Chapter 127 Mesencephalic Tractotomy and Anterolateral Cordotomy for Intractable Pain
Mesencephalic Tractotomy
Anatomic Background
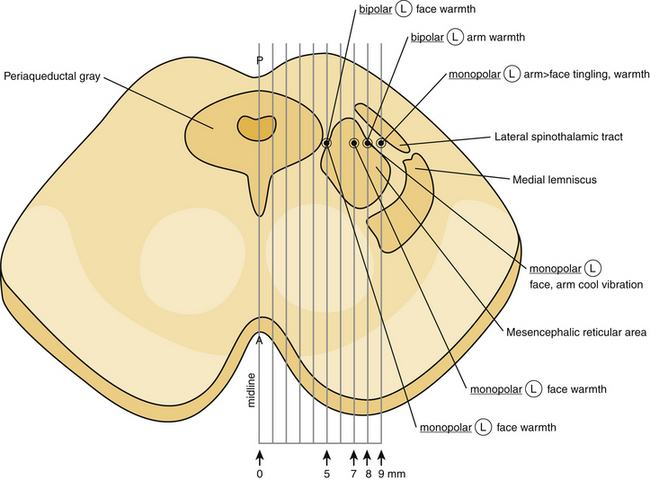
It is well known that the mesencephalon is topographically divided into three distinct regions: tectum, tegmentum, and basal portion.1 The tectum, which is a mixture of gray and white matter, is located dorsally to the central gray matter in cross-section, occupying the dorsal surface of the midbrain. It includes the superior and the inferior colliculi. The tegmentum constitutes the main, central part of the mesencephalon and lies anterior to the central gray. It contains all the ascending and descending neuronal tracts, including the brachium conjuctivum (superior cerebellar peduncle), the rubrospinal tract, the medial lemniscus, the trigeminal lemniscus, the spinothalamic and spinotectal tracts, the medial longitudinal fasciculus, the central tegmental tract, the lateral lemniscus, and the dorsal longitudinal fasciculus. The brachium conjuctivum represents a massive bundle of fibers arising from the deep cerebellar nuclei, decussating at the level of the inferior colliculus in the tegmentum and terminating on the red nucleus and the ventrolateral thalamic nucleus. The rubrospinal tract lies anteriorly to the brachium conjuctivum and conveys fibers from the red nucleus to the spinal cord and the inferior olive. The medial lemniscus lies lateral to the brachium conjuctivum, and conveys kinesthesia and discriminative touch from the spinal cord to the thalamus. The fibers of the medial lemniscus are somatotopically organized with the cervical fibers being more medial, while sacral fibers are more lateral.1 The trigeminal lemniscus lies medially to the medial lemniscus. The spinothalamic and spinotectal tracts lie laterally to the medial lemniscus and convey pain and temperature sensation from the contralateral side of the body. The fibers of the spinothalamic and spinotectal tracts are somatotopically organized with cervical fibers being more medial, while sacral most lateral (Fig. 127-1). The medial longitudinal fasciculus lies dorsally to the brachium conjuctivum. The central tegmental tract lies dorsally to the brachium conjuctivum and laterally to the medial longitudinal fasciculus, conveying fibers from the basal ganglia and the mesencephalic nuclei to the inferior olive. The lateral lemniscus lies dorsally and laterally to the spinothalamic tract, conveying auditory fibers. The dorsal longitudinal fasciculus (fasciculus of Schutz) is a periaqueductal ascending and descending fiber system arising from the hypothalamus and terminating to the autonomic nuclei of the pons and the medulla, conveying autonomic fibers.
The basal portion of the mesencephalon includes the cerebral peduncles and the substantia nigra, a pigmented nuclear mass, which lies between the dorsal surface of the cerebral peduncle and the tegmentum.1 In addition, several nuclear groups can be found in the mesencephalon at the level of the inferior colliculi. These include the mesencephalic nucleus of the trigeminal nerve, the nucleus of the trochlear nerve, the interpeduncular nucleus, the nucleus parabrachialis pigmentosus, the dorsal tegmental nucleus, the ventral tegmental nucleus, the pedunculopontine and the lateral dorsal tegmental nuclei, the dorsal raphe nucleus, the parabigeminal nucleus, the nucleus pigmentosus (locus ceruleus), and the substantia nigra. The red nucleus, the oculomotor nucleus including the Edinger-Westphal and the Perlia’s nuclei, the interstitial nucleus of Cajal, the rostral interstitial nucleus of the medial longitudinal fasciculus, the Darkschewitsch’s nucleus, and the nucleus of the posterior commissure can be found at the level of the superior colliculi (Fig. 127-2).
The central gray of the mesencephalon represents a complex anatomical structure. It surrounds the Sylvian aqueduct and contains several nuclei and a mixture of myelinated and unmyelinated fibers.1 It is well known that the central gray is implicated in pain conduction. Enkephalin, a neuropeptide associated with pain alleviation, has been identified in large quantities in the central gray. It has been postulated that stimulation of ventrolateral regions of the central gray produces enkephalins, which subsequently act on serotonergic neurons in the medulla oblongata, which in turn project on afferent axons (concerned with pain) in the dorsal horn of the spinal cord to produce analgesia. Contrariwise, stimulation of the lateral and rostral areas of the central gray facilitates pain conduction. Furthermore, the production of several other neuropeptides by different areas of the central gray, such as neurotensin, substance P, cholecystokinin, serotonin, somatostatin, and dynorphin has been demonstrated in numerous studies.1 The central gray has been also involved in vocalization process, modulation of medullary respiratory centers, vertical gaze, aggressive behavior, and control of reproductive behavior.
