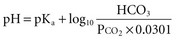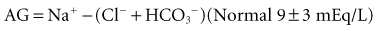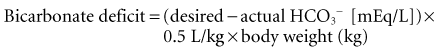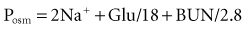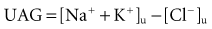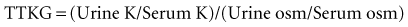109 Metabolic Acidosis and Alkalosis
 Acid-Base Disorders
Acid-Base Disorders
Normal Acid-Base Homeostasis
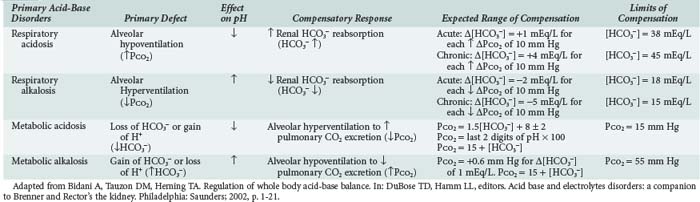
Under most circumstances, CO2 production and excretion are matched, and the usual steady-state PaCO2 is maintained at 40 mm Hg. Primary changes in PaCO2 can cause acidosis or alkalosis, depending on whether PaCO2 is above or below the normal value (respiratory acidosis or alkalosis, respectively). Underexcretion of CO2 produces hypercapnia, and overexcretion causes hypocapnia, both of which will affect the systemic pH. The PaCO2 is regulated primarily by neural respiratory factors and is not subject to regulation by the rate of CO2 production; therefore, hypercapnia is usually the result of hypoventilation rather than of increased CO2 production. Increases or decreases in PaCO2 may represent primary derangements of the ventilatory function of the lungs (under neural respiratory control) or may be due to compensatory changes in response to a primary alteration in the plasma [HCO3−].1
Primary alteration of PaCO2 evokes two metabolic mechanisms to limit change in systemic pH: the fast-acting cellular buffering and the renal-adaptive response, a slower process that becomes more efficient with time. This metabolic response would be secondary (or compensatory) to the primary respiratory disorder. A primary change in the plasma [HCO3−] as a result of metabolic or renal factors results in compensatory changes in ventilation that blunt the changes in blood pH that would occur otherwise. Such respiratory alterations are referred to as secondary or compensatory changes because they occur in response to primary metabolic alterations.1
The kidneys regulate plasma [HCO3−] through three main processes: (1) “reabsorption” of filtered HCO3−, (2) generation of “new” HCO3−, which is accomplished by formation of titratable acid, and (3) excretion of NH4+ in the urine. The kidney filters approximately 4000 mEq of HCO3− per day, and between 80% and 90% of HCO3− is reabsorbed in the proximal tubule. The distal nephron reabsorbs the remaining HCO3− and more importantly, secretes protons generated from dietary protein intake to defend systemic pH. Metabolism of the average diet rich in protein produces fixed acids that consume bicarbonate on entry into the extracellular fluid. Although the quantity of protons from dietary protein metabolism is small (40-60 mEq/day), it must be secreted to prevent chronic positive H+ balance and metabolic acidosis. This quantity of secreted protons (net acid) is represented in the urine as titratable acid and NH4+. Metabolic acidosis in the presence of normal renal function augments net acid excretion by markedly increasing NH4+ production and excretion. It is important to note that this vital compensatory mechanism is impaired in chronic renal failure, hyperkalemia, and renal tubular acidosis.1,2
 Diagnosis of Types of Disturbances
Diagnosis of Types of Disturbances
Historically, two different conceptual frameworks have evolved among clinicians and physiologists for interpreting acid-base phenomena. The traditional or bicarbonate-centered framework relies quantitatively on the Henderson-Hasselbalch equation (see later), whereas the Stewart or strong-ion approach utilizes the original Stewart equation to calculate the H+ concentration. The traditional approach has not only proven to be a mechanistic formulation that reflects the acid-base status at the tissue level but is also considerably easier to use in daily clinical practice, given the complexity of the Stewart theory and its associated fromulas.3
The most common clinical disturbances are simple acid-base disorders—that is, one of the metabolic disturbances (metabolic acidosis or alkalosis) or one of the respiratory disturbances (respiratory acidosis or alkalosis) occurring alone rather than in combination. Because physiologic compensation is not complete and cannot achieve a normal pH, the pH remains abnormal in simple disturbances. More complicated clinical situations can give rise to mixed acid-base disturbances through simultaneous expression of more than one simple disturbance, and in this setting the pH may be at a dangerous extreme or appear normal.1,3
Simple Acid-Base Disorders
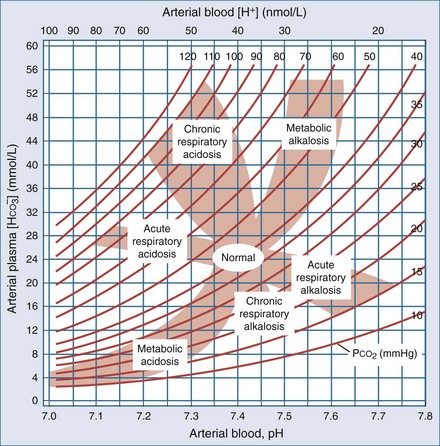
The degree of primary alteration and secondary compensation in either or both of these two variables (acidosis or alkalosis) determines the systemic pH (acidemia or alkalemia). For example, metabolic acidosis due to an increase in endogenous acids (e.g., ketoacidosis) lowers extracellular fluid [HCO3−] and decreases systemic pH. This stimulates the medullary chemoreceptors to increase ventilation and to return the ratio of [HCO3−] to PaCO2, and thus pH, toward normal, although not to normal. Table 109-1 contains the acid-base disturbances along with the appropriate compensatory response for simple disorders. The degree of respiratory compensation expected in a simple form of metabolic acidosis can be predicted from the relationship PaCO2 = (1.5 × [HCO3−]) + 8 ± 2; that is, the PaCO2 is expected to decrease 1.25 mm Hg for each mEq/L per liter decrease in [HCO3−]. Thus, a patient with metabolic acidosis and [HCO3−] of 12 mEq/L would be expected to have a PaCO2 between 24 and 28 mm Hg. Values for PaCO2 below 24 or greater than 28 mm Hg define a mixed disturbance (metabolic acidosis plus respiratory alkalosis or metabolic acidosis plus respiratory acidosis, respectively). A readily available (though not as reliable) method of determining the nature and degree of compensatory response is the use of nomograms (Figure 109-1).1,4 If the arterial acid-base value falls within one of the shaded bands in Figure 109-1, one may assume that a simple acid-base disorder is present, and a tentative diagnostic category can be assigned. Values that fall outside the shaded area suggest the presence of a mixed disorder. These nomograms, though helpful, are not substitutes for an appreciation of the limits of compensation as displayed in Table 109-1.4
Mixed Acid-Base Disorders
Mixed acid-base disorders, defined as independently coexisting disorders, not merely compensatory responses, are more often seen in patients in ICUs and can lead to dangerous extremes of pH. A patient with diabetic ketoacidosis (DKA; high-AG metabolic acidosis) may develop an independent and superimposed respiratory problem leading to respiratory acidosis or alkalosis. Patients with underlying pulmonary disease may not respond to metabolic acidosis with an appropriate ventilatory response because of insufficient respiratory reserve. Such imposition of respiratory acidosis on metabolic acidosis can lead to severe acidemia and a poor outcome. When metabolic acidosis and metabolic alkalosis coexist in the same patient, the pH may be normal or near normal. When the pH is normal, an elevated AG (see later) denotes the presence of a metabolic acidosis. Patients who have ingested an overdose of drug combinations such as sedatives and salicylates may have mixed disturbances as a result of the acid-base response to the individual drugs (metabolic acidosis mixed with respiratory acidosis or respiratory alkalosis, respectively). Even more complex are triple acid-base disturbances. For example, patients with metabolic acidosis due to alcoholic ketoacidosis may develop metabolic alkalosis due to vomiting and superimposed respiratory alkalosis due to the hyperventilation of hepatic dysfunction or alcohol withdrawal.1 In general, a normal arterial pH in face of abnormal bicarbonate level, PaCO2, or AG is highly suggestive of a complex and mixed acid base disorder.
Pathway to Diagnosis of Acid-Base Disorders
A stepwise approach to the diagnosis of acid-base disorders follows and is summarized in Table 109-2. In the determination of arterial blood gases by the clinical laboratory, both pH and PaCO2 are measured, and the [HCO3−] is calculated from the Henderson-Hasselbalch equation. This calculated value should be compared with the measured [HCO3−] (or total CO2) on the electrolyte panel. These two values should agree within 2 mEq/L. If they do not, the values may not have been drawn simultaneously, a laboratory error may be present, or an error could have been made in calculating the [HCO3−]. After verifying the blood acid-base values, one can then identify the precise acid-base disorder.1
|
2 Compare [HCO3−] on ABG and electrolytes to rule out error due to specimen handling or measurements.
|
Adapted in part from DuBose TD Jr. Acid-base disorders. In: Brenner BM, editor Brenner and Rector’s the kidney 8th ed. Philadelphia: Saunders; 2008, p. 513.
Blood for electrolytes and arterial blood gases should be drawn simultaneously before therapy, because an increase in [HCO3−] occurs with metabolic alkalosis and respiratory acidosis. Conversely, a decrease in [HCO3−] occurs in metabolic acidosis and respiratory alkalosis.1,2
Metabolic acidosis often leads to hyperkalemia as a result of cellular shifts in which H+ is exchanged for K+ or Na+. For each decrease in blood pH of 0.10, the plasma [K+] should rise by 0.6 mEq/L. This relationship is not invariable, however. DKA, lactic acidosis, diarrhea, and renal tubular acidosis are regularly associated with potassium depletion because of urinary K+ wasting.1
Anion Gap
Calculation of the anion gap is a key step in evaluation of acid-base disorders (see Table 109-2). Because normally the total unmeasured anions exceed the total unmeasured cations as indicated by the electrolyte panel, there exists an AG of 9 ± 3 mEq/L in plasma. The concentration of potassium in the blood usually is relatively small compared with that of sodium, chloride, and bicarbonate, so many clinicians omit this variable when calculating the AG.5
Simply put, AG represents unmeasured anions in plasma and is calculated as shown in the following equation. The various contributors to plasma AG in normal physiologic state and in metabolic acidosis are depicted in Figure 109-2. Patients with underlying pulmonary disease may not respond to metabolic acidosis with an appropriate ventilatory response because of insufficient respiratory reserve. Such imposition of respiratory acidosis on metabolic acidosis can lead to severe acidemia and a poor outcome. When metabolic acidosis and metabolic alkalosis coexist in the same patient, the pH may be normal or near normal. When the pH is normal, an elevated AG (see later) denotes the presence of a metabolic acidosis.
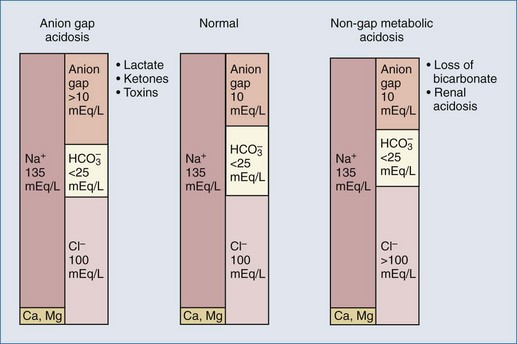
Figure 109-2 Contributors to plasma anion gap in normal physiologic state and in metabolic acidosis.
