CHAPTER 5 Metabolism
Endocrinology
Diabetes
Pathophysiology
CNS. Neuropathy impairs neuromuscular transmission. Decreased response to tetanic stimulation.
GI. Delayed gastric emptying and gastroparesis are secondary to autonomic neuropathy.
Alberti regimen (Alberti and Thomas 1979). Safe because glucose and insulin are provided together.
Guidelines for the Management of Diabetic Patients Undergoing Surgery
British National Formulary 59
Cushing’s syndrome
Postoperative problems. Sleep apnoea (20%), steroids risk wound breakdown and infection.
Acromegaly
This is characterized by excess growth hormone secretion resulting in soft tissue overgrowth.
Surgery. Via transfrontal craniotomy or transethmoidal approach.
Perioperative: 25% have enlarged thyroid which may compress the trachea.
Postoperative. Addison’s disease, hypothalamic damage, CSF leak, diabetes insipidus, sleep apnoea.
Thyroid disease
Hyperthyroidism
Symptoms. Excitability, tremor, sweating, weight loss, palpitations, exophthalmos.
Diagnosis. Thyroid-stimulating hormone (TSH), free T3/T4, resin uptake, thoracic inlet X-ray/CT.
Hypoparathyroidism
Severe hypocalcaemia indicated by:
Trousseau’s sign. Tourniquet inflated above arterial pressure causes carpopedal spasm.
Chvostek’s sign. Percussion of the facial nerve produces facial muscle contraction.
Hyperparathyroidism
Carcinoid tumour
Perioperative Steroid Supplementation
Anaesthetic implications
There is no evidence that aiming for cortisol levels higher than normal baseline values is of any benefit in patients with suppressed HPA function (i.e. those on steroid therapy). The current recommendations are summarized in Table 5.1.
Table 5.1 Recommendations for perioperative steroid supplementation
| Preoperative | Additional steroid cover | |
|---|---|---|
| Patients currently taking steroids | ||
| <10 mg/day | Assume normal HPA function | Additional steroid cover not required |
| >10 mg/day | Minor surgery | 25 mg hydrocortisone on induction |
| Moderate surgery | Usual preoperative steroids + 25 mg hydrocortisone on induction + 100 mg/day for 24 h | |
| Major surgery | Usual preoperative steroids + 25 mg hydrocortisone on induction + 100 mg/day for 48–72 h | |
| Patients stopped taking steroids | ||
| <3 months | Treat as if on steroids | |
| >3 months | No perioperative steroids necessary | |
Alberti K.G., Thomas D.J. The management of diabetes during surgery. Br J Anaesth. 1979;51:693-703.
Annane D., Bellissant E., Bollaert P.E., et al. Corticosteroids in the treatment of severe sepsis and septic shock in adults: a systematic review. JAMA. 2009;301:2362-2375.
Bacuzzi A., Dionigi G., Del Bosco A., et al. Anaesthesia for thyroid surgery: perioperative management. Int J Surg. 2008;6:S8.
British National Formulary 59. http://bnf.org.bnf/extra/current/450062.htm. The reader is reminded that the BNF is constantly revised; for the latest guidelines please consult the current edition at www.bnf.org
Holdcroft A. Hormones and the gut. Br J Anaesth. 2000;85:58-68.
Lipshutz A.K.M., Gropper M.A. Perioperative glycemic control – an evidence-based review. Anesthesiology. 2009;110:408-421.
Malhotra S., Sodhi V. Anaesthesia for thyroid and parathyroid surgery. Contin Edu Anaesth, Crit Care Pain. 2007;7:55-58.
Mihai R., Farndon J.R. Parathyroid disease and calcium metabolism. Br J Anaesth. 2000;85:29-43.
NICE. Intraoperative nerve monitoring during thyroid surgery, 2008. March National Institute for Health and Clinical Excellence www.nice.org.uk/IPG255distributionlist ©c
Nicholson G., Burrin J.M., Hall G.M. Peri-operative steroid supplementation. Anaesthesia. 1998;53:1091-1104.
Pace N., Buttigieg M. Phaeochromocytoma. BJA CEPD Rev. 2003;3:20-23.
Robertshaw H.J., Hall G.M. Diabetes mellitus: anaesthetic management. Anaesthesia. 2006;61:1187-1190.
Smith M., Hirsh N.P. Pituitary disease and anaesthesia. Br J Anaesth. 2000;85:3-14.