Historical Evolution
The first open mesencephalic tractotomy for managing medically intractable pain was performed by Dogliotti in 1938.2 Although he described his surgical procedure as a surgical section of the “lemniscus lateralis” at the level of the rostral pons, it should be considered certain that the level of the incision was at the lower mecencephalon (inferior colliculi).2,3 His surgical methodology consisted of surgical exposure of the lower part of the midbrain and introduction of an electric coagulator into the lateral sulcus.2 His initial series included four patients with medically refractory pain.2 He reported pain abolishment postoperatively in two of them, while there was significant pain reduction in one patient, and the other patient died immediately after surgery.2 In all his reported cases, there was some degree of hemihypoalgesia associated with paresthesia postoperatively.2
Bailey and his coworkers described their experience from performing an open mesencephalic tractotomy for managing medically intractable pain.3 They performed their tractotomy by making an incision, approximately 8 mm in length and 4 mm deep, extending from the lateral sulcus of the midbrain to the posterior edge of the inferior colliculus, on a plane between the superior cerebellar artery and the trochlear nerve.3 They reported excellent pain control postoperatively, while they observed some transient postoperative drowsiness and aphasia in one of their cases, most probably due to surgical manipulation of the dominant hemisphere of their patient.3
Similarly, Drake and McKenzie reported their experience from a series of six patients, undergoing mesencephalic tractotomy due to drug-resistant upper extremity or facial pain.4 In all their cases, the patient was in a sitting position and the incision was made through a small occipital bone flap, placed between the sagittal sinus medially and the transverse sinus inferiorly. The overlying occipital lobe was gently retracted superiorly and laterally, and the tentorium was incised along the straight sinus. The posterolateral aspect of the midbrain was exposed after opening the arachnoid overlying the cisterna ambiens. A tractotomy incision was placed by using a cordotomy knife. The incision usually extended from the lateral sulcus to the lower pole of the superior colliculus, and it was 5 mm deep. They reported that all their patients had no pain and temperature sensation in the contralateral half of the body, postoperatively. However, all their patients developed progressively quite bothersome dysesthesias after surgery, while one patient developed postoperatively severe burning, dysesthetic pain. Interestingly, touch, vibration, and position sensations remained unaffected after surgery in all their cases. They observed in five sixths6 of their cases temporary complete homonymous hemianopsia postoperatively, apparently due to the retraction of the occipital lobe and/or the sacrifice of the cortical occipital veins. The observed hemianopsia was spontaneously resolved with no further sequelae.4
Stereotactic mesencephalotomy for pain management was introduced by Spiegel and Wycis in 1947.5,6 In their first attempts, they combined this procedure with thalamotomy for treating patients with chronic, medically refractory pain of noncancerous origin, which showed no response to previous surgical procedures, such as retrogasserian rhizotomy, sympathectomy, and cordotomy.5,6 In their initial cases, they aimed not only at the spinothalamic and the quintothalamic tracts, lateral to the Sylvian aqueduct, but also the tegmentum adjacent areas, dorsally to the red nucleus.6 Pneumoencephalography was routinely employed for the surgical planning. Both patients in their initial report demonstrated satisfactory long-term pain relief.5,6
In a later study, they described their modified technique, in which the entry point was through a burr hole, placed on a paramedian plane (7.5 mm lateral to the midline) at the interaural level, and the trajectory was inclining 34 degrees posteriorly, passing through the posterior commissure, which was serving as a reference point.7 A lesion, approximately 5.5 to 9.5 mm in dorsoventral diameter, was placed 2 to 3 mm posterior and 0 to 3.5 mm inferior to the posterior commissure, destroying the spinothalamic and the reticulothalamic tracts at this level, after applying anodal direct electrical current for 60 seconds. Intraoperative electrical stimulation was usually employed in order to physiologically verify the anatomical target and thus minimize the possibility of any procedure-related complications. They reported immediate complete pain relief in 72.2% (39/54 patients) and partial relief in 31%, while 20.3% demonstrated no response.7 In the case of proximity of the inserted electrode to the deeply sited oculomotor fibers, ipsilateral ocular movements were observed, while tinnitus could occur due to stimulation of the adjacent lateral geniculate body in cases of laterally placed electrode. Their indications included atypical or postherpetic facial pain, pain secondary to tabes dorsalis, chronic pain secondary to spinal cord and spinal roots trauma, cancer-related pain, and thalamic and phantom-limb pain.7 They reported a 7.4% (4/54 patients) mortality rate, limited to patients with thalamic or cancerous pain. They also reported 14.8% permanent postoperative dysesthesia, while ocular disturbances occurred in 3.7%, and permanent motor deficits in 1.8%.7
Torvik reported Leksell’s experience with stereotactic mesencephalic tractotomy (STM).8 They performed their procedures under local anesthesia, while the Leksell stereotactic apparatus was used.8 A small burr hole was placed in the parieto-occipital region and an electrode was stereotactically inserted utilizing the Sylvain aqueduct and the posterior commissure as reference points.8 An electrical stimulation study was routinely performed for verifying the anatomic target, and subsequently a spherical lesion was placed via a bipolar coagulation (lesioning temperature 52 to 60 °C), extending from the level of posterior commissure to the rostral level of the trochlear nucleus in the rostro-caudal direction. A microscopic examination of the lesioned midbrain area in a postmortem autopsy study in their patients, showed a sharply circumscribed cavity filled with debris and macrophages surrounded by a thin and sparse gliotic rim, with no evidence of retrograde or anterograde corticospinal fiber degeneration. Interestingly, the ipsilateral medial lemniscus and the spinothalamic tract were completely destroyed; the adjacent reticulothalamic tract was massively damaged, while the superior colliculus and the red nucleus were partially destroyed. Torvic reported in his article that despite the extensive lesioning of the reticulothalamic and spinothalamic tracts at the midbrain, there was still postoperatively some residual pain sensation. He postulated that tracts other than the reticulothalamic and spinothalamic may be involved in pain propagation.8
Orthner and Roeder9 reported their surgical technique in stereotactic mesencephalic tractotomy in managing patients with trigeminal, postherpetic, phantom-limb, and Dejerine-Roussy syndrome pain. Their anatomic target was at the level of the posterior commissure, 7.5 mm lateral to the midsagittal plane.9 They routinely employed an electrode and a radiofrequency generator at 30 mA for 30 seconds for making a lesion. They emphasized the importance of partially destroying the spinoreticular along with the spinothalamic tract, in order to maximize the pain relief effect.9
