends on radiographic findings, histologic features, microscopic comparison of the current lesion with any previous malignancies, and the use of ancillary pathologic studies, such as immunohistochemistry, cytochemistry, molecular biologic techniques, cytogenetic methods,82 and electron microscopy. If paraffin-embedded tissue from previous tumor material is available, immunopathologic studies of the previous tumor and the current specimen can be obtained comparatively.
Useful information for the distinction between primary and secondary neoplasms in the lung may be derived from details of the clinical evaluation and physical findings.83,84 For example, the characteristically “spiculated” appearance of primary lung cancers (Fig. 17-11) on imaging studies of the chest distinguishes them from the more rounded and well-delimited appearance of metastases. Unfortunately, such information is often not made available to pathologists, even though it is well known to enhance diagnostic accuracy.85 A high index of suspicion must be maintained, and communication with the radiologist should occur whenever the pathologist has increased concern, histologic or otherwise, that a tumor may be a metastasis. Open communication is essential if the patient has a history of oncologic disease.
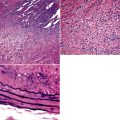
The light microscopic features of any given lesion are the cornerstone of pathologic diagnosis. The appearance of the lesion after hematoxylin and eosin staining is often sufficient to determine whether the tumor is primary or metastatic. Carcinomas arising in the lung typically have evolved over several years before coming to clinical attention. As a consequence, the host responds to such lesions by surrounding them with an irregular cuff of fibroinflammatory tissue (Fig. 17-12). The mixture of proliferation and degeneration that characterizes primary carcinomas commonly causes central zones of fibrosis, with entrapment of some residual native pulmonary structures. In contrast, metastases to the lung parenchyma have a “clean” interface with the surrounding tissue and are not associated with peripheral zones of fibroinflammatory response. Because they are rapidly growing vis-à-vis bronchogenic neoplasms, metastatic carcinomas also lack centrally sclerotic regions. These “rules” do not apply universally to all tumor types. Specifically, primary and metastatic sarcomas, adenocarcinomas with a “lepidic” growth pattern, and small cell neuroendocrine carcinomas are essentially superimposable morphologically.
Adenocarcinoma Variants
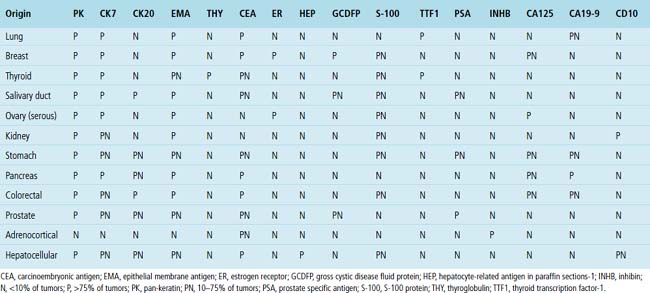
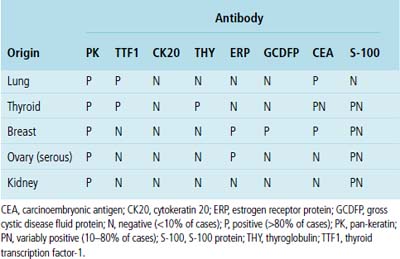
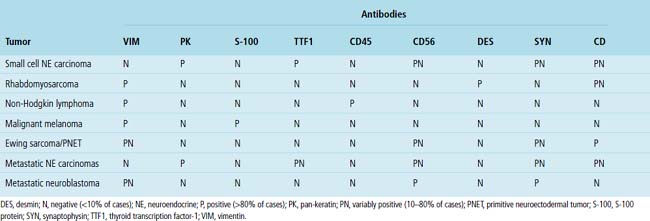
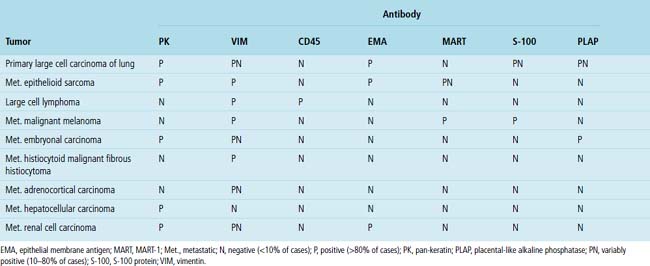
Adenocarcinoma is the most common form of primary lung cancer. In patients who have a history of an extrapulmonary adenocarcinoma, the distinction between a primary and secondary lesion may be challenging. In some cases, morphologic appearances alone are sufficient to accomplish that task, as discussed later. Immunopathology is also helpful in the recognition of some of these tumors. Table 17-1 shows the immunohistologic profile of specific adenocarcinomas based on their site of origin.86
Papillary Adenocarcinomas
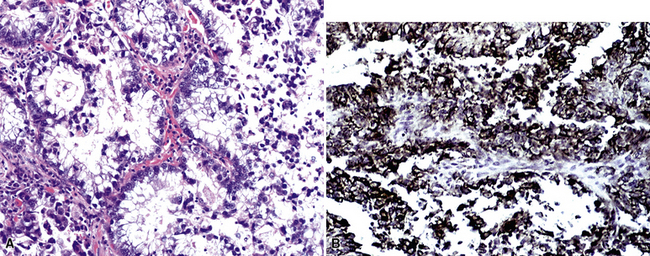
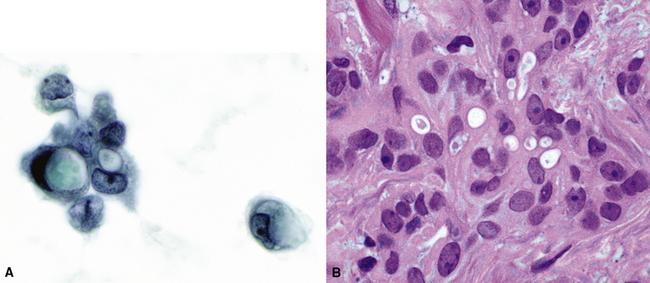
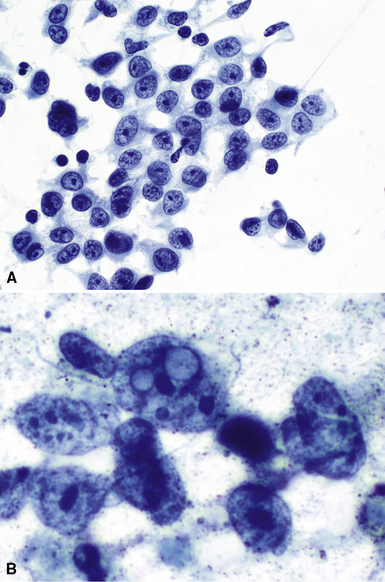
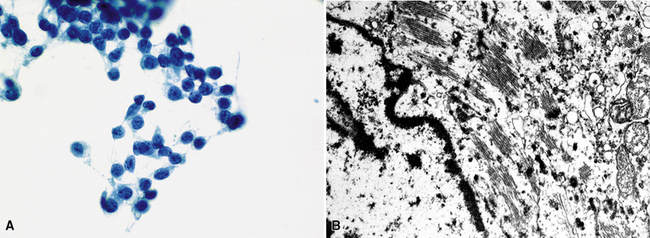
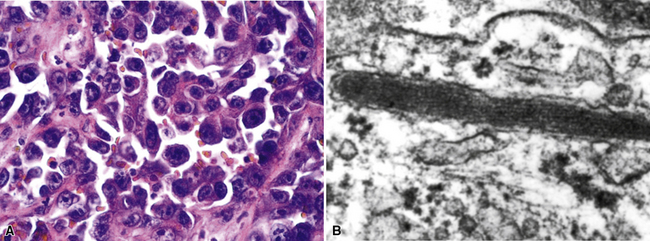
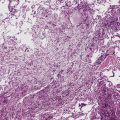
Silver and Askin reported that primary papillary pulmonary adenocarcinomas—defined as such if 75% or more of the neoplasm shows micropapillary architecture—are not uncommon87; moreover, micropapillae may be seen in conventional adenocarcinomas of the lung88,89 (Fig. 17-13). Metastatic adenocarcinomas in the lung also may contain micropapillary structures. In FNA specimens, such tumors show fibrovascular fragments covered by cuboidal or low-columnar neoplastic cells.
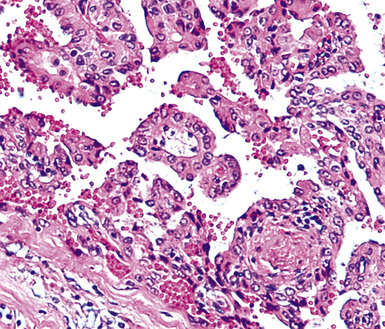
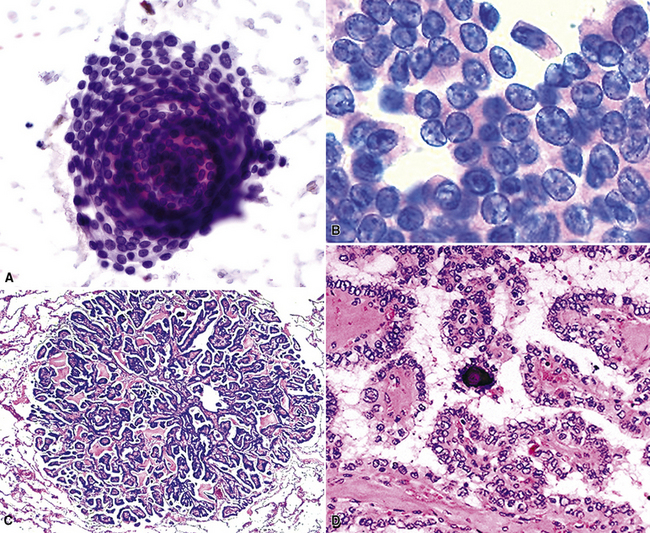
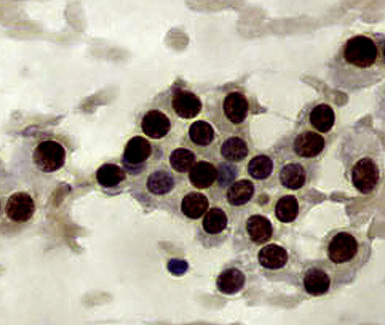
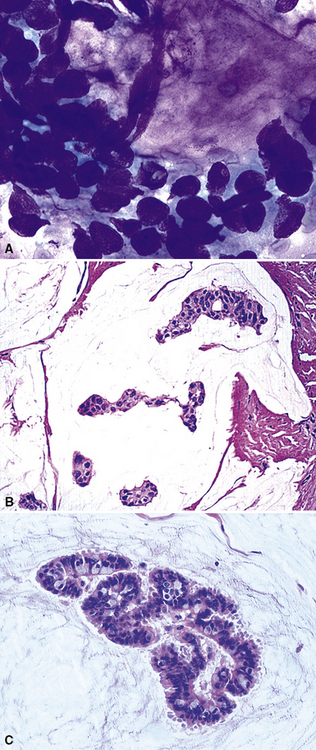
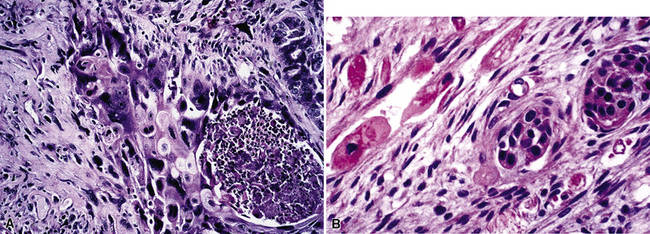
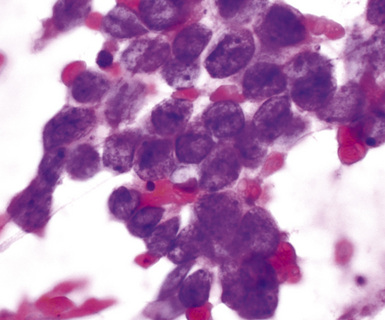
Metastatic papillary adenocarcinomas may originate in the thyroid, breast, ovary, or kidney (Fig. 17-14). Thyroid carcinomas of all histologic subtypes have the potential to metastasize to the lung. Of all thyroid carcinomas that spread to distant sites other than lymph nodes, up to 50% involve the pulmonary parenchyma.90–92 Papillary thyroid carcinoma almost always metastasizes to regional cervical lymph nodes beforehand90; in addition, this tumor type may directly invade the trachea and produce an endoluminal mass. Anaplastic thyroid carcinoma shares the latter potential. Hilar intrathoracic and mediastinal lymph nodes are also involved by papillary thyroid carcinoma in half of patients with lung metastasis.93 Secondary papillary thyroid carcinoma may grow very slowly and remain solitary for extended periods, simulating the biologic characteristics of a primary pulmonary neoplasm.90 In addition to its papillary substructure, other cytologic clues to metastatic papillary thyroid carcinoma include its characteristic nuclear features—including nuclear grooves, nuclear membrane irregularities, cytoplasmic invaginations (pseudoinclusions), and nuclear overlap—as well as the formation of colloid and psammoma bodies (Fig. 17-15).
All types of ovarian carcinoma may metastasize to the lungs, and up to 50% of stage IV cases feature pulmonary involvement.94 The papillary serous form of ovarian cancer is the most common subtype. The pleura is often involved early, by lymphatic spread through the diaphragm, and the peripheral lung parenchyma is then affected. Malignant pleural effusions caused by papillary serous carcinomas are seen in 40% of all cases with metastases,94 and solitary pulmonary nodules are present in 7%.95 Lymphangitic intrapulmonary growth of ovarian malignancies is associated with a rapid demise.96,97
Immunopathologic studies to determine the site of origin of a papillary carcinoma in the lung are outlined in Table 17-2.86 The authors recommend using at least one marker (e.g., vimentin or pan-keratin) that should be positive in each of the differential diagnostic possibilities to establish the antigenic integrity of the tissue.98 In this setting, the highest specificity of immunopathologic identification is associated with tumors of thyroid, pulmonary, or mammary origin. Metastatic papillary tumors arising in the kidneys or ovaries are more difficult to distinguish from others definitively with immunohistology, although CA-125 reactivity is a consistent feature of ovarian epithelial neoplasms (Fig. 17-16).
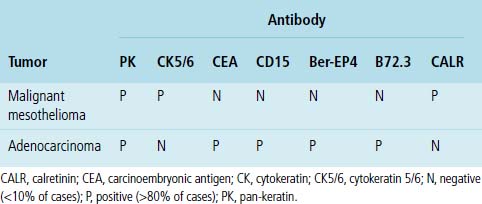
Another malignancy that often has a papillary “pseudocarcinomatous” appearance, especially in pleural fluid specimens, is the epithelioid variant of malignant mesothelioma (Fig. 17-17). Renshaw and colleagues estimated that the sensitivity of pleural fluid cytology for the diagnosis of mesothelioma was only 32%99 because epithelioid tumor cells often have a bland appearance and the sarcomatoid variant of mesothelioma rarely sheds into the pleural space. Many reports have considered the diagnostic distinction of mesothelioma from metastatic adenocarcinoma. This topic is discussed in detail in Chapter 20.100–102 Immunopathologic and electron microscopic studies are generally used to make this distinction.100,103 Table 17-3 shows a typical immunopathologic antibody panel that can be used to distinguish mesothelial proliferations from epithelial tumors.100,104,105 Imlay and Raab examined the utility of immunohistochemistry in this context in hospital practice.45 They reported that immunopathologic techniques were applied to 2.6% of all cases with pleural fluid specimens. In 71.9% of these cases, a firm interpretation was facilitated by the results of such analyses.45 However, none of the diagnoses in that series were based solely on immunopathology.45 The low prevalence of mesothelioma in the general population explains the rarity of this interpretation in the experience of most practicing pathologists.
Clear Cell Adenocarcinomas
Clear cell features are best seen in histologic specimens, in which the cytoplasm of the neoplastic cells is lucent and only the cell borders are apparent. Clear cell change is often an artifact of formalin fixation, and in cytologic specimens, the cytoplasm of the neoplastic cells has a more vacuolated appearance. Primary clear cell tumors of the lung are rare and have variable cellular lineage; they are usually peripherally located106 (see Chapters 16 and 19). Clear cell change also may be seen focally in common tumor types. For example, biopsy specimens of primary squamous cell carcinomas may show that alteration. That phenomenon is seen less frequently in cytologic specimens, in which the cytoplasm of the neoplastic cells maintains a classic “metaplastic” appearance.
