CHAPTER 8 METABOLIC AND ENDOCRINE PROBLEMS
SODIUM
(Normal range serum sodium 135–145 mmol/L.)
Hyponatraemia
| Excess water intake | Hypotonic fluids TURP syndrome Water intoxication |
| Reduced free water clearance | Stress response with raised ADH Syndrome of inappropriate ADH secretion Renal impairment Cardiac failure |
| Loss of body sodium | GI tract losses Renal losses including diuretic therapy Adrenal insufficiency Hyperpyrexia and sweating (inadequate salt replacement) |
Hyponatraemia is most commonly due to an excess of extracellular fluid (rather than sodium loss). This is often the result of excessive use of hypotonic intravenous fluids. Hyponatraemia may also result from the chronic use of some diuretic drugs; this is more commonly seen in the elderly. More rarely, hyponatraemia is associated with other forms of organ dysfunction, including renal dysfunction and hepatic cirrhosis (where it is seen in association with secondary hyperaldosteronism). Treatment is not usually necessary unless the serum sodium falls below 130 mmol/L. Serum sodium below 120 mmol/L may be associated with altered conscious level and fits. Symptoms are related as much to the speed of change in concentration as to the actual measured level.
Hypernatraemia
 Give additional free water as 5% dextrose (e.g. 1 L over 6–12 h) or water via NG tube. Consider diluting enteral feeds with sterile water.
Give additional free water as 5% dextrose (e.g. 1 L over 6–12 h) or water via NG tube. Consider diluting enteral feeds with sterile water.POTASSIUM
(Normal range serum potassium 3.5–5 mmol/L.)
Potassium is primarily an intracellular ion. Small changes in serum concentration have significant effects on nerve conduction and muscle contraction.
Hypokalaemia
Causes of hypokalaemia are shown in Box 8.1.
Hypokalaemia is relatively common in the ICU. ECG changes include ST depression, flattening of the T wave and prominent U wave. If severe (<2 mmol/L), cardiac arrhythmias, including supraventricular and ventricular extrasystoles, tachycardias, atrial fibrillation, and ventricular fibrillation, may occur (Fig. 8.1).
If additional potassium is required:
 Give 20 mmol of K+ in 20–40 mL of saline over 1 h via a central venous catheter using an infusion pump. Repeat as necessary.
Give 20 mmol of K+ in 20–40 mL of saline over 1 h via a central venous catheter using an infusion pump. Repeat as necessary.Hyperkalaemia
Causes of hyperkalaemia are shown in Box 8.2.
Box 8.2 Causes of hyperkalaemia
Spurious (e.g. haemolysed blood sample, check result)
Iatrogenic (excess administration)
Muscle injury (including suxamethonium, crush injury, compartment syndrome)
ECG changes include peaked T waves, broad QRS complexes and conduction defects (Fig. 8.2). Asystole may occur. Urgent treatment is usually required, although patients with long-term end-stage renal failure may be more tolerant of hyperkalaemia than the general intensive care patient population.
CALCIUM
(Normal range standard serum calcium 2.12–2.62 mmol/L.)
(Normal range ionized serum calcium 0.84–1 mmol/L.)
Most laboratories measure total calcium, which includes bound and unbound fractions. The unbound fraction (ionized Ca2+), which is the physiologically active component, varies with the albumin concentration. Therefore, look at the corrected figure, which takes account of protein binding. Alternatively, many blood gas analysers now measure ionized Ca2+ directly.
Hypocalcaemia
Hypocalcaemia is common on the ICU. Typical causes are shown in Box 8.3.
Hypercalcaemia
This occurs less commonly, and is generally due to an underlying disease process. Typical causes are listed in Box 8.4.
 Give 0.9% saline to rehydrate the patient. Check plasma osmolality is within the normal range (280–290 mosmol/L).
Give 0.9% saline to rehydrate the patient. Check plasma osmolality is within the normal range (280–290 mosmol/L). A forced diuresis may be used to aid excretion. Furosemide (frusemide) is administered and the urine output replaced with alternating 0.9% saline and 5% dextrose.