Mazars et al.10 reported in 1976 their experience with a different and more accurate approach (through a transcortical posterior parietal lobe trajectory) for mesencephalic stereotactic tractotomy (MST). The proposal of a more precise anatomic target in their clinical series had as a consequence equally good pain relief rates with significantly reduced morbidity.10 The occurrence of postoperative dysesthesia and anesthesia dolorosa was significantly diminished; on the other hand, the occurrence of oculomotor palsies was increased due to the passage of the lesioning electrode through the quadrigeminal plate.10
At approximately the same time Amano et al.11 reported their technique and their results from treating medically intractable pain of cancerous origin with MST. They performed their procedures under local anesthesia by utilizing either Sano’s or Todd-Wells’ stereotactic head frame.11 The surgical planning was based on pneumoencephalography and the Schaltenbrand-Bailey stereotactic atlas.11 The dorsal portion of mesencephalic tegmentum near the central gray at the level of the rostral end of the superior colliculus was used as anatomic target. The stereotactic coordinates for their selected target was P 14, H –5 to –8, and L 5 to 8, while the laterality of the target was measured from the midline of the Sylvain aqueduct.11 They emphasized that the usage of frontal entry point could minimize the incidence of Parinaud’s syndrome postoperatively. A radiofrequency lesion was made after electrophysiologic confirmation of the target. They reported significant information regarding the electrophysiologic profile of the mesencephalic reticular formation by employing detailed microelectrode recordings.11
Nashold and his coworkers employed a slightly different surgical technique for performing stereotactic mesencephalic tractotomy.12–15 The surgical planning was based on a contrast ventriculography for visualizing the Sylvian aqueduct and the anterior (AC) and posterior commissures (PC). The procedure was routinely performed under local anesthesia. A burr hole was placed 1 cm anterior to the coronal suture and 1.5 cm from the midsagittal plane. Nashold et al. had developed a mesencephalon stereotactic atlas that could be used for this anterior approach.12–15 After accurate recognition of the rostral end of the Sylvian aqueduct and the AC-PC line, an electrode was inserted in a trajectory crossing the AC-PC line at a 65- to 70-degree angle. The lateromedial angulation of the electrode was 2 to 4 degrees off the midsagittal plane. Electrical stimulation could be performed for verifying the anatomic target and identify the fine somatotopic organization of the spinothalamic and reticulothalamic tracts. A radiofrequency generator was used for 30 seconds for placing a spherical lesion. Nashold initially in his series utilized a target located at the level of the PC, 3 mm posterior to that (“superior colliculus target”), while later he utilized a target located 5 mm posterior, inferior, and lateral to the PC (“inferior colliculus target”).12–15 They greatly emphasized in their reports the importance of intraoperative stimulation for verifying the anatomic target and thus maximizing the physiologic effect.12–15
Indications for Stereotactic Mesencephalic Tractotomy
The recent advances in neuropharmacology, the development of multidisciplinary pain management strategies, and the emerging of novel neuromodulative surgical techniques have limited the role of SMT, making it a valid treatment option only for patients with medically refractory pain due to extensive carcinoma involving the head, neck, and/or arm, with likely survival time on the order of 6 (±3) months. Several previous reports confirm pain relief and also pain-associated anxiety after performing SMT and lesioning the spinoreticular along with the spinothalamic tracts in cases of extensive head and neck cancer.9,12 The performance of SMT carries the advantage of reducing the patient’s emotional reaction to pain. It has been reported that the postoperative calming effect of the patient’s fear and anxiety after a SMT closely resembles that of bilateral cingulotomy.12
In cases of unilateral facial, neck, and/or arm medically intractable pain of cancerous origin, a SMT on the opposite side should be performed. In cases of bilateral pain, the side of the greatest involvement should be managed. Whisler and Voris reported no serious postoperative side effects from placing bilateral SMT lesions in patients with bilateral pain.12,13
Patients suffering from phantom-limb pain following avulsion of the brachial plexus, postcordotomy dysesthesia, or central pain secondary to thalamic syndrome, who failed medical or other surgical treatment, may be candidates for SMT. Although the analgesic effect of SMT wears off in the vast majority of the reported cases there are reports of long-lasting pain relief.9
Surgical Technique
Preoperative Preparation
The surgical procedure is carefully explained to the patient. The importance of the patient’s cooperation cannot be overemphasized. The patient remains fasted for a minimum of 8 hours before the procedure. The required dose of analgesics is administered parenterally. Antibiotics are begun intravenously on the morning of surgery and are continuing for 24 hours after surgery. Midazolam is given intravenously for preoperative sedation. The procedure is routinely performed with neuroleptanalgesia using intravenous alfentanil and propofol. The area of the burr hole is clipped and saved. The skin is thoroughly prepped and appropriately draped in a standard sterile fashion. A field block of the scalp is performed, and then a mixture of 20 ml of 0.25% bupivacaine hydrochloride with epinephrine, 20 ml of 0.5% lidocaine with epinephrine, and 4 ml of sodium bicarbonate 8.4% is used.17 The bicarbonate neutralizes acidity and considerably reduces the discomfort of the block.
Operative Procedure
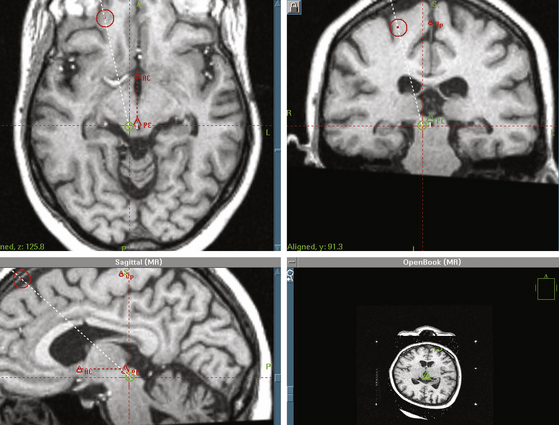
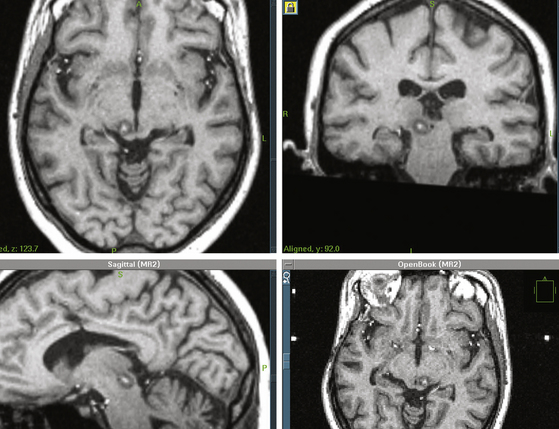
The MRI/CT to compatible Leksell base ring and localizer (Elektra, Stockholm, Sweden) are fixed to the patient’s skull.17 Carbon fiber posts and MRI/CT-compatible pins are used. The MRI scan consists of a contrast enhanced T-1–weighted volume acquisition and MPRAGE pulse sequences, using axial 1.3-mm slices with zero slice gaps. This is followed by a whole head CT scan, using 3-mm slices with zero slice gaps. The two data sets are imported over the local network to the computer workstation. After fusing the MRI data to the CT data, targets, and trajectories are defined. If an automatic fusion software program is not available, a visual anatomic point-to-point matching between CT and MRI studies may be performed for ruling out any magnetic field-generated distortion. A probe-view algorithm is used to maximize the distance between any surface cortical veins and electrode at the cortical entry points.
After defining the anterior commissure (AC), the posterior commissure (PC), and the AC-PC line, the mesencephalic target is set 5 mm inferior to the AC-PC line, and 5 to 8 mm lateral to the midsagittal plane, at the level of the superior colliculus17 (Fig. 127-3). The entry point and a transcortical trajectory are selected, and a frontal stab incision is appropriately made. A 7/64-inch twist–drill hole is usually placed, approximately 1 cm anterior to the coronal suture and 1.5 cm lateral to the midsagittal plane. The underlying dura and pia are carefully cauterized with monopolar cautery. A 1.1-mm diameter bipolar straight stimulation-lesioning thermo-coupled electrode (F.L. Fischer, Freiburg, Germany) (Fig. 127-4) is inserted and proper positioning is confirmed with intraoperative fluoroscopy. Neuroleptanalgesia must be reversed as much as possible, at this point, in order to maximize patient cooperation. Stimulation studies may be performed at this point for physiologic verification of the anatomic target (Fig. 127-1).