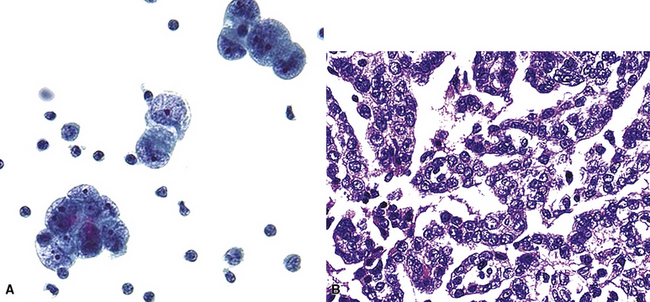
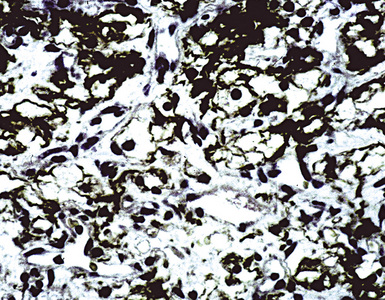
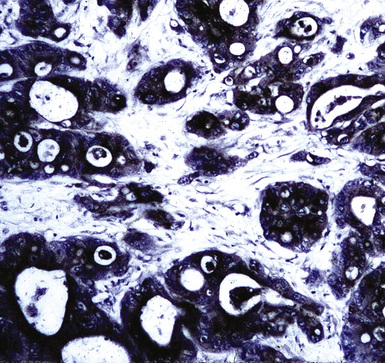
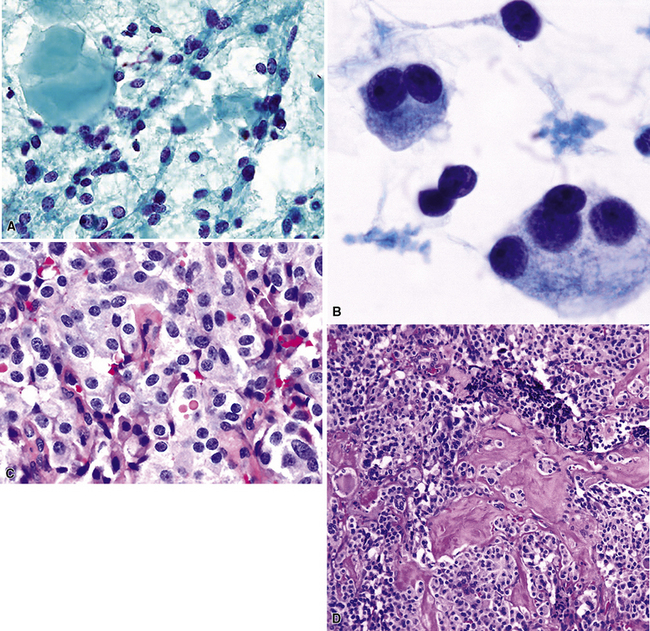
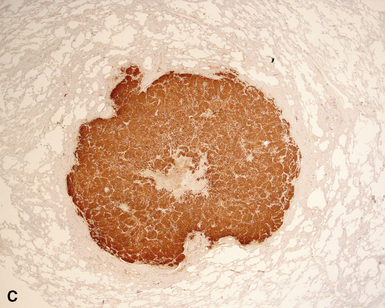
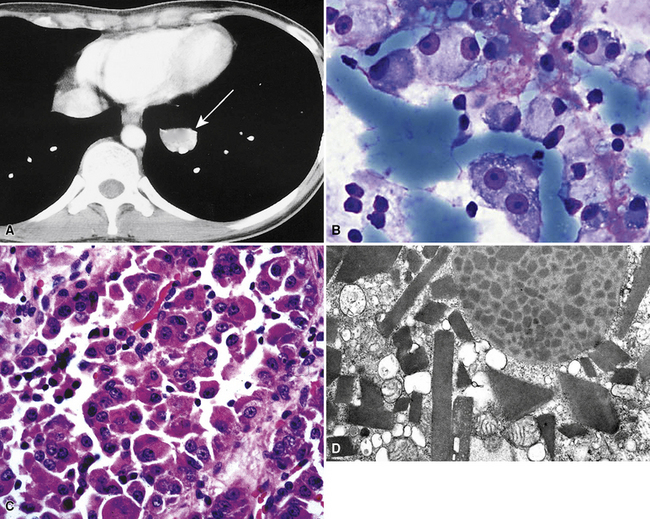
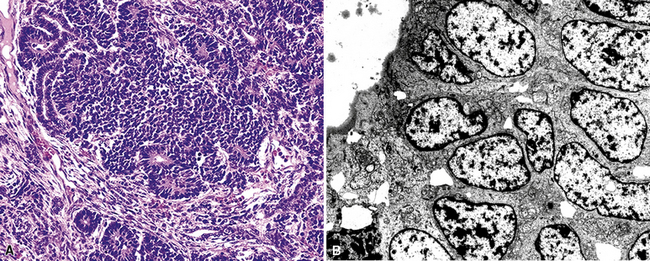
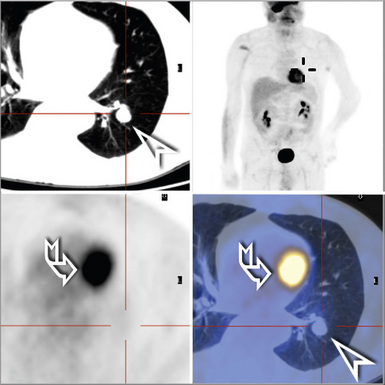
Metastatic clear cell adenocarcinomas in the lungs may emanate from the kidney, breast, adrenal cortex, salivary gland, or other locations; primary clear cell malignancies have been described in practically every organ. The most common clear cell neoplasm in the lung is metastatic renal cell adenocarcinoma (Figs. 17-18 and 17-19).107 Hughes and associates reported that among 12 lung FNA specimens with clear cell features, 10 originated in the kidney, 1 in the cervix, and 1 in an undetermined site.108 Clear cell carcinomas are only a subset of renal tumors that may metastasize to the lungs; papillary, oncocytic, and sarcomatoid neoplasms may also do so. Because epithelial malignancies of the kidney have a proclivity to invade the renal veins and bypass the hepatic circulation, the first site of secondary disease may be in the lungs.107,109 The pulmonary parenchyma is involved in up to 75% of cases of metastatic renal cell carcinoma.109,110 Almost half of these patients have no symptoms that suggest extrathoracic disease110 (Fig. 17-20). Metastatic disease in the lungs may take several radiographic forms, including a solitary mass, multiple nodules, miliary spread (innumerable small nodules), large- or small-vessel emboli, lymphatic space disease, hilar or mediastinal lymph nodal disease, and endobronchial tumor.111–115 Metastatic renal cell carcinoma is an example of a neoplasm that may grow very slowly and become clinically evident only many years after the primary diagnosis.116
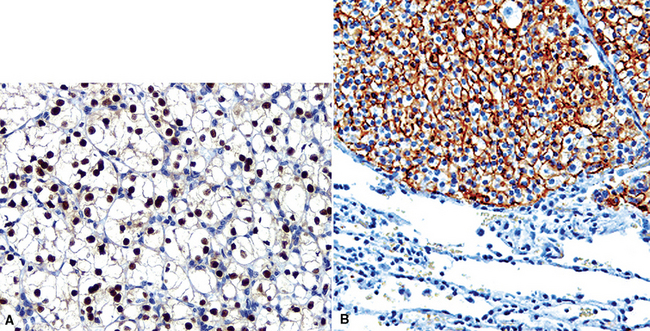
Like ovarian carcinomas, clear cell carcinomas of the kidney are difficult to identify definitively by immunohistologic studies. Most are reactive for CD10, PAX2, adipophilin, RCC antigen, and cytokeratin 8 (Figs. 17-21 and 17-22). In combination, these are highly suggestive of the diagnosis.117–120
Signet Ring Cell Adenocarcinomas
A “signet ring” cell is relatively small and has an eccentrically placed nucleus indented by a large cytoplasmic vacuole or multiple vacuoles. Such cells generally are considered part of the spectrum of poorly differentiated mucin-forming adenocarcinoma, and some tumors of that type are composed almost entirely of signet ring cell forms. Signet ring cell differentiation is uncommon in most primary pulmonary adenocarcinomas. If it is present, a secondary malignancy is favored (Fig. 17-23). Sources of metastases with that appearance include the stomach and other gastrointestinal sites, breast, and pancreas. Metastatic signet ring cell carcinomas are usually associated with a dismal prognosis; they characteristically spread initially to regional lymph nodes before involving the lungs. Some esophageal signet ring cell tumors originating in foci of Barrett esophagus may directly invade the lung or pleura. Metastatic pancreatic adenocarcinomas, including signet ring cell variants, often involve the liver and lungs.121 Multiple pulmonary nodules are virtually always seen rather than a solitary secondary lesion.121
Well-Differentiated Adenocarcinomas
The authors use the term well-differentiated adenocarcinoma in more than just a descriptive fashion to mean a malignant glandular proliferation that is morphologically difficult to differentiate from benign or reactive pulmonary proliferations; the nosologic term “minimal-deviation adenocarcinoma” also has been used in this context. The recognition of these tumors is often extremely difficult in cytologic specimens because of the lack of contextual architecture. Well-differentiated primary adenocarcinomas of the lung include some “conventional” (acinar) adenocarcinomas, selected bronchioloalveolar adenocarcinomas, and salivary gland-type adenocarcinomas (see Chapter 16). These show relatively low nuclear-to-cytoplasmic ratios and lack the degree of nuclear atypia seen in more overtly malignant lesions. Bronchioloalveolar adenocarcinomas, in particular, often pose a diagnostic conundrum, especially in cytologic preparations where the malignant cells lining the alveolar septa in a “lepidic” manner cannot be seen. However, well-differentiated adenocarcinomas in the lung may also be metastases, especially when sharply defined mass lesions are seen macroscopically. Sites of origin for secondary adenocarcinomas with these attributes include the breast, pancreas, kidney, thyroid, and salivary glands.
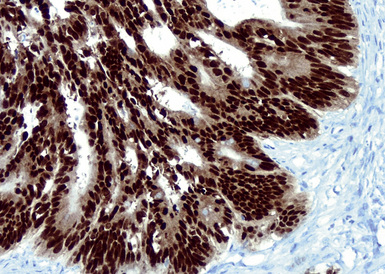
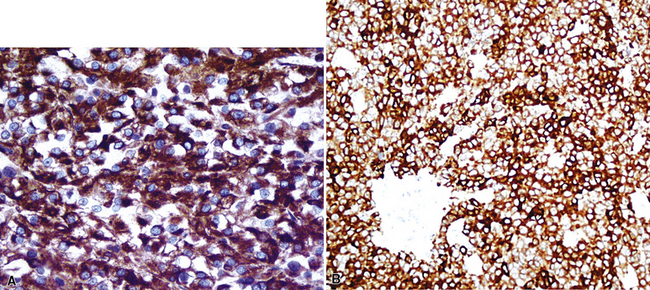
Mammary carcinomas may metastasize to the lungs, pleura, or both. In most cases, the malignant cells are easily identified, but in some FNA or pleural fluid specimens, the malignant cells are bland (Fig. 17-24). Intrapulmonary metastatic foci are often nodular, but other presentations, such as endobronchial lesions, lymphangitic spread, and intravascular tumor emboli, may be seen.122–125 Approximately 50% of metastatic breast cancers are associated with pleural effusions.33,126 Casey and colleagues reported that 3% of primary mammary carcinomas were associated with a lung mass at the time of initial diagnosis; 43% of the pulmonary lesions were metastases and 52% were concurrent primary lung cancers. The rest were non-neoplastic.125 The lungs and pleura are the first sites of tumor recurrence in 10% of cases of mammary carcinoma.124 In patients with a history of breast carcinoma and an adenocarcinoma in the lung, Raab and coworkers showed, with immunohistochemical studies (for estrogen receptor, gross cystic disease fluid protein-15, S-100 protein, and carcinoembryonic antigen) that 50% of the pulmonary lesions were metastatic mammary tumors, 37% were primary pulmonary carcinomas, and 13% were indeterminate (Figs. 17-25 and 17-26).127 Dabbs and associates found that some primary lung cancers may label for hormone receptor proteins, but not for the other specified markers.128 Mammaglobin is another breast-related polypeptide that is valuable in the immunohistochemical recognition of metastatic mammary carcinoma.129 On the other hand, positivity for thyroid transcription factor-1 (Fig. 17-27) is compelling evidence in favor of pulmonary derivation in this context, as discussed later.
Oncocytic and Granular Cell Carcinomas
Neoplasms composed of cells containing granular cytoplasm may be oncocytic or nononcocytic. Both subtypes contain cells that have an eosinophilic appearance on conventional stains. In oncocytic cells, this reflects the presence of numerous cytoplasmic mitochondria. Nononcocytic cells instead contain a preponderance of other cytoplasmic organelles, especially lysosomes. Primary pulmonary malignancies that may have a granular cell constituency include conventional adenocarcinomas and salivary gland-type adenocarcinomas. However, this cytologic feature is rare in lung tumors. Secondary neoplasms with granular cytoplasm include carcinomas of the kidney, thyroid, and liver (Fig. 17-28).
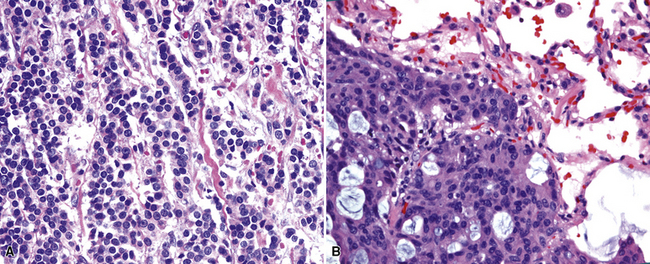
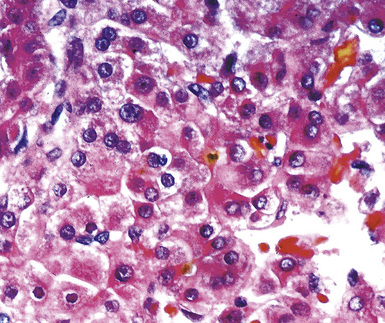
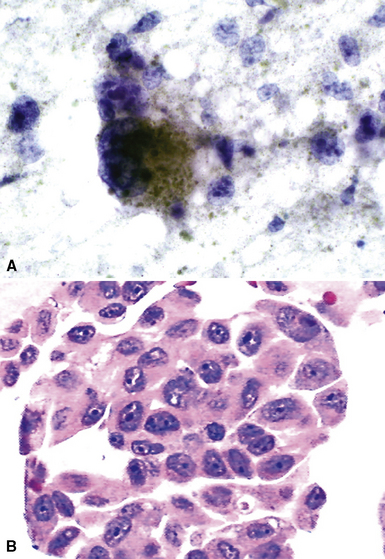
The lungs are involved in up to 70% of cases of metastatic hepatocellular carcinoma (HCC).130–132 Several patterns of intrapulmonary spread have been reported; through transdiaphragmatic lymphatics, HCC may enter the right lower lobe. In this setting, several parenchymal mass lesions and pleural involvement are typically seen.133,134 Alternatively, HCC may transit the venous system through the hepatic vein and inferior vena cava, presenting as a large intravascular mass or “showering” the lungs with small emboli that appear as miliary tumors.133 Cytologically, the cells of this neoplasm often show multinucleation; this feature is uncommon in most primary pulmonary malignancies. Moreover, bile formation may be seen in metastatic HCC (Fig. 17-29). In addition, the tumor cells cluster around intralesional blood vessels and “stripped” nuclei are seen.135 Cytopathologists must avoid misinterpretation of FNA specimens obtained from the right lower pulmonary lobe as well-differentiated oncocytic or granular cell carcinomas; these specimens may simply represent normal liver that has been mistakenly sampled instead of the lung. A monoclonal antibody raised against paraffin-embedded tissue from HCC, and designated “Hep-PAR1,” has shown reasonably good discrimination in labeling that tumor.136
Largely Necrotic Adenocarcinomas
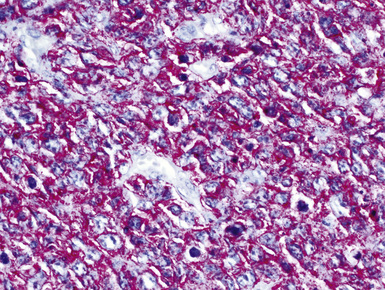
In up to 50% of metastatic colorectal adenocarcinomas, the lungs are involved.137 Most of these cases show multiple pulmonary masses radiographically,137 but approximately 40% of all solitary metastases in the lung are also derived from the large intestine.21,22 Right-sided colonic tumors may produce lung metastases without liver metastases.138–141 These lesions are often cystic, and FNA specimens may be mistakenly interpreted as showing cavitary squamous cell carcinoma. In histologic sections, zones of necrosis may be surrounded by limited numbers of viable tumor cells; in cytologic preparations, rare viable cells may be seen. Flint and Lloyd suggested that “dirty” (karyorrhectic) necrosis (Figs. 17-30 and 17-31) was more often seen in metastatic colorectal tumors than in primary adenocarcinomas of the lung.142 Immunopathologic studies are often helpful in distinguishing secondary colonic malignancies from primary adenocarcinomas of the lung. Colorectal tumors generally are reactive for cytokeratin 20 but negative for cytokeratin 7 and thyroid transcription factor-1; the converse is true for primary pulmonary adenocarcinomas, even those that have an “enteric” morphologic image on conventional microscopy.143–146 A cytoskeletal protein known as “villin” is also selectively seen in gastrointestinal malignancies, as are the plasmalemmal glycoprotein recognized by monoclonal antibody CA-19-9 and the nuclear transcription factor CDX2 (Figs. 17-32 and 17-33).147–149
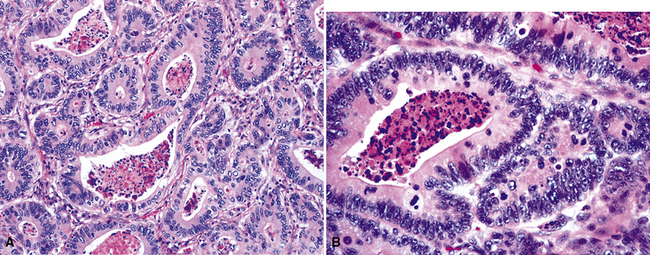
Figure 17-31 A and B, “Dirty” necrosis is apparent in the centers of tumoral glands in metastatic colonic adenocarcinoma.