A forced diuresis may be used to aid excretion. Furosemide (frusemide) is administered and the urine output replaced with alternating 0.9% saline and 5% dextrose. Calcitonin reduces the rate of calcium and phosphate release from the bones. It is useful in patients with hypercalcaemia associated with malignancy, and generally reduces the calcium level within 2 h.
Calcitonin reduces the rate of calcium and phosphate release from the bones. It is useful in patients with hypercalcaemia associated with malignancy, and generally reduces the calcium level within 2 h.PHOSPHATE
(Normal range serum phosphate 0.7–1.25 mmol/L.)
Hyperphosphataemia
Hyperphosphataemia is caused by excessive intake or decreased excretion (e.g. renal failure). Maintain adequate hydration with 5% dextrose. If severe, consider the need for renal replacement therapy. Phosphate is effectively removed by continuous RRT techniques, with haemodialysis and haemodiafiltration performing better than simple filtration techniques.
MAGNESIUM
(Normal range serum magnesium 0.7–1 mmol/L.)
Hypomagnesaemia
Magnesium is the second most common intracellular cation and as such, serum levels are a poor guide to the need for replacement. Serum magnesium is frequently depleted in critical illness. Common causes of hypomagnesaemia are shown in Box 8.5.
ALBUMIN
Hypoalbuminaemia
Low serum albumin is common in critically ill patients. Common causes of hypoalbuminaemia are shown in Box 8.6.
Box 8.6 Causes of hypoalbuminaemia
Impaired protein synthesis (liver disease)
Use of 20% albumin
Given as a bolus, 20% albumin effectively raises the plasma oncotic pressure and expands the intravascular space by factor of up to 5 times the volume given, by drawing fluid in from extravascular spaces. Depending on vascular permeability and metabolism of albumin, this effect is likely to be transient (lasting less than 4 h). This effect has, however, been used in conjunction with diuretics in an attempt to correct oedema secondary to severe hypoalbuminaemia. Similar strategies have been used in treatment of hepatorenal failure (together with terlipressin) and to try to avert impending renal failure. Although relatively widely practised, the evidence for such strategies is limited. Twenty percent albumin is used to replace protein losses in the nephrotic syndrome.
METABOLIC ACIDOSIS
(See Interpretation of blood gases, p. 114, and Sepsis, p. 331.)
Common causes of metabolic acidosis classified according to anion gap are shown in Box 8.7.
Box 8.7 Causes of metabolic acidosis
| Accumulation of H+ (anion gap > 18 mmol/L) | Loss of bicarbonate (anion gap < 18 mmol/L) |
|---|---|
| Lactic acidosis (shock and tissue ischaemia) | Vomiting or diarrhoea |
| Ketoacidosis | Small bowel fistula |
| Liver failure | Renal tubular acidosis |
| Acute renal failure | Hyperchloraemic acidosis |
| Salicylate poisoning |
The effects of acidosis are increased respiratory drive (unless the patient is sedated/paralysed), and at low pH < 7.1 reduced CO, and reduced response to inotropes. Hydrogen ions move into cells and K+ moves out in an attempt to buffer the acidosis, so hyperkalaemia may occur. Treatment depends on the severity, underlying cause and speed of response to interventions. In most cases, the metabolic acidosis will correct as the underlying condition improves.
 Treat the underlying cause. (See Lactic acidosis below, Diabetic ketoacidosis, p. 218, and Poisoning p. 230.)
Treat the underlying cause. (See Lactic acidosis below, Diabetic ketoacidosis, p. 218, and Poisoning p. 230.) If pH < 7.1 or the patient’s clinical condition is deteriorating, either give 50 mL 8.4% (50 mmol) sodium bicarbonate i.v., or for lower body weight individuals, calculate the dose of bicarbonate as follows:
If pH < 7.1 or the patient’s clinical condition is deteriorating, either give 50 mL 8.4% (50 mmol) sodium bicarbonate i.v., or for lower body weight individuals, calculate the dose of bicarbonate as follows:Lactic acidosis
Type A
 Consider the need for renal replacement therapy. If already on RRT using lactate buffered replacement/dialysis fluid, consider changing to bicarbonate buffered solutions. Seek senior advice.