The topographic distribution of thermal or pain response and/or the observation of any side effects may require repositioning of the electrode and redefining of the target. Tinnitus would indicate that the inserted electrode is too laterally, within or adjacent to the inferior quadrigeminal brachium. Limb paresthesias would indicate too anterior placement in the medial lemniscus. Ipsilateral ocular movement would also indicate that the inserted electrode is too medial and inferior. It has been demonstrated that physiological responses are best produced by bipolar electrical stimulation with a square wave generator, using various pulse widths (1 or 0.5 millisecond), and pulse frequencies (5, 15, 30, 60, 120, 300 Hz).12 It is apparent that electrophysiologic stimulation requires an awake, cooperative patient with minimal pain medication. Initially Spiegel and Wycis6 and Nashold13 later showed that a definite topographical organization of the observed paresthesia existed, and they also noted painful paresthesias and ocular and auditory phenomena occurring during mesencephalic stimulation.
It has to be emphasized that the response of the patient to intraoperative electrical stimulation is related not only to the anatomic location of the tip of the inserted electrode but also to the electrical parameters of the employed stimulation. A detailed description of the observed responses after intraoperative mesencephalic electric stimulation may be found in the work of previous investigators.11,12 A side-searching, extruding electrode may also be used for more extensive electrophysiologic mapping.17 This is a monopolar electrode, the outer diameter of which is 2 mm, while the extruding noninsulated tip has a 0.5-mm diameter.17 The lateral-extruding searching tip of the electrode can extrude up to a maximum of 8.5 mm, under a 30-degree angle. A millimetric Vernier scale at the proximal end of the electrode controls the lateral extrusion of the side-searching electrode (Fig. 127-4). We have been using the searching electrode for electrophysiologic mapping and target localization, in order to minimize the passages of straight electrodes for stimulation, with subsequent minimization of the passage-associated trauma to the brain parenchyma. It has to be emphasized, that the avoidance of even the slightest rotary movement of the side-searching electrode when its tip is extruding, is of paramount importance for avoiding any shear injuries. The usual stimulation parameters include monopolar stimulation, frequency of 60 Hz, duration of 0.5 milliseconds, and amplitude range between 0.1 and 0.4 V. A milimetric Vernier scale is used and the lateral extrusion of the side-searching electrode is plotted.
At the completion of the procedure, the previously inserted electrode is carefully withdrawn. The surgical wound is thoroughly irrigated with antibiotic irrigation, and is closed with a single 3/0 nylon suture. A sterile dressing is applied and the patient remains in the postanesthesia care unit for approximately an hour, and then is transferred to the ward for further observation and treatment. A postoperative MRI study is usually obtained for verifying the accurate location of the lesion (Fig. 127-5).
Results and Complications
Satisfactory pain relief has been reported in the vast majority of cases with various recurrence rates.2–17 Shieff and Nashold reported in their series of 27 patients suffering medically intractable thalamic pain 66.7% long-term relief.14,15 They noted that 77.8% of their patients were pain free at their discharge from the hospital; however, pain progressively recurred in 11.1% of them.14,15 Amano et al.11 reported excellent pain control in both their cases for 12 months, with no ocular or other complications. Similarly, Frank et al.18 reported their experience from treating a large series of 202 patients with pharmacoresistant pain of cancerous origin. The vast majority of their cases suffered face or upper body pain.18 They observed pain recurrence during the first postoperative month in 15% of their patients, while late (4–14 postoperative months) pain recurrence occurred in 4.2%.18 Their immediate transient postoperative complications included gaze palsy (9%), dysesthesia (5%), and anesthesia dolorosa (1%), while their permanent complications were limited to dysesthesias (6%) and gaze palsy (3%).18
The potential SMT-associated complications have to be thoroughly discussed preoperatively with the patient. Various complications such as postoperative altered mental status, dysphasia, dysarthria, paresis, or paralysis of the contralateral lower extremity, dysesthesias and/or painful dysesthesias of the affected area, paralysis of the vertical gaze, ocular convergence defects, divergent paralysis, skew deviation, and miotic pupils have been reported in the literature.12 The occurrence of contralateral leg weakness significantly diminished with the evolution of the stereotactic SMT, while the incidence of postoperative dysesthesia seemed to be diminishing with the employment of smaller size mesencephalic lesions. The postoperative ocular motility changes may be transient, resolving within a short period of time, or permanent. Most of the observed ocular complications are temporary and result in no significant disability. The vertical gaze paralysis is usually permanent, however does not cause any significant disability.
Anterolateral Cordotomy
Anatomic Background
It is well known that pain and temperature as well as tactile information are conveyed by the ascending lateral spinothalamic tract (LST), which is located in the anterolateral part of the spinal cord.1 It has been demonstrated that the LST is organized in such a way that superficial pain is conveyed in the outer part of the tract, temperature more inward, and deep pain even more centrally.1 In addition, LST is characterized by somatotopic organization with fibers from the upper extremities and upper body located anteromedially, while fibers from the lower extremities and the lower body posterolaterally.1 The sensory segmentation and the somatotopic organization of the LST allow its selective destruction and thus provide a surgical treatment option for patients suffering localized, medically intractable pain. Sectioning of the LST produces loss of pain and thermal sense on the opposite side of the body, beginning approximately one dermatome level below the section level.1
The reticulospinal tract is located anteromedially to the LST and its accidental lesioning, due to its close relationship, may be responsible for the observed respiratory complications in cases of bilateral cordotomy.1 Likewise, the anterior spinocerebellar tract is located laterally to the LST and its accidental injury may cause ataxia after a cordotomy. The rest of the ascending and descending spinal tracts are usually not in close proximity with the LST. However, it has to be emphasized that there is much variation in the size and the location of the spinal tracts (particularly of the anterior corticospinal tract), and there have been much fewer anatomic stereotactic atlas measurements of the spinal cord than that of the brain.