(Data from Gamble JL. Chemical anatomy, physiology, and pathology of extracellular fluid. 6th ed. Cambridge: Harvard University Press; 1954; and from Stewart PA. How to understand acid-base. New York: Elsevier; 1981.)
The unmeasured anions include predominately anionic proteins such as albumin but also phosphate, sulfate, and organic anions. An increase in the AG is most often due to an increase in unmeasured anions and less commonly is caused by a decrease in unmeasured cations (calcium, magnesium, potassium) (Table 109-3).1 When endogenously produced acid anions such as acetoacetate and lactate accumulate in extracellular fluid, the AG increases, causing a high-AG acidosis. In addition, the AG may increase with an increase in anionic albumin, either because of increased albumin concentration due to profound volume depletion or alkalosis, which alters albumin charge (increased negative charge).6
TABLE109-3 Anion Gap in the Diagnosis of Metabolic Acidosis Anion Gap = Na+ − (Cl− + HCO3−) = 9 + 3 mEq/L
| Decreased Anion Gap | Increased Anion Gap |
|---|---|
| Increased cations (not Na+): | Increased anions (not Cl− or HCO3−): |
| ↑ Ca++, Mg++ | ↑ Albumin concentration |
| ↑ Li+ | Alkalosis |
| ↑ IgG | ↑ Inorganic anions: |
| Decreased anions: | Phosphate |
| (not Cl− or HCO3−) | Sulfate |
| Hypoalbuminemia* | |
| Acidosis | ↑ Organic anions: |
| Laboratory error: | L-Lactate D-Lactate` |
| Hyperviscosity | Ketones |
| Bromism | Uremic |
| ↑ Exogenously supplied anions: | |
| Toxins: | |
| Salicylate | |
| Paraldehyde | |
| Ethylene glycol | |
| Methanol Toluene Pyroglutamic acid |
|
| ↑ Unidentified anions: | |
| Uremic | |
| Hyperosmolar, nonketotic states | |
| Myoglobinuric acute renal failure | |
| Decreased cations (not Na+): | |
| ↓ Ca++, Mg++ |
* Albumin is the major unmeasured anion. A decline in serum albumin of 1.0 g/dL from the normal value of 4.5 g/dL decreases the anion gap by 2.3-2.5 mEq/L. Correction is very important to diagnose anion gap acidosis in setting of hypoalbuminemia.
Adapted from Emmett M, Narins RG. Clinical use of the anion gap. Medicine 1997;56:38-54; from Oh MS, Carroll HJ. The anion gap. N Engl J Med 1977;297:814-7; and from Kraut JA, Madisa NE. Serum anion gap: its uses and limitations in clinical medicine. Clin J Am Soc Nephrol 2007;2:162-74. Epub 2006 Dec 6.
A low serum AG is not an uncommon occurrence and most frequently is the result of severe hypoalbuminemia. Albumin is the major contributor to serum AG, and a decline of 1 g/dL in serum albumin from the normal value of 4.5 g/dL will cause a reduction of 2.3 to 2.5 mEq/L in AG. Given the pivotal role of AG in formulating differential and treatment plans in acid-base disorders, it is extremely important to correct for low albumin when calculating AG in setting of hypoalbuminemia (see Table 109-3). Other causes of a low AG include:
Besides hypoalbuminemia, polyclonal gammopathy and monoclonal gammopathy with excessive accumulation of cationic immunoglobulin (Ig)G are the most common clinical disorders associated with a low serum AG. Therefore, once laboratory error and hypoalbuminemia have been excluded, a search for accumulation of IgG should be initiated. In patients with disturbed mentation or unexplained clinical findings, the possibility of lithium ingestion, bromism, or iodide intoxication should be considered. When the serum AG is negative in the absence of laboratory error, an extremely uncommon situation, bromide intoxication and iodide intoxication, should be excluded.5
In the face of a normal serum albumin, a high AG is usually due to non–chloride-containing acids that contain inorganic (phosphate, sulfate), organic (ketoacids, lactate, uremic organic anions), exogenous (salicylate or ingested toxins with organic acid production), or unidentified anions. As mentioned earlier, a high-AG acidosis has two identifying features: a low [HCO3−] and an elevated AG. The latter is present even if an additional acid-base disorder is superimposed to modify the [HCO3−] independently. Metabolic acidosis of the high-AG variety, concomitant with either chronic respiratory acidosis or metabolic alkalosis, represents a situation for which [HCO3−] may be normal or increased. Nevertheless, the AG is elevated, signaling the presence of the acidosis (see Table 109-3). In a typical simple AG metabolic acidosis, one would expect an equal but reciprocal change in serum bicarbonate and the AG, but this relationship does not hold when mixed disorders are present. Therefore, ΔAG versus ΔHCO3− is an important tool to search for a concealed acid-base disorder. For example, the combination of metabolic acidosis and metabolic alkalosis is expected to be present in a patient with advanced renal failure with several days’ history of vomiting. This mixed disorder would be most easily recognized when the AG is elevated but the HCO3− concentration and pH are near normal (ΔAG > ΔHCO3−). In general, a ΔAG/ΔHCO3− value of 1 is typical of pure high-AG acidosis such as lactic or DKA acidosis.7 A ratio significantly greater than 1 suggests the presence of metabolic acidosis and metabolic alkalosis, whereas a ratio less than 1 suggests the presence of mixed gap and non-gap metabolic acidosis. However, studies have indicated variability in the ΔAG/ΔHCO3−.5 This observation undercuts the ability to use this ratio alone to detect complex acid-base disorders, thus emphasizing the need to consider additional information to obtain the appropriate diagnosis.
 Metabolic Acidosis
Metabolic Acidosis
Metabolic acidosis can occur because of an increase in endogenous acid production (such as lactate and ketoacids), loss of bicarbonate (as in diarrhea), or accumulation of endogenous acids (as in renal failure). Metabolic acidosis along with an elevated AG have profound effects on patient survival.8
Effects of Acidosis
The effects of acidemia on the body are multiple (Table 109-4). The fall in blood pH is accompanied by a characteristic increase in ventilation, especially the tidal volume (Kussmaul respiration). Intrinsic cardiac contractility may be depressed, but inotropic function can be normal because of catecholamine release. Both peripheral arterial vasodilation and central venoconstriction can be present; the decrease in central and pulmonary vascular compliance predisposes to pulmonary edema with even minimal volume overload. CNS function is depressed, with headache, lethargy, stupor, and in some cases, even coma. Glucose intolerance may also occur.1
| Neurologic |
Adapted in part from Whitney GM, Szerlip HM. Acid-base disorder in critical care setting. In: DuBose TD, Hamm LL, editors. Acid-base and electrolytes disorders: a companion to Brenner and Rector’s the kidney. Philadelphia: Saunders; 2002, p. 165-83.
General Approach in Treatment of Metabolic Acidosis
The treatment of metabolic acidosis with alkali should be reserved for severe acidemia, except when the patient has no “potential [HCO3−]” in plasma. Potential [HCO3−] can be estimated from the increment (Δ) in the AG (ΔAG = patient’s AG − 10).1,9 It must be determined if the acid anion in plasma is metabolizable (i.e., β-hydroxybutyrate, acetoacetate, and lactate) or non-metabolizable (anions that accumulate in chronic renal failure and after toxin ingestion with subsequent kidney injury). The latter requires return of renal function to replenish the [HCO3−] deficit, a slow and often unpredictable process. Consequently, patients with a normal AG acidosis (hyperchloremic acidosis), a slightly elevated AG (mixed hyperchloremic and AG acidosis), or an AG attributable to a non-metabolizable anion in the presence of renal failure should receive alkali therapy, either orally (NaHCO3 or Shohl’s solution) or intravenously (NaHCO3), in an amount necessary to slowly increase the plasma [HCO3−] into the 20- to 22-mEq/L range. Controversy exists, however, in regard to the use of alkali in patients with a pure AG acidosis from accumulation of a metabolizable organic acid anion (ketoacidosis or lactic acidosis).1 In general, severe acidosis (pH < 7.15) warrants the intravenous (IV) administration of 50 to 100 mEq of NaHCO3 over 30 to 45 minutes during the initial 1 to 2 hours of therapy. Provision of such modest quantities of alkali in this situation seems to provide an added measure of safety, but it is essential to monitor plasma electrolytes during the course of therapy, because the [K+] may decline as pH rises. The goal is to increase the [HCO3−] to no more than 15 mEq/L and the pH to 7.25. The goal is never to increase these values to the normal values of 25 mEq/L and 7.40, respectively. It is important to point out that studies in humans and animals have failed to conclusively show any significant and positive effect with bicarbonate therapy on hemodynamic parameters or patient outcome in the ICU setting.1 However, the use of alkali remains a common practice in patients with profound acidosis or acidemia. There are two major clinical categories of metabolic acidosis: high AG and normal AG.
 High–Anion Gap Acidosis
High–Anion Gap Acidosis
High-AG acidosis is the most common form of metabolic acidosis encountered in the ICU. There are four principal causes of a high-AG acidosis (Figure 109-3; Tables 109-5 and 109-6)1,4:
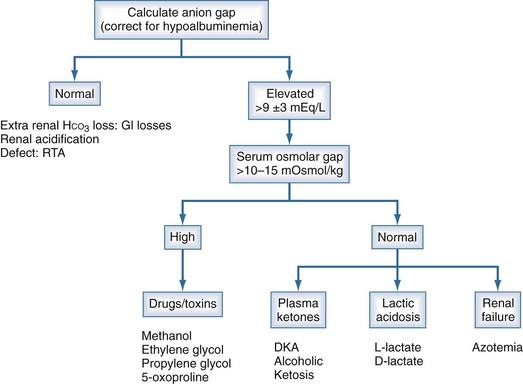
Figure 109-3 Anion gap acidosis workup.
(Data from Finkle KW, DuBose TD Jr. Metabolic acidosis. In: Dubose TD Jr, Hamm LL, editors. Acid-base and Electrolyte disorders: a companion to Brenner and Rector’s the kidney. Philadelphia: Saunders; 2002, p. 55-66.)
TABLE109-5 Clinical Causes of High Anion Gap and Normal Anion Gap Acidosis
| High Anion Gap |
Adapted in part from DuBose TD Jr. Acid-base disorders. In: Brenner BM, editor. Brenner and Rector’s the kidney. 8th ed. Philadelphia: Saunders; 2008, p. 513-46.
TABLE109-6 Etiologies of Lactic Acidosis
| L-Lactic Acidosis |
| D-Lactic Acidosis |
Adapted in part from DuBose TD Jr. Acid-base disorders. In: Brenner BM, editor. Brenner and Rector’s the kidney. 8th ed. Philadelphia: Saunders; 2008, p. 513-46.