Mucinous Adenocarcinomas
Primary mucin-producing carcinomas of the lung include some bronchioloalveolar carcinomas, selected mucoepidermoid carcinomas, and other rare primary mucinous tumors, some of which have the appearance of “colloid” carcinomas (Fig. 17-34).150 Most of these tumors have relatively specific radiologic attributes, and when these images are absent and a mucinous carcinoma is seen microscopically, metastasis may be suspected. However, large amounts of postobstructive mucin production by the lung may surround nonmucinous malignancies. Consequently, cytopathologists should be cautious in interpreting FNA specimens containing carcinoma cells and abundant mucin as bona fide mucinous carcinomas. The most common sites of origin for metastatic mucinous adenocarcinomas are the intestine (including the vermiform appendix), ovary, and breast. Pathologic specimens of these secondary tumors in the lung may show rare malignant cells and copious mucin pools. The neoplastic cells are often well differentiated and are arranged in small clusters. The immunopathologic features of metastatic colorectal adenocarcinoma of the “colloid” type are essentially the same as those of ordinary colon cancers, and that also applies to secondary mucinous carcinoma of the breast. However, mucinous ovarian carcinomas differ from other epithelial malignancies of the ovary immunophenotypically; they typically lack CA-125 and instead exhibit an enteric antigenic profile similar to that of intestinal neoplasms.151
Other Metastatic Malignancies That Mimic Adenocarcinomas of the Lung
The immunopathologic profiles of prostatic, endometrial, and adrenocortical carcinomas are shown in Table 17-1. Prostatic and endometrial tumors rarely metastasize selectively to the lungs152,153; only 10% of prostatic carcinomas yield lung metastases,154,155 and fewer than 3% of endometrial carcinomas do so.153 Both of these malignancies usually first involve other sites, such as lymph nodes, bones, or liver.152 When these lesions spread to the lungs, multiple masses usually are apparent.156 Metastatic prostatic carcinomas may produce endobronchial masses, lymphangitic carcinomatosis, or thoracic lymph nodal spread.157–159 Antibodies to prostate-specific antigen, prostate-specific acid phosphatase, and prostate-specific membrane antigen are highly specific for tumors of prostatic origin (Figs. 17-35 to 17-37).160 No such specific markers are currently available to identify endometrial neoplasms. Biopsy specimens of metastatic adrenocortical carcinoma may show lipidized cytoplasm in the tumor cells and extensive cellular dyshesion. The immunoprofile of this neoplasm is unusual in that it features scant keratin production (if any), vimentin reactivity, and labeling for inhibin, MART-1/Melan-A (Figs. 17-38 and 17-39), or both, despite S-100 protein negativity. Inhibin and MART-1 are typically associated with ovarian stromal tumors and melanocytic proliferations, respectively. Why they should be present in an epithelial tumor is unknown, but their presence makes adrenocortical carcinoma a singularly identifiable form of metastasis in the lung.
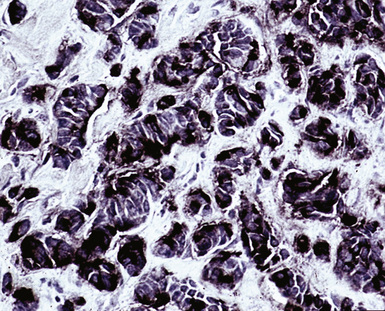
Figure 17-37 Immunoreactivity for prostate-specific membrane antigen in metastatic prostatic adenocarcinoma.
“Gene Chip” Analyses
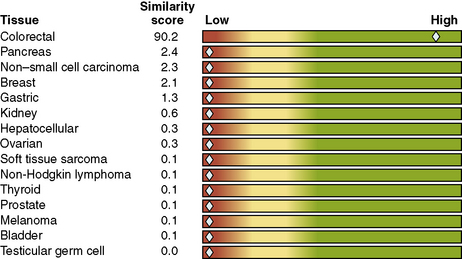
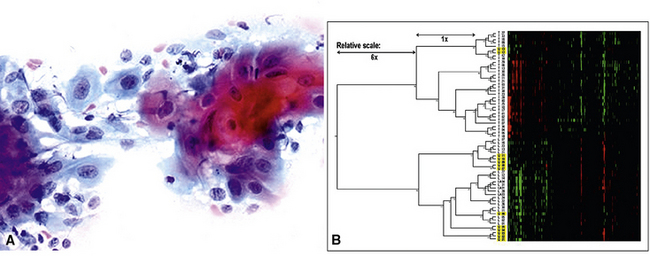
In the last several years, a number of publications have addressed the possibility that MCUOs might be identified through analysis of their genetic profile, in addition to, or in lieu of, evaluation of their immunophenotype.161–169 That approach has yielded the commercial availability of such assessments, offered by a growing number of biomedical firms. The general background of the technique is predicated on the evaluation of predefined and sizable gene sets in a number of metastases of primary carcinomas. In this way, a characteristic “gene fingerprint” can usually be identified for each histologic tumor type. An “unknown” clinical case is then similarly assessed for the same gene groups, and using computer algorithms for comparison, statistical synonymities to known carcinoma identities are calculated. The submitting physician is given a visual report that shows the relative likelihood of several origins for the lesion (Fig. 17-40).
Spindle Cell and Sarcomatoid Tumors
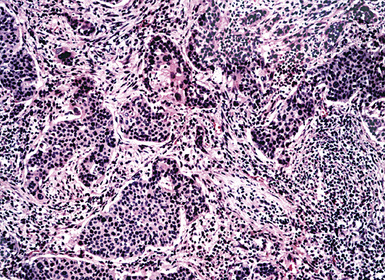
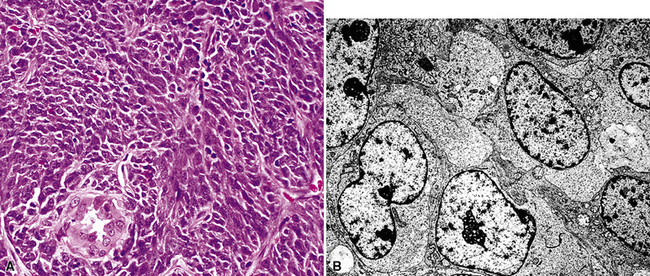
The most common primary pulmonary malignancy with a spindle cell or pleomorphic growth pattern is sarcomatoid bronchogenic carcinoma. It must be distinguished from primary and metastatic sarcomas and other types of malignant spindle cell tumors (e.g., sarcomatoid mesothelioma). Primary sarcomatoid carcinomas of the lung are extremely poorly differentiated, and many have been classified simply as “non–small cell carcinomas” in the past. Pathologic specimens of these tumors often show foci of spindle cell growth admixed with areas of more obvious epithelial differentiation (“biphasic” sarcomatoid carcinoma), but monomorphic examples with no epithelioid components are also seen (“monophasic” sarcomatoid carcinoma [Fig. 17-41]; see Chapter 14). Sarcomatoid carcinomas may contain homologous or heterologous foci of divergent mesenchymal-like differentiation. The latter resembles osteosarcoma, myogenous sarcoma, chondrosarcoma, and other forms of sarcoma (Figs. 17-42 and 17-43), potentiality further confusing the diagnostic picture. Primary neuroendocrine carcinoma, especially “spindle cell carcinoid,” may also enter the differential diagnosis, but sarcomatoid carcinomas do not show the nuclear features seen in neuroendocrine lesions.
Table 17-4 shows the immunophenotypes of specific spindle cell tumors in the lung.170 In practice, the number of antibodies applied in immunohistologic studies varies according to the clinicopathologic setting. The ultimate diagnosis of sarcomatoid pulmonary lesions may require extensive adjunctive pathologic analysis.
Most patients with clinically apparent, metastatic, intrapulmonary spindle cell malignancies have metastatic sarcomas. Secondary spindle cell carcinoma is unusual in the lungs. However, sarcomas are uncommon as well; only 5000 to 6000 new cases of sarcoma are seen each year in the United States.171 In most cases of metastatic sarcoma in the lungs, a history of the tumor is well known when pulmonary involvement becomes apparent. Thus, there is no need to institute a search for the primary lesion. Solitary sarcomatous lesions of the lung and pleura are more difficult to recognize diagnostically because the sarcoma morphotypes that occur primarily in these locations are also seen in extrathoracic tissues and organs. That topic is discussed in detail in Chapter 14.
Extrathoracic sarcomas are associated with a high incidence of pulmonary metastasis overall. Autopsy series considering that point have found involvement of the lungs in up to 95% of cases.172,173 Most metastatic sarcomas form multiple nodules in the pulmonary parenchymal or pleural surfaces, although solitary or multifocal endobronchial disease occasionally is seen.174,175 Lymphatic spread of sarcomas is extremely unusual. Metastatic subpleural mesenchymal malignancies also may cavitate; this eventuality potentially causes pneumothorax formation or results in bronchopleural fistulae.176–179 Foci of metastatic sarcoma may show a varied morphologic appearance, even if the primary lesion did not; this phenomenon, termed “clonal evolution” is well documented and may indicate aggressive tumor growth.180 Irradiation and chemotherapy may facilitate its appearance.
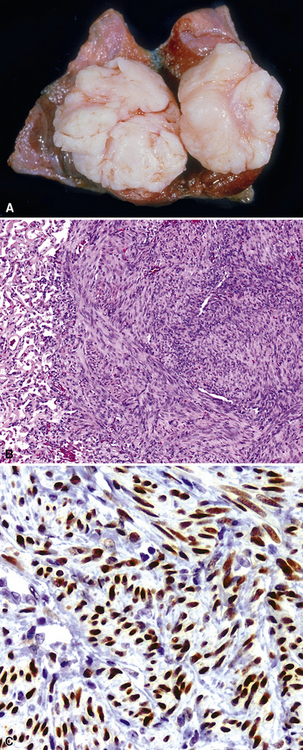
Metastatic uterine smooth muscle tumors deserve special mention. Both high-grade leiomyosarcomas and low-grade myogenous tumors of the uterus (sometimes termed “metastasizing leiomyomas”) may spread secondarily to the lungs.181,182 In either instance, multiple and occasionally cystic nodular lesions are seen in the parenchyma and pleura; in rare cases, the metastatic tumor assumes a miliary pattern of spread.183,184 Metastases of uterine smooth muscle neoplasms are usually seen in women of reproductive age or older,185,186 but secondary intrapulmonary leiomyosarcoma has also been reported in men with primary soft tissue tumors.185 Many of these patients have no symptoms, although dyspnea, cough, and cyanosis can be present. Some lesions may lead to respiratory failure.185,187 Large smooth muscle tumors can yield neoplastic emboli and tumor-related pulmonary infarction.188
Grossly, metastatic smooth muscle tumors are white and well circumscribed, with a “whorled” cut surface. Histologically, the constituent spindle cells may have a bland appearance, especially in “metastasizing uterine leiomyomas.” In these lesions, mitoses are rare or absent (Fig. 17-44).185,186 Other examples of secondary leiomyosarcoma are easily recognized as malignant lesions because of the degree of nuclear atypia and mitotic activity.
Some authors have suggested that “metastasizing leiomyomas” are actually multifocal pulmonary hamartomas rather than metastatic tumors. That argument has focused on the bland appearance of the smooth muscle cells, the lack of mitoses, and the occasional presence of admixed glandular elements. Even so, the authors believe that “metastasizing leiomyomas” are a separate pathologic entity,185 based partly on aggregated clinicopathologic information. Most patients with such lesions have a history of surgical removal of uterine smooth muscle tumors (usually diagnosed as leiomyomas) or neoplasms of that type are found at autopsy. Synchronous metastatic implants have also been seen in abdominal, retroperitoneal, and pelvic soft tissue and lymph nodes in women who have “metastasizing leiomyomas” in the lungs.189 The argument that these intrapulmonary smooth muscle tumors are derived from uterine lesions gained strong support from a study by Patton and colleagues.190 Through paired analysis of uterine and pulmonary neoplasms in the same patients, they showed that the lesions were clonal by assessing a nucleotide sequence from the human androgen receptor gene and measuring telomere lengths.
In postmenopausal women, metastatic lesions tend to be slowly growing or static. They are associated with more rapid evolution and pulmonary morbidity in premenopausal patients. Some may even cause death. “Metastasizing leiomyomas” must be distinguished from lymphangioleiomyomatosis of the lungs, a condition that is discussed in detail in Chapter 7.
Malignant Small Round Cell Tumors
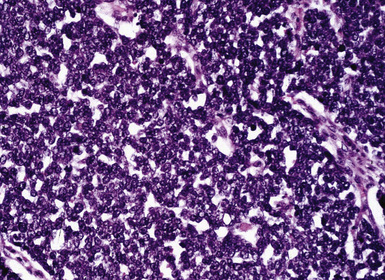
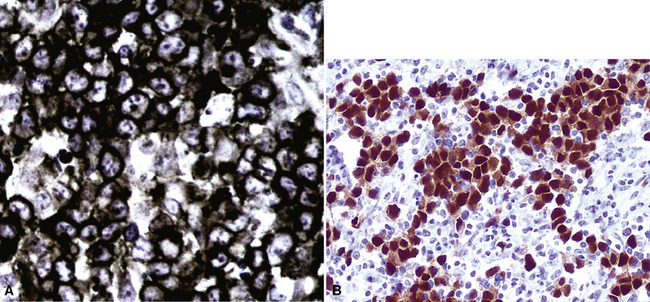
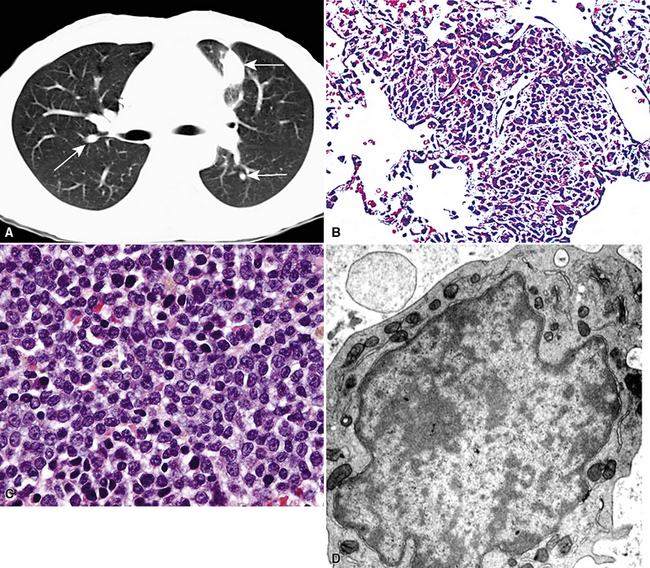
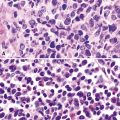
Malignant small round cell tumors prototypically are composed of cells with round to oval nuclei, scant cytoplasm, and extensive cellular dissociation. Scanning microscopy often shows a “sheet” of nuclei; these neoplasms are sometimes termed “small blue cell” malignancies. An organoid growth pattern is also potentially apparent (Figs. 17-45 and 17-46). The primary pleuropulmonary lesions in this category include neuroendocrine carcinomas, malignant lymphomas, and rare malignancies, such as Askin tumor or small cell mesothelioma. Metastatic small round cell tumors also include neuroendocrine carcinomas of extrapulmonary sites and malignant lymphomas, but additional sarcomas and other tumor types must also be considered (e.g., malignant melanoma, rhabdomyosarcoma, mesenchymal chondrosarcoma, small cell osteosarcoma, hepatoblastoma, neuroblastoma, and Wilms tumor). Selected primary and secondary non-neuroendocrine carcinomas also may have a small cell composition.191,192
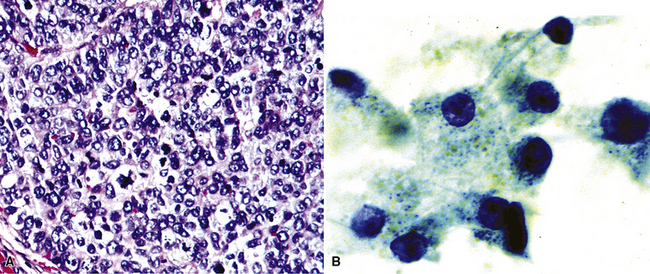
The most common primary intrathoracic small round cell malignancy is small cell neuroendocrine carcinoma of the lung (SCNCL).192 This tumor is histologically and cytologically identical to small cell carcinoma originating in other sites (Figs. 17-47 and 17-48). Clinically, most SCNCLs are centrally located and accompanied by enlarged mediastinal lymph nodes; they quickly metastasize and are usually advanced in stage at diagnosis. Regardless of their anatomic origins, metastatic foci of small cell neuroendocrine carcinoma (SCNC) are typically characterized radiographically by multiple intrapulmonaory masses; mediastinal involvement is often lacking when the tumor has arisen outside the lungs. The morphologic features of these lesions include granular dispersed nuclear chromatin, nuclear fragility, nuclear molding, and cellular clumping. Those attributes are generally associated with neuroendocrine differentiation. Immunopathologically, SCNCs often show a characteristic reactivity pattern for keratin, with dot-like perinuclear labeling. They may or may not show additional positivity for synaptophysin, chromogranin-A, CD56, CD57, and neuron-specific enolase. Byrd-Gloster and coworkers reported that 97% of SCNCLs were reactive for thyroid transcription factor-1, whereas most (but not all) SCNCs of nonpulmonary derivation lacked that marker.193 On the other hand, cytokeratin 20 positivity is potentially seen in primary extrathoracic SCNCs, but it is uncommon in primary pulmonary small cell carcinoma.194,195 SCNC of the lung and other sites may also contain a non–small cell component (“composite” or “combined” SCNCL; see Chapter 13).