Consider the need for renal replacement therapy. If already on RRT using lactate buffered replacement/dialysis fluid, consider changing to bicarbonate buffered solutions. Seek senior advice.Type B
 Treat the underlying condition if possible. The acidosis should be corrected. This may be possible simply by removing the underlying precipitating cause, but may require vigorous resuscitation.
Treat the underlying condition if possible. The acidosis should be corrected. This may be possible simply by removing the underlying precipitating cause, but may require vigorous resuscitation.Renal tubular acidosis (RTA)
This is a metabolic acidosis arising from renal tubular dysfunction, in which there is excess loss of bicarbonate through the kidneys, with a corresponding increased serum chloride (normal anion gap). This may present as a feature of renal disease, or may result as a side-effect from drugs (e.g. amphotericin). If you suspect renal tubular acidosis, test the urine pH. A systemic acidosis accompanied by a non acid urine (pH > 6) is highly suggestive. Type 1 (distal convoluted tubule) and type 2 (proximal convoluted tubule) are associated with hypokalaemia. Type 4 results from hypoaldosteronism or aldosterone resistance, and is associated with hyperkalaemia and metabolic acidosis. (Type 3 is no longer recognized.)
DISTURBANCES OF BLOOD GLUCOSE
Tight glycaemic control regimens have become standard practice in critical care units in recent years because of the apparent reduction in overall intensive care mortality, particularly in post-surgical patients. They are associated, however with a greatly increased risk of hypoglycaemia (due to the use of insulin to control hyperglycaemia), and more recent studies have challenged their efficacy.
Hypoglycaemia
Hypoglycaemia can be defined as a blood glucose <3 mmol/L. It occurs most commonly as a consequence of insulin or oral hypoglycaemic therapy in diabetic patients, but may also be associated with some disease states. Typical causes are shown in Box 8.8.
DIABETIC EMERGENCIES
Most diabetic emergencies are managed on general wards or HDU rather than ICU. Occasionally patients are moribund or have associated features, such as sepsis, which require intensive care. The source of sepsis may be occult (e.g. renal abscess). Abdominal ultrasound or a CT scan may be required.
DIABETIC KETOACIDOSIS
Management
 Give oxygen. Despite profound acidosis, most patients manage to compensate well by hyperventilation and do not require artificial ventilation. If there is evidence of hypoxia, hypoventilation or compromised airway, intubate and establish ventilation, but beware of cardiovascular collapse!
Give oxygen. Despite profound acidosis, most patients manage to compensate well by hyperventilation and do not require artificial ventilation. If there is evidence of hypoxia, hypoventilation or compromised airway, intubate and establish ventilation, but beware of cardiovascular collapse! The need for invasive cardiovascular monitoring will depend on the severity of the condition but in general arterial access and CVP lines are appropriate.
The need for invasive cardiovascular monitoring will depend on the severity of the condition but in general arterial access and CVP lines are appropriate. Take base line investigations. FBC, urea and electrolytes (sodium and potassium), glucose, and blood gases. Monitor glucose, potassium and arterial blood gases hourly.
Take base line investigations. FBC, urea and electrolytes (sodium and potassium), glucose, and blood gases. Monitor glucose, potassium and arterial blood gases hourly. Look for a precipitating cause. Send blood, urine and sputum for culture. ECG, CXR and troponin if indicated. Abdominal pain is a common feature of DKA; consider abdominal ultrasound/CT and send blood for amylase.
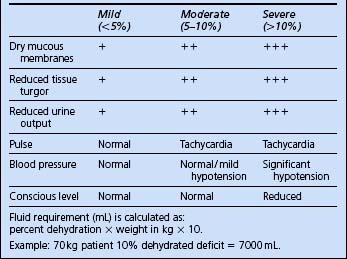
Look for a precipitating cause. Send blood, urine and sputum for culture. ECG, CXR and troponin if indicated. Abdominal pain is a common feature of DKA; consider abdominal ultrasound/CT and send blood for amylase. Start i.v. infusion of 0.9% normal saline and aim to correct dehydration over 24 h. The volume required can be estimated from the percent dehydration (Table 8.2). These are estimates only and should be viewed in the context of the overall clinical picture.