Initial screening to identify the cause of the high-AG acidosis should include (1) a search in the history for evidence of drug or toxin ingestion (ethylene glycol, methyl alcohol, salicylates); (2) determination of whether diabetes mellitus is present (DKA); (3) a search for evidence of alcoholism or increased levels of β-hydroxybutyrate (alcoholic ketoacidosis); (4) observation for clinical signs of uremia and determination of the blood urea nitrogen and creatinine (uremic acidosis); (5) inspection of the urine for oxalate crystals (ethylene glycol); and (6) recognition of the common clinical settings in which lactate levels may be increased (hypotension, septic or hemorrhagic shock, cardiac failure, leukemia, cancer, and drug or toxin ingestion).1
Lactic Acidosis
Lactic acidosis is one the most common causes of high-AG acidosis in the ICU. An increase in plasma L-lactate is most commonly due to increased production of lactate in setting of an imbalance in oxygen supply and demand at the tissue level (type A). Thus type A lactic acidosis is thought to be caused by tissue hypoperfusion and/or severe hypoxemia, although in recent years this view is thought to be an oversimplification. Non-hypoxic conditions can also generate significant lactic acidosis (type B) in a variety of clinical settings such as malignancies, hepatic failure, or ingestion of drugs/toxins. The following are some of the causes of lactic acidosis in clinical setting (also see Table 109-6):
Among the most common causes of lactic acid acidosis in medical ICUs is unrecognized bowel ischemia or infarction in a patient with severe atherosclerosis or cardiac decomposition receiving vasopressors.10 Moreover, independent of the cause of hemodynamic instability, use of catecholamines, especially epinephrine, also results in lactic acidosis, presumably by stimulating cellular metabolism such as hepatic glycolysis.9
D-Lactic acid acidosis is due to formation of D-lactate by gut bacteria and may cause both an increased AG and hyperchloremia (see Table 109-6).9,10 This condition is caused by overgrowth of intestinal flora and may be associated with jejunoileal bypass or intestinal obstruction.
Lactic acidosis is among the most frequent and critical of all AG acidoses observed in the acute care setting. A study of 50 ICUs revealed an incidence of elevated lactate levels in over 60% of patients.11 Whether lactic acidosis represents a unique entity or is a consequence of a variety of other conditions common to the ICU has been debated. Lactate concentrations are mildly elevated in nonpathologic states (such as exercise), but the magnitude of elevation is generally small. For purposes of definition, a serum L-lactate level greater than 4 mEq/L (normal being 1 mEq/L) is thought to represent a clinically significant lactic acidosis and is the initiating point for resuscitative protocols in many critical care units. Nevertheless, some patients in the ICU maintain serum lactate levels between 2 to 4 mEq/L, and it is uncertain whether such patients progress to frank lactic acidosis, but studies are suggestive of higher mortality for patients with even intermediate rises in serum lactate.12
Whereas the lactic acid acidoses have been classified by Huckabee and Cohen into two types—type A (hypoxic) and type B (non-hypoxic) as noted earlier13—it has been recognized that lactic acidosis is often the result of the simultaneous existence of both hypoxic and non-hypoxic factors, and in many cases the precise etiology is difficult to establish. Decreased arterial perfusion to peripheral tissues in shock despite adequate arterial oxygen content, for example, results in L-lactic acid accumulation. Severe acidemia decreases portal blood flow and hepatic clearance of lactic acid.1 Moreover, in sepsis there is both a decrease in tissue perfusion and a decrease in oxygen utilization. Technically, therefore, the classification of lactic acidosis is primarily of conceptual interest.
Numerous drugs have been implicated in the occurrence of lactic acidosis (see Table 109-6). Of particular note is biguanide (metformin) and antiretroviral therapy, specifically the nucleoside reverse transcriptase inhibitors (NRTIs) used in treatment of HIV infection. The biguanide family of hypoglycemic agents, which includes metformin, have been associated with mild elevation in serum lactate (usually <2 mEq/L) in patients with otherwise normal renal and hepatic function. Cases of severe lactic acidosis associated with use of metformin are typically accompanied by presence of sepsis and/or profound renal failure. The mechanism is largely unknown,14 and the current recommendations are that the drug not be used in patients with congestive heart failure, liver disease, and significant renal insufficiency (creatinine >1.5 mg/dL in men, or >1.4 mg/dL in women).9,15 Nucleoside analogs used in treatment of HIV infections inhibit mitochondrial polymerase and lead to lactic acid accumulation. Hyperlactemia is common with NRTI therapy, especially stavudine and zidovudine, but the serum lactate is mildly elevated and well compensated. Risk factors for NRTI therapy–associated lactic acidosis include a creatinine clearance less than 70 mL/min and a low CD4+ T-lymphocyte count.9,16
Carbon monoxide poisoning produces lactic acidosis by reducing the oxygen-carrying capacity of the hemoglobin, resulting in tissue hypoxia. A newly recognized and not too uncommon cause of lactate accumulation is propylene glycol. This agent is used as carrier for a variety of IV medications used in ICU setting, most notably diazepam, lorazepam, nitroglycerin, and etomidate. Metabolism of propylene glycol by alcohol dehydrogenase in the liver results in lactate formation, which is then converted to pyruvate and shunted to glycolytic pathways. There have been numerous reports of high-AG acidosis and elevated serum osmolarity in patients receiving benzodiazepine infusions in the critical care setting.17
Critically ill patients with a significantly elevated AG or low serum bicarbonate should be suspected of having a lactic acidosis, particularly in the presence of hepatic insufficiency. A high index of suspicion must be maintained, however, because the AG is a relatively insensitive reflection of lactic acidosis. Iberti and coworkers reported a poor correlation between arterial pH, the AG, and serum lactate levels.18 Fifty percent of patients with serum lactate levels above 5 and less than 9.9 mmol/L displayed a normal AG.
Several investigators have sought to characterize the prognostic value of serum lactic acid levels. Studies have found an inverse correlation between mortality and L-lactate levels above 2.0 to 2.5 mmol/L.19–21 Prognosis is related to lactate concentration, as well as the ability to metabolize a lactic acid load after a resuscitative effort. Although lactic acidemia is associated with adverse outcome during critical illness, it does not appear to be a direct causative agent, rather a marker of poor prognosis. Therapeutic interventions that target the primary pathophysiology rather than the lactic acidosis per se have been shown to have outcome benefits.22 Moreover, normalization of elevated lactic acid levels regardless of causative source is associated with better outcome in patients with sepsis, but this may be a surrogate for the acuity of the illness and index of organ dysfunction.23 Falk and associates observed that the ability to lower serum lactate levels by 50% within 18 hours after resuscitation correlated with a significantly greater rate of survival.24 Other studies have reinforced these findings, revealing both significantly lower lactate levels and increased ability to clear lactate in survivors as opposed to nonsurvivors.25 To add to this argument, dichloroacetate, which indirectly decreases lactic acid level, has not been shown to improve survival, suggesting that elevated lactate level is an epiphenomenon with varying prognostic value in different clinical settings.26
Treatment of Lactic Acidosis
Therapy of lactic acidosis has two distinct goals. The first is to identify and remedy the defect in the oxidative metabolism (hypoperfusion and hypoxemia being the most common causes) in order to halt further production of lactate. The second is to raise the serum pH toward normal. If the underlying pathophysiologic state is effectively treated, the excess lactate production and acidemia often correct without specific interventions. As mentioned earlier, there is controversy as to whether lactic acidosis directly contributes to mortality or is simply a marker of the severity of the underlying illness,9,10 and this has led to added debate on recommendations for use of buffers in the management of lactic acidosis. Nevertheless, the basic principle and most effective therapy for L-lactic acidosis is that the underlying condition disrupting the normal lactate metabolism must first be corrected.
Fluid overload occurs rapidly with NaHCO3 administration because of the massive amounts required in some cases, along with high sodium content of the solution. In addition, central venoconstriction and decreased cardiac output are common, which lead to tissue hypoperfusion and further end-organ dysfunction—specifically, reduced glomerular filtration. As such, the volume overload and acidemia may precipitate the need for renal replacement therapy which can simultaneously deliver HCO3−, remove lactate and excess extracellular fluid (ECF) volume, and correct electrolyte abnormalities. Although use of continuous hemofiltration (HF) in critically ill patients provides minimal additional lactate clearance, perhaps due to the high rate of its production,27 the ultrafiltration accomplished with continuous renal replacement therapy may offer additional benefits in terms of volume and electrolyte management. The use of bicarbonate-based replacement fluid or dialysate is thought to convey better acid-base control when compared to lactate based solutions, though controlled studies have shown controversial results.28,29 There are theoretical advantages of extracorporeal therapies such as HF or hemodiafiltration in treatment of sepsis with lactic acidosis. These include enhanced removal of inflammatory mediators and endotoxin while providing early and effective management of volume and acid-base disturbances. However, as yet, the evidence in humans is too limited to recommend HF as an adjunctive therapy for critically ill patients with sepsis or systemic inflammatory response syndrome (SIRS). Regarding the many uncertainties about optimal volume (high or very high) and type of membrane, clinical studies should first focus on endpoints as recovery from organ failure and length of treatment before survival studies are started.30 Recently, smaller uncontrolled trials have shown promising effects of HF in refractory shock,31 which raises the need for additional large-scale studies to further define the role of renal replacement therapy in treatment of sepsis and shock.
If the underlying cause of the L-lactic acidosis can be remedied, it is anticipated that the lactate will be reconverted to HCO3−. HCO3− derived from lactate conversion, and any new HCO3− generated by renal mechanisms during acidosis and IV-administered bicarbonate are all additive and may result in overshoot alkalosis.32
Sodium Bicarbonate
Despite highly debated and somewhat discouraged practice of its use, bicarbonate therapy remains the most commonly administered agent in treatment of lactic acidosis. Two major factors contribute to this common practice: bicarbonate is readily available to physicians who feel obliged to react to a low pH; and bicarbonate use is often associated with some degree of rather immediate improvement in measured pH which may, often unrealistically, be interpreted as a sign of improvement in the metabolic derangements and patient condition. Although severe acidosis is classically thought to have a deleterious effect on cardiopulmonary performance, recent studies have actually shown an improvement in cardiac performance in the presence of a mild to moderate acidosis.10 Accordingly, the pH below which most clinicians feel obligated to use NaHCO3 has declined. Therefore, the recommendation for administration of NaHCO3 in the treatment of severe acidosis when the pH is less than 7.15 seems reasonable. At this pH, as predicted by the Henderson-Hasselbalch equation, minor changes in bicarbonate or PCO2 will result in a large decrease in pH.33 However, there are no data supporting a specific pH at which therapy must be instituted.
A prospective study evaluating NaHCO3 in patients with lactic acidosis showed an increase in serum pH but no improvement in hemodynamics when compared with normal saline.34 The use of NaHCO3 also failed to increase hemodynamic responsiveness to circulating catecholamines concomitant with a decrease in serum ionized calcium.35–38 It is important to note, however, the numerous deleterious effects of bicarbonate therapy in treatment of lactic acidosis. NaHCO3 therapy can cause fluid overload and hypertension, because the amount required can be massive when accumulation of lactic acid is relentless. Fluid administration is poorly tolerated because of central venoconstriction, especially in the oliguric patient, and can worsen the state of volume overload. If the underlying cause of the lactic acidosis can be remedied, blood lactate will be converted to HCO3− and may result in an overshoot alkalosis. Sodium bicarbonate may also result in impaired utilization of oxygen and increase anaerobic metabolism through stimulation of phosphofructokinase (by raising cellular pH), which leads to further lactate accumulation. Finally, sodium bicarbonate IV administration generates CO2 (HCO3− + H+ → H2O + CO2). With depressed cardiac output or ventilatory capacity of lungs, CO2 can accumulate, causing intracellular acidosis and a further reduction in cardiac output. Other possible adverse effects of bicarbonate infusion include hypernatremia, hyperosmolarity, hypocalcaemia, and hypokalemia.
This must be viewed as a very rough estimate with considerable variability in the clinical setting. Constant infusion of hypertonic bicarbonate has many disadvantages and is discouraged to minimize the rapid development of fluid overload. The use of continuous renal replacement therapy is common in patients with overt shock and lactic acidosis, as frequently simultaneous acute kidney injury exists in such a setting. Though the extracorporeal removal of lactate is negligible and likely bears no effect on treatment of underlying cause,27 effective alkali therapy can be provided with minimal volume overload using the newer bicarbonate-based dialysate or replacement fluid formulations.