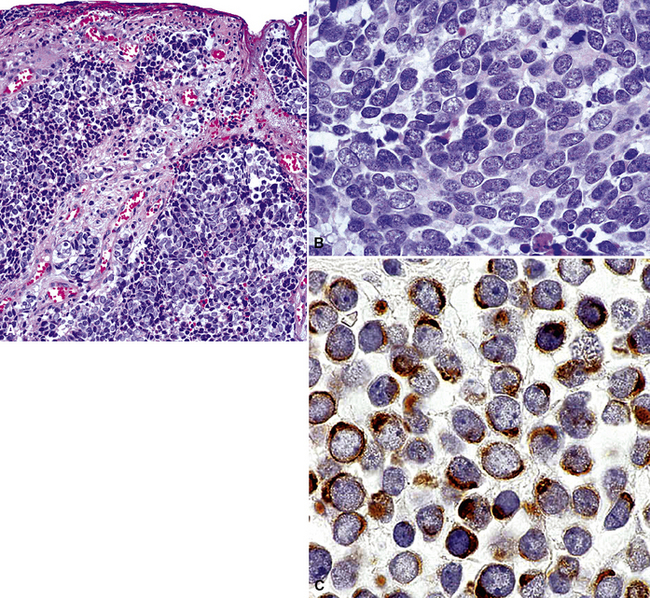
Figure 17-48 A and B, The primary tumor of the skin is seen, corresponding to the lesion shown in Figure 17-47. C, Both the primary and secondary lesions expressed cytokeratin-20, shown here.
Metastatic well- or moderately differentiated neuroendocrine carcinomas in the lung include “carcinoids” of gastrointestinal, uterine cervical, or other topographic derivations; neuroendocrine pancreatic tumors; and medullary thyroid carcinomas (Fig. 17-49).196,197 Many of those tumors are associated with specific symptoms because of their production of various neuropeptides or amines. However, in histologic or cytologic specimens, such lesions are potentially identical to primary neuroendocrine neoplasms of the lung. Data on discriminating immunostains are still evolving for this group of tumors, but thyroid transcription factor-1 reactivity again may favor a pulmonary origin.198 Histochemical evaluations are still helpful in a proportion of cases; most pulmonary neuroendocrine tumors are argyrophilic (e.g., with the Grimelius method) but nonargentaffinic (with Fontana-Masson stain).199 Hence, strong argentaffinity in a carcinoid tumor suggests an origin outside the lungs.
Parathyroid carcinoma is a rare, progressive, but often indolent neoplasm that metastasizes to the lung in approximately 33% of cases.200,201 Metastatic parathyroid carcinoma may mimic a primary lung carcinoid tumor. Although most patients with metastatic parathyroid carcinoma to the lung have a history of primary parathyroid carcinoma and these cases are not diagnostically challenging, in some patients, lung metastases may occur several years after surgical resection of the primary parathyroid tumor. When a clinical history of parathyroid carcinoma is not apparent, metastatic parathyroid carcinoma to the lung may be confused with a primary lung carcinoid tumor. Immunostain with parathyroid hormone should be positive in tumor cells, and serum calcium levels may be elevated.200,201
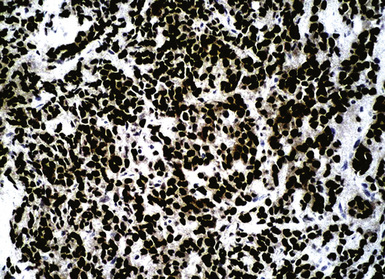
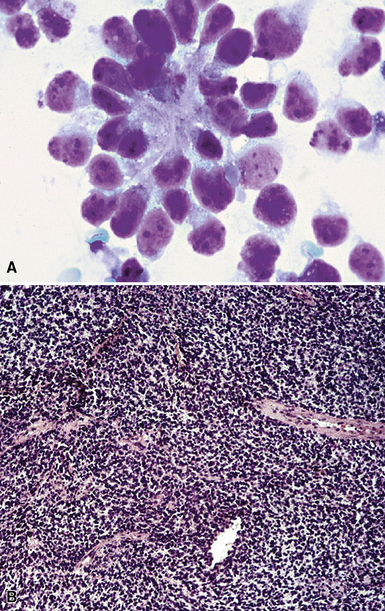
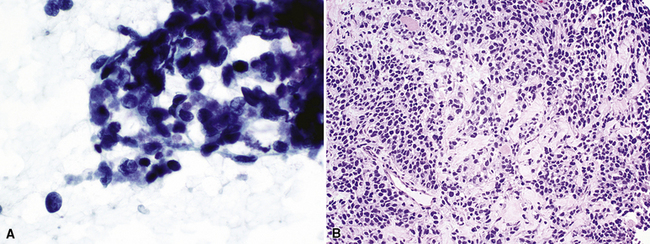
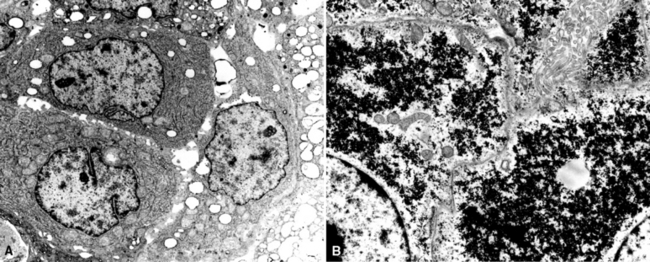
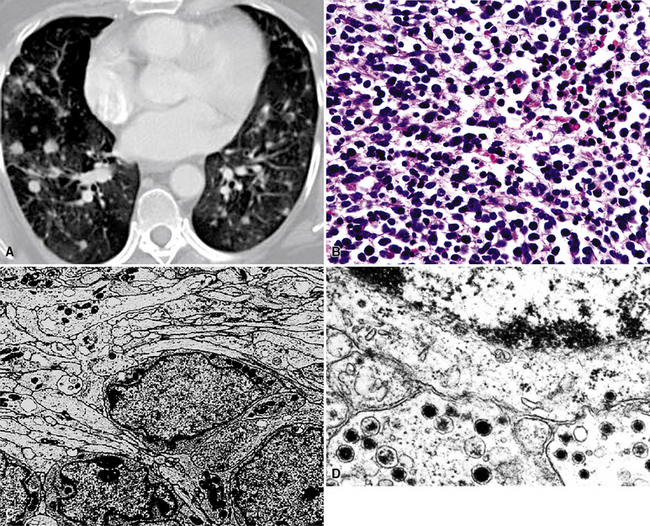
Primary pulmonary lymphomas are discussed in Chapter 15, but most malignant lymphoid tumors of the lung are secondary lesions that occur in the context of systemic dissemination. Primary malignant lymphomas in this organ are typically low grade, whereas secondary hematolymphoid malignancies may represent any histologic subtype and grade. For example, 30% of patients with mediastinal Hodgkin lymphoma have lung involvement by direct extension.202 Malignant lymphomas that secondarily affect the pulmonary parenchyma are not believed to be truly metastatic because they populate lymphoid structures that are normally present in the lungs and pleura. One form of lymphomatous involvement is characterized by diffuse interstitial lymphocytic permeation, with focal formation of micronodules (Fig. 17-50).203,204 When the constituent cells are relatively mature, the image of “lymphocytic interstitial pneumonia” may be obtained (see Chapters 7 and 15). However, discrete nodules also may occur, especially with high-grade large-cell neoplasms (Fig. 17-51), and these commonly simulate metastatic nonhematopoietic lesions clinically and pathologically.
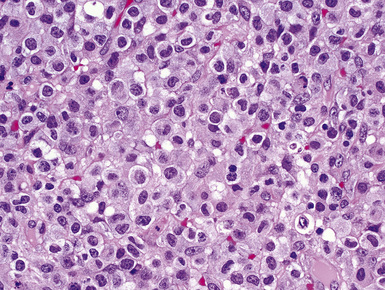
Figure 17-51 Nodules of anaplastic polygonal cells are seen in this case of large-cell non-Hodgkin lymphoma involving the lungs.
Involvement of the pleura may be unilateral or bilateral in malignant lymphoma, but lymphoma and metastatic carcinoma are the two most common causes of malignant bilateral pleural effusion.39 In cytologic practice, it is difficult to be certain that effusion specimens containing atypical lymphoid cells are definitively positive for lymphoma; such cells may represent contamination of the sample by lymphocytes from the peripheral blood, and especially when they are relatively mature, discrimination from other causes of lymphoid pleural effusion depends mainly on the findings of adjunctive studies.205 Flow cytometry is probably optimally suited for this application.206
In the pediatric age group, some small round cell malignancies may metastasize to the lungs, as cited earlier (Fig. 17-52). Immunohistochemical studies and electron microscopy are helpful in differentiating these lesions in difficult cases.191 Cytogenetic analyses may also be extremely valuable. Table 17-5 shows the immunopathologic profile of several primary and secondary small round cell tumors.191
Squamous Cell Carcinomas and Morphologic Simulants
Based on morphologic findings alone, the site of origin for squamous cell carcinoma (SCC) is impossible to determine with certainty (Figs. 17-53 and 17-54). Most squamous carcinomas in the lung have arisen there, and although multifocal SCCs suggest metastatic disease, that picture is also seen with multiple primary synchronous bronchogenic carcinomas. The usual sources of metastastic SCC in the lung are mucosal sites of the head and neck, esophagus, uterine cervix, and skin.
Squamous cell carcinomas of the head and neck that involve the lungs may originate in the larynx, nasopharynx, oropharynx, or hypopharynx, and lesions in the lungs may be single or multiple.83,207,208 Because all of these tumors are associated with the use of alcohol or tobacco or with infection with human papillomavirus, patients with squamous cell carcinoma in one of the specified locations also commonly have separate metachronous or synchronous primary squamous malignancies in other mucosal sites or in the lungs.209 In patients with both pulmonary lesions and SCC in the head or neck, Malfetto and colleagues reported that 53% had separate primary bronchogenic carcinomas and 19% had metastatic SCC in the lung.209 Cervical lymph nodal involvement is also present in up to 80% of cases of metastatic intrapulmonary SCC.83,84 The cumulative incidence of second malignancies in patients who have SCC of the head and neck is approximately 4% per year, and 30% of these tumors arise in the lungs.210
Sostman and Matthay reported that uterine cervical squamous cell carcinomas spread to the lungs less frequently (4%) than cervical adenocarcinomas do (20%).211 In most cases, single or multiple lesions are present, and as with all squamous cell carcinomas, there is a tendency toward cavitation.212,213 Metastatic cervical SCC also may involve pulmonary hilar or mediastinal lymph nodes, endobronchial mucosal sites,214,215 or the intrapulmonary lymphatics.29,216
“Gene profiling” has also been used in this context to differentiate metastatic SCC from primary pulmonary SCC. Vachani and coworkers217 used a selected 10-gene panel, achieving 96% accuracy among 122 cases. A hierarchical segregation of the genes in that data set is shown in Figure 17-54. Girard and associates showed that the same end can be achieved by scrupulous histologic assessment, focusing on tumor grade, cytologic features, stromal patterns, and the extent of necrosis.218 In that study, the concordance between histologic predictions and genomic profiling was approximately 90%.
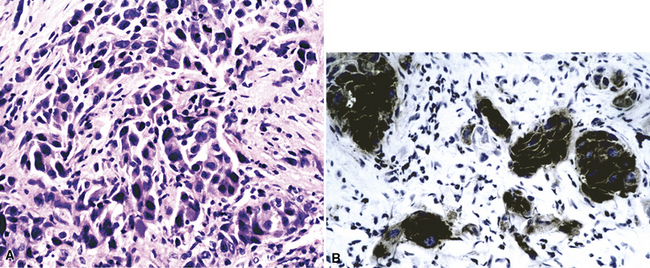
Metastatic malignant melanoma has a myriad of histologic and cytologic appearances. For this reason, it may be considered in the differential diagnosis with selected adenocarcinomas, malignant small round cell tumors, spindle cell neoplasms, large polygonal cell malignancies, and some squamous cell carcinomas. The appearance of single cells with “metaplastic” cytoplasm, large ovoid nuclei, and prominent nucleoli is common in the cytologic evaluation of malignant melanoma (Figs. 17-55 and 17-56). Melanin production is a helpful clue to the identity of this tumor (Fig. 17-57), but it may be confused with other pigments, such as hemosiderin, and it requires histochemical verification. A history of ocular, cutaneous, or mucosal melanoma is often known when that neoplasm involves the lungs metastatically. However, some metastatic melanomas represent the initial signs of that tumor.
Virtually all examples of melanoma in the lungs and pleura are metastatic; only anecdotal examples of primary pulmonary malignancies with melanocytic differentiation have been documented.219 Metastatic melanomas usually involve multiple organs, but DasGupta and Brasfield found that the lungs were involved in 70% of cases.220 Secondary lesions usually have multiple intrapulmonary nodules, but solitary lesions, lymphangitic or miliary disease, or endobronchial implants may also be seen.221–224 Balch and coworkers reported that a solitary pulmonary mass was the first evidence of metastatic disease in 38% of cases.225 Conversely, Pogrebniak and colleagues found that 33% of lung nodules in patients with melanoma were unrelated and benign.226
Metastatic transitional cell carcinomas (TCCs) of the urinary tract often have a squamoid cytoplasmic appearance, and true squamous differentiation also may be seen. Cytologic specimens show extensive cellular dyshesion and necrosis. Some authors have reported that cercariform cells—containing nucleated globular bodies and bulbous cytoplasmic processes—suggest metastatic TCC.99,227 These tumors often form mass lesions in the lungs and also can spread lymphangitically.
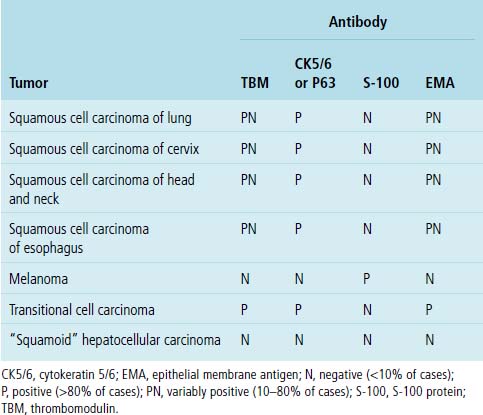
Table 17-6 shows the immunopathologic profile of squamous cell carcinomas, based on their sites of origin; the immunophenotypes of their morphologic mimics are also included. If metastatic malignant melanoma is included in the differential diagnosis, it can be easily characterized by its reactivity for S-100 protein, HMB-45, MART-1, and tyrosinase.228 TCCs may label for cytokeratin 20, but are negative for cytokeratin 7 in most cases; this profile is the opposite of that seen in primary pulmonary adenocarcinoma.145,229–231 Thrombomodulin (CD141) is potentially shared by both of the latter tumors, but it is more commonly present in TCC. Similarly, p63 protein is present in TCC, but not in most adenocarcinomas. Squamous cell carcinomas of all sites share with TCC the potential for thrombomodulin and p63 reactivity, but the two may be distinguished by selective immunoreactivity for uroplakin in TCC.232
Undifferentiated Large Polygonal Cell Malignancies
Ultrastructural studies have shown that primary large cell carcinomas of the lung often show squamous or glandular differentiation; these features may be seen focally in histologic or cytologic preparations. As their name implies, the cells that make up these tumors contain a relatively large amount of cytoplasm and correspondingly large nuclei. In cytologic specimens, some large cell carcinomas show significant cellular dyshesion (Fig. 17-58). Clinically, they are often bulky masses associated with mediastinal lymphadenopathy. Primary large cell carcinomas of the lung have an immunopathologic profile that is similar to that of other primary non–small cell carcinomas.