Start i.v. infusion of 0.9% normal saline and aim to correct dehydration over 24 h. The volume required can be estimated from the percent dehydration (Table 8.2). These are estimates only and should be viewed in the context of the overall clinical picture. Typically give 1 L in the first 30 min and then 1 L/h over the next 2 h, and assess response. Give the rest over the remaining 22 h. If serum sodium is >150 mmol/L consider 0.45% saline as an alternative.
Typically give 1 L in the first 30 min and then 1 L/h over the next 2 h, and assess response. Give the rest over the remaining 22 h. If serum sodium is >150 mmol/L consider 0.45% saline as an alternative. In addition to resuscitation fluid volume above, give maintenance fluids to provide the patient’s normal daily requirements. Give this as a separate infusion. Initially give as 0.9% normal saline. This can be changed to a dextrose solution when the blood glucose is under control (see below).
In addition to resuscitation fluid volume above, give maintenance fluids to provide the patient’s normal daily requirements. Give this as a separate infusion. Initially give as 0.9% normal saline. This can be changed to a dextrose solution when the blood glucose is under control (see below). Give 10 units of short-acting insulin i.v. Follow this with an insulin infusion of 6–10 units/h as necessary. The aim should be to reduce the blood sugar slowly over a number of hours.
Give 10 units of short-acting insulin i.v. Follow this with an insulin infusion of 6–10 units/h as necessary. The aim should be to reduce the blood sugar slowly over a number of hours. The acidosis will usually correct as the patient’s overall condition improves. If there is a severe metabolic acidosis pH < 7.1, and the patient’s condition is not improving, consider bicarbonate (see above).
The acidosis will usually correct as the patient’s overall condition improves. If there is a severe metabolic acidosis pH < 7.1, and the patient’s condition is not improving, consider bicarbonate (see above). Monitor the potassium carefully. As the acidosis corrects, the potassium will fall. Start a potassium infusion at 20 mmol/h as required.
Monitor the potassium carefully. As the acidosis corrects, the potassium will fall. Start a potassium infusion at 20 mmol/h as required.HYPEROSMOLAR NON-KETOTIC STATES
Management
 Use 0.9% saline as rehydration fluid (0.45% saline in severe cases). Correct dehydration more slowly than in DKA, typically over 24–48 h. Rapid correction may be associated with cerebral oedema.
Use 0.9% saline as rehydration fluid (0.45% saline in severe cases). Correct dehydration more slowly than in DKA, typically over 24–48 h. Rapid correction may be associated with cerebral oedema.ADRENAL INSUFFICIENCY
Addison’s disease
 If possible perform a short Synacthen test (Synacthen is an ACTH analogue). Give Synacthen 0.25 mg i.m. Measure plasma cortisol before and 30 min after. Cortisol level should rise by 2–3 times the basal level.
If possible perform a short Synacthen test (Synacthen is an ACTH analogue). Give Synacthen 0.25 mg i.m. Measure plasma cortisol before and 30 min after. Cortisol level should rise by 2–3 times the basal level.Patients on long-term steroid therapy
Many patients will already be on long-term corticosteroids for management of various disease processes. The true significance of pituitary adrenal suppression by longer term steroid therapy is still debatable but most authorities recommend increasing doses of steroids during critical illness and major surgical interventions. Equivalent anti-inflammatory doses for a change from oral steroids to i.v. hydrocortisone are:
PHAEOCHROMOCYTOMA
This adrenal secretory tumour is a rare cause of hypertension/heart failure in young adults and may occasionally be a presenting diagnosis in critical care. Patients are most likely to been seen in ICU situation in the postoperative period. Patients are typically volume depleted due to the long-term effects of endogenous catecholamine secretion. The diagnosis is made by measurement of serum or urinary catecholamines or metabolites, and CT imaging to find the tumour.
Management
 Patients are commenced on α-blocking agents preoperatively. These block the hypertensive effects of catecholamines. Relative hypovolaemia is unmasked and fluid loading is required until postural changes in blood pressure are abolished.
Patients are commenced on α-blocking agents preoperatively. These block the hypertensive effects of catecholamines. Relative hypovolaemia is unmasked and fluid loading is required until postural changes in blood pressure are abolished. High dose magnesium may be administered prophylactically to stabilize catecholamine secreting cells.