Dichloroacetate
Dichloroacetate stimulates the activity of pyruvate dehydrogenase, thereby increasing the rate of oxidation of pyruvate and limiting the generation of lactate. Initial animal studies showed improved aerobic glucose utilization and an increase in intracellular adenosine triphosphate. A large multicenter trial showed a significant reduction in serum lactate, an increase in arterial pH, and an increase in the number of patients able to resolve hyperlactemia from 43% to 58%.26 Nevertheless, although dichloroacetate was effective in improving lactic acidosis, there was no decrease in mortality. Chronic use of dichloroacetate has been associated with neurologic toxicity, including limb paralysis and neuropathies.39 In summary, dichloroacetate is not recommended in the therapy for lactic acid acidosis.
Other Agents
Tromethamine (THAM) is a non–sodium containing buffer that accepts proton and generates bicarbonate. It does not raise the CO2 content of the blood, thereby avoiding a fall in intracellular and cerebrospinal fluid pH. Despite this theoretical advantage, this agent has not proven to be any better than bicarbonate in managing lactic acidosis.9 THAM is excreted in the urine and should be avoided in renal insufficiency. Severe hyperkalemia, hypoglycemia, ventilatory depression, and hepatic necrosis in neonates have been reported.40 Given the risks of serious side effects, THAM should be used only after careful consideration or not at all. Other buffers such as Carbicarb (mixture of sodium bicarbonate and sodium carbonate) or Tribonat (a mixture of THAM, acetate, NaHCO3, and phosphate) have not shown any survival advantage. Methylene blue was once advocated as a means of reversing the altered redox state to enhance lactate metabolism, but there is no evidence from controlled studies for its use.1
D-Lactic Acidosis
D-Lactic acidosis should be considered in patients with a history of intestinal disease who present with confusion and AG metabolic acidosis. Overproduction of D-lactate may occur when there is overgrowth of gut bacteria.41 Patients present with an AG acidosis, normal L-lactate levels, and neurologic findings such as confusion, ataxia, and loss of memory (see Table 109-5). Symptoms are worsened after high-carbohydrate meals or oral hyperalimentation or tube feedings. In patients with short bowel syndrome or who have undergone jejunoileal bypass, there not only is an overgrowth of bacteria but also accumulation of carbohydrate in the colon. Sufficient D-lactate can be produced to overwhelm enzymatic clearance. Treatment is directed at decreasing the overgrowth of bacteria with antibiotics and the avoidance of high-carbohydrate feeding. D-Lactate is not measured on routine laboratory testing unless specifically ordered. Serum D-lactate levels of greater than 3 mmol/L confirm the diagnosis.1
Ketoacidosis
Diabetic Ketoacidosis
Treatment of Diabetic Ketoacidosis
The general principles of treatment of DKA include (1) frequent monitoring and recording of electrolyte values, (2) fluid replacement to correct the consequences of the preceding osmotic diuresis, (3) identification of the precipitating cause of the ketoacidosis (commonly an infectious process), and (4) anticipation of the consequences of therapy, especially if alkali therapy is included in the regimen. Most patients with DKA require correction of the volume depletion that almost invariably accompanies the osmotic diuresis and ketoacidosis. Extreme caution needs to be exercised in patients with history of end-stage renal disease (ESRD), as the hyperglycemia has limited to no diuretic effect in such patients, and aggressive fluid therapy may have a deleterious effect. The serum Na+ concentration may be arithmetically corrected for the degree of hyperglycemia to determine the type of IV fluid needed (i.e., correct Na+ by 1.6 to 1.8 mEq/L for each 100 mg/dL increment in plasma glucose). In general, it seems prudent to initiate therapy with isotonic saline at a rate of 1000 mL IV per hour. When the pulse and blood pressure have stabilized and the corrected serum Na+ concentration is in the range 130 to 135 mEq/L, switch to 0.45% sodium chloride. Use of lactated Ringer’s should be avoided. If the blood glucose level falls below 300 mg/dL, 0.45% sodium chloride with 5% dextrose should be administered.42
Low-dose IV insulin therapy (0.1 U/kg/h) smoothly corrects the biochemical abnormalities and minimizes hypoglycemia and hypokalemia.42 Usually, in the first hour, a loading dose of the same amount is given initially as an IV bolus. Although regular insulin may also be administered intramuscularly (0.1 U/kg initially, then 0.1 U/kg/h), it should be noted that intramuscular insulin may not be effective in patients with volume depletion, which often occurs in ketoacidosis.
The arguments for and against alkali therapy have been summarized previously. The young patient with a pure AG acidosis (ΔAG = ΔHCO3−) usually does not require exogenous alkali, because the metabolic acidosis should be entirely reversible. Elderly patients, patients with severe high-AG acidosis (pH < 7.15), or patients with a superimposed hyperchloremic component may receive small amounts of sodium bicarbonate by slow IV infusion (no more than 44-88 mEq in 60 minutes). Thirty minutes after this infusion is completed, arterial blood gas analysis should be repeated. Alkali administration can be repeated if the pH is 7.20 or less or if the patient exhibits a significant hyperchloremic component, but it is rarely necessary. The AG should be followed closely during therapy, because it is expected to decline as ketones are cleared from plasma and herald an increase in plasma HCO3− as the acidosis is repaired. Therefore, it is not necessary to monitor blood ketone levels continuously. Hypokalemia and other complications of alkali therapy dramatically increase when amounts of sodium bicarbonate exceeding 400 mEq are administered. However, the effect of alkali therapy on arterial blood pH needs to be reassessed regularly and the total administered kept at a minimum if necessary.42
Routine administration of PO4−3 (usually as potassium phosphate) is not advised because of the potential for hyperphosphatemia and hypocalcemia.42 A significant proportion of patients with DKA have significant hyperphosphatemia before initiation of therapy. In the volume-depleted, malnourished patient, however, a normal or elevated PO4−3 concentration on admission may be followed by a rapid fall in plasma PO4−3 levels within 2 to 6 hours after initiation of therapy.
Alcoholic Ketoacidosis
Chronic alcoholics who discontinue solid food intake while continuing alcohol use can develop ketoacidosis when alcohol consumption is abruptly curtailed. Usually, the onset of vomiting and abdominal pain leads to cessation of alcohol use prior to presentation to the hospital. The glucose concentration is low or normal, and acidosis may be severe because of elevated ketones, predominantly β-hydroxybutyrate. Mild lactic acidosis may coexist because of alteration in the redox state. The nitroprusside ketone reaction (Acetest) can detect acetoacetic acid but not β-hydroxybutyrate, so the degree of ketosis and ketonuria can be underestimated. Typically, insulin levels are low and concentrations of triglyceride, cortisol, glucagon, and growth hormone are increased. This disorder is not rare and is underdiagnosed. The clinical presentation of alcoholic ketoacidosis (AKA) is complex owing to the fact that mixed disorders frequently exist. The vomiting can lead to a metabolic alkalosis, respiratory alkalosis may be present in setting of chronic liver disease, hypoperfusion due to volume depletion can cause mild lactic acidosis, and hyperchloremic acidosis can be present due to renal excretion of ketoacids. Moreover, the osmolar gap may be elevated if blood alcohol level is elevated, though the differential in such settings should always include ethylene glycol, methanol, or other ingestions.1,43
Treatment of Alcoholic Ketoacidosis
Extracellular fluid deficits should be repleted by IV administration of saline and glucose (5% dextrose in 0.9% NaCl), and insulin should be avoided. Glucose in isotonic solution, not normal saline, is the mainstay of therapy. Hypophosphatemia, hypokalemia, and hypomagnesemia may coexist and should be corrected. Hypophosphatemia usually emerges 12 to 24 hours after admission, may be exacerbated by glucose infusion, and if severe may induce rhabdomyolysis, aspiration, and platelet dysfunction. Therefore, serum electrolytes—especially serum phosphorus, magnesium, and potassium—should be checked frequently. One such schedule may be blood draws at 4, 6, 12, and 18 hours post admission. Upper gastrointestinal (GI) hemorrhage, pancreatitis, and pneumonia may accompany this disorder.42,44
Ingestion-Induced Acidosis
(See Tables 109-5 and 109-6.)
Salicylates
Salicylate intoxication in adults usually causes respiratory alkalosis (most common), mixed metabolic acidosis–respiratory alkalosis, or a pure high-AG metabolic acidosis. In the latter example, which is less common, only a portion of the AG is due to the salicylates. Lactic acid production is also often increased. Generally, the presentation is one of respiratory alkalosis with metabolic acidosis. The uncoupling of oxidative phosphorylation is thought to be the cause of the metabolic acidosis. Acidosis can lead to further movement of salicylate into the CNS. High ketone concentration is reported in as many as 40% of adult salicylate poisoning patients, which is thought to be as a result of salicylate-induced hypoglycemia.45 Treatment should begin with vigorous gastric lavage with isotonic saline (not NaHCO3) followed by administration of activated charcoal. In the acidotic patient, to facilitate removal of salicylate, IV NaHCO3 is administered in amounts adequate to alkalinize the urine and maintain urine output (urine pH >7.5). While this form of therapy is straightforward in acidotic patients, a coexisting respiratory alkalosis may make this approach hazardous. Acetazolamide may be administered when an alkaline diuresis cannot be achieved, but this drug can cause systemic metabolic acidosis if HCO3− is not replaced. Hypokalemia may occur with an alkaline diuresis from NaHCO3 and should be treated promptly and aggressively. Glucose-containing fluids should be administered because of the danger of hypoglycemia. Excessive insensible fluid losses may cause severe volume depletion and hypernatremia. Hemodialysis may be necessary for severe poisoning, especially if renal failure coexists, and is preferred with severe intoxication (level >100 mg/dL). Hemodialysis is superior to other dialytic modalities such as hemofiltration for simultaneous management of electrolyte abnormalities.10,45
Ethylene Glycol
Ingestion of ethylene glycol (commonly used in antifreeze) leads to a metabolic acidosis and severe damage to the CNS, heart, lungs, and kidneys. Early on, the patient appears intoxicated and may develop seizures or frank coma. In the next 12 to 24 hours, signs of cardiopulmonary collapse ensue, along with development of renal failure due to intratubular obstruction by oxalate crystals. The increased AG and osmolar gap are due to ethylene glycol and its metabolites, oxalic acid, glycolic acid, and other organic acids. Lactic acid production increases secondary to inhibition of the tricarboxylic acid cycle and altered intracellular redox state.10,46 Recognizing oxalate crystals in the urine, the presence of serum osmolar gap and high-AG acidosis facilitate diagnosis. Treatment should not be delayed while awaiting measurement of ethylene glycol levels in this setting. Treatment includes the prompt institution of a saline or osmotic diuresis, thiamine and pyridoxine supplements, fomepizole or ethanol, and hemodialysis.10,47 The IV administration of the alcohol dehydrogenase inhibitor, fomepizole (4-methylpyrazole; 7 mg/kg as a loading dose), or IV ethanol to achieve a level of 22 mmol/L (100 mg/dL) serves to lessen toxicity, because both compete with ethylene glycol for metabolism by alcohol dehydrogenase. Fomepizole offers the advantages of a predictable decline in ethylene glycol levels without the adverse effects, such as excessive obtundation, associated with ethyl alcohol infusion. Once the above measures are undertaken, hemodialysis is performed to remove ethylene glycol and its metabolites from the blood.