Many of the other neoplasms in the differential diagnosis were discussed previously. Sarcomas that imitate primary large cell carcinomas have an epithelioid appearance, but except for epithelioid sarcoma and epithelioid synovial sarcoma, they are nonreactive for keratin immunohistochemically. Mesenchymal malignancies usually do not present with pulmonary metastases, with the possible exception of alveolar soft tissue sarcoma and some examples of malignant fibrous histiocytoma. The primary foci of those tumors may be occult, yet produce extensive distant disease in the lungs, brain, and other organs.232–234
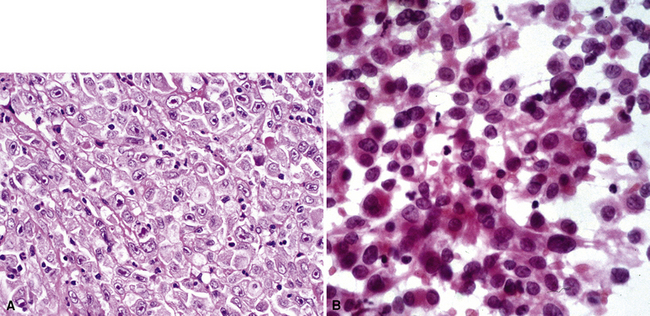
Poorly differentiated adrenocortical carcinomas may show lipidized cytoplasm, which is an unusual feature in primary large cell carcinoma. The most common metastatic germ cell malignancies that simulate primary pulmonary large cell carcinoma are embryonal carcinoma (Fig. 17-59) and choriocarcinoma. When these neoplasms metastasize, they are often admixed with other germ cell components, such as seminoma or teratoma, features that help to distinguish them from other large polygonal cell tumors. The clinical history of patients with secondary germ cell lesions is often distinctive, vis-à-vis that which accompanies primary lung cancers. For example, metastatic choriocarcinomas are most commonly seen in young women,235 whereas primary large cell carcinomas more often occur in older patients with a significant smoking history.236 Furthermore, individuals with metastatic choriocarcinoma typically have multiple lung lesions237 and an elevated level of β-human chorionic gonadotropin in the serum. Secondary embryonal carcinomas are usually seen in men, who also have gonadal, intracranial, mediastinal, or retroperitoneal masses.
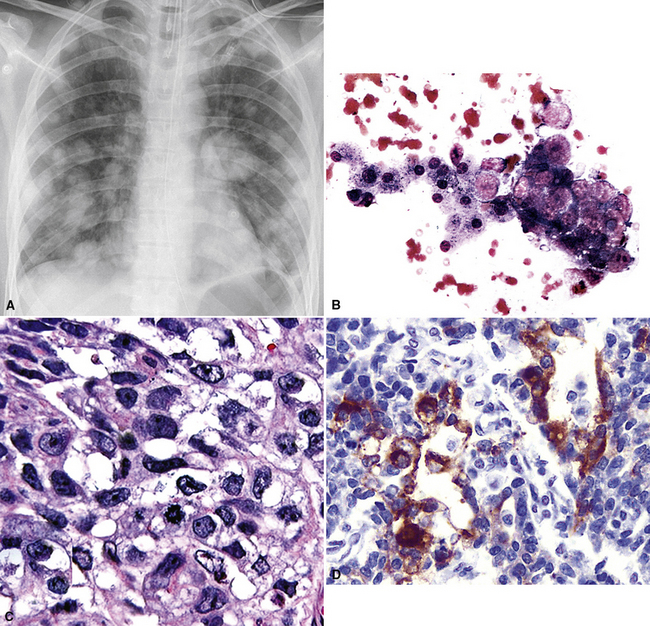
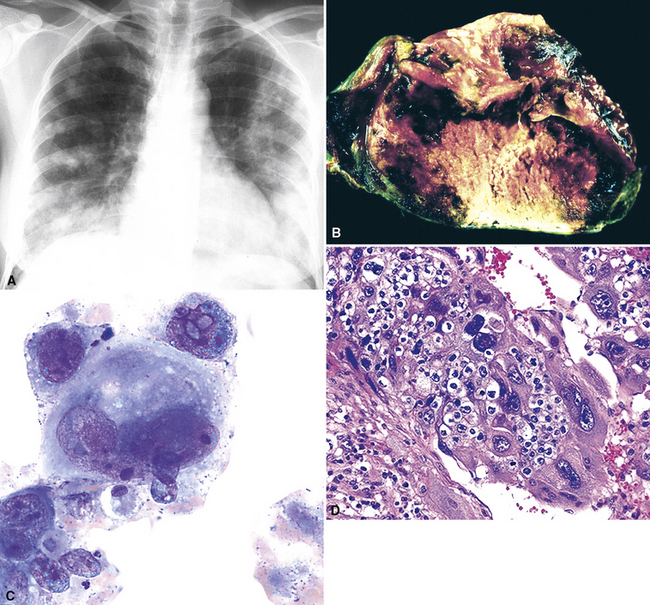
Figure 17-59 A, Chest radiograph showing metastatic “solid” embryonal carcinoma of the testis involving the lung. Findings on fine-needle aspiration (B) and subsequent biopsy (C) led to a diagnosis of metastatic embryonal carcinoma. The tumor is morphologically similar to that shown in Figure 17-58, but immunoreactivity for alpha-fetoprotein (D) and the patient’s history confirmed metastatic embryonal carcinoma.
The histologic image of metastatic germ cell tumors is often sufficient for their definitive recognition, especially when it includes two or more morphologic subtypes of such lesions. Moreover, yolk sac tumor commonly shows distinctive intercellular and intracytoplasmic eosinophilic globules, and choriocarcinoma is singular in its biphasic composition by cytotrophoblastic and syncytiotrophoblastic elements (Fig. 17-60). Nevertheless, the existence of primary somatic carcinomas of the lung with areas resembling germ cell tumor confounds the evaluation of these lesions.238
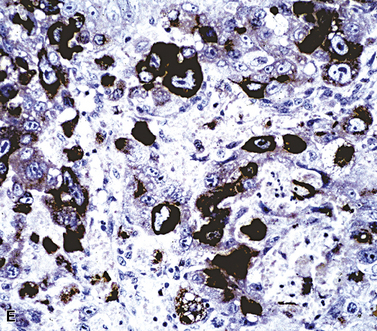
Table 17-7 shows the immunopathologic profile of specific large polygonal cell tumors. The panel of antibody reagents is particularly helpful in distinguishing primary large cell carcinoma from its diagnostic alternatives. Placental-like alkaline phosphatase is seen in most germ cell tumors, and it is occasionally present in primary large cell carcinomas.239 CD117 and CD30 are also selective markers for seminoma and embryonal carcinoma, respectively (Fig. 17-61). Those lesions may be distinguished from somatic tumors by their differential expression of epithelial membrane antigen; it is present in primary lung cancers, but not in germ cell malignancies.86,239 CD45 is restricted to large cell lymphoma (Fig. 17-62). MART-1 is limited to melanoma and adrenocortical carcinoma, and except for epithelioid sarcoma and clear cell sarcoma, metastatic mesenchymal neoplasms are typified by positivity for vimentin but are devoid of keratin and melanocyte-related markers.
Other Adjunctive Pathologic Techniques for the Diagnosis of Metastatic Carcinoma
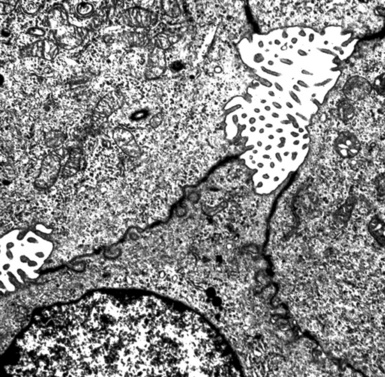
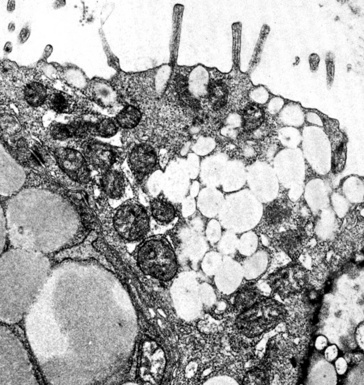
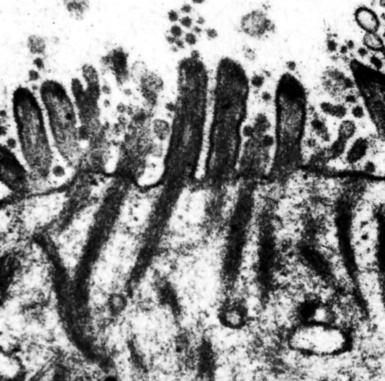
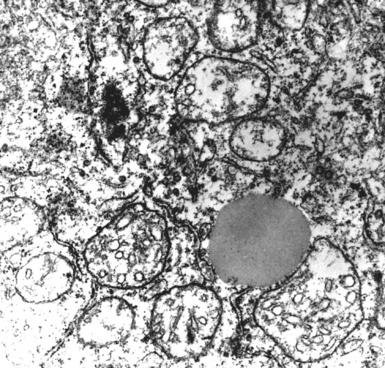
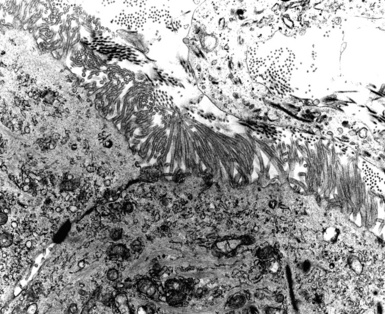
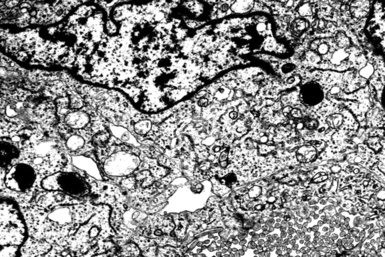
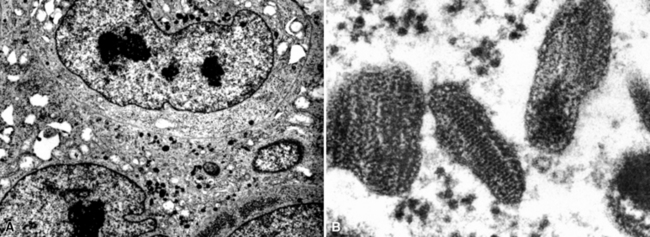
Although electron microscopy has been used less and less in recent years in the diagnosis of tumors in surgical pathology, it still has considerable value in that context. There are several settings in the evaluation of possibly metastatic tumors in the lung where ultrastructural studies are useful.240–250 Adenocarcinomas—including those arising in the pulmonary parenchyma—are characterized generically by short, nonbranched plasmalemmal microvilli with a length-to-diameter ratio of less than 10:1 (Figs. 17-63 and 17-64).251–253 Specialized features of specific lesions in that category include laminated cytoplasmic granules (primitive surfactant bodies) in some examples of primary adenocarcinoma of the lung (Fig. 17-65); cytoplasmic mucin granules and “terminal webs” of intermediate filaments that insert into the microvilli in adenocarcinomas with enteric differentiation (Fig. 17-66); glycogen pools and lipid droplets in metastatic renal cell carcinomas (Fig. 17-67); and tubular cristae in the mitochondria of steroid-producing tumors, such as adrenocortical carcinoma (Fig. 17-68).243,244,248,249 Malignant mesothelioma, a simulant of adenocarcinoma at the light microscopic level, is typified by elongated, bushy, branching microvilli with an length-to-diameter ratio of greater than 10:1, together with complex desmosomal complexes and cytoplasmic tonofilaments (Figs. 17-69 and 17-70).251–253 Mucin granules and surfactant bodies are absent in that tumor type.
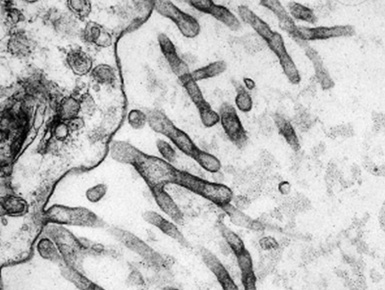
Figure 17-70 The branching nature of plasmalemmal microvilli in mesothelioma is well seen in an electron photomicrograph.
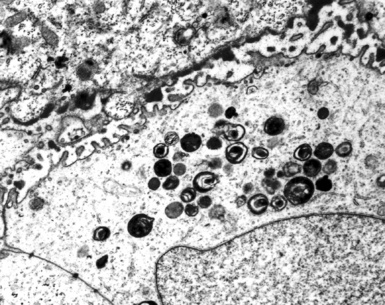
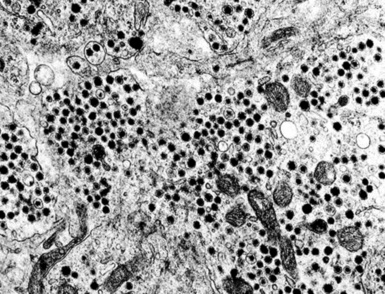
Neuroendocrine carcinomas may be identified with certainty because of their synthesis of dense-core (neurosecretory) granules measuring 150 to 400 nm in diameter. Those inclusions have peripheral zones of lucency and tend to be clustered in the cytoplasm (Fig. 17-71), often near the Golgi apparatus.240–243 Macular intercellular junctional complexes are also evident in such lesions, and small whorls of perinuclear intermediate filaments are common. These characteristics are the same, regardless of the site of origin of neuroendocrine tumors; therefore, electron microscopy cannot be used to distinguish primary pulmonary lesions from metastases.
Germ cell tumors exhibit electron microscopic characteristics that potentially simulate those of somatic carcinomas. Seminomas are undifferentiated at a fine structural level, showing only primitive appositional intercellular junctional complexes, prominent nucleoli, and the usual constituency of basic metabolic organelles (Fig. 17-72). One salient feature of these tumors is the presence of cytoplasmic glycogen pools, but those are shared by many nongerminal tumors.243 Embryonal carcinomas largely resemble somatic adenocarcinomas, including the formation of plasmalemmal microvilli and intercellular gland-like spaces (Fig. 17-73). Yolk sac tumors share the same potentialities. Choriocarcinomas are relatively distinctive ultrastructurally and show cytoplasmic tonofibrils reminiscent of those seen in squamous tumors.247 The latter lesions are not part of the light microscopic differential diagnosis of choriocarcinomas; that discrepancy may provide a clue to the interpretation.
Nonepithelial malignancies with specific electron microscopic attributes include rhabdomyosarcoma, leiomyosarcoma, alveolar soft tissue sarcoma, neuroblastoma, melanoma (and clear cell sarcoma), and endothelial sarcoma.254 These neoplasms show primitive sarcomeric differentiation, with thick and thin cytoplasmic myofilaments (Fig. 17-74); cytoplasmic skeins of thin filaments punctuated by dense bodies; paracrystalline cytoplasmic inclusions (Fig. 17-75); complex interdigitating cytoplasmic extensions containing microtubules (Fig. 17-76); premelanosomes (Fig. 17-77); and Weibel-Palade bodies (Fig. 17-78), respectively. All of these structures are absent in primary pulmonary tumors, except for sarcomatoid carcinomas. These lesions show a mixture of cells with epithelial characteristics (e.g., intercellular junctions, tonofibrils, microvilli) and others with mesenchymal attributes.
The electron microscopic attributes of other mesenchymal neoplasms are nondescript or even misleading diagnostically. For example, primitive neuroectodermal tumors (and other blastomas, such as hepatoblastoma or Wilms tumor) are composed of primordial round cells joined by macular attachment plaques and often containing only basic organelles (Fig. 17-79).245,254 In some cases, glycogen deposits or nascent cytoplasmic extensions may be seen, the latter of which contain neurosecretory granules or synaptic-type vesicles. Although they are soft tissue neoplasms, epithelioid sarcomas and synovial sarcomas mirror their immunohistologic features ultrastructurally because they show polygonal cells joined by well-formed intercellular attachment plaques (Fig. 17-80) and may even show microvillous differentiation.254
Hematopoietic proliferations are perhaps the most primitive at an electron microscopic level. They show only basic cytoplasmic constituents—often containing abundant dispersed ribosomes, with or without rough endoplasmic reticulum—but lacking other distinguishing features (Fig. 17-81).249,255
Cytogenetic information is rapidly accumulating on a variety of tumor types, and it holds the promise of serving as helpful differential diagnostic data. For example, several neoplasms have unique chromosomal abnormalities that exclude other possibilities. These include deletions of the short arm of chromosome 3 in renal cell carcinoma; an unrelated deletion in the same chromosomal segment in primary SCNCL; the t(11;22) translocation in primitive neuroectodermal tumor/Ewing sarcoma; the t(1;13) or t(2;13) translocation in alveolar rhabdomyosarcoma; the t(12;22) translocation in clear cell sarcoma; the der(17)t(X;17) translocation in alveolar soft tissue sarcoma; the t(X;18) translocation in synovial sarcoma; isochromosome 12p in germ cell malignancies; and a group of semispecific or specific karyotypic abnormalities in malignant lymphomas and leukemias.256–267 These markers are best assessed with fresh tissue, but assays based on polymerase chain reaction or fluorescent in situ hybridization studies are also becoming available for use in hospital practice.
Outcomes Analysis
Most decision-related analytic studies that have attempted to determine optimal testing strategies have focused on patients with solitary pulmonary nodules, and the authors generally have assumed that there was no known history of malignancy. In these publications, the optimal testing paradigm was also equated with the most cost-effective strategy, meaning that it resulted in the greatest increase in population-related life expectancy for the lowest cost. Evaluations of this type have reached contradictory conclusions, with some stating that open biopsy or excision is the procedure of choice and others suggesting that sputum cytology should precede other testing methods.268–272
Potential reasons for these disagreements include study bias, assumptions based on incomplete data, and the overall complexity of analytic modeling. One study that included theoretical patient preferences showed that the cost-effectiveness of testing strategies was variable, depending on patient values such as risk aversion (aversion to a false-negative diagnosis or a testing complication).273–276 Although these factors have yet to be measured in practice, the results of the latter study showed that a single testing strategy may not apply for all patient populations, including those with potential metastatic disease in the lungs.