High dose magnesium may be administered prophylactically to stabilize catecholamine secreting cells.THYROID DYSFUNCTION
Sick euthyroid syndrome
Thyroid function tests are often abnormal in the critically ill patient. Most sick patients will have results consistent with the so-called sick euthyroid syndrome. The pattern is low T3, low T4, and inappropriately low/normal TSH. This pattern persists until recovery occurs. The current consensus is that it does not reflect true clinical hypothyroidism so thyroid replacement therapy is not usually warranted. Seek expert advice if unclear.
TEMPERATURE CONTROL
Hyperthermia
Hyperthermia is important as a marker for infection or other disease processes. Causes of hyperthermia are shown in Box 8.10.
Box 8.10 Causes of hyperthermia
Systemic inflammatory response syndrome
Adverse reactions to drugs or blood products
Neuroleptic malignant syndrome*
* Rare
The exact mechanisms that produce hyperthermia are not known but in many cases it can be viewed as a physiological response to critical illness rather than a significant part of the disease process. There is debate, therefore, about the need to treat a mildly raised temperature <39°C except in brain-injured patients, where increased temperature is associated with a worse outcome. (See Brain injury, p. 272 and Management of cardiac arrest, p. 109.)In general, however, measures such as regular paracetamol and tepid sponging for low-grade pyrexia may improve patient comfort.
 Give regular rectal paracetamol (ibuprofen is also effective if there are no contraindications to NSAIDs).
Give regular rectal paracetamol (ibuprofen is also effective if there are no contraindications to NSAIDs). Institute passive cooling: wet drapes, ice packs, fans, and gastric, peritoneal or bladder lavage with cold fluids.
Institute passive cooling: wet drapes, ice packs, fans, and gastric, peritoneal or bladder lavage with cold fluids.Hypothermia
Hypothermia is defined as a core temperature below 35°C. To avoid missing hypothermia, you should always have a high index of suspicion and measure core temperature in at-risk patients. Common causes are given in Box 8.11.
Box 8.11 Common causes of hypothermia
Environmental (particularly elderly)
Exposure (e.g. trauma victims)
Prolonged surgery with massive fluid/blood losses
In severely hypothermic patients from other causes:
 Exclude other injuries, drug ingestions, head injury, myxoedema, pressure necrosis of limbs, compartment syndromes, rhabdomyolysis and renal failure (measure CK). Treat appropriately (see Trauma, p. 306).
Exclude other injuries, drug ingestions, head injury, myxoedema, pressure necrosis of limbs, compartment syndromes, rhabdomyolysis and renal failure (measure CK). Treat appropriately (see Trauma, p. 306). Most patients respond to passive slow rewarming with a slow rise in core temperature of about 1°C per hour. Utilize a warm environment, hot-air warming blankets, and warm i.v. fluids for volume replacement.
Most patients respond to passive slow rewarming with a slow rise in core temperature of about 1°C per hour. Utilize a warm environment, hot-air warming blankets, and warm i.v. fluids for volume replacement. For severely hypothermic patients, with a core temperature below 32°C, consider more active warming measures such as peritoneal lavage with warm fluids, instillation of warm fluids into the bladder, or partial (femoral–femoral) bypass with a heat exchanger.
For severely hypothermic patients, with a core temperature below 32°C, consider more active warming measures such as peritoneal lavage with warm fluids, instillation of warm fluids into the bladder, or partial (femoral–femoral) bypass with a heat exchanger. Patients may develop dysrhythmias on rewarming, usually at around 31°C, and may need repeated cardioversion. It may, however, be difficult to restore sinus rhythm while the patient remains hypothermic. Occasionally under these circumstances profoundly hypothermic patients can be successfully warmed utilizing cardiopulmonary bypass.
Patients may develop dysrhythmias on rewarming, usually at around 31°C, and may need repeated cardioversion. It may, however, be difficult to restore sinus rhythm while the patient remains hypothermic. Occasionally under these circumstances profoundly hypothermic patients can be successfully warmed utilizing cardiopulmonary bypass.




 Rapid correction of severe hyponatraemia can cause central pontine demyelination (brainstem damage) and death. It is recommended that sodium should not rise more than 2 mmol / L per hour and by not more than 12 mmol / L in 24 h, to achieve an initial plasma sodium level of 120–130 mmol / L. The use of hypertonic saline solutions is controversial. Seek advice.