Isopropyl Alcohol
Rubbing alcohol poisoning is usually the result of accidental oral ingestion or absorption through the skin. Although isopropyl alcohol is metabolized by the enzyme alcohol dehydrogenase, as is methanol and ethanol, isopropyl alcohol is not metabolized to a strong acid. Isopropyl alcohol is metabolized to acetone, and the osmolal gap increases as the result of accumulation of both acetone and isopropyl alcohol. Despite a positive nitroprusside reaction from acetone, the AG, as well as the blood glucose, is typically normal, not elevated, and the plasma HCO3− is not depressed. Thus, isopropyl alcohol intoxication does not typically cause metabolic acidosis. Treatment is supportive, with attention to removal of unabsorbed alcohol from the GI tract and IV fluids. Hemodialysis is effective but not usually necessary. Patients with severe isopropyl alcohol intoxication (blood levels >100 mg/dL) may develop cardiovascular collapse and lactic acidosis. Such severe intoxication may benefit from more aggressive therapy, including hemodialysis.10
Paraldehyde
Intoxication with paraldehyde is now very rare but is due partly to acetic acid, the metabolic product of the drug from acetaldehyde and other organic acids.48
Pyroglutamic Acidosis
Pyroglutamic acid, or 5-oxoproline, is an intermediate in the synthesis of glutathione. Acetaminophen ingestion rarely depletes glutathione stores, resulting in an imbalance in the precursors of this compound and excess formation of pyroglutamic acid.49 Most of the cases of 5-oxoproline-induced acidosis has been in patients with sepsis who were receiving full doses of acetaminophen, with all showing an elevated plasma level of pyroglutamic acid and elevated AG. It is conceivable that heterozygosity for glutathione synthase deficiency could be the underlying risk factor for development of this newly appreciated form of metabolic acidosis. It is important to note that only a minority of critically ill patients on acetaminophen develop this condition.1,49
 Non–Anion Gap (Hyperchloremic) Metabolic Acidoses
Non–Anion Gap (Hyperchloremic) Metabolic Acidoses
Alkali can be lost from the GI tract in diarrhea or from the kidneys (renal tubular acidosis [RTA]). Because a reduced plasma [HCO3−] and elevated [Cl−] can also occur in chronic respiratory alkalosis as compensatory response, it is important to confirm the presence of acidemia by measuring the arterial pH. In hyperchloremic metabolic acidosis, reciprocal changes in [Cl−] and [HCO3−] result in a normal AG (Table 109-7). In pure hyperchloremic acidosis, therefore, the increase in [Cl−] above the normal value approximates the decrease in [HCO3−]. The absence of such a relationship suggests a mixed disturbance.
TABLE109-7 Differential Diagnosis of Hyperchloremic Metabolic Acidosis
| Gastrointestinal Bicarbonate Loss |
Adapted in part from DuBose TD Jr. Acid-base disorders. In: Brenner BM, editor. Brenner and Rector’s the kidney. 8th ed. Philadelphia: Saunders; 2008, p. 513-46.
Gastrointestinal Tract Loss
With diarrhea, stools contain a higher [HCO3−] and decomposed HCO3− than plasma, so metabolic acidosis develops along with volume depletion. Instead of an acid urine pH (as anticipated with systemic acidosis), urine pH is usually around 6, because metabolic acidosis and hypokalemia increase renal synthesis and excretion of NH4+, thus providing a urinary buffer that increases urine pH despite increased net acid excretion. Metabolic acidosis due to GI losses with a high urine pH can be differentiated from RTA, because urinary NH4+ excretion is typically low in RTA and high with diarrhea.50 Urinary NH4+ levels can be estimated by calculating the urine AG (UAG):
When [Cl−]u is greater than [Na+ + K+], and the UAG is negative, the urine ammonium level is appropriately increased, suggesting an extrarenal cause of the hyperchloremic acidosis. In such a setting, urinary fractional excretion of Na will also be less than 1% to 2% owing to volume loss from the GI tract. Conversely, when the UAG is positive, the urine ammonium level is low, suggesting a renal cause of the acidosis. Note that this qualitative test is useful in differential diagnosis of a hyperchloremic metabolic acidosis. Furthermore, it is not reliable in the presence of large amounts of other anions in the urine (ketonuria, penicillins, or aspirin).51 Gastrointestinal HCO3− loss, as well as proximal RTA (type 2) and cDRTA (type 1), results in ECF contraction and stimulation of the renin-aldosterone system, leading typically to hypokalemia. The serum K+ concentration, therefore, serves to distinguish these disorders with a low K+ from either generalized distal nephron dysfunction (e.g., type 4 RTA) in which the renin-aldosterone–distal nephron axis is abnormal and hyperkalemia exists, and the acidosis of progressive chronic kidney disease in which normokalemia is common (see later discussion of different types of RTA).
In addition to GI tract HCO3− loss, external loss of pancreatic and biliary secretions can cause a hyperchloremic acidosis. Cholestyramine, calcium chloride, and magnesium sulfate ingestion can also result in a hyperchloremic metabolic acidosis (see Table 109-7), especially in patients with renal insufficiency. Coexistent L-lactic acid acidosis is common in severe diarrheal illnesses but will increase the AG.
Renal Tubular Acidosis
Loss of functioning renal parenchyma due to progressive renal disease leads to hyperchloremic acidosis when the glomerular filtration rate (GFR) is between 20 and 50 mL/min and typically changes into a high-AG acidosis when the GFR falls to less than 20 mL/min.52 Such a progression occurs commonly with tubulointerstitial forms of renal disease, but hyperchloremic metabolic acidosis can persist with advanced glomerular disease. In advanced renal failure, ammoniagenesis is reduced in proportion to the loss of functional renal mass, and ammonium accumulation and trapping in the outer medullary collecting tubule may also be impaired. Because of adaptive increases in K+ secretion by the collecting duct and colon, the acidosis of chronic renal insufficiency is typically normokalemic52 (see Table 109-7).
Proximal RTA (type 2 RTA) is commonly due to generalized proximal tubular dysfunction manifested by glycosuria, generalized aminoaciduria, and phosphaturia (Fanconi’s syndrome). With a low plasma [HCO3−], the urine pH is acid (pH < 5.5); the serum HCO3− concentration usually reaches a nadir of 15 to 18 mEq/L, which limits further filtration and delivery of bicarbonate, so systemic acidosis is not progressive. The fractional excretion of [HCO3−] may exceed 10% to 15% when the serum HCO3− is greater than 20 mEq/L. Because HCO3− is not reabsorbed normally in the proximal tubule, therapy with NaHCO3 will enhance renal potassium wasting and hypokalemia. The two most common causes of acquired proximal RTA in adults are multiple myeloma, in which increased excretion of immunoglobulin light chains injures the proximal tubule epithelium, and chemotherapeutic drug injury of the proximal tubule (ifosfamide). Table 109-8 lists disorders associated with renal tubular acidosis.
TABLE109-8 List of Select Disorders Associated with Renal Tubular Acidosis*
| Renal Defect in Net Acid Excretion, Classic Distal Renal Tubular Acidosis (RTA 1) |
| Systemic or Tubulointerstitial Disease |
* See following source for complete list of disorders.
Adapted in part from DuBose TD Jr. Acid-base disorders. In: Brenner BM, editor. Brenner and Rector’s the kidney. 8th ed. Philadelphia: Saunders; 2008, p. 513-46.
Classic distal RTA (RTA type 1) is characterized by inability to acidify urine appropriately during spontaneous or chemically induced acidosis. The defect limits the ability of the collecting duct to excrete NH4+ and other titratable acids, resulting in a net positive acid balance. The typical findings in classic distal RTA include hypokalemia, hyperchloremic acidosis, low urinary NH4+ excretion (positive UAG), and inappropriately high urine pH (urine pH > 5.5 despite systemic acidosis). Most patients have hypocitraturia and hypercalciuria, so nephrolithiasis, nephrocalcinosis, and bone disease are common. If bicarbonate administration has been high in an attempt to repair the acidosis, the bicarbonaturia will drive kaliuresis, and the hypokalemia may be severe.2 Most studies suggest that the acquired or inherited forms of cDRTA are due to defects in the basolateral Cl−/HCO3 exchanger or subunits of the H+-ATPase. Other examples include an abnormal leak pathway (e.g., amphotericin B)2,53 or abnormalities of the H+/K+-ATPase (see Table 109-8). Correction of chronic metabolic acidosis can usually be achieved readily in patients with cDRTA by administration of alkali in an amount sufficient to neutralize the production of metabolic acids derived from the diet.2 In adult patients with distal RTA, this is may be equal to no more than 1 to 3 mEq/kg/d.54
In type 4 RTA, generalized distal nephron (collecting tubules) dysfunction is manifested by coexistence of hyperchloremic acidosis and hyperkalemia. In the differential diagnosis, it is important to evaluate the functional status of the renin-aldosterone system and ECF volume, which can effect renal perfusion and function. The specific disorders causing hyperkalemic hyperchloremic metabolic acidosis are outlined in detail in Table 109-8.55
When the TTKG is low in a hyperkalemic patient (<8), it reveals that the collecting tubule is not responding appropriately to the prevailing hyperkalemia and that potassium secretion is impaired. In contrast, in hyperkalemia of nonrenal origin, the kidney should respond by increasing K+ secretion, as evidenced by a sharp increase in the TTKG. An important point to consider is that with high urine flow rates, the TTKG underestimates K+ secretory capacity in the hyperkalemic patient.1
The underlying abnormalities that result in RTA type 4 are mineralocorticoid deficiency/resistance or renal tubular dysfunction (voltage defect). The former is most commonly present in older adults with diabetes mellitus or tubulointerstitial disease and renal insufficiency, although other conditions or medications can induce aldosterone deficiency or interfere with its effects (see Tables 109-8 and 109-9).2 Independent of the tubular defect, hyperkalemia inhibits ammoniagenesis at the proximal tubule and contributes to development of metabolic acidosis. The importance of hyperkalemia as a cause of metabolic acidosis is underscored by the frequent observation that correction of it is associated with a marked increase in net acid excretion and a parallel correction in acidosis.55
TABLE109-9 Causes of Drug-Induced Hyperkalemia
| Impaired Renin-Aldosterone Elaboration/Function |
| Inhibitors of Renal Potassium Secretion |
| Altered Potassium Distribution |
Adapted in part from DuBose TD Jr. Acid-base disorders. In: Brenner BM, editor. Brenner and Rector’s the kidney. 8th ed. Philadelphia: Saunders; 2008, p. 513-46.
A variety of clinical conditions and medications result in hyperkalemia with or without associated metabolic acidosis (Table 109-9). Commonly encountered disorders associated with type 4 RTA include diabetic kidney disease, obstructive uropathy, tubulointerstitial disease, and human immunodeficiency virus (HIV)-associated nephropathy (see Table 109-8).