Raab and coworkers assessed the sensitivity and cost-effectiveness of percutaneous FNA in a group of patients with known extrapulmonary malignancies and solitary lung masses.277 Using data from two hospitals with active FNA programs, they showed that pathologists correctly classified 87% of the pulmonary lesions as primary or secondary malignancies. More than 90% were metastases, indicating that the probability of a primary lung tumor is low in this clinical setting. As mentioned earlier, the distinction of primary and secondary malignancies depended on light microscopic features; morphologic comparison with previous specimens, when available; and the judicious use of immunocytochemistry.
Raab and coworkers found that the latter method was needed in only 20% of cases, but it yielded a definitive diagnosis in 78% of cases in which it was used.277 The cost-effectiveness of percutaneous FNA—compared with bronchoscopy and thoracoscopy—depends on several factors, such as test sensitivity and the pre-FNA probability of malignancy. An underlying assumption is that patients with metastatic disease do not need pulmonary resection, although these procedures may be appropriate in some cases. At pre-FNA probability of malignancy of more than 50% and FNA sensitivity of greater than 75%, FNA was more cost-effective than thoracoscopy in the cited series.277 In most clinical scenarios, percutaneous FNA was also more cost-effective than bronchoscopy. Nonetheless, clinicians still tend to use a wide variety of testing strategies in the diagnostic evaluation of pulmonary masses.274
Another question of increasing importance is the utilitarian value of ancillary studies, such as immunopathology, in tumor subtyping. Raab showed that immunocytochemistry was cost-effective in three theoretical scenarios: increasing patient life expectancy, diagnostic certainty, and the ability to predict patient prognosis.277 However, as Wick and colleagues indicated, it has never been proven formally that the correct immunohistologic diagnosis of a specific tumor type, particularly concerning nonhematolymphoid malignancies, produces a benefit in patient outcome.197 Accurate pathologic diagnosis is believed to be advantageous in some instances, such as those concerning small-cell pediatric malignancies. Nonetheless, for the most part, patient survival generally depends more on nonpathologic variables, such as tumor stage, patient age, and overall health.276–280 The actual effect of accurate diagnosis was not reported in the latter study, which investigated the cost-effectiveness of FNA in evaluation of lung masses, but Imlay and Raab separately showed that the use of immunopathology had no effect on patient survival in reference to pleural fluid evaluation.45
Some immunologic markers have a potentially predictive role in secondary pulmonary malignancies, such as estrogen receptor protein and progesterone receptor protein in cases of metastatic breast cancer. Those determinants may forecast the clinical response to appropriate treatment regimens, the response to which, in turn, may be prognostic (indicative of overall patient survival).197 The cost-effectiveness of most other markers of that type has not yet been thoroughly assessed.197
1 Crow J., Slavin G., Kreel L. Pulmonary metastasis: a pathologic and radiologic study. Cancer. 1981;47:2595-2603.
2 Johnson R.M., Lindskog G.E. 100 cases of tumor metastatic to the lung and mediastinum. JAMA. 1967;202:94-98.
3 Abrams H.J., Spiro R., Goldstein N. Metastates in carcinoma, analysis of 1000 autopsied cases. Cancer. 1950;3:74-75.
4 Farrell J.T. Pulmonary metastasis: a pathologic, clinical, roentgenologic study based on 78 cases seen at necropsy. Radiology. 1935;24:444-451.
5 Matthay R., Coppage L., Shaw C., Filderman A. Malignancies metastatic to the pleura. Invest Radiol. 1990;25:601-619.
6 Filderman A.E., Coppage L., Shaw C., Matthay R.A. Pulmonary and pleural manifestations of extrathoracic malignancies. Clin Chest Med. 1989;10:747-807.
7 Fidler I.J. Review: Biologic heterogeneity of cancer metastases. Breast Cancer Res Treat. 1987;17:17.
8 Fidler I.J. The evolution of biologic heterogeneity in metastatic neoplasms. In: Nicholson G.L., Milas L., editors. Cancer Invasion and Metastases: Biologic and Therapeutic Aspects. New York: Raven Press; 1984:5.
9 Liotta L.A. Editorial. H-ras p21 and the metastatic phenotype. J Natl Cancer Inst. 1988;80:468.
10 Cotran R.S., Kumar V., Collins T., Robbins S.L. Neoplasia. In: Robbins Pathologic Basis of Disease. Philadelphia: WB Saunders; 1999:241-304.
11 Braman S.S., Whitcomb M.E. Endobronchial metastasis. Arch Intern Med. 1975;135:543-547.
12 Winterbauer R.H., Elfenbein I.B.Jr. WCB. Incidence and clinical significance of tumor embolization to the lungs. Am J Med. 1968;45:271-290.
13 Goldhaber S.Z., Dricker E., Buring J.E. Clinical suspicion of autopsy-proven thrombotic and tumor pulmonary embolism in cancer patients. Am Heart J. 1987;114:1432-1435.
14 Chan C.K., Hutcheon M.A., Hyland R.H., et al. Pulmonary tumor embolism: a critical review of clinical, imaging, and hemodynamic features. J Thorac Imaging. 1987;2:4-14.
15 Gonzalez-Vitale J.C., Garcia-Bunuel R. Pulmonary tumor emboli and cor pulmonale in primary carcinoma of the lung. Cancer. 1976;38:2105-2110.
16 Abbondanzo S.L., Klappenbach R.S., Tsou E. Tumor cell embolism to pulmonary alveolar capillaries. Arch Pathol Lab Med. 1986;110:1197-1198.
17 Kane R.D., Hawkins H.K., Miller J.A. Microscopic pulmonary tumor emboli associated with dyspnea. Cancer. 1975;36:1473-1482.
18 Pinckard J.K., Wick M.R. Tumor-related thrombotic pulmonary microangiopathy: review of pathologic findings and pathophysiologic mechanisms. Ann Diagn Pathol. 2000;4:154-157.
19 Soares F.A., Landell G.A.M., deOliveira J.A.M. Pulmonary tumor embolism to alveolar septal capillaries: a prospective study of 12 cases. Arch Pathol Lab Med. 1991;115:127-130.
20 Scholten E.T., Kreel L. Distribution of lung metastases in the axial plane. Radiol Clin North Am. 1977;46:248-265.
21 Steele J.D. The solitary pulmonary nodule. J Thorac Cardiovasc Surg. 1963;46:21-39.
22 Toomes H., Delphendahl A., Manke H., Vogt-Moykopf I. The coin lesion of the lung: a review of 955 resected coin lesions. Cancer. 1983;51:534-537.
23 Viggiano R.W., Swensen S.J., Rosenow E.C. Evaluation and management of solitary and mulitple pulmonary nodules. Clin Chest Med. 1992;13:83-95.
24 Quint L.E., Park C.H., Iannettoni M.D. Solitary pulmonary nodules in patients with extrapulmonary neoplasms. Radiology. 2000;217:257-261.
25 Dodd G.D., Boyle J.J. Excavating pulmonary metastases. Am J Roentgenol. 1961;85:277-293.
26 D’Angio G.J., Iannaccone G. Spontaneous pneumothorax as a complication of pulmonary metastases in malignant tumors of childhood. Am J Roentgenol. 1961;86:1092-1102.
27 Fichera G., Hagerstrand I. The small lymph vessels of the lungs in lymphangiosis carcinomatosa. Acta Pathol Microbiol Scand. 1965;65:505-513.
28 Harold J.T. Lymphangitis carcinomatosa of the lungs. Q J Med. 1952;21:353-360.
29 Yang S.P., Lin C.C. Lymphangitic carcinomatosis of the lungs: the clinical significance of its roentgenologic classification. Chest. 1972;62:179-187.
30 Trapnell D.H. The radiological appearance of lymphangitic carcinomatosa of the lung. Thorax. 1964;19:251-260.
31 Winterbauer R.H., Belic N., Moores K.D. A clinical interpretation of bilateral hilar adenopathy. Ann Intern Med. 1973;78:65-71.
32 Janower M.L., Blennerhassett J.B. Lymphangitic spread of metastatic cancer to the lung: a radiologic–pathologic classification. Radiology. 1971;101:267-273.
33 Goldsmith H.S., Bailey H.D., Callahan E.L., Beattie E.J. Pulmonary lymphangitic metastases from breast cancer. Arch Surg. 1967;94:483-488.
34 Thurlbeck W.M. Neoplasia of the pulmonary vascular bed. In: Moser K.M., editor. Pulmonary Vascular Disease. New York: Marcel Dekker; 1979:629-649.
35 Canto-Armengod A. Macroscopic characteristics of pleural metastases arising from the breast and observed by diagnostic thorascopy. Am Rev Respir Dis. 1990;142:616-618.
36 Hsu C. Cytologic detection of malignancy in pleural effusion: review of 5,255 samples from 3,811 patients. Diagn Cytopathol. 1987;3:8-12.
37 Johnson W.W. The malignant pleural effusion: a review of cytopathologic diagnoses of 584 specimens from 472 consecutive patients. Cancer. 1985;56:905-909.
38 Venrick M.G., Sidaway M.K. Cytologic evaluation of serous effusions: processing techniques and optimal number of smears for routine preparation. Am J Clin Pathol. 1993;99:182-186.
39 DeMay R.M. Fluids. In: The Art and Science of Cytopathology. Chicago: ASCP Press; 1996:257-325.
40 Chernow B., Sahn S.A. Carcinomatous involvement of the pleura: an analysis of 96 patients. Am J Med. 1977;63:695-702.
41 Edoute Y., Kuten A., Ben-Haim S.A. Symptomatic pericardial effusion in breast cancer patients: the role of fluid cytology. J Surg Oncol. 1990;45:265-269.
42 Sahn S.A. Malignant pleural effusions. Semin Respir Med. 1987;9:43-53.
43 Canto A., Ferrer G., Romagosa V., et al. Lung cancer and pleural effusion. Clincal significance and study of pleural metastic locations. Chest. 1985;87:649-852.
44 DiBonito L., Falconieri G., Colautti I. Cytopathology of malignant mesothelioma: a study of its patterns and histological bases. Diagn Cytopathol. 1993;9:25-31.
45 Imlay S.P., Raab S.S. Pleural fluid cytology: immunocytochemistry usage patterns and significance of nondefinitive diagnosis. Diagn Cytopath. 1999;22:281-285.
46 VandeMolengraft F.J.J.M., Vooijs G.P. Survival of patients with malignancy-associated effusions. Acta Cytol. 1989;33:911-916.
47 Chretien J., Jaubert F. Pleural responses in malignant metastatic tumors. In: Chretien J., Bignon J., Hirsch A., editors. The Pleura in Health and Disease. New York: Marcel Dekker; 1985:489-505.
48 Sahn S.A. Malignant pleural effusion. In: Fishman A.P., editor. Pulmonary Diseases and Disorders. 2nd ed. New York: McGraw-Hill; 1988:2159-2169.
49 Spriggs A.I. Malignant cells in serous effusions complicating bronchial carcinoma. Thorax. 1954;9:26-34.
50 Smith-Purslow M.J., Kini S.R., Naylor B. Cells of squamous cell carcinoma in pleural, peritoneal and pericardial fluids: origina and morphology. Acta Cytol. 1989;84:125-128.
51 Light RW, Tumors of the pleura In: Murray JF,.Nadel JA, eds. Textbook of Respiratory Medicine. Philadelphia: WB Saunders; 1770–1780
52 Bourke S.A., Henderson A.F., Stevenson R.D., Banham S.W. Endobronchial metastases simulating primary carcinoma of the lung. Respir Med. 1989;83:151-152.
53 King D.S., Castleman B. Bronchial involvement in metastatic pulmonary malignancy. J Thorac Surg. 1943;12:305-315.
54 Katsimbri P.P., Bamias A.T., Froudarakis M.E., et al. Endobronchial metastases secondary to solid tumors: report of eight cases and review of the literature. Lung Cancer. 2000;28:163-170.
55 Casino A.R., Bellmunt J., Salud A., et al. Endobronchial metastases in colorectal adenocarcinoma. Tumori. 1992;78:270-273.
56 Schoenbaum S., Viamonte M. Subepithelial endobronchial metastases. Diagn Radiol. 1971;101:63-69.
57 Fitzgerald R.H. Endobronchial metastases. South Med J. 1977;79:440-443.
58 Heitmiller R., Marasco W., Hruban R., Marsh B. Endobronchial metastasis. J Thorac Cardiovasc Surg. 1993;106:537-542.
59 Argyros G., Torrington K. Fiberoptic bronchoscopy in the evaluation of carcinoma metastatic to the lung. Chest. 1994;105:454-457.
60 Wang Y.H., Wong S.L., Lai Y.F., et al. Endobronchial metastatic disease. Chang Keng I Hsueh Tsa Chih. 1999;22:240-245.
61 Salud A., Porcel J.M., Rovirosa A., Bellmunt J. Endobronchial metastatic disease: analysis of 32 cases. J Surg Oncol. 1996;62:249-252.
62 Mehta A.C., Marty J.J., Lee F.W.Y. Sputum cytology. Lung Cancer. 1993;14:36-85.
63 Koss L., Melamed M., Goodner J. Pulmonary cytology: a brief survey of diagnostic results from July 1st, 1952 until December 31st, 1960. Acta Cytol. 1964;8:104-113.
64 Arroliga A., Matthay R. The role of bronchoscopy in lung cancer. Lung Cancer. 1993;14:87-98.
65 Harrow E.M., Wang K.P. The staging of lung cancer by bronchoscopic transbronchial needle aspiration. Chest Surg Clin North Am. 1996;6:223-235.
66 Wang K.P. Transbronchial needle aspiration and percutaneous needle aspiration for staging and diagnosis of lung cancer. Clin Chest Med. 1995;16:535-552.
67 Salazar A., Westcott J. The role of thoracic needle biopsy for the diagnosis and staging of lung cancer. Lung Cancer. 1993;14:99-110.
68 Garcia R.F., Lobato S.D., Pino J.M. Value of CT-guided fine needle aspiration in solitary pulmonary nodules with negative fiberoptic bronchoscopy. Acta Radiol. 1994;35:478-480.
69 Sonett J. Pulmonary metastases: biologic and historical justification for VATS. Video assisted thoracic surgery. Eur J Cardiothorac Surg. 1999;16(suppl):S13-S15.
70 Coosemans W., Lerut T., Raemdonck D.V. Thoracoscopic surgery: the Belgian experience. Ann Thorac Surg. 1993;56:621-660.
71 Miller J. The present role and future considerations of video-assisted thorascopy in general thoracic surgery. Ann Thorac Surg. 1993;56:804-806.
72 Nathan M., Colloing V., Adams R. Differentation of benign and malignant pulmonary nodules by growth rate. Radiology. 1962;79:221-232.
73 Nathan M. Management of solitary pulmonary nodules: an organized approach based on growth rate and statistics. JAMA. 1974;227:1141-1144.
74 Godwin J.P., Speckman J.M., Fram E.K. Distinguishing benign from malignant pulmonary nodules by computed tomography. Radiology. 1982;144:349-352.
75 Layfield L.J., Coogan A., Johnston W.W., Patz E.F. Transthoracic fine needle aspiration biopsy. Sensitivity in relation to guidance technique and lesion size and location. Acta Cytol. 1996;40:687-690.
76 Pilotti S., Rilke F., Gribaudi G., Damascelli B. Fine needle aspiration biopsy cytology of primary and metastatic pulmonary tumors. Acta Cytol. 1982;26:661-666.
77 Zarbo R., Fenoglio-Preiser C. Interinstitutional database for comparison of performance in lung fine-needle aspiration cytology. A College of American Pathologists Q-Probe Study of 5264 cases with histologic correlation. Arch Pathol Lab Med. 1992;116:463-470.
78 Kern W.H., Schweizer C.W. Sputum cytology of metastatic carcinoma of the lung. Acta Cytol. 1976;20:514-520.
79 Perchalski J.E., Hall K.L., Dewar M.A. Metastasis of unknown orgin. Prim Care. 1992;19:747-757.
80 Fizazi K., Culine S. Metastatic carcinoma of unknown orgin. Bull Cancer. 1998;85:609-617.
81 Schapira D.V., Jerrett A.R. The need to consider survival, outcome, and expense when evaluating and treating patients with unknown primary carcinoma. Arch Intern Med. 1995;155:2050-2054.
82 Heim S., Mitleman F. Solid tumors. In: Cancer Cytogenetics. New York: Alan R Liss; 1987:227-261.
83 Papec R. Distant metastases from head and neck. Cancer. 1984;53:342-345.
84 Probert J., Thompson R., Bagshaw M. Patterns of spread of distant metastases in head and neck cancer. Cancer. 1974;33:127-133.
85 Raab S.S., Oweity T., Hughes J.H., et al. The effect of patient history on diagnostic accuracy in the interpretation of bronchial brush specimens. Am J Clin Pathol. 2000;114:78-83.
86 DeYoung B.R., Wick M.R. Immunohistologic evaluation of metastatic carcinomas by unknown origin: an algorithmic approach. Semin Diagn Pathol. 2000;17:184-193.
87 Silver S.S., Askin F.B. True papillary carcinoma of the lung. A distinct clinicopathologic entity. Am J Surg Pathol. 1997;21:43-51.
88 Nakamura S., Koshikawa T., Sato T., et al. Extremely well differentiated papillary adenocarcinoma of the lung with prominent cilia formation. Acta Pathol Jpn. 1992;42:745-750.
89 Salisbury J.R., Darby A.J., Whimster W.F. Papillary adenocarcinoma of lung with psammoma bodies: report of a case derived from type II pneumocytes. Histopathology. 1986;10:877-884.