Vaughan D.J., Brunner M.D. Anesthesia for patients with carcinoid syndrome. Int Anesthesiol Clin. 1997;35:129-142.
Webster N.R., Galley H.F. Does strict glucose control improve outcome? Br J Anaesth. 2009;103:331-334.
Malignant hyperthermia
Signs
Early. Tachypnoea, rise in end-tidal CO2, tachycardia, hypoxaemia, fever (>2°C.h−1), masseter spasm.
Triggering factors
Pathophysiology
Muscle contraction results from flooding of the cytoplasm by Ca2+ entering across the plasma membrane through voltage-gated Ca2+ channels and released from the sarcoplasmic reticulum (SR) through ryanodine-sensitive Ca2+ channels (Fig. 5.1). These channels occur in pairs where folds in the SR meet the sarcolemma of the t-tubule. The ryanodine (Ry1) receptor is a large protein molecule comprising four identical monomers that sits between the two Ca2+ channels. Depolarization results in charge movement in the voltage-operated Ca2+ channels which activates the Ry1 receptor to open and Ca2+ is released into the myoplasm. Volatile anaesthetic agents may increase the leak of Ca2+ through the Ry1 protein, which does not cause clinical symptoms. In myopathic muscle, this leak may be sufficient to trigger a final common pathway with activation of contractile elements, ATP hydrolysis, O2 consumption, CO2 production, lactate and heat generation, uncoupling of oxidative phosphorylation, and cell breakdown with loss of myoglobin, CPK and K+ to cause the clinical picture of MH.
Guidelines for the Management of a Malignant Hyperthermia Crisis
Association of Anaesthetists of Great Britain and Ireland 2007
Take measures to halt the MH process
Perioperative management of patients with known MH
Dental patient. Any major surgery must be performed in hospital.
Adnet P., Lestavel P., Krivosic-Horber R. Neuroleptic malignant syndrome. Br J Anaesth. 2000;85:129-135.
Association of Anaesthetists of Great Britain and Ireland. Guidelines for the management of a malignant hyperthermia crisis, 2007. Reproduced with the kind permission of the Association of anaesthetists of Great Britain and Ireland
Hopkins P.M. Malignant hyperthermia. Advances in clinical management and diagnosis. Br J Anaesth. 2000;85:118-128.
Robinson R.L., Hopkins P.M. A breakthrough in the genetic diagnosis of malignant hyperthermia. Br J Anaesth. 2001;86:166-168.
Anaesthesia for the morbidly obese patient
Definition
Body mass index (BMI) = weight (kg)/height (m)2. Normal = 22–28 kg/m2.
Pharmacokinetic and pharmacodynamic changes
Drug doses
Some drug dosages may need to be altered for morbidly obese patients (see Table 5.2).
| Unchanged dose per total weight | Unchanged dose per lean weight | Larger absolute dose but smaller dose per total weight |
|---|---|---|
| Midazolam | Vecuronium | Thiopentone |
| Diazepam | Propofol | |
| Suxamethonium | Remifentanil | |
| Pancuronium | ||
| Atracurium | ||
| Fentanyl | ||
| Alfentanil | ||
| Lignocaine |
Perioperative management
Regional/local anaesthesia
Never perform regional/local block unless able and ready to convert to a GA.
Perioperative Management of the Morbidly Obese Patient
Association of Anaesthetists of Great Britain and Ireland 2007
Association of Anaesthetists of Great Britain and Ireland: Peri-Operative Management of the Morbidly Obese Patient. Reproduced with the kind permission of the Association of Anaesthetists of Great Britain and Ireland, 2007.
Cheah M.H., Kam P.C.A. Obesity: basic science and medical aspects relevant to anaesthetists. Anaesthesia. 2005;60:1009-1021.
Lotia S., Bellamy M.C. Anaesthesia and morbid obesity. Contin Edu Anaesth, Crit Care Pain. 2008;8:151-156.
NICE. Obesity – guidance on the prevention, identification, assessment and management of overweight and obesity in adults and children, 2006. December National Institute for Health and Clinical Excellence www.nice.org.uk/CG043
Ogunnaike B.O., Jones S.B., Jones D.B. Anesthetic considerations for bariatric surgery. Anesth Analg. 2002;95:1793-1805.
Pieracci F.M., Barie P.S., Pomp A. Critical care of the bariatric patient. Crit Care Med. 2006;34:1796-1804.
Saravanakumar K., Rao S.G., Cooper G.M. Obesity and obstetric anaesthesia. Anaesthesia. 2006;61:36-48.