Dilutional and Total Parenteral Nutrition–Associated Acidosis
A rapid increase in extracellular volume (ECV) or addition of exogenous acid (or acid equivalents) to blood can result in the development of hyperchloremic metabolic acidosis (see Table 109-7).10 Examples of exogenous acid loads include infusion of arginine or lysine during parenteral hyperalimentation. This effect is thought to be secondary to an excess of cationic amino acids as compared with anionic amino acids present in these formulas. The severity of the acidosis associated with the use of protein solutions is less than that encountered with the older protein hydrolysate formulations.10 Large amounts of normal saline infusion, especially in patients with limited renal function, can cause a decline in serum bicarbonate concentration and is referred to as dilutional acidosis. This phenomenon is thought to occur as a result of a change in the volume of distribution of bicarbonate which leads to a decrease in its serum concentration, with reciprocal increase in serum chloride concentration.53 Nevertheless, Garella and associates have demonstrated that the serum bicarbonate is only diluted modestly by large increases in ECV.56 Thus, a clinically significant metabolic acidosis would occur only with massive fluid administration. Dilutional acidosis, however, is not uncommon in the ICU, and the intensivist should recognize this phenomenon and consider using solutions with lower chloride concentrations if large amounts of IV fluids are administered. A similar situation may arise from endogenous addition of ketoacids during recovery from ketoacidosis when the sodium salts of ketones may be excreted by the kidneys and lost as potential HCO3−.42
 Metabolic Alkalosis
Metabolic Alkalosis
Metabolic alkalosis in its simplest from is revealed by an elevated arterial pH (alkalemia) and an increase in PaCO2 as a result of compensatory alveolar hypoventilation. It is often accompanied by hypochloremia and hypokalemia. The patient with a high [HCO3−] and a low [Cl−] has either metabolic alkalosis or chronic respiratory acidosis. The arterial pH establishes the diagnosis, as it will be increased in metabolic alkalosis but decreased or normal in respiratory acidosis. As shown in Table 109-1, the PaCO2 increases 6 mm Hg for each 10 mEq/L increase in the [HCO3−] above normal. Stated differently, in the range of [HCO3−] from 10 to 40 mEq/L, the predicted PaCO2 is approximately equal to the patient’s [HCO3−] ± 15 mEq/L. Metabolic alkalosis is one of the more common acid-base disorders in hospitalized patients and occurs as both a simple and a mixed disorder.57 Metabolic alkalosis is also frequently observed not as a pure or simple acid-base disturbance, but in association with other disorders such as respiratory acidosis, respiratory alkalosis, and metabolic acidosis (mixed disorders). Mixed metabolic alkalosis–metabolic acidosis can be appreciated only if the accompanying metabolic acidosis is a high-AG acidosis. The mixed disorder can be appreciated by comparison of the increment in the AG above the normal value of 10 mEq/L (ΔAG = Patient’s AG − 10), with the decrement in the [HCO3−] below the normal value of 25 mEq/L (ΔHCO3− = 25 − Patient’s HCO3−). A mixed metabolic alkalosis–high-AG metabolic acidosis is recognized because the delta values are not similar, and the delta/delta ratio is significantly greater than 1. Often, there is no bicarbonate deficit, yet the AG is significantly elevated. Thus, in a patient with an AG of 20 but near-normal bicarbonate, mixed metabolic alkalosis–metabolic acidosis should be considered. Common examples include renal failure acidosis (uremic) with vomiting or DKA with vomiting.1
Pathogenesis and Differential Diagnosis
Metabolic alkalosis occurs as a result of net gain of [HCO3−] or loss of nonvolatile acid (usually HCl by vomiting) from the extracellular fluid. Because it is unusual for alkali to be added to the body, the disorder involves a generative stage in which the loss of acid usually causes alkalosis, and a maintenance stage in which the kidneys fail to compensate (by excreting HCO3−) because of limiting factors such as volume contraction, a low GFR, or depletion of Cl− or K+.1,58
To establish the cause of metabolic alkalosis (Table 109-10), it is necessary to assess the status of the ECV (orthostatic vitals), the serum [K+], and the renin-aldosterone system.1 For example, the presence of chronic hypertension and chronic hypokalemia in an alkalotic patient suggests either some type of primary mineralocorticoid excess or that the hypertensive patient is receiving diuretics. Low plasma renin activity and normal urine [Na+] and [Cl−] in a patient who is not taking diuretics indicate a primary mineralocorticoid excess syndrome. The combination of hypokalemia and alkalosis in a normotensive, nonedematous patient can be a challenging problem. The possible causes to consider include Bartter or Gitelman syndrome, Mg2+ deficiency, surreptitious vomiting, exogenous alkali, and diuretic ingestion. Determination of urine electrolytes (especially the urine [Cl−]) and screening of the urine for diuretics may be helpful (Table 109-11). If the urine is alkaline with an elevated [Na+] and [K+] but low [Cl−], the diagnosis is usually either prolonged vomiting (overt or surreptitious) or alkali ingestion. If the urine is relatively acid and has low concentrations of Na+, K+, and Cl−, the most likely possibilities are prior vomiting, the posthypercapnic state, or prior diuretic ingestion. If, on the other hand, urine sodium, potassium, or chloride concentrations are not depressed, magnesium deficiency, Bartter’s or Gitelman’s syndrome, or current diuretic ingestion should be considered. Bartter’s syndrome is distinguished from Gitelman’s syndrome by hypocalciuria and hypomagnesemia in the latter disorder. The genetic and molecular basis of these two disorders has been elucidated.1,57
| Exogenous HCO3− Loads |
TABLE109-11 Diagnosis of Metabolic Alkalosis
| Saline-Responsive Alkalosis | Saline-Unresponsive Alkalosis |
|---|---|
| LOW URINARY [Cl−] | HIGH OR NORMAL URINARY [Cl−] |
Metabolic Alkalosis Associated with Extracellular Fluid Volume Contraction, Hypokalemia, and Hyperreninemic Hyperaldosteronism
Gastrointestinal Origin
Gastrointestinal loss of H+ from vomiting or gastric aspiration results in retention of HCO3−. The loss of fluid and NaCl in vomitus or nasogastric suction results in contraction of the ECV and an increase in the secretion of renin and aldosterone. Volume contraction causes a reduction in GFR and an enhanced capacity of the renal tubule to reabsorb HCO3−. During active vomiting, there is continued addition of HCO3− to plasma in exchange for Cl−, and the plasma [HCO3−] exceeds the reabsorptive capacity of the proximal tubule. The excess NaHCO3 reaches the distal tubule, where H+ secretion is enhanced by aldosterone and the delivery of the poorly reabsorbed anion HCO3−.57 Because of contraction of the ECV and hypochloremia, Cl− is avidly conserved by the kidney, as recognized by a low urinary chloride concentration (see Table 109-11). Correction of the contracted ECV with NaCl and repair of K+ deficits corrects the acid-base disorder. Metabolic alkalosis has been described in cases of villous adenoma and is ascribed to adenoma-derived high K+ secretion. The K+ and volume depletion likely causes the alkalosis, because colonic secretion is alkaline.
Renal Origin
Bartter’s Syndrome
Three types of Bartter’s syndrome have been described, and all are inherited as autosomal recessive disorders. Both classic Bartter’s syndrome and the antenatal Bartter’s involve impaired Cl− absorption, which results in salt wasting, volume depletion, and activation of the renin-angiotensin system. Excessive prostaglandin elaboration commonly found with this disorder is in response to volume depletion, hypokalemia, and high angiotensin II levels.59–64 These phenotypes are the result of loss-of-function mutations of one of the genes that encode three transporters involved in vectorial NaCl absorption in the thick ascending limb of Henle’s loop.65 The most prevalent disorder is a mutation of the gene that encodes the bumetanide-sensitive Na+ 2Cl− K+ co-transporter (NKCC2 or BSC1) on the apical membrane. A second mutation has been discovered in the gene that encodes the apical K+ conductance channel (ROMK),65 which operates in parallel with the Na+ 2Cl− K+ transporter to recycle K+.66 A third defect, in the basolateral Cl− channel which transports Cl− out of the cell, has been described. All three defects have the same net effect: loss of Cl− transport in the thick ascending limb of Henle’s loop.67,68 Such defects would predictably lead to extracellular fluid contraction, hyperreninemic hyperaldosteronism, and increased delivery of Na+ to the distal nephron and thus alkalosis and renal K+ wasting and hypokalemia. Secondary overproduction of prostaglandins, juxtaglomerular apparatus hypertrophy, and vascular pressor unresponsiveness would then ensue.
Distinction from surreptitious vomiting, diuretic administration, and laxative abuse is necessary to make the diagnosis of Bartter’s syndrome.1 The finding of a low urinary Cl− concentration is helpful in identifying the vomiting patient (see Table 109-11).69,70 The urinary Cl− concentration in Bartter’s syndrome would be expected to be normal or increased rather than depressed.
Treatment of Bartter’s syndrome is generally focused on the repair of hypokalemia by inhibition of the renin-angiotensin-aldosterone or the prostaglandin-kinin system. K+ supplementation,71 Mg++ repletion,72,73 propranolol,74,75 spironolactone,74,75 prostaglandin inhibitors, and angiotensin-converting enzyme inhibitors76,77 have all been advocated, but each has met with limited success.
Gitelman’s Syndrome
Gitelman’s syndrome, which occurs more often in adults, is distinguished from Bartter’s syndrome, which occurs more commonly in children, by the presence of hypocalciuria, hypermagnesuria, and hypomagnesemia.78–80 These unique features mimic the effect of chronic thiazide diuretic administration. Gitelman’s syndrome is the result of missense mutations (several have been described) in the gene SLC12A3, which encodes the thiazide-sensitive distal convoluted tubule Na+/Cl− co-transporter (NCCT).80–82 Loss of activity of the NaCl co-transporter increases tubule Ca++ absorption, leading to the classic finding of hypocalciuria. A large study of adults with proven Gitelman’s syndrome and NCCT mutations showed that salt craving, nocturia, cramps, and fatigue were more common than in sex- and age-matched controls.82 Women experienced exacerbation of symptoms during menses, and many had complicated pregnancies. Treatment of Gitelman’s syndrome, as with Bartter’s syndrome, consists of liberal dietary sodium and potassium salts, but with the addition of magnesium supplementation in most patients. Angiotensin-converting enzyme inhibitors have been suggested to be helpful in selected patients but can cause frank hypotension.