90 Massin J-P, Savoie J-C, Garnier H., et al. Pulmonary metastases in differentiated thyroid carcinoma. Study of 58 cases with implications for the primary tumor treatment. Cancer. 1984;53:982-992.
91 Samaan N.A., Schultz P.N., Haynie T.P., Ordonez N.G. Pulmonary metastasis of differential thyroid carcinoma: treatment results in 101 patients. J Clin Endocrinol Metab. 1985;60:376-380.
92 Venkatesh Y.S.S., Ordonez N.G., Schultz P.N., et al. Anaplastic carcinoma of the thyroid: a clinicopathologic study of 121 cases. Cancer. 1990;66:321-330.
93 Shepherd M.P. Endobronchial metastatic disease. Thorax. 1982;37:362-365.
94 Kerr V.E., Cadman E. Pulmonary metastases in ovarian cancer. Cancer. 1985;56:1209-1213.
95 Dvoretsky P.M., Richards K.A., Angel C. Distribution of disease at autopsy in 100 women with ovarian cancer. Hum Pathol. 1988;19:57-63.
96 Oosterlee J. Peritoneovenous shunting for ascites in cancer patients. Br J Surg. 1980;67:663-666.
97 Fildes J., Narvarez G.P., Baig K.A., et al. Pulmonary tumor embolization after peritoneovenous shunting for malignant ascites. Cancer. 1988;61:1973-1976.
98 Werner M., Chott A., Fabiano A., Battifora H. Effect of formalin tissue fixation and processing on immunohistochemistry. Am J Surg Pathol. 2000;24:1016-1019.
99 Renshaw A.A., Dean B.R., Antman K.H., et al. The role of cytologic evaluation of pleural fluid in the diagnosis of malignant mesothelioma. Chest. 1997;111:106-109.
100 Moran C.A., Wick M.R., Suster S. The role of immunohistochemistry in the diagnosis of malignant mesothelioma. Semin Diagn Pathol. 2000;17:178-183.
101 Moch H., Oberholzer M., Dalquen P. Diagnostic tools for differentiating between pleural mesothelioma and lung adenocarcinoma in paraffin embedded tissue. Virch Arch A. 1993;423:19-27.
102 Moch H., Oberholzer M., Christen H. Diagnostic tools for differentiating plural mesothelioma from lung adenocarcinoma in paraffin embedded tissue. Part II. Virch Arch A. 1993;423:493-496.
103 Wang N-S. Electron microscopy in the diagnosis of pleural mesotheloma. Cancer. 1973;31:1046-1054.
104 Batiffora H., Kopinski M.I. Distinction of mesothelioma from adenocarcinoma. An immunohistochemical approach. Cancer. 1985;55:655-662.
105 Strickler J.G., Hemdier B.G., Rouse R.V. Immunohistochemical staining in malignant mesotheliomas. Am J Clin Pathol. 1987;88:610-614.
106 Gaffey M., Mills S., Askin F., et al. Clear cell tumor of the lung. A clinicopathologic, immunohistochemical, and ultrastructural study of eight cases. Am J Surg Pathol. 1990;14:248-259.
107 Greenberg B.E., Young J.M. Pulmonary metastasis from occult primary sites resembling bronchogenic carcinoma. Dis Chest. 1958;33:496-505.
108 Hughes J.H., Jensen C.S., Donnelly A.D., et al. The role of fine-needle aspiration cytology in the evaluation of metastatic clear cell tumors. Cancer. 1999;87:380-389.
109 Saitoh H. Distant metastasis of renal adenocarcinoma in patients with a tumor thrombus in the renal vein and/or vena cava. J Urol. 1982;127:652-653.
110 Latour A., Shulman H.S. Thoracic manifestations of renal cell carcinoma. Radiology. 1976;121:43-48.
111 Coppage L., Shaw C., Curtis A.M. Metastatic disease to the chest in patients with extrathoracic malignancy. J Thoracic Imag. 1987;2:24-37.
112 Gerle R., Felson B. Metastatic endobronchial hypernephroma. Dis Chest. 1963;44:225-233.
113 Noy S., Michowitz M., Lazebnik N., Baratz M. Endobronchial metastasis of renal cell carcinoma. J Surg Oncol. 1986;31:268-270.
114 Amer E., Guy J., Vaze B. Endobronchial metastasis from renal adenocarcinoma simulating a foreign body. Thorax. 1981;36:183-184.
115 King T.E., Fisher J., Schwarz M.I., Patzelt L.H. Bilateral hilar adenopathy: an unusual presentation of renal cell carcinoma. Thorax. 1982;37:317-318.
116 Katzenstein A-LA, Purvis R.W., Gmelich J.T., Askin F.B. Pulmonary resection for metastatic renal adenocarcinoma. Cancer. 1978;41:712-723.
117 Chu P., Arber D.A. Paraffin section detection of CD10 in 505 nonhematopoietic neoplasms: frequent expression in renal cell carcinoma and endometrial stromal sarcoma. Am J Clin Pathol. 2000;113:374-382.
118 Scarpatetti M., Tsybrovskyy O., Popper H.H. Cytokeratin typing as an aid in the differential diagnosis of primary versus metastatic lung carcinoma, and comparison with normal lung. Virchows Arch. 2002;440:70-76.
119 Avery A.K., Beckstead J., Renshaw A.A., Corless C.L. Use of antibodies to RCC and CD10 in the differential diagnosis of renal neoplasms. Am J Surg Pathol. 2000;24:203-210.
120 Ostler D.A., Prieto V.G., Reed J.A., et al. Adipophilin expression in sebaceous tumors and other cutaneous lesions with clear-cell histology: an immunohistochemical study of 117 cases. Mod Pathol. 2010;23:567-573.
121 Lisa J.R., Trinidad S., Rosenblatt M.B. Pulmonary manifestations of carcinoma of the pancreas. Cancer. 1964;17:395-401.
122 Cutler S.J., Asire A.J., Taylor S.G. Classification of patients with disseminated cancer of the breast. Cancer. 1969;24:861-869.
123 DeBeer R.A., Garcia R.L., Alexander S.C. Endobronchial metastasis from cancer of the breast. Chest. 1978;73:94-96.
124 Winchester D.P., Sener S.F., Khandekar J.D. Symptomatology as an indicator of recurrent or metastatic breast cancer. Cancer. 1979:43.
125 Casey J.J., Stempel B.G., Scanlon E.F., Fry W.A. The solitary pulmonary nodule in the patient with breast cancer. Surgery. 1984;96:801-805.
126 Fracchia A.A., Knapper W.H., Carey J.T. Intrapleural chemotherapy for effusion from metastatic breast carcinoma. Cancer. 1970;26:626-629.
127 Raab S.S., Berg L.C., Swanson P.E., Wick M.R. Adenocarcinoma in the lung in patients with breast cancer. A prospective analysis of the discriminatory value of immunohistology. Am J Clin Pathol. 1993;100:27-35.
128 Dabbs D.J., Landreneau R.J., Liu Y., et al. Detection of estrogen receptor by immunohistochemistry in pulmonary adenocarcinoma. Ann Thorac Surg. 2002;73:403-406.
129 Wang Z., Spaulding B., Sienko A., et al. Mammaglobin: a valuable diagnostic marker for metastatic breast carcinoma. Int J Clin Exp Pathol. 2009;2:384-389.
130 MacDonald R.A. Primary carcioma of the liver. A clinicopathologic study of one hundred eight cases. Arch Intern Med. 1957;99:266-279.
131 Katyal S., Oliver J.H., Peterson M.S., et al. Extrahepatic metastases of hepatocellular carcinoma. Radiology. 2000;216:698-703.
132 Patton R.B., Horn R.C. Primary liver carcinoma. Autopsy study of 60 cases. Cancer. 1964;17:757-768.
133 Tsai G.L., Liu J.D., Siauw C.P., Chen P.A. Thoracic roentgenologic manifestations in primary carcinoma of the liver. Chest. 1984;86:430-434.
134 Levy J.I., Geddes E.W., Kew M.C. The chest radiograph in primary liver cancer. An analysis of 449 cases. S Afr Med J. 1976;50:1323-1326.
135 Cohen M.B., Haber M.M., Holly E.A., et al. Cytologic criteria to distinguish hepatocellular carcinoma from nonneoplastic liver. Am J Clin Pathol. 1991;95:125-130.
136 Wieczorek T.J., Pinkus J.L., Glickman J.N., Pinkus G.S. Comparison of thyroid transcription factor-1 and hepatocyte antigen immunohistochemical analysis in the differential diagnosis of hepatocellular carcinoma, metastatic adenocarcinoma, renal cell carcinoma, and adrenal cortical carcinoma. Am J Clin Pathol. 2002;118:911-921.
137 August D.A., Ottow R.T., Sugarbaker P.H. Clinical perspective of human colorectal cancer metastasis. Cancer Metastasis Rev. 1984;5:303-324.
138 Dionne L. The pattern of blood-borne metastasis from carcinoma of rectum. Cancer. 1965;18:775-781.
139 Taylor F.W. Cancer of the colon and rectum: a study of routes of metastases and death. Surgery. 1962;52:302-308.
140 Langer B. Managing distant metastases. Can J Surg. 1985;28:419-421.
141 McCormack P.M., Attiyeh F.F. Resected pulmonary metastases from colorectal cancer. Dis Colon Rectum. 1979;22:553-556.
142 Flint A., Lloyd R.V. Colon carcinoma metastatic to the lung. Cytologic manifestations and distinction from primary pulmonary adenocarcinoma. Acta Cytol. 1992;36:230-235.
143 Harlamert H.A., Mira J., Bejarano P.A. Thyroid transcription factor-1 and cytokeratins 7 and 20 in pulmonary and breast carcinoma. Acta Cytol. 1998;42:1382-1388.
144 Bohinski R.J., Bejarano P.A., Balko G. Determination of lung as the primary site of cerebral metastatic adenocarcinomas using monoclonal antibody to thyroid transcription factor-1. J Neurooncol. 1998;40:227-231.
145 Wauters C.C., Smedts F., Gerrits L.G., et al. Keratins 7 and 20 as diagnositc markers of carcinomas metastatic to the ovary. Hum Pathol. 1995;26:852-855.
146 Tsao M.S., Fraser R.S. Primary pulmonary adenocarcinoma with enteric differentiation. Cancer. 1991;68:1754-1757.
147 Bacchi C.E., Gown A.M. Distribution and pattern of expression of villin, a gastrointestinal-associated cytoskeletal protein, in human carcinomas: a study employing paraffin-embedded tissue. Lab Invest. 1991;64:418-424.
148 Gatalica Z., Miettinen M. Distribution of carcinoma antigens CA19-9 and CA15-3: an immunohistochemical study of 400 tumors. Appl Immunohistochem. 1994;2:205-211.
149 Saad R.S., Silverman J.F., Khalifa M.A., Rowsell C. CDX2, cytokeratin 7 and 20 immunoreactivity in rectal adenocarcinoma. Appl Immunohistochem Mol Morphol. 2009;17:196-201.
150 Moran C.A., Hocchholzer L., Fishback N., et al. Mucinous (so-called colloid) carcinomas of lung. Mod Pathol. 1992;5:634-638.
151 Cathro H.P., Stoler M.H. Expression of cytokeratins 7 and 20 in ovarian neoplasia. Am J Clin Pathol. 2002;117:944-951.
152 Ware J.L. Prostate tumor progression and metastasis. Biochim Biophys Acta. 1987;907:279-298.
153 Ballon S.C., Donaldson R.C., Growdon W.A. Pulmonary metastases in endometrial carcinoma. In: Weiss L., Gilbert H.W., editors. Pulmonary Metastasis. Boston: GK Hall, 1978.
154 Mintz E.R., Smith G.G. Autopsy finding in 100 cases of prostatic cancer. N Engl J Med. 1934;211:479-487.
155 Elkin M., Mueller H.P. Metastasis from cancer of the prostate: autopsy and roentgenological findings. Cancer. 1954;7:1246-1248.
156 Kume H., Takai K., Kameyama S., Kawabe K. Multiple pulmonary metastasis of prostatic carcinoma with little or no bone or lymph node metastasis. Report of two cases and review of the literature. Urol Int. 1999;62:44-47.
157 Scherz H., Schmidt J.D. Endobronchial metastasis from prostate carcinoma. Prostate. 1986;8:319-324.
158 Legge D.A., Good C.A., Ludwig J. Roentgenologic features of pulmonary carcinomatosis from carcinoma of the prostate. AJR. 1971;11:360-364.
159 Apple J.S., Paulson D.F., Baber C., Putman C.E. Advanced prostatic carcinoma: pulmonary manifestations. Radiology. 1985;54:601-604.
160 Miller G.J. The use of histochemistry and immunohistochemistry in evaluating prostatic neoplasia. Prog Surg Pathol. 1982;5:115-126.
161 Ross J.S., Mazumder A. Tissue microarrays and gene chips. In: Wick M.R., editor. Metastatic Carcinomas of Unknown Origin. New York: Demos; 2008:177-190.
162 van Laar R.K., Ma X.J., de Jong D., et al. Implementation of a novel microarray-based diagnostic test for cancer of unknown primary. Int J Cancer. 2009;125:1390-1397.
163 Varadhachary G.R., Talantov D., Raber M.N., et al. Molecular profiling of carcinoma of unknown primary and correlation with clinical evaluation. J Clin Oncol. 2008;26:4442-4448.
164 Horlings H.M., van Laar R.K., Kerst J.M., et al. Gene expression profiling to identify the histogenetic origin of metastatic adenocarcinomas of unknown primary. J Clin Oncol. 2008;26:4435-4441.
165 Oien K.A., Evans T.R. Raising the profile of cancer of unknown primary. J Clin Oncol. 2008;26:4373-4375.
166 Pentheroudakis G., Golfinopoulos V., Pavlidis N. Switching benchmarks in cancer of unknown primary: from autopsy to microarray. Eur J Cancer. 2007;43:2026-2036.
167 Ullmann R., Morbini P., Halbwedl I., et al. Protein expression profiles in adenocarcinomas and squamous cell carcinomas of the lung generated using tissue microarrays. J Pathol. 2004;203:798-807.
168 Dennis J.L., Vass J.K., Wit E.C., et al. Identification from public data of molecular markers of adenocarcinoma characteristic of the site of origin. Cancer Res. 2002;62:5999-6005.
169 Buckhaults P., Zhang Z., Chen Y.C., et al. Identifying tumor origin using a gene expression-based classification map. Cancer Res. 2003;63:4144-4149.
170 Suster S. Recent advances in the application of immunohistochemical markers for the diagnosis of soft tissue tumors. Semin Diagn Pathol. 2000;17:225-235.
171 Lewis J., Brennan M. Soft tissue sarcomas. Curr Probl Surg. 1996;33:817-872.
172 Scranton P.E., DeCicco F.A., Totten R.S. Prognostic factors in osteosarcoma. A review of 20 year’s experience at the University of Pittsburgh Health Center Hospitals. Cancer. 1975;36:2179-2191.
173 Vezeridis M.P., Moore R., Karakousis C.P. Metastatic patterns in soft-tissue sarcomas. Arch Surg. 1983;118:915-918.
174 Flynn K.J., Kim H.S. Endobronchial metastasis of uterine leiomyosarcoma. JAMA. 1978:1978.
175 Aronchick J.M., Palevsky H.I., Miller W.T. Cavitary pulmonary metastases in angiosarcoma. Diagnosis by trans-thoracic needle aspiration. Am Rev Respir Dis. 1989;139:252-253.
176 Shaw A.B. Spontaneous pneumothorax from secondary sarcoma of lung. Br Med J. 1951;1:278-280.
177 Spittle M.F., Heal J., Harmer C., White W.F. The association of spontaneous pneumothorax with pulmonary metastases in bone tumours of children. Clin Radiol. 1968;19:400-403.
178 Lodmell E.A., Capps S.C. Spontaneous pneumothorax associated with metastatic sarcoma. Radiology. 1949;52:88-93.
179 Dines D.E., Cortese D.A., Brennan M.D., et al. Malignant pulmonary neoplasms predisposing to spontaneous pneumothorax. Mayo Clin Proc. 1973;48:541-544.
180 Brooks J.J. The significance of double phenotypic patterns and markers in human sarcomas: a new model of mesenchymal differentiation. Am J Pathol. 1986;125:113-123.
181 Gal A.A., Brooks J.S.J., Pietra G.G. Leiomyomatous neoplasms of the lung: a clinical, histologic and immunohistochemical study. Mod Pathol. 1989;2:209-216.
182 Cho K.R., Woodrumm J.D., Epstein J.I. Leiomyoma of the uterus with multiple extrauterine smooth muscle tumors: a case report suggesting multifocal orgin. Hum Pathol. 1989;20:80-83.
183 Sherman R.S., Brant E.E. An x-ray of spontaneous pneumothorax due to cancer metastases to the lungs. Chest. 1954;26:328-337.
184 Lipton J.H., Fong T.C., Burgess K.R. Miliary pattern as presentation of leiomyomatosis of the lung. Chest. 1987;91:781-782.
185 Wolff M., Kaye G., Silva F. Pulmonary metastases (with admixed epithelial elements) from smooth muscle neoplasms. Report of nine cases, including three males. Am J Surg Pathol. 1979;3:325-342.
186 Horstmann J.P., Pietra G.G., Harman J.A. Spontaneous regression of pulmonary leiomyomas during pregnancy. Cancer. 1977;39:314-321.