Historical Evolution
Interruption of the LST in the anterolateral spinal cord is known as cordotomy. The introduction of this term has to be credited to Schuller, while the first open cordotomy was performed by Martin.19–21 There are reports of open anterior cordotomies performed by Collis in 1963 and Cloward in 1964.22,23 At approximately the same time, Mullan et al. performed the first radioisotope percutaneous cervical cordotomy, which was the first stereotactic spinal procedure.24,25 In their technique, a strontium needle was introduced posterolaterally under fluoroscopic guidance to the subarachnoid space of the anterior lateral quadrant of the cervical spinal cord, between the posterior arch of C1 and the lamina of C2.24 The radioisotope was left in place and a lesion was gradually developed affecting the underlying LST and producing analgesia and pain relief.24 This first stereotactic cordotomy carried several disadvantages: the lesioning could not be controlled, the effect was gradual, and there was radiation exposure for both the patient and the performing surgeon.24
All these limitations led to the development of a radiofrequency-lesioning percutaneous cordotomy by Rosomoff et al. in 1965.26 They performed their procedures under local anesthesia with the patient awake. They introduced a 3-inch lumbar puncture stylet into the anterolateral quadrant of the cervical spinal cord under fluoroscopic guidance. Then, a radiofrequency electrode was inserted through the stylet and a lesion was placed in a fractionated manner, guided by the patient’s physiologic response.26
The occurrence of respiratory impairment as a complication in cases of percutaneous cervical cordotomies due to accidental lesioning of the descending respiratory fibers of the adjacent reticulospinal tract27,28 led to the development of a lower cervical cordotomy technique by Lin et al. in 1966.29 In their technique, the 3-inch stylet was introduced diagonally through the disc at a lower cervical level for avoiding the upper cervical pulmonary fibers, and thus minimizing the chance of postoperative respiratory impairment.29 The procedure was performed with the patient awake, in a supine position and under dual fluoroscopic guidance (anteroposterior and lateral views).29 They used the lowermost cervical disc that could be seen on the lateral fluoroscopic view, and the stylet was introduced so that its tip lies in the intervertebral disc near the midline.29
Hitchcock reported his stereotactic technique for performing percutaneous cordotomies in 1972.30 He used his own stereotactic frame with the patient awake in a sitting position. AP and lateral x-rays were routinely obtained for verifying the midline, and a needle was inserted into the subarachnoid space through the C1–C2 interspace. Then, a small amount of emulsified Pantopaque was injected through the needle for identifying the dentate ligament and the obex under fluoroscopy. A sharpened 0.015-inch, insulated stainless-steel electrode (with a noninsulated 2- mm tip) was introduced through the needle into the spinal cord. Electrical stimulation studies were performed for physiologically identifying the LST (tingling sensation). After appropriately identifying the LST, a radiofrequency lesion was placed.30
Todd and his coworkers employed a similar stereotactic technique for performing percutaneous cervical cordotomies.31,32 The only modifications were that the patient was in a prone position, and the stereotactic apparatus was the Todd-Wells frame.31,32 They routinely employed electrophysiologic stimulation studies for verifying their target and making radiofrequency lesions.31,32
The wide application of CT imaging allowed better visualization of the spinal cord and led to the development of CT-guided percutaneous stereotactic cordotomy (PSC) by Kanpolat and his team in 1986.33,34 They approached their anatomic target, the LST, through a C1–2 approach.33,34 With their technique the obtaining of coronal and sagittal spinal cord diameter measurements was possible, while high-resolution cord imaging could be accomplished, giving a new dimension to cordotomy practice and making stereotactic cordotomy a valid treatment option for medically intractable pain management until now.
Indications for Stereotactic Percutaneous Cordotomy
Despite the recent advances in neurostimulation procedures and implantable neuropharmacologic delivery systems, stereotactic percutaneous cordotomy (SPC) still remains a considerable management option for patients suffering terminal cancer, pharmacoresistant pain.35 Ideal candidates for SPC are patients with unilateral localized intractable cancer pain or severe localized pain of noncancerous origin.34–37 Patients with medically intractable pain due to chest wall mesothelioma, Pancoast tumors, pulmonary malignancies, gastrointestinal malignancies, or terminal lower extremity carcinoma may be relieved by SPC.35 Even patients with bilateral lower extremity pain of cancerous origin may be offered the option of SPC for pain relief.35 Contrariwise, the performance of SPC in patients with bilateral upper body pain is not advocated, mainly due to the associated high complication risk.35
The presence of severe pulmonary dysfunction, preoperative oxygen saturation of 80% or less, life expectancy less than 3 months, advanced patient’s age, and poor general medical condition are considered as contraindications for performing SPC.35 Additionally, a patient’s neuropsychological condition (especially in patients receiving long-term opiate alkaloid analgesics) or the existence of another dysfunctional patient–family relationship may be relative contraindications.35 Furthermore, SPC requires an awake, emotionally stable patient that can cooperate during the procedure.35
Controversial remains the issue of performing SPC before or after initiating morphine therapy for pain management.35 There is a general consensus that SPC should be considered after morphine treatment failure.38 However, Kanpolat et al. consider that SPC may be offered in good candidates even before initiating any morphine treatment.35 We prefer to consider a neuroablative procedure such as SPC only in those cases that any acceptable medical treatment has been adequately employed and has failed.
Surgical Technique
The surgical technique that we recommend is the one described and widely employed by Kanpolat and his associates.33,37,39–43
Preoperative Preparation
The patient remains fasted for a minimum of 8 hours before the procedure. The required dose of analgesics is administered parenterally. Antibiotics are begun intravenously on the morning of surgery and are continuing for 24 hours after surgery. Midazolam is given intravenously for preoperative sedation. The procedure is routinely performed with neuroleptanalgesia using intravenous alfentanil and propofol. The surgical procedure is carefully explained to the patient. The importance of the patient’s cooperation cannot be overemphasized. The patient is positioned prone on a rotating table and the lower lumbar area is prepped and draped in a standard sterile fashion. Iohexol is administered to the patient by a lumbar puncture and the table is repositioned to Trendelenburg position in order to visualize the cervical spinal cord. If a lumbar puncture cannot be performed contrast material may be injected through a C1–C2 puncture.
Operative Procedure
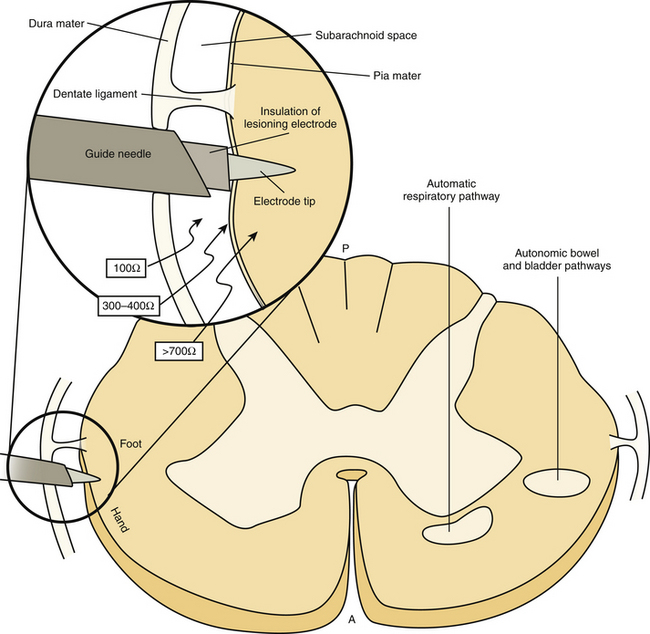
After the contrast administration, the patient is transported to the computed tomography (CT) unit, where the procedure is performed under direct CT guidance. After positioning the patient on the CT table in a supine position, the head is positioned on the headrest and is flexed and fixed with a Velcro band. An upper cervical spine CT scan is obtained, covering with 1-mm thick slices with no gap, the C1–C2 area. The sagittal and transverse diameters of the cervical cord are measured as well as the distance between the skin and the dura. Based on the obtained measurements, a cordotomy needle is inserted after infiltrating the skin with local anesthetic and is propagated until puncturing the dura. The dura may be very thick and fibrotic, especially in cancer patients with previous radiotherapy.35 A new CT study is obtained for localizing the tip of the inserted needle. The ideal position for the tip of the inserted needle is 1 mm anterior to the dentate ligament when the lumbosacral fibers of the LST are the target, or 2 to 3 mm anterior to the dentate ligament when the thoracic or cervical fibers are the target.35 After verifying the proper position of the inserted needle with a new CT scan, a straight electrode is inserted through the needle.