Metabolic response to stress
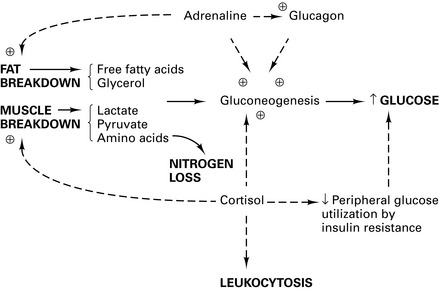
The metabolic response is initiated by:
This neurohumoral response (Fig. 5.2) converges on the hypothalamus to trigger:
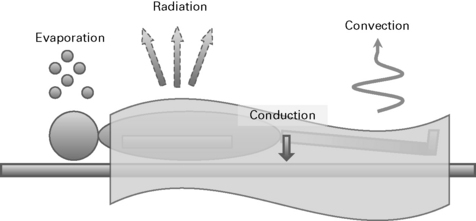
Thermoregulation
Physiology
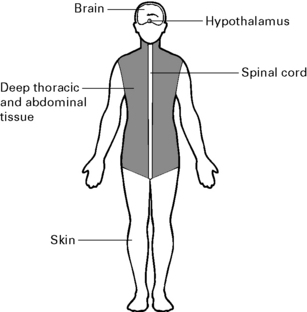
Afferent input
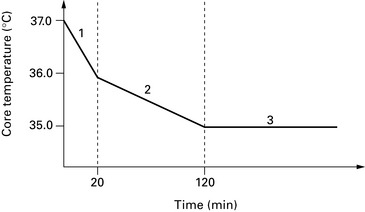
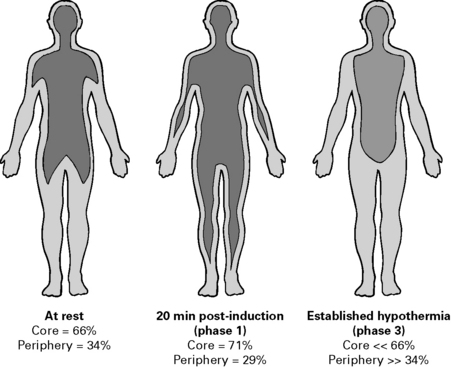
Perioperative hypothermia
Perioperative hypothermia develops in three phases (see Figs 5.4 and 5.5):
Effects of general anaesthesia
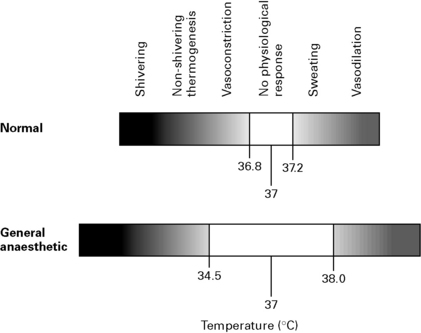
GA inhibits behavioural responses to hypothermia, decreases metabolic rate, inhibits hypothalamic function and attenuates homeostatic reflexes. The threshold at which compensatory mechanisms to hypothermia are activated is lowered by about 2.5°C and the threshold for mechanisms protecting from hyperthermia is increased by about 1.0°C, i.e. widening of thresholds with ↑ MAC (Fig. 5.7).
Thermoregulatory thresholds vary depending upon the anaesthetic agents used:
Effects of hypothermia
CVS
Metabolism
Techniques to avoid heat loss
Inadvertent Perioperative Hypothermia
NICE Clinical Guideline 65, April 2008
Preoperative phase
Intraoperative phase
Buggy D.J., Crossley A.W. Thermoregulation, mild perioperative hypothermia and post-anaesthetic shivering. Br J Anaesth. 2000;84:615-628.
NICE. Inadvertent perioperative hypothermia. NICE clinical guideline 65 www.nice.org.uk/CG065, 2008.
Sessler D.I. Temperature monitoring and perioperative thermoregulation. Anesthesiology. 2008;109:318-338.

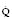 mismatch and hypoxia, obstructive sleep apnoea progressing to Pickwickian syndrome (cor pulmonale, hypoxia, hypercapnia, polycythaemia). Increased O2 consumption and low FRC cause faster desaturation.
mismatch and hypoxia, obstructive sleep apnoea progressing to Pickwickian syndrome (cor pulmonale, hypoxia, hypercapnia, polycythaemia). Increased O2 consumption and low FRC cause faster desaturation.