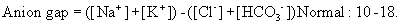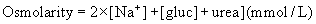Chapter 27 Metabolic disorders
ELECTROLYTE EMERGENCIES
Theory
Causes/assessment
Clinical effects
Management
Treat the patient, not just the abnormality.
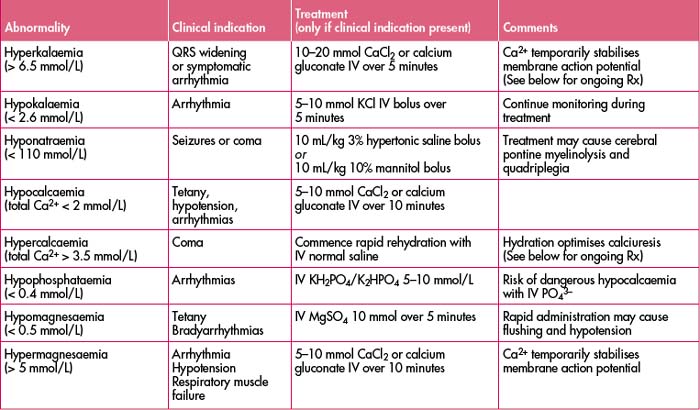
(See Table 27.3 for a list of electrolyte abnormalities, clinical indications and treatment.)
ACID–BASE DISTURBANCES
Pearls/pitfalls
Determining the acid–base abnormality
Metabolic acidosis
(Mild: [HCO3–] < 18 mmol/L; moderate: < 15 mmol/L; severe: < 12 mmol/L)
Metabolic alkalosis
(Mild: [HCO3–] > 25 mmol/L; moderate: > 30 mmol/L; severe: > 35 mmol/L)
Respiratory acidosis
(Mild: pCO2 > 45 mmHg; moderate: > 55 mmHg; severe > 65 mmHg)
HYPONATRAEMIA
(Mild < 130 mmol/L; severe < 110 mmol/L)
HYPERNATRAEMIA
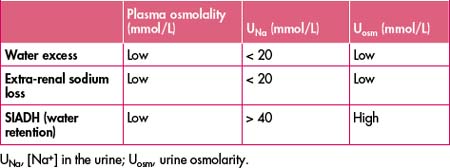
URINE OSMOLARITY AND OTHER URINE ELECTROLYTES
HYPOKALAEMIA
(Mild < 3.5 mmol/L; severe < 2.4 mmol/L)
Management
Successful treatment is dependent on addressing concurrent hypomagnesaemia (see ‘Hypomagnesaemia’).
HYPERKALAEMIA
(Mild > 5 mmol/L; severe > 7.5 mmol/L)
Management
HYPOCALCAEMIA
(Symptomatic: total Ca2+ < 1.8 mmol/L, ion Ca2+ < 0.7 mmol/L)
HYPERCALCAEMIA
(See also Chapter 42, ‘The immunosuppressed patient’, ‘Hypercalcaemia’).
HYPOPHOSPHATAEMIA
HYPERPHOSPHATAEMIA
HYPERMAGNESAEMIA
(Mild > 2 mmol/L; moderate > 3.5 mmol/L; severe > 5 mmol/L)
Management
Note: All therapies take time to work.
Table 27.2 Approximate correction factors for electrolyte abnormalities
| Situation | Correction∗ |
|---|---|
| Hyperglycaemia | True [Na+] = measured [Na+] + ([Gluc] ÷ 4) |
| Metabolic acidosis | Reduce measured [K+] by 0.5 for every ↓ pH 0.1 |
| Metabolic alkalosis | Increase measured [K+] by 0.5 for every ↑ pH 0.1 |
| Hypoalbuminaemia | Add 0.1 total [Ca2+] for every 4 g/dL decrease in albumin |
ACUTE RENAL FAILURE
Early emergency department assessment and management
Pre-renal (dehydration/hypovolaemia) or established (acute tubular necrosis) renal failure?
(See Table 27.4, ‘Pre-renal vs renal failure’.)
| Cause/mechanism | Pre-renal failure | Established renal failure (i.e. ATN) |
|---|---|---|
| Summary | Maximal water and sodium retention and maximally concentrated urine to maintain circulatory volume (UNa ↓ but Uurea,, Uosm,,Ucreat ↑↑↑) | Loss of concentrating power volume (UNa ↑ and Uurea, Uosm,,Ucreat ↔) |
| UNa (mmol/L) | < 20 | > 40 |
| Uurea | > 450 | > 300 |
| Uurea😛urea | > 20:1 | < 10:1 |
| Uosm > Posm | > 2:1 | < 1:2 |
| Ucreat😛creat | 40:1 | 20:1 |
ATN, acute tubular necrosis/renal failure; Pcreat, plasma creatinine; Posm, plasma osmolarity; Purea, plasma urea; Ucreat, urinary creatinine; Uosm, urinary osmolarity; UNa, urinary sodium.
Hyperlactataemia or ‘lactic acidosis’—clinical use, prognostication, disposition
[Normal value < 2 mmol/L (‘significant’ > 5 mmol/L)]
Broder G., Weil M. Excess lactate: an index of reversibility of shock in human patients. Science. 1964;143:1457-1459.
Cooper SC, Nathan DM, Snyder PJ, eds. Endocrinology and diabetes [e-book]. Waltham, MA; UptoDate; 2008. Online. Available from: UptoDate. http://www.uptodate.com/home/index.html.
Stacpoole P., Wright E., Baumgauter T., et al. Natural history and course of acquired lactic acidosis in adults. Am J Med. 1994;97:47-54.
Walmsley R.N., White G.H.. A guide to diagnostic clinical chemistry, 3rd edn. Blackwell Scientific Publications, Melbourne, 1994.