Nonreabsorbable Anions and Magnesium Deficiency
Administration of large quantities of nonreabsorbable anions such as penicillin or carbenicillin can enhance distal acidification and K+ secretion by increasing the transepithelial potential difference (lumen negative). Mg++ deficiency results in hypokalemic alkalosis by enhancing distal acidification through stimulation of renin and hence aldosterone secretion.83
Potassium Depletion
Pure K+ depletion causes metabolic alkalosis, although generally of only modest severity. One reason the alkalosis is usually mild is that K+ depletion also causes positive sodium chloride balance with or without mineralocorticoid administration. The salt retention in turn antagonizes the degree of alkalemia. When access to salt as well as to K+ is restricted, more severe alkalosis develops. Activation of the renal H+/K+-ATPase in the collecting duct by chronic hypokalemia likely plays a major role in maintenance of the alkalosis. Specifically, chronic hypokalemia has been shown to markedly increase the abundance of the colonic H+/K+-ATPase mRNA and protein in the outer medullary collecting duct. In animals, the alkalosis is maintained in part by reduction in GFR without a change in tubule HCO3− transport. In humans, the pathophysiologic basis of the alkalosis has not been well defined. Alkalosis associated with severe K+ depletion, however, is resistant to salt administration. Repair of the K+ deficiency is necessary to correct the alkalosis.83
After Treatment of Lactic Acidosis or Ketoacidosis
When an underlying stimulus for the generation of lactic acid or ketoacid is removed rapidly, as with repair of circulatory insufficiency or with insulin therapy, the lactate or ketones are metabolized to yield an equivalent amount of HCO3−. Other sources of new HCO3− are additive with the original amount generated by organic anion metabolism to create an excess of HCO3−. The sources of the additional alkali include (1) new HCO3− added to the blood by the kidneys as a result of enhanced acid excretion during the preexisting period of acidosis and (2) alkali therapy during the treatment phase of the acidosis. Acidosis-induced contraction of the ECV and K+ deficiency act to sustain the alkalosis.57
Metabolic Alkalosis Associated with Hypervolemia, Hypertension, And Hyperaldosteronism
Mineralocorticoid administration or excess production (primary aldosteronism of Cushing’s syndrome and adrenal cortical enzyme defects) increases net acid excretion and tends to result in metabolic alkalosis. The degree of alkalosis is augmented by the simultaneous increase in K+ excretion leading to K+ deficiency and hypokalemia. Salt intake for sufficient distal Na+ delivery is also a prerequisite for the development of both the hypokalemia and the alkalosis. Hypertension develops partly as a result of ECF expansion from salt retention. The alkalosis is not progressive and is generally mild. Volume expansion tends to antagonize the decrease in GFR and/or increase in tubule acidification induced by hypermineralocorticoidism and K+ deficiency. The kaliuresis persists and causes continued K+ depletion with polydipsia, inability to concentrate the urine, and polyuria. Increased aldosterone levels may be the result of autonomous primary adrenal overproduction or of secondary aldosterone release due to renal overproduction of renin. In both situations, the normal feedback of ECV on net aldosterone production is disrupted, and hypertension from volume retention can result (see Table 109-10). States associated with inappropriately high renin levels include renovascular disease and accelerated and malignant hypertension. Estrogens increase renin substrate and, hence, angiotensin II formation. Primary tumor overproduction of renin is another rare cause of hyperreninemic hyperaldosterone–induced metabolic alkalosis.83
Ingestion of licorice, carbenoxolone, chewer’s tobacco, or nasal spray can cause a typical pattern of hypermineralocorticoidism. These substances inhibit 11β-hydroxysteroid dehydrogenase (which normally metabolizes cortisol to an inactive metabolite), and the cortisol buildup results in interaction and activation of type 1 renal mineralocorticoid receptors, mimicking aldosterone.1
 Treatment of Metabolic Alkalosis
Treatment of Metabolic Alkalosis
The maintenance of metabolic alkalosis represents a failure of the kidney to excrete bicarbonate efficiently because of chloride or potassium deficiency, or continuous mineralocorticoid elaboration, or both. Treatment is primarily directed at correcting the underlying stimulus for HCO3− generation and restoring the ability of the kidney to excrete the excess bicarbonate.1,58 Assistance is gained in the diagnosis and treatment of metabolic alkalosis by paying attention to the urinary chloride concentration, the arterial blood pressure, and the volume status of the patient (particularly the presence or absence of orthostasis) (see Table 109-11 and Figure 109-4).1 Particularly helpful in the history is the presence or absence of vomiting, diuretic use, or alkali therapy. A high urine chloride concentration and hypertension suggests that mineralocorticoid excess is present. If primary aldosteronism is present, correction of the underlying cause will reverse the alkalosis (adenoma, bilateral hyperplasia, Cushing’s syndrome). Patients with bilateral adrenal hyperplasia may respond to spironolactone. Normotensive patients with a high urine chloride may have Bartter’s or Gitelman’s syndrome if diuretic use or vomiting can be excluded. A low urine chloride and relative hypotension suggests a chloride-responsive metabolic alkalosis such as vomiting or nasogastric suction. [H+] loss by the stomach or kidneys can be mitigated by the use of proton pump inhibitors or the discontinuation of diuretics. The second aspect of treatment is to remove the factors that sustain HCO3− reabsorption, such as ECV contraction or K+ deficiency. Although K+ deficits should be repaired, NaCl therapy is usually sufficient to reverse the alkalosis if ECV contraction is present, as indicated by low urine [Cl−].
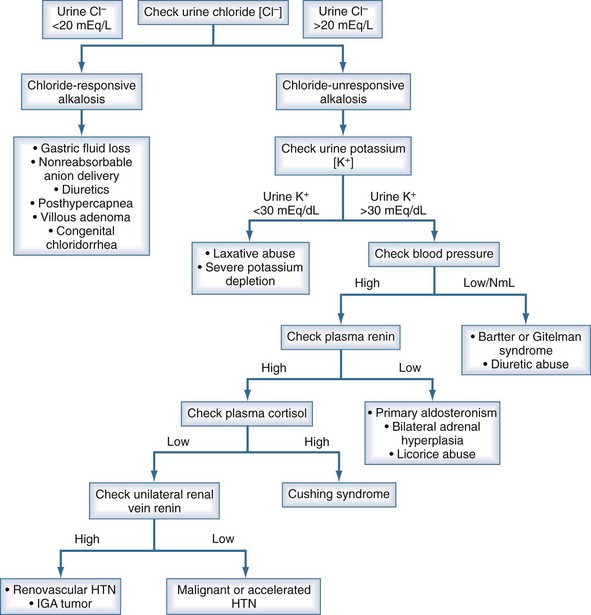
Figure 109-4 Workup of metabolic alkalosis.
(Data from DuBose TD Jr. Acid-base disorders. In: Brenner BM, editor. Brenner and Rector’s the kidney. 8th ed. Philadelphia: Saunders; 2008, p. 513.)
Patients with congestive heart failure or unexplained volume overexpansion represent special challenges in the ICU. Patients with a low urine chloride concentration, which is usually indicative of a “chloride-responsive” form of metabolic alkalosis, may not tolerate normal saline infusion. Renal HCO3− loss can be accelerated by administration of acetazolamide (250-500 mg IV), a carbonic anhydrase inhibitor, if associated conditions preclude infusion of saline (elevated pulmonary capillary wedge pressure, or evidence of CHF.1 Acetazolamide is usually very effective in patients with adequate renal function but can exacerbate urinary K+ losses. Dilute hydrochloric acid (0.1 N HCl) is also effective but can cause hemolysis and may be difficult to titrate. If used, the goal should be to not restore the pH to normal but to a pH of approximately 7.50. Alternatively, acidification can also be achieved with oral NH4Cl, which should be avoided in the presence of liver disease. Hemodialysis against a dialysate low in [HCO3−] and high in [Cl−] can be effective when renal function is impaired. Patients receiving continuous renal replacement therapy in the ICU are prone to development of metabolic alkalosis due to bicarbonate-based replacement fluid/dialysate or when citrate regional anticoagulation is employed.
Key Points
Bonnet F, Bonarek M, Morlat P, et al. Risk factors for lactic acidosis in HIV-infected patients treated with nucleoside reverse-transcriptase inhibitors: A case-control study. Clin Infect Dis. 2003;36:1324-1328.
Bouman CS, Oudemans-van Straaten HM, Schultz MJ, Vroom MB. Hemofiltration in sepsis and systemic inflammatory response syndrome: the role of dosing and timing. J Crit Care. 2007;22:1-12. Epub 2007 Jan 31
Halperin ML, Hammeke M, Jose RG, et al. Metabolic acidosis in the alcoholic: a pathophysiologic approach. Metabolism. 1983;32:308.
Ogedegbe AE, Thomas DL, Diehl AM. Hyperlactatemia syndromes associated with HIV therapy. Lancet Infect Dis. 2003;3:329-337.
Mizock BA, Belyaev S, Mecher C. Unexplained metabolic acidosis in critically ill patients: the role of pyroglutamic acid. Intensive Care Med. 2004;30:502-505.
Stacpoole PW, Nagaraja NJ, Hutson AD. Efficacy of dichloroacetate as a lactate-lowering drug. J Clin Pharmacol. 2003;43:683-691.
1 DuBose TD. Acid-base disorders. edited by Brenner and Rector’s. In: Brenner. Philadelphia: WB Saunders; 2008:513-546.
2 DuBose TD, Alpern RJ. Renal tubular acidosis. The Metabolic and Molecular Bases of Inherited Disease, 8th ed. New York: McGraw-Hill; 2001.
3 Kurtz I, Kraut J, Ornekian V, Nguyen MK. Acid-base analysis: a critique of the Stewart and bicarbonate-centered approaches. Am J Physiol Renal Physiol. 2008;294:F1009-F1031.
4 Bidani A, Tauzon DM, Heming TA. Regulation of whole body acid-base balance. In: Dubose TDJr, Hamm L, editors. Acid-Base and Electrolyte Disorders. Philadelphia: Saunders; 2002:1-22.
5 Kraut JA, Madias NE. Serum anion gap: its uses and limitations in clinical medicine. Clin. J Am Soc Nephrol. 2007;2:162-174.
6 Oh MS, Carroll HJ. The anion gap. N Engl J Med. 1977;297:814-817.
7 Oh MS, Carroll HJ, Goldstein DA, Fein IA. Hyperchloremic acidosis during the recovery phase of diabetic ketosis. Ann Intern Med. 1978;89:925-927.
8 Kaplan LJ, Kellum JA. Initial pH, base deficit, lactate, anion gap, strong ion difference, and strong ion gap predict outcome from major vascular injury. Crit Care Med. 2004;32:1120-1124.
9 Laski ME, Wesson DE. Lactic acidosis. In: Dubose TDJr, Hamm L, editors. Acid-Base and Electrolyte Disorders. Philadelphia: WB Saunders; 2002:83-108.
10 Whiteney GM, Szerlip HM. Acid-base disorders in the critical care setting. In: Dubose TDJr, Hamm L, editors. Acid-Base and Electrolyte Disorders. Philadelphia: WB Saunders; 2002:165-188.
11 Moviat M, van HF, van der HH. Conventional or physicochemical approach in intensive care unit patients with metabolic acidosis. Crit Care. 2003;7:R41-R45.
12 Mikkelsen ME, Miltiades AN, Gaieski DF, Goyal M, Fuchs BD, Shah CV, et al. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med. 2009;37:1670-1677.
13 Huckabee WE. Abnormal resting blood lactate. I. The significance of hyperlactatemia in hospitalized patients. Am J Med. 1961;30:840-848.
14 Brown JB, Pedula K, Barzilay J, Herson MK, Latare P. Lactic acidosis rates in type 2 diabetes. Diabetes Care. 1998;21:1659-1663.
15 Sulkin TV, Bosman D, Krentz AJ. Contraindications to metformin therapy in patients with NIDDM. Diabetes Care. 1997;20:925-928.
16 John M, Mallal S. Hyperlactatemia syndromes in people with HIV infection. Curr Opin Infect Dis. 2002;15:23-29.
17 Wilson KC, Reardon C, Theodore AC, Farber HW. Propylene glycol toxicity: a severe iatrogenic illness in ICU patients receiving IV benzodiazepines: a case series and prospective, observational pilot study. Chest. 2005;128:1674-1681.
18 Iberti TJ, Leibowitz AB, Papadakos PJ, Fischer EP. Low sensitivity of the anion gap as a screen to detect hyperlactatemia in critically ill patients. Crit Care Med. 1990;18:275-277.
19 Peretz DI, Scott HM, Duff J, Dossetor JB, MacLean LD, McGregor M. The significance of lacticacidemia in the shock syndrome. Ann N Y Acad Sci. 1965;119:1133-1141.
20 Cady LDJr, Weil MH, Afifi AA, Michaels SF, Liu VY, Shubin H. Quantitation of severity of critical illness with special reference to blood lactate. Crit Care Med. 1973;1:75-80.
21 Kruise JA, Mehta KC, Carlson RW. Definition of clinically significant lactic acidosis. Chest. 1987;92:S100.
22 Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368-1377.
23 Nguyen HB, Rivers EP, Knoblich BP, Jacobsen G, Muzzin A, Ressler JA, et al. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit Care Med. 2004;32:1637-1642.
24 Falk JL, Rackow EC, Leavy J. Delayed lactate clearance in patients surviving circulatory shock. Acute Care. 1985;11:212-215.
25 Parker MM, Shelhamer JH, Natanson C, Alling DW, Parrillo JE. Serial cardiovascular variables in survivors and nonsurvivors of human septic shock: heart rate as an early predictor of prognosis. Crit Care Med. 1987;15:923-929.