Electrical impedance studies may be performed for verifying the exact position of the inserted electrode. The impedance measurements are approximately 100 Ω when the tip is in the cerebrospinal fluid (CSF), 300 to 400 Ω when the tip is against the cord surface, and more than 700 Ω when it is inside the cord.35 Stimulation electrophysiologic studies may also be performed for verifying the position of the electrode, although the obtained CT provides accurate localization of the inserted electrode (Fig. 127-6). The process of stimulation is begun. When little or no motor response is obtained at low frequency and stimulation at 50 to 100 Hz produces a warm or cool thermal sensation, or less likely, pain or paresthesias on the contralateral body side, this indicates proper electrode position. The observations of sensory responses, which involve the entire contralateral body below the neck level or the hand, suggest that satisfactory analgesia below C5 dermatome will be obtained.
After reaching the target point and verifying the ideal position, a radiofrequency lesion is placed by applying 60 °C for 30 seconds. The patient is monitored during the whole process and motor function, pain perception, and temperature sensation are meticulously examined. The final lesion is placed by applying 80 °C for 60 seconds, and the physiologic result is tested again. If the obtained level of analgesia is not satisfactory a second or rarely a third lesion may be necessary.35
Results and Complications
Sanders and Zuurmond reported their results from a series of 80 patients with medically intractable pain of malignant etiology, for which they underwent unilateral (62 patients) and bilateral (18 patients) fluoroscopically guided PSC.44 They reported initial complete pain relief in 87.1%, partial pain response in 9.7%, and no response in 3.2% of their unilateral cases. The respective percentages for the bilateral PSC group were 50%, 33.3%, and 16.7%. The observed permanent complications in the unilateral group were hemiparesis occurring in 8.1%, urinary retention in 6.5%, and new postoperative mirror-image pain in 6.5%. The observed permanent complications in the bilateral group were hemiparesis occurring in 11.1%, urinary retention in 11.1%, and new postoperative mirror-image pain in 5.6%. They concluded that unilateral PSC was a safe and efficacious surgical procedure for patients with intractable malignant pain, while bilateral PSC had a high complication rate in their series.44
Crul et al. presented their results after performing unilateral fluoroscopically-guided PSC in 43 terminally ill cancer patients.45 They reported 95% initial good results, while good results remained until death in 69% of these patients. A quantitative measurement of the pain relief was performed by employing the Numeric Rating Scale (NRS), showing that there was an initial postoperative mean decrease from 7.2 to 1.1.45 At the end of life in their patients, the mean NRS score was 2.9. They observed transient postoperative new mirror-image pain in 16.3%, transient muscle weakness in 4.6%, transient short-lasting apnea in 2.3%, transient bladder dysfunction in 2.3%, and permanent ipsilateral leg paresis in 2.3%.45
Kanpolat reported his 20-year experience in performing CT-guided PSC in a series of 207 patients (232 procedures).35,46 The vast majority of these patients (93.2%) suffered medically intractable pain of cancerous etiology, while the remaining 6.7% had chronic, pharmacoresistant pain of benign etiology. The reported initial success rate was as high as 95%, while this rate was even higher among patients with cancerous pain. There was a subgroup of 12 patients in this series, who underwent bilateral PSC with great pain relief rates. In the cases of bilateral PSC, the procedures were performed at different settings, 1 week apart from each other, for minimizing the chance of postoperative respiratory impairment (night sleep apnea), which constitutes the most serious complication. It has to be emphasized that all the reported bilateral cases suffered lower body pain. There was no mortality in this series, while there was a 2.4% incidence of temporary motor deficits, and 2.4% occurrence of temporary ataxia.35,46 These symptoms were completely resolved within 3 weeks postoperatively with no further consequences. Permanent dysesthesia was postoperatively developed in 1.9% of the reported cases. In the group of bilateral PSC, there was no respiratory impairment, while there was postoperative temporary hypotension in 1.4% and temporary urinary retention in 0.9%.35,46
Kanpolat et al. reported another series of five patients suffering intractable bilateral lower trunk and extremity pain for which they underwent bilateral CT-guided PSC.47 The success rate was 100%, while the only observed complications were transient Horner’s syndrome in one of five patients, and transient urinary retention in one of five patients.47
Raslan recently reported his results from a series of 41 terminal cancer patients undergoing unilateral CT-guided PSC.48 The initial postoperative pain relief rate was 98%, and at 6 months 80%. Furthermore, the mean preoperative Karnofsky Performance Scale score was 55.5±6.7, while the mean postoperative score was 76.9±7.6. Similarly, the mean preoperative Visual Analog Scale score was 8.5±0.8, while the immediate postoperative was 1.2±1.0 increasing to 1.7±1.2 at 1 month after the procedure, 1.8±1.1 at 3 months, and 2.3±0.6 at 6 months. The preoperative mean sleeping time in this series was 3.2 during a 24-hour period, while the immediate postoperative mean increased to 7 hours, and progressively decreased to 4.8 hours, 6 months after the procedure. Raslan concluded that CT-guided PSC remains a valuable and safe surgical option for managing patients with cancer pharmacoresistant pain.48
Future of Neuroablative Stereotactic Pain Procedures
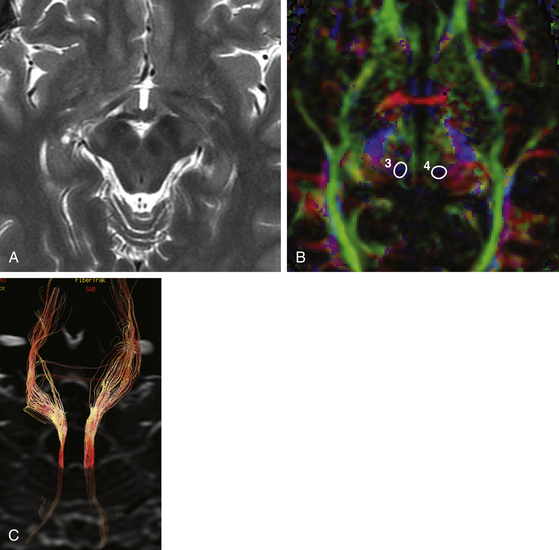
The introduction of high-magnetic-field MRI units in the daily neurosurgical practice (3 or 7 Tesla) allow better visualization and delineation of the mesencephalic and high cervical cord anatomy, and identification of the ascending and descending neuronal pathways, as well as more accurate, submillimetric definition of their anatomic relationships. Moreover, the emergence of newer MR imaging techniques such as diffusion tensor imaging may provide better visualization of the lateral spinothalamic, the corticospinal, and the reticulospinal tracts.49 These recent imaging advances along with the designing and manufacturing of more precise frame-based or frame-less stereotactic devices may further increase the efficacy and the safety of SMT and PSC.