Rapid correction of severe hyponatraemia can cause central pontine demyelination (brainstem damage) and death. It is recommended that sodium should not rise more than 2 mmol / L per hour and by not more than 12 mmol / L in 24 h, to achieve an initial plasma sodium level of 120–130 mmol / L. The use of hypertonic saline solutions is controversial. Seek advice.





 Rapid correction of hypernatraemia, particularly if serum sodium is >160 mmol / L, can result in cerebral oedema as water enters the brain. As with hyponatraemia, correct slowly over 24–48 h.
Rapid correction of hypernatraemia, particularly if serum sodium is >160 mmol / L, can result in cerebral oedema as water enters the brain. As with hyponatraemia, correct slowly over 24–48 h.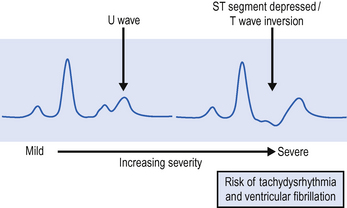


 Inadvertent and inappropriate injection of strong potassium solutions has been associated with death. These solutions should be treated as a controlled drug, and use should be governed by local protocols. Ideally, strong potassium infusions should be prepared by a pharmacy aseptic service. If you are required to prepare potassium infusions, ensure that fluids are thoroughly mixed before administration. Strong potassium chloride solution has a higher specific gravity than standard i.v. solutions and can ‘layer’ at the bottom of a bag / syringe. Administration should be via a central venous cannula to avoid the risk of extravasation injury. During infusion continuous ECG monitoring and regular blood sampling are mandatory.
Inadvertent and inappropriate injection of strong potassium solutions has been associated with death. These solutions should be treated as a controlled drug, and use should be governed by local protocols. Ideally, strong potassium infusions should be prepared by a pharmacy aseptic service. If you are required to prepare potassium infusions, ensure that fluids are thoroughly mixed before administration. Strong potassium chloride solution has a higher specific gravity than standard i.v. solutions and can ‘layer’ at the bottom of a bag / syringe. Administration should be via a central venous cannula to avoid the risk of extravasation injury. During infusion continuous ECG monitoring and regular blood sampling are mandatory.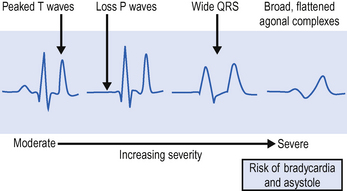

















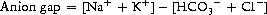
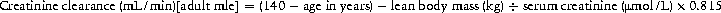

 The evidence of benefit from bicarbonate solutions to treat acidosis is limited with some evidence that it actually worsens intracellular pH. Other disadvantages include large sodium load (particularly if used repeatedly). Therefore only consider if the pH < 7.2 in an inotrope resistant, hypotensive patient.
The evidence of benefit from bicarbonate solutions to treat acidosis is limited with some evidence that it actually worsens intracellular pH. Other disadvantages include large sodium load (particularly if used repeatedly). Therefore only consider if the pH < 7.2 in an inotrope resistant, hypotensive patient.





 If assisted ventilation is considered, beware of cardiovascular collapse when using sedative agents in profoundly dehydrated patients. Avoid hyperventilation, which will exacerbate the alkalosis.
If assisted ventilation is considered, beware of cardiovascular collapse when using sedative agents in profoundly dehydrated patients. Avoid hyperventilation, which will exacerbate the alkalosis. When measuring blood glucose, be careful that the sampling line used is not contaminated with glucose solutions. There have been cases of samples drawn from lines that have been contaminated with glucose inadvertently used to flush the line, leading to spuriously high serum glucose levels, followed by excessive insulin administration and profound hypoglycaemia.
When measuring blood glucose, be careful that the sampling line used is not contaminated with glucose solutions. There have been cases of samples drawn from lines that have been contaminated with glucose inadvertently used to flush the line, leading to spuriously high serum glucose levels, followed by excessive insulin administration and profound hypoglycaemia.