26 Stacpoole PW, Wright EC, Baumgartner TG, Bersin RM, Buchalter S, Curry SH, et al. Dichloroacetate-Lactic Acidosis Study Group. A controlled clinical trial of dichloroacetate for treatment of lactic acidosis in adults. N Engl J Med. 1992;327:1564-1569.
27 Levraut J, Ciebiera JP, Jambou P, Ichai C, Labib Y, Grimaud D. Effect of continuous venovenous hemofiltration with dialysis on lactate clearance in critically ill patients. Crit Care Med. 1997;25:58-62.
28 Barenbrock M, Hausberg M, Matzkies F, de la MS, Schaefer RM. Effects of bicarbonate- and lactate-buffered replacement fluids on cardiovascular outcome in CVVH patients. Kidney Int. 2000;58:1751-1757.
29 Heering P, Ivens K, Thumer O, Morgera S, Heintzen M, Passlick-Deetjen J, et al. The use of different buffers during continuous hemofiltration in critically ill patients with acute renal failure. Intensive Care Med. 1999;25:1244-1251.
30 Bouman CS, Oudemans-van Straaten HM, Schultz MJ, Vroom MB. Hemofiltration in sepsis and systemic inflammatory response syndrome: the role of dosing and timing. J Crit Care. 2007;22:1-12.
31 Honore PM, Joannes-Boyau O, Boer W, Collin V. High-volume hemofiltration in sepsis and SIRS: current concepts and future prospects. Blood Purif. 2009;28:1-11.
32 Stacpoole PW, Nagaraja NV, Hutson AD. Efficacy of dichloroacetate as a lactate-lowering drug. J Clin Pharmacol. 2003;43:683-691.
33 Narins RG, Cohen JJ. Bicarbonate therapy in severe acidosis. Ann Intern Med. 1988;108:311.
34 Mathieu D, Neviere R, Billard V, Fleyfel M, Wattel F. Effects of bicarbonate therapy on hemodynamics and tissue oxygenation in patients with lactic acidosis: a prospective, controlled clinical study. Crit Care Med. 1991;19:1352-1356.
35 Arieff AI, Park R, Leach WJ, Lazarowitz VC. Pathophysiology of experimental lactic acidosis in dogs. Am J Physiol. 1980;239:F135-F142.
36 Arieff AI, Gertz EW, Park R, Leach W, Lazarowitz VC. Lactic acidosis and the cardiovascular system in the dog. Clin Sci (Lond). 1983;64:573-580.
37 Bersin RM. Effects of sodium bicarbonate on myocardial metabolism and circulatory function during hypoxia. In: Hypoxia, Metabolic Acidosis, and the Circulation. Oxford: Oxford University Press; 1992:139-174.
38 Cooper DJ, Walley KR, Wiggs BR, Russell JA. Bicarbonate does not improve hemodynamics in critically ill patients who have lactic acidosis. A prospective, controlled clinical study. Ann Intern Med. 1990;112:492-498.
39 Stacpoole PW, Moore GW, Kornhauser DM. Toxicity of chronic dichloroacetate. N Eng J Med. 1979;300:372.
40 Nahas GG, Sutin KM, Fermon C, Streat S, Wiklund L, Wahlander S, et al. Guidelines for the treatment of acidaemia with THAM. Drugs. 1998;55:191-224.
41 Halperin ML, Kamel KS. D-lactic acidosis: turning sugar into acids in the gastrointestinal tract. Kidney Int. 1996;49:1-8.
42 Halperin ML, Kamel KS, Cherney DZ. Ketoacidosis. In: DuBose TDJr, Hamm LL, editors. Acid-Base and Electrolyte Disorders: A companion to Brenner and Rector’s The Kidney. Philadelphia: WB Saunders; 2002:67-82.
43 Halperin ML, Hammeke M, Josse RG, Jungas RL. Metabolic acidosis in the alcoholic: a pathophysiologic approach. Metabolism. 1983;32:308-315.
44 Umpierrez GE, DiGirolamo M, Tuvlin JA, Isaacs SD, Bhoola SM, Kokko JP. Differences in metabolic and hormonal milieu in diabetic- and alcohol-induced ketoacidosis. J Crit Care. 2000;15:52-59.
45 Proudfoot AT, Krenzelok EP, Brent J, Vale JA. Does urine alkalinization increase salicylate elimination? If so, why? Toxicol Rev. 2003;22:129-136.
46 Fraser AD. Clinical toxicologic implications of ethylene glycol and glycolic acid poisoning. Ther Drug Monit. 2002;24:232-238.
47 Brent J, McMartin K, Phillips S, Aaron C, Kulig K. Fomepizole for the treatment of methanol poisoning. N Engl J Med. 2001;344:424-429.
48 Gutman RA, Burnell JM. Paraldehyde acidosis. Am J Med. 1967;42:435-440.
49 Mizock BA, Belyaev S, Mecher C. Unexplained metabolic acidosis in critically ill patients: the role of pyroglutamic acid. Intensive Care Med. 2004;30:502-505.
50 Alpern RJ, Hamm LL. Urinary calcifications. In: DuBose TDJr, Hamm LL, editors. Acid-Base and electrolyte disorders: A companion to Brenner and Rector’s The Kidney. Philadelphia: WB Saunders; 2002:129-146.
51 Morris RCJr. Renal tubular acidosis. Mechanisms, classification and implications. N Engl J Med. 1969;281:1405-1413.
52 Gautheir P. Acidosis of chronic renal failure. In: DuBose TDJr, Hamm LL, editors. A companion to Brenner and Rector’s The Kidney. Philadelphia: WB Saunders; 2002:207-216.
53 Krapfr AR, Seldin DW. Clinical syndromes of metabolic acidosis. In: Seldin DW, Giebisch G, editors. The Kidney. 3rd ed. Philadelphia: Lippincott Williams and Wilkins; 2000:2055-2072.
54 Morris RCJr. Sebastian A: Alkali therapy in renal tubular acidosis: who needs it? J Am Soc Nephrol. 2002;13:2186-2188.
55 DuBose TD, McDonald GA. Renal tubular acidosis. In: DuBose TDJr, Hamm LL, editors. Acid-Base and Electrolyte Disorders: A companion to Brenner and Rector’s The Kidney. Philadelphia: WB Saunders; 2002:189-206.
56 Garella S, Chang BS, Kahn SI. Dilution acidosis and contraction alkalosis: review of a concept. Kidney Int. 1975;8:279-283.
57 Galla JH. Metabolic alkolosis. In: DuBose TDJr, Hamm LL, editors. Acid-base and Electrolyte disorders; A companion to Brenner and Rector’s The Kidney. Philadelphia: WB Saunders; 2002:109-128.
58 Galla JH. Metabolic alkalosis. J Am Soc Nephrol. 2000;11:369-375.
59 Gardner JD, Simopoulos AP, Lapey A, Shibolet S. Altered membrane sodium transport in Bartter’s syndrome. J Clin Invest. 1972;51:1565-1571.
60 Mongeau JG, Garay R, de MM, Broyer M, Meyer P. Erythrocyte Na+ and K+ transport systems in children with Bartter syndrome: increase in passive sodium permeability. Kidney Int. 1983;23:530-535.
61 Baehler RW, Work J, Kotchen TA, McMorrow G, Guthrie G. Studies on the pathogenesis of Bartter’s syndrome. Am J Med. 1980;69:933-938.
62 Gill JRJr, Bartter FC. Evidence for a prostaglandin-independent defect in chloride reabsorption in the loop of Henle as a proximal cause of Bartter’s syndrome. Am J Med. 1978;65:766-772.
63 Kurtzman NA, Gutierrez LF. The pathophysiology of Bartter syndrome. JAMA. 1975;234:758-759.
64 Bartter FC. On the pathogenesis of Bartter’s syndrome. Miner Electrolyte Metab. 1980;3:61.
65 Simon DB, Karet FE, Rodriguez-Soriano J, Hamdan JH, DiPietro A, Trachtman H, et al. Genetic heterogeneity of Bartter’s syndrome revealed by mutations in the K+ channel, ROMK. Nat Genet. 1996;14:152-156.
66 Simon DB, Bindra RS, Mansfield TA, Nelson-Williams C, Mendonca E, Stone R, et al. Mutations in the chloride channel gene, CLCNKB, cause Bartter’s syndrome type III. Nat Genet. 1997;17:171-178.
67 Derst C, Konrad M, Kockerling A, Karolyi L, Deschenes G, Daut J, et al. Mutations in the ROMK gene in antenatal Bartter syndrome are associated with impaired K+ channel function. Biochem Biophys Res Commun. 1997;230:641-645.
68 Herbert SC, Gullans SR. The molecular basis of inherited hypokalemic alkalosis: Bartter’s and Gitelman’s syndromes. Am J Physiol. 1996;271:F957-F959.
69 Rosenblum M, Simpson DP, Evenson M. Factitious Bartter’s syndrome. Arch Intern Med. 1977;137:1244-1245.
70 Ogedegbe AE, Thomas DL, Diehl AM. Hyperlactataemia syndromes associated with HIV therapy. Lancet Infect Dis. 2003;3:329-337.
71 Cannon PJ, Leeming JM, Sommers SC, Winters RW, Laragh JH. Juxtaglomerular cell hyperplasia and secondary hyperaldosteronism (Bartter’s syndrome): a re-evaluation of the pathophysiology. Medicine (Baltimore). 1968;47:107-131.
72 Gullner HG, Bartter FC, Gill JRJr, Dickman PS, Wilson CB, Tiwari JL. A sibship with hypokalemic alkalosis and renal proximal tubulopathy. Arch Intern Med. 1983;143:1534-1540.
73 Fujita T, Sakaguchi H, Shibagaki M, Fukui T, Nomura M. The pathogenesis of Bartter’s syndrome. Functional and histologic studies. Am J Med. 1977;63:467-474.
74 Solomon RJ, Brown RS. Bartter’s syndrome. New insights into pathogenesis and treatment. Am J Med. 1975;59:575-583.
75 Modlinger RS, Nicolis GL, Krakoff LR, Gabrilove JL. Some observations on the pathogenesis of Bartter’s syndrome. N Engl J Med. 1973;289:1022-1024.
76 Hene RJ, Koomans HA, Boer P, Dorhout Mees EJ. Long-term treatment of Bartter’s syndrome with captopril. Br Med J (Clin Res Ed). 1982;285:695.
77 Aurell M, Rudin A. Effects of captopril in Bartter’s syndrome. N Engl J Med. 1982;304:1609.
78 Silverberg AB, Mennes PA, Cryer PE. Resistance to endogenous norepinephrine in Bartter’s syndrome: reversion during indomethacin administration. Am J Med. 1978;64:231-235.
79 Simon DB, Lifton RP. Ion transporter mutations in Gitelman’s and Bartter’s syndromes. Curr Opin Nephrol Hypertens. 1998;7:43-47.
80 Shaer AJ. Inherited primary renal tubular hypokalemic alkalosis: a review of Gitelman and Bartter syndromes. Am J Med Sci. 2001;322:316-332.
81 Simon DB, Nelson-Williams C, Bia MJ, Ellison D, Karet FE, Molina AM, et al. Gitelman’s variant of Bartter’s syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat Genet. 1996;12:24-30.
82 Monkawa T, Kurihara I, Kobayashi K, Hayashi M, Saruta T. Novel mutations in thiazide-sensitive Na-Cl cotransporter gene of patients with Gitelman’s syndrome. J Am Soc Nephrol. 2000;11:65-70.
83 DuBose TD. Metabolic alkalosis. In: Greenberg A, editor. Primer on Kidney Diseases. Philadelphia: Elsevier Saunders; 2005:90-96.