Afifi A.K., Bergman R.A. Functional Neuroanatomy, 2nd ed., New York: Lange Medical Books/McGraw-Hill, 2005.
Amano K., Kitamura K., Sano K., Sekino H. Relief of intractable pain for neurosurgical point of view with reference to present limits and clinical indications. A review of 100 cases. Neurol Med Chir. 1976;16:141-153.
Bailey R.A., Glees P., Oppenheimer D.R. Midbrain tractotomy: a surgical and clinical report, with observations an ascending and descending tract degeneration. Eur Neurol. 1954;127:316-335.
Crul B.J.P., Blok L.M., Egmond J.V., et al. The present role of percutaneous cervical cordotomy for the treatment of cancer pain. J Headache Pain. 2005;6:24-29.
Dogliotti M. First surgical sections, in man, of the lemniscus lateralis (pain-temperature path) at the brain stem, for the treatment of diffuse rebellious pain. Curr Res Anesth. 1938;17:143-145.
Drake C.G., McKenzie K.G. Mesencephalic tractotomy for pain. Experience with six cases. J Neurosurg. 1953;10:457-462.
Fountas K.N., Lane F.J., Jenkins P.D., Smith J.R. MR-based stereotactic mesencephalic tractotomy. Stereotact Funct Neurosurg. 2004;82:230-234.
Frank F., Fabrizi A.P., Gaist G. Tractotomy in the treatment of chronic cancer pain. Acta Neurochir. 1989;99:38-40.
Gildenberg P.L. Spinal stereotaxic procedures. In: Schaltenbrand G., Walker A.E. Stereotaxy of the Human Brain. 2nd ed. Stuttgart: Verlag; 1982:469-474.
Kanpolat Y., Akyar S., Caglar S. Diametral measurements of the upper spinal cord for Stereotactic pain procedures; experimental and clinical study. Surg Neurol. 1995;43:478-483.
Kanpolat Y., Caglar Ugur H., Ayten M., et al. Computed tomography-guided percutaneous cordotomy for intractable pain in malignancy. Neurosurgery. 2009;64:187-194.
Kanpolat Y., Deda H., Akyar S., et al. CT-guided percutaneous cordotomy. Acta Neurochir Suppl (Wien). 1989;46:67-68.
Kanpolat Y., Savas A., Caglar S., et al. Computerized tomography-guided percutaneous bilateral selective cordotomy. Neurosurg Focus. 1997;15(2):e4.
Kanpolat Y. Percutaneous destructive pain procedures on the upper spinal cord and brain stem in cancer pain: CT-guided techniques, indications and results. Adv Tech Stand Neurosurg. 2007;32:147-173.
Kanpolat Y. The surgical treatment of chronic pain: destructive therapies in the spinal cord. Neurosurg Clin North Am. 2004;15:307-317.
Lin P.M., Gildenberg P.L., Polakoff P.P. An anterior approach to percutaneous lower cervical cordotomy. J Neurosurg. 1966;25:553-560.
Nashold B.S. Brainstem stereotaxic procedures. In: Schaltenbrand G., Walker A.E. Stereotaxy of the Human Brain. 2nd ed. Stuttgart: Verlag; 1982:475-483.
Orthner H., Roeder F. Further clinical and anatomical experiences with stereotactic operations for relief of pain. Confin Neurol. 1966;27:418-430.
Raslan A. Percutaneous computed tomography-guided radiofrequency ablation of upper spinal cord pain pathways for cancer-related pain. Neurosurgery. 2008;62:226-234.
Sanders M., Zuurmond W. Safety of unilateral and bilateral percutaneous cervical cordotomy in 80 terminally III cancer patients. J Clin Oncol. 1995;13:1509-1512.
Shieff C., Nashold B.S. Stereotactic mesencephalic tractotomy for thalamic pain. Neurol Res. 1987;9:101-104.
Shieff C., Nashold B.S. Thalamic pain and stereotactic mesencephalotomy. Acta Neurochir. Suppl. 1988;42:239-242.
Spiegel E.A., Wycis H.T. Mesencephalothalamotomy for relief of pain: in Anniversary volume of O. Petzl. Vienna: Wagner; 1948. 437–443
Torvik A. Sensory, motor, and reflex changes in two cases of intractable pain after stereotactic mesencephalic tractotomy. J. Neurol Neurosurg Psychiatry. 1959;22:299-305.
Wycis H.T., Spiegel E.A. Long-range results in the treatment of intractable pain by stereotaxic midbrain surgery. J Neurosurg. 1962;9:101-107.
1. Afifi A.K., Bergman R.A. Functional Neuroanatomy, 2nd ed, New York: Lange Medical Books/McGraw-Hill, 2005.
2. Dogliotti M. First surgical sections, in man, of the lemniscus lateralis (pain-temperature path) at the brain stem, for the treatment of diffuse rebellious pain. Curr Res Anesth. 1938;17:143-145.
3. Bailey R.A., Glees P., Oppenheimer D.R. Midbrain tractotomy: a surgical and clinical report, with observations an ascending and descending tract degeneration. Eur Neurol. 1954;127:316-335.
4. Drake C.G., McKenzie K.G. Mesencephalic tractotomy for pain. Experience with six cases. J Neurosurg. 1953;10:457-462.
5. Spiegel E.A., Wycis H.T. Pain: mesencephalotomy and mecencephalo-thalamotomy. In: Spiegel E.A., Wycis H.T. Stereoencephalotomy. New York: Grune and Stratton; 1962:205-244. vol. 2
6. Wycis H.T., Spiegel E.A. Long-range results in the treatment of intractable pain by stereotaxic midbrain surgery. J Neurosurg. 1962;9:101-107.
7. Spiegel E.A., Wycis H.T. Mesencephalothalamotomy for relief of pain. In: Anniversary volume of O. Petzl. Vienna: Wagner; 1948:437-443.
8. Torvik A. Sensory, motor, and reflex changes in two cases of intractable pain after stereotactic mesencephalic tractotomy. J Neurol Neurosurg Psychiatry. 1959;22:299-305.
9. Orthner H., Roeder F. Further clinical and anatomical experiences with stereotactic operations for relief of pain. Confin Neurol. 1966;27:418-430.
10. Mazars G., Merienne L., Cioloca C. Etat actuel de la chirurgie de la douler. Neurochirurgie. 1976;22:53-61.
11. Amano K., Kitamura K., Sano K., Sekino H. Relief of intractable pain for neurosurgical point of view with reference to present limits and clinical indications. A review of 100 cases. Neurol Med Chir. 1976;16:141-153.