187 Kaplan C., Katoh A., Shamoto M. Multiple leiomyomas of the lung: benign or malignant. Am Rev Respir Dis. 1973;108:656-659.
188 Norris H.J., Parmley T. Mesenchymal tumors of the uterus.V: Intravenous leiomyomatosis. A clinical and pathologic study of 14 cases. Cancer. 1975;36:2164-2178.
189 Bachman D., Wolff M. Pulmonary metastases from benign smooth muscle tumors of the uterus. AJR. 1976;127:441-446.
190 Patton K.T., Cheng L., Papavero V., et al. Benign metastasizing leiomyoma: clonality, telomere length, and clinicopathologic analysis. Mod Pathol. 2006;19:130-140.
191 Devoe K., Weidner N. Immunohistochemistry of small round-cell tumors. Semin Diagn Pathol. 2000;17:216-224.
192 Meis-Kindblom J.M., Stenman G., Kindblom L.G. Differential diagnosis of small round cell tumors. Semin Diagn Pathol. 1996;13:213-241.
193 Byrd-Gloster A.L., Khoor A., Glass L.F., et al. Differential expression for thyroid transcription factor 1 in small cell lung carcinoma and Merkel cell tumor. Hum Pathol. 2000;31:58-62.
194 Moll R., Lower A., Laufer J. Cytokeratin 20 in human carcinomas. A new histodiagnostic marker detected by monoclonal antibodies. Am J Pathol. 1992;140:427-447.
195 Schmidt U., Muller U., Metz K.A. Cytokeratin and neurofilament protein staining in Merkel cell carcinoma and of the small cell type and small cell carcinoma of the lung. Am J Dermatopathol. 1998;20:346-351.
196 Wick M.R. Immunohistology of neuroendocrine and neuroectodermal tumors. Semin Diagn Pathol. 2000;17:194-203.
197 Wick M.R., Ritter J.H., Swanson P.E. The impact of diagnostic immunohistochemistry on patient outcomes. Clin Lab Med. 1999;19:797-814.
198 Oliveira A.M., Tazelaar H.D., Myers J.L., et al. Thyroid transcription factor-1 distinguishes metastatic pulmonary from well-differentiated neuroendocrine tumors of other sites. Am J Surg Pathol. 2001;25:815-819.
199 Corrin B. Lung endocrine tumors. Invest Cell Pathol. 1980;3:195-206.
200 Ikeda K., Tate G., Suzuki T., Mitsuya T. Cytologic comparison of a primary parathyroid cancer and its metastatic lesions: a case report. Diagn Cytopathol. 2005;34:50-55.
201 Iihara M., Okamoto T., Suzuki R., et al. Functional parathyroid carcinoma: long-term treatment outcome and risk factor analysis. Surgery. 2007;142:936-943.
202 Juhl J. Tumors of the lungs and bronchi. In: Essentials of Radiologic Imaging. Philadelphia: Lippincott-Raven; 1998:1–95.
203 Colby T.V., Carrington C.B. Malignant lymphoma simulating lymphomatoid granulomatosis. Am J Surg Pathol. 1982;6:19-32.
204 Colby T.V., Carrington C.B. Lymphoreticular tumors and infiltrates of the lung. Pathol Annu. 1983;18:27-70.
205 Melamed M.R. The cytological presentation of malignant lymphomas and related diseases in effusions. Cancer. 1963;16:413-431.
206 Meda B.A., Buss D.H., Woodruff R.D., et al. Diagnosis and subclassification of primary and recurrent lymphoma. The usefulness and limitations of combined fine-needle aspiration cytomorphology and flow cytometry. Am J Clin Pathol. 2000;113:688-689.
207 Demington M., Carter D., Meyers A. Distant metastases in head and neck epidermoid carcinoma. Laryngoscope. 1980;90:196-201.
208 O’Brien P.H., Carlson R., Steubner E.A. Distant metastases in epidermoid cell carcinoma of the head and neck. Cancer. 1971;27:204-307.
209 Malefetto J.P., Kasimis B.S., Moran E.M., et al. The clinical significance of radiographically detected pulmonary neoplastic lesions in patients with head and neck cancer. J Clin Oncol. 1984;2:625-630.
210 Leon X., Quer M., Diez S., et al. Second neoplasm in patients with head and neck cancer. Head Neck. 1999;21:204-210.
211 Sostman H.D., Matthay R.A. Thoracic metastases from cervical carcinoma: current status. Invest Radiol. 1980;15:113-119.
212 D’Orsi C.J., Bruckman J., Mauch P., Smith E.H. Lung metastases in cervical and endometrial carcinoma. Am J Roentgenol. 1979;133:719-722.
213 Kirubakaran M.G., Pulimood B.M., Ray D. Excavating pulmonary metastases in carcinoma of the cervix. Postgrad Med J. 1975;51:243-245.
214 Scott I., Bergin C.J., Muller N.L. Mediastinal and hilar lymphadenopathy as the only manifestation of metastatic carcinoma of the cervix. J Can Assoc Radiol. 1986;37:52-53.
215 King T.E., Neff T.A., Ziporin P. Endobronchial metastasis from the uterine cervix: presentation as primary lung abscess. JAMA. 1979;242:1651-1652.
216 Buchsbaum H.J. Lymphangitis carcinomatosis secondary to carcinoma of cervix. Obstet Gynecol. 1970;36:850-860.
217 Vachani A., Nebozhyn M., Singhal S., et al. A 10-gene classifier for distinguishing head and neck squamous cell carcinoma and lung squamous cell carcinoma. Clin Cancer Res. 2007;13:2905-2915.
218 Girard N., Deshpande C., Lau C., et al. Comprehensive histologic assessment helps to differentiate multiple lung primary non-small cell carcinomas from metastases. Am J Surg Pathol. 2009;33:1752-1764.
219 Wilson R.W., Moran C.A. Primary melanoma of the lung: a clinicopathologic and immunohistochemical study of eight cases. Am J Surg Pathol. 1997;21:1196-1202.
220 DasGupta T., Brasfield R. Metastic melanoma: a clinopathological study. Cancer. 1964;17:1323-1339.
221 Harpole D.H., Johnson C.M., Wolfe W.G., et al. Analysis of 945 cases of pulmonary metastatic melanoma. J Thorac Cardiovasc Surg. 1992;103:743-750.
222 Chen J.T.T., Dahmash N.S., Ravin C.E. Metastatic melanoma to the thorax: report of 130 patients. Am J Roentgenol. 1981;137:293-298.
223 Dwyer A.J., Reichert C.M., Woltering E.A., Flye M.W. Diffuse pulmonary metastasis in melanoma: radiographic–pathologic correlation. Am J Roentgenol. 1984;143:983-984.
224 Webb W.R., Gamsu G. Thoracic metastasis in malignant melanoma. Chest. 1977;71:176-181.
225 Balch C.M., Soong S.J., Murad T.M., et al. A multifactorial analysis of melanoma: prognostic factors in 200 melanoma patients with distant metastases. J Clin Oncol. 1983;1:126-134.
226 Pogrebniak H.W., Stovroff M., Roth J.A., Pass H.I. Resection of pulmonary metastases from malignant melanoma: results of a 16-year experience. Ann Thorac Surg. 1988;46:20-23.
227 Renshaw A., Madge R. Cercariform cells for helping distinguish transitional cell carcinoma from non-small cell lung carcinoma in fine needle aspirates. Acta Cytol. 1997;41:999-1007.
228 Gown A.M., Vogel A.M., Hoak D., et al. Monoclonal antibodies specific for melanocytic tumors distinguish subpopulations of melanocytes. Am J Pathol. 1986;123:195-203.
229 Wang N.P., Zee S., Zarbo R.J., et al. Coordinate expression of cytokeratins 7 and 20 defines unique subsets of carcinomas. Appl Immunohistochem. 1995;3:99-107.
230 Baars J.H., DeRuijter J.L., Smedts F., et al. The applicability of a keratin 7 monoclonal antibody in routinely Papanicolaou-stained cytologic specimens for the differential diagnosis of carcinomas. Am J Clin Pathol. 1994;101:257-261.
231 Sack M.J., Roberts S.A. Cytokeratins 20 and 7 in the differential diagnosis of metastatic carcinoma in cytologic specimens. Diagn Cytopathol. 1997;16:132-136.
232 Lotan T.L., Ye H., Melamed J., et al. Immunohistochemical panel to identify the primary site of invasive micropapillary carcinoma. Am J Surg Pathol. 2009;33:1037-1041.
233 Munk P.L., Connell D.G., Muller N.L., Lentle B.C. Alveolar soft parts sarcoma with pulmonary metastases. Skeletal Radiol. 1988;17:454-457.
234 Weiss S.W., Enzinger F.M. Malignant fibrous histiocytoma: an analysis of 200 cases. Cancer. 1978;41:2250-2266.
235 Hendin A.S. Gestational trophoblastic tumors metastatic to the lungs. Cancer. 1984;53:58-61.
236 Hatch K.D., Shingleton H.M., Gore H., et al. Human chorionic gonadotropin-secreting large cell carcinoma of the lung detected during follow-up of a patient previously treated for gestational trophoblastic disease. Gynecol Oncol. 1980;10:98-104.
237 Kumar J., Ilancheran A., Ratnam S.S. Pulmonary metastases in gestational trophoblastic disease: a review of 97 cases. Br J Obstet Gynecol. 1988;95:70-74.
238 Siegel R.J., Bueso-Ramos C., Cohen C., Koss M. Pulmonary blastoma with germ cell (yolk sac) differentiation: report of two cases. Mod Pathol. 1991;4:566-570.
239 Wick M.R., Swanson P.E., Manivel J.C. Placenta-like alkaline phosphatase reactivity in human tumors: an immunohistochemical study of 520 cases. Hum Pathol. 1987;18:946-954.
240 Hammar S. The use of electron microscopy and immunohistochemistry in the diagnosis and understanding of lung neoplasms. Clin Lab Med. 1987;7:1-30.
241 Hammar S.P., Bolen J.W., Bockus D., et al. Ultrastructural and immunohistochemical features of common lung tumors: an overview. Ultrastruct Pathol. 1985;9:283-318.
242 Mennemeyer R., Hammar S.P., Bauermeister D.E., et al. Cytologic, histologic, and electron microscopic correlations in poorly-differentiated primary lung carcinoma: a study of 43 cases. Acta Cytol. 1979;23:297-302.
243 Mackay B., Silva E.G. Diagnostic electron microscopy in oncology. Pathol Annu. 1980;15(part II):241-270.
244 Tucker J.A. The continuing value of electron microscopy in surgical pathology. Ultrastruct Pathol. 2000;24:383-389.
245 Mierau G.W., Berry P.J., Malott R.L., Weeks D.A. Appraisal of the comparative utility of immunohistochemistry and electron microscopy in the diagnosis of childhood round cell tumors. Ultrastruct Pathol. 1996;20:507-517.
246 Erlandson R.A., Rosai J. A realistic approach to the use of electron microscopy and other ancillary diagnostic techniques in surgical pathology. Am J Surg Pathol. 1995;19:247-250.
247 Lombardi L., Orazi A. Electron microscopy in an oncologic institution: diagnostic usefulness in surgical pathology. Tumori. 1988;74:531-535.
248 Williams M.J., Uzman B.G. Uses and contributions of diagnostic electron microscopy in surgical pathology: a study of 20 Veterans Administration hospitals. Hum Pathol. 1984;15:738-745.
249 Azar H.A., Espinoza C.G., Richman A.V., et al. Undifferentiated’ large cell malignancies: an ultrastructural and immunocytochemical study. Hum Pathol. 1982;13:323-333.
250 Seymour A.E., Henderson D.W. Electron microscopy in surgical pathology: a selective review. Pathology. 1981;13:111-135.
251 Warhol M.J., Corson J.M. An ultrastructural comparison of mesotheliomas with adenocarcinomas of the lung and breast. Hum Pathol. 1985;16:50-55.
252 Warhol M.J., Hickey W.F., Corson J.M. Malignant mesothelioma: ultrastructural distinction from adenocarcinoma. Am J Surg Pathol. 1982;6:307-314.
253 Warhol M.J., Hunter N.J., Corson J.M. An ultrastructural comparison of mesotheliomas and adenocarcinoma of the ovary and endometrium. Int J Gynecol Pathol. 1982;1:125-134.
254 Wick M.R., Swanson P.E., Manivel J.C. Immunohistochemical analysis of soft tissue sarcomas. Comparisons with electron microscopy. Appl Pathol. 1988;6:169-196.
255 Gonzalez-Crussi F., Mangkornkanok M., Hsueh W. Large-cell lymphoma: diagnostic difficulties and case study. Am J Surg Pathol. 1987;11:59-65.
256 Weinstein M.H., Dal Cin P. Genetics of epithelial tumors of the renal parenchyma in adults and renal cell carcinoma in children. Anal Quant Cytol Histol. 2001;23:362-372.
257 Argani P., Antonescu C.R., Illei P.B., et al. Primary renal neoplasms with the ASPL-TFE3 gene fusion of alveolar soft parts sarcoma: a distinctive tumor entity previously included among renal cell carcinomas of children and adolescents. Am J Pathol. 2001;159:179-192.
258 Cerasoli S., Spada F., Buda R., et al. Cytogenetic analysis of 19 renal cell tumors. Pathologica. 2001;93:118-123.
259 Dennis T.R., Stock A.D. A molecular cytogenetic study of chromosome 3 rearrangements in small cell lung cancer: consistent involvement of chromosome band 3q13.2. Cancer Genet Cytogenet. 1999;113:134-140.
260 Onuki N., Wistuba I.I., Travis W.D., et al. Genetic changes in the spectrum of neuroendocrine lung tumors. Cancer. 1999;85:600-607.
261 Kovatich A., Friedland D.M., Druck T., et al. Molecular alterations to human chromosome 3p loci in neuroendocrine lung tumors. Cancer. 1998;83:1109-1117.
262 Sandberg A.A. Cytogenetics and molecular genetics of bone and soft-tissue tumors. Am J Med Genet. 2002;115:189-193.
263 Parham D.M. Neuroectodermal and neuroendocrine tumors principally seen in children. Am J Clin Pathol. 2001;115(suppl):S113-S128.
264 Summersgill B., Goker H., Osin P., et al. Establishing germ cell origin of undifferentiated tumors by identifying gains of 12p material using comparative genomic hybridization analysis of paraffin-embedded samples. Diagn Mol Pathol. 1998;7:260-266.
265 Ahmed S., Siddiqui A.K., Rai K.R. Low-grade B-cell bronchial associated lymphoid tissue (BALT) lymphoma. Cancer Invest. 2002;20:1059-1068.
266 Hedvat C.V., Hegde A., Chaganti R.S., et al. Application of tissue microarray technology to the study of non-Hodgkin’s and Hodgkin’s lymphoma. Hum Pathol. 2002;33:968-974.
267 Mehra S., Messner H., Minden M., Chaganti R.S. Molecular cytogenetic characterization of non-Hodgkin lymphoma cell lines. Genes Chromosomes Cancer. 2002;33:225-234.
268 Minna J.D. Neoplasms of the lung. In: Favei A.S., Braunwald E., Isselbacher K.J., editors. Harrison’s Principles of Internal Medicine. New York: McGraw-Hill; 1998:552-562.
269 Snyder C.L., Saltzman D.A., Ferrell K.L., et al. A new approach to the resection of pulmonary osteosarcoma metastases. Results of aggressive metastasectomy. Clin Orthop. 1991;270:247-253.
270 Todd T.R. The surgical treatment of pulmonary metastases. Chest. 1997;112:287S-290S.
271 Downey R.J. Surgical treatment of pulmonary metastases. Surg Oncol Clin North Am. 1999;8:341.
272 Raab S.S., Hornberger J., Raffin T. The importance of sputum cytology in the diagnosis of lung cancer. A cost-effectiveness analysis. Chest. 1997;112:937-945.
273 Barlow P.B., Beck J.R. The solitary pulmonary nodule: a decision analysis [Abstract]. Chest. 1985;88:45S.
274 Cummings S.R., Lillington G.A., Richards R.J. Managing solitary pulmonary nodules: the choice of strategy is a “close call”. Am Rev Respir Dis. 1986;134:453-460.
275 Kunstaetter R., Wolkove N., Kreisman H. The solitary pulmonary nodule: decision analysis. Med Decis Making. 1985;5:61-75.
276 Raab S.S., Hornberger J. The effect of a patient’s risk-taking attitude on the cost effectiveness of testing strategies in the evaluation of pulmonary lesions. Chest. 1997;111:1583-1590.
277 Raab S.S., Slagel D.D., Hughes J.H., et al. Sensitivity and cost-effectiveness of fine needle aspiration with immunocytochemistry in the evaluation of patients with a pulmonary malignancy and a history of cancer. Arch Pathol Lab Med. 1997;121:695-700.
278 Raab S., Gross T., Grzybicki D., Silverman J. Clinical perception of the utility of sputa and other tests in patients with lung masses. Mod Pathol. 1999;12:188A.
279 Raab S.S. The cost-effectiveness of immunohistochemistry. Arch Pathol Lab Med. 2000;124:1185-1191.
280 Alberts A.S., Falkson G., Falkson H.C., Merwe M.P. Treatment and prognosis of metastatic carcinoma of unknown primary: analysis of 100 patients. Med Pediatr Oncol. 1989;17:188-192.