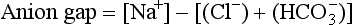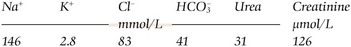Metabolic acid–base disorders
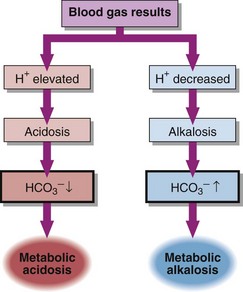
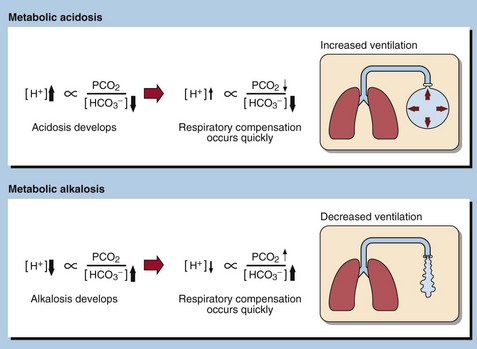
Metabolic acid–base disorders are caused by an increase in H+ production or a loss of H+ triggering compensatory mechanisms that result in the loss or gain of HCO3−. Direct loss or gain of HCO3− will also cause metabolic acid-base disorders. Primary metabolic acid–base disorders are recognized by inspecting the bicarbonate concentration (Fig 21.1). Respiratory compensation takes place quickly so patients with metabolic acid–base disorders will usually show some change in blood PCO2 because of hyperventilation or hypoventilation (Fig 21.2).
Metabolic acidosis
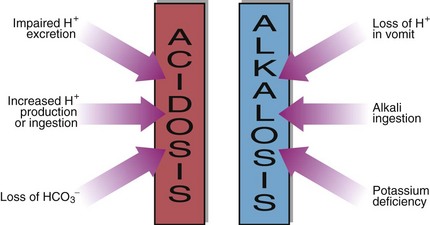
In a metabolic acidosis the primary problem is a reduction in the bicarbonate concentration of the extracellular fluid. The main causes of a metabolic acidosis are shown in Figure 21.3. These are:
 increased production of hydrogen ions
increased production of hydrogen ions
 ingestion of hydrogen ions, or of drugs that are metabolized to acids
ingestion of hydrogen ions, or of drugs that are metabolized to acids
 impaired excretion of hydrogen ions by the kidneys
impaired excretion of hydrogen ions by the kidneys
 loss of bicarbonate from the gastrointestinal tract or in the urine.
loss of bicarbonate from the gastrointestinal tract or in the urine.
Causes of metabolic acidosis
Metabolic acidosis with an elevated anion gap occurs in:
 Renal disease. Hydrogen ions are retained along with anions such as sulphate and phosphate.
Renal disease. Hydrogen ions are retained along with anions such as sulphate and phosphate.
 Diabetic ketoacidosis. Altered metabolism of fatty acids, as a consequence of the lack of insulin, causes endogenous production of acetoacetic and β-hydroxybutyric acids.
Diabetic ketoacidosis. Altered metabolism of fatty acids, as a consequence of the lack of insulin, causes endogenous production of acetoacetic and β-hydroxybutyric acids.
 Lactic acidosis. This results from a number of causes, particularly tissue anoxia. In acute hypoxic states such as respiratory failure or cardiac arrest lactic acidosis develops within minutes and is life-threatening. Lactic acidosis may also be caused by liver disease. The presence of a lactic acidosis can be confirmed, if necessary, by the measurement of plasma lactate concentration.
Lactic acidosis. This results from a number of causes, particularly tissue anoxia. In acute hypoxic states such as respiratory failure or cardiac arrest lactic acidosis develops within minutes and is life-threatening. Lactic acidosis may also be caused by liver disease. The presence of a lactic acidosis can be confirmed, if necessary, by the measurement of plasma lactate concentration.
 Certain cases of overdosage or poisoning. The mechanism common to all of these is the production of acid metabolites. Examples include salicylate overdose where build-up of lactate occurs, methanol poisoning when formate accumulates, or ethylene glycol poisoning where oxalate is formed.
Certain cases of overdosage or poisoning. The mechanism common to all of these is the production of acid metabolites. Examples include salicylate overdose where build-up of lactate occurs, methanol poisoning when formate accumulates, or ethylene glycol poisoning where oxalate is formed.
Clinical effects of acidosis
A raised [H+] leads to increased neuromuscular irritability. There is a hazard of arrhythmias progressing to cardiac arrest, and this is made more likely by the presence of hyperkalaemia, which will accompany the acidosis (pp. 22–23). Depression of consciousness can progress to coma and death.
Metabolic alkalosis
The causes of a metabolic alkalosis are shown in Figure 21.3. The condition may be due to:
 Loss of hydrogen ion in gastric fluid during vomiting. This is especially seen when there is pyloric stenosis preventing parallel loss of bicarbonate-rich secretions from the duodenum.
Loss of hydrogen ion in gastric fluid during vomiting. This is especially seen when there is pyloric stenosis preventing parallel loss of bicarbonate-rich secretions from the duodenum.
 Ingestion of an absorbable alkali such as sodium bicarbonate. Very large doses are required to cause a metabolic alkalosis unless there is renal impairment.
Ingestion of an absorbable alkali such as sodium bicarbonate. Very large doses are required to cause a metabolic alkalosis unless there is renal impairment.
 Potassium deficiency. In severe potassium depletion, often a consequence of diuretic therapy, hydrogen ions are retained inside cells to replace the missing potassium ions. In the renal tubule more hydrogen ions, rather than potassium, are exchanged for reabsorbed sodium. So, despite there being an alkalosis, the patient passes an acid urine. This is often referred to as a ‘paradoxical’ acid urine, because in other causes of metabolic alkalosis urinary [H+] usually falls.
Potassium deficiency. In severe potassium depletion, often a consequence of diuretic therapy, hydrogen ions are retained inside cells to replace the missing potassium ions. In the renal tubule more hydrogen ions, rather than potassium, are exchanged for reabsorbed sodium. So, despite there being an alkalosis, the patient passes an acid urine. This is often referred to as a ‘paradoxical’ acid urine, because in other causes of metabolic alkalosis urinary [H+] usually falls.