12. Nashold B.S. Brainstem stereotaxic procedures. In: Schaltenbrand G., Walker A.E. Stereotaxy of the Human Brain. 2nd ed. Stuttgart: Verlag; 1982:475-483.
13. Nashold B.S., Wilson W.P., Slaughter D.G. Sensations evoked by stimulation in the midbrain of man. J Neurosurg. 1969;3:14-24.
14. Shieff C., Nashold B.S. Stereotactic mesencephalic tractotomy for thalamic pain. Neurol Res. 1987;9:101-104.
15. Shieff C., Nashold B.S. Thalamic pain and stereotactic mesencephalotomy. Acta Neurochir Suppl. 1988;42:239-242.
16. Whisler W.W., Voris H.C. Mesencephalotomy for intractable pain due to malignant disease. Appl Neurophysiol. 1978;41:52-56.
17. Fountas K.N., Lane F.J., Jenkins P.D., Smith J.R. MR-based stereotactic mesencephalic tractotomy. Stereotact Funct Neurosurg. 2004;82:230-234.
18. Frank F., Fabrizi A.P., Gaist G. Tractotomy in the treatment of chronic cancer pain. Acta Neurochir (Wien). 1989;99:38-40.
19. Kanpolat Y. The surgical treatment of chronic pain: destructive therapies in the spinal cord. Neurosurg Clin North Am. 2004;15:307-317.
20. Schuller A. Uber operative Durchtrennung der ruckenmarksstrange (Chordotomie). Wien Med Wschr. 1910;60:2291.
21. Spiller W.G., Martin E. The treatment of persistent pain of organic origin in the lower part of the body by division of the antero-lateral column of the spinal cord. JAMA. 1912;58:1489-1490.
22. Collis J.S.Jr. Anterolateral cordotomy by an anterior approach: report of a case. J Neurosurg. 1963;20:445-446.
23. Cloward R.B. Cervical cordotomy by an anterior approach: report of a case. J Neurosurg. 1925;21:1964.
24. Mullan S. Percutaneous cordotomy. J Neurosurg. 1971;35:360.
25. Gildenberg P.L. Spinal stereotaxic procedures. In: Schaltenbrand G., Walker A.E. Stereotaxy of the Human Brain. 2nd ed. Stuttgart: Verlag; 1982:469-474.
26. Rosomoff H.L., Carroll F., Brown J., et al. Percutaneous radiofrequency cervical cordotomy technique. J Neurosurg. 1965;23:639-644.
27. Fox J.L. Localization of the respiratory motor pathway in the upper cervical spinal cord following percutaneous cordotomy. Neurology. 1969;19:1115-1118.
28. Rosomoff H.L., Kringer A.J., Kupman A.S. Effects of percutaneous cervical cordotomy on pulmonary function. J Neurosurg. 1969;31:620-627.
29. Lin P.M., Gildenberg P.L., Polakoff P.P. An anterior approach to percutaneous lower cervical cordotomy. J Neurosurg. 1966;25:553-560.
30. Hitchcock E.R. Stereotaxis of the spinal cord. Confin Neurol. 1972;34:299-310.
31. Crue B.L., Todd E.M., Carregal E.J.A. Posterior approach for high cervical percutaneous radiofrequency cordotomy. Confin Neurol. 1968;30:41-52.
32. Todd E.M., Crue B.L., Carregal E.J.A. Posterior percutaneous tractotomy and cordotomy. Confin Neurol. 1969;31:106-115.
33. Kanpolat Y., Atalag M., Deda H., et al. CT-guided extralemniscal myelotomy. Acta Neurochir (Wien). 1988;91:151-152.
34. Kanpolat Y., Deda H., Akyar S., et al. CT-guided percutaneous cordotomy. Acta Neurochir Suppl (Wien). 1989;46:67-68.
35. Kanpolat Y. Percutaneous destructive pain procedures on the upper spinal cord and brain stem in cancer pain: CT-guided techniques, indications and results. Adv Tech Stand Neurosurg. 2007;32:147-173.
36. Kanpolat Y., Caglar S., Akyar S., et al. CT-guided pain procedures for intractable pain in malignancy. Acta Neurochir Suppl. 1995;64:88-91.
37. Kanpolat Y. Cordotomy for pain. In: Schulder M., editor. Handbook of Stereotactic and Functional Neurosurgery. New York: Markel & Dekker; 2003:459-472.
38. Gybels L.M. Indications for the use of neurosurgical techniques in pain control. In: Bond M.R., Charlton L.E., Wolf J. Proceedings of the Sixth World Congress on Pain. Amsterdam: Elsevier; 1995:475.
39. Kanpolat Y., Deda H., Akyar S., et al. CT-guided trigeminal tractotomy. Acta Neurochir (Wien). 1989;100:112-114.
40. Kanpolat Y. Percutaneous cordotomy, tractotomy and midline myelotomy, minimally invasive stereotactic pain procedures. Semin Neurosurg. 2004;2/3:203-220.
41. Kanpolat Y. Percutaneous stereotactic pain procedures: percutaneous cordotomy, extralemniscal myelotomy, trigeminal tractotomy-nucleotomy. In: Burchiel K., editor. Surgical Management of Pain. Stuttgart: Thieme; 2002:745-762.
42. Kanpolat Y. Percutaneous cervical cordotomy for persistent pain. In: Gildenberg P.L., Tasker R.R. Textbook of Stereotactic and Functional Neurosurgery. New York: McGraw-Hill; 1998:1485-1490.
43. Kanpolat Y., Akyar S., Caglar S. Diametral measurements of the upper spinal cord for Stereotactic pain procedures; experimental and clinical study. Surg Neurol. 1995;43:478-483.
44. Sanders M., Zuurmond W. Safety of unilateral and bilateral percutaneous cervical cordotomy in 80 terminally III cancer patients. J Clin Oncol. 1995;13:1509-1512.
45. Crul B.J.P., Blok L.M., Egmond J.V., et al. The present role of percutaneous cervical cordotomy for the treatment of cancer pain. J Headache Pain. 2005;6:24-29.
46. Kanpolat Y., Caglar Ugur H., Ayten M., et al. Computed tomography–guided percutaneous cordotomy for intractable pain in malignancy. Neurosurgery. 2009;64:187-194.
47. Kanpolat Y., Savas A., Caglar S., et al. Computerized tomography-guided percutaneous bilateral selective cordotomy. Neurosurg Focus. 1997;15(2):e4.
48. Raslan A. Percutaneous computed tomography-guided radiofrequency ablation of upper spinal cord pain pathways for cancer-related pain. Neurosurgery. 2008;62:226-234.
49. Ciccarelli O., Toosy A.T., Parker G.J., et al. Diffusion tractography based group mapping of major white-matter pathways in the human brain. Neuroimage. 2003;19:1545